Abstract
Steinert’s disease or Myotonic Dystrophy type 1 (DM1) is an autosomal dominant multisystemic disorder characterized by myotonia, muscle and facial weakness, cataracts, cognitive, endocrine and gastrointestinal involvement, and cardiac conduction abnormalities. Although mild myocardial dysfunction may be detected in this syndrome with age, overt myocardial dysfunction with heart failure is not frequent. Cardiac resynchronization therapy is an effective treatment to improve morbidity and reduce mortality in patients with DM1 showing intra-ventricular conduction delay and/or congestive heart failure. We report the case of a patient with Steinert disease showing an early onset ventricular dysfunction due to chronic right ventricular apical pacing, in which an epicardial left ventricular lead implantation was performed following the failure of the percutaneous attempt. As no relief in symptoms of heart failure, nor an improvement of left ventricular ejection fraction and reverse remodelling was observed six months later, the patient was addressed to the heart transplantation.
Key words: cardiac resynchronization, epicardial left ventricular implantation, Steinert disease
Introduction
Myotonic dystrophy type 1 (DM1) or Steinert’s disease,is the most common muscular dystrophy in adult life with an estimated prevalence of 1/8000. Cardiac involvement, including conduction abnormalities with arrhythmias and conduction disorders, contributes significantly to the morbidity and mortality of the disease. It is recorded in about 80% of cases, and may precede the involvement of skeletal muscles (1-3). The characteristic impairment of His-Purkinje system is the most common cardiac abnormality. Mild ventricular dysfunction has also been reported associated with conduction disorders, but severe ventricular systolic dysfunction is not frequent and usually occurs late in the course of the disease as the final stage of cardiomyopathy (1). Cardiac resynchronization therapy (CRT) is able to restore physiological pattern of ventricular depolarization, resulting in reduction of mitral regurgitation and improvement of left ventricular (LV) systolic function (4-6). CRT has demonstrated reduction in morbidity and mortality in patients with severe refractory heart failure (HF) and intraventricular conduction delay (4-6). The technique of choice for left ventricular pacing in ventricular resynchronization is the insertion of a lead through the coronary sinus, into the postero-lateral vein. The epicardial placement of ventricular leads is considered at present, a salvage technique for patients in whom the percutaneous procedure fails (7).
We report the case of a patient with Steinert disease showing an early onset ventricular dysfunction caused by chronic right ventricular apical pacing, in which an epicardial left ventricular lead implantation was performed following the failure of the standard percutaneous attempt. As no relief in symptoms of heart failure, nor an improvement of left ventricular ejection fraction and reverse remodelling was observed six month later, the patient has been addressed to the heart transplantation.
Case report
A 43-year-old man – affected by Steinert disease and regularly followed at Cardiomyology and Medical Genetics Service since the time of his diagnosis (2003) – was hospitalised for an exacerbation of signs and symptoms of congestive heart failure [fatigue, muscle weakness, dyspnea, ortophnea, edema and palpitations, New York Heart Association (NYHA) class III]. His blood pressure (BP) was 100/60 mmHg and heart rate (HR) 60/bpm. Crackles at the basal field of lungs and pretibial edema were detected. Chest X-ray revealed cardiac dilation and pulmonary congestion.
The diagnosis of DM1, at first based on the family history (one affected brother) and clinical features (myotonic phenomenon, mild distal skeletal muscle dysfunction, cataract, gastrointestinal disturbances, endocrine deficiency), was subsequently confirmed by molecular testing, that showed a pathological expansion of CTG triplets (E1 class). In 2005, a bicameral pacemaker (PM) was implanted because evidence of first degree (PR interval ≥ 255ms) plus second-degree type2 atrio-ventricular block (8-13), and concomitant paroxysmal atrial flutter (AF) episodes. The implant was made according to the current guidelines (14) and was followed by an improvement of symptoms and quality of life. To be noted that atrial arrhythmias are not rare in this population (15-17).
In 2013, the PM – according to the current guidelines (18) – was uploaded to a cardioverter defibrillator (ICD) due to the finding of not sustained ventricular tachycardia (NSVT) in pacemaker stored electrograms to prevent the high risk of sudden cardiac death, frequently observed in these patients as in others muscular dystrophies (19). The ICD was placed in the right position, because of the occlusion of left subclavian vein (20-22).
In 2016 during a routine clinical and instrumental follow-up, signs of congestive heart failure (CHF) were detected. The ECG showed a sinus rhythm and a wide QRS interval (165 ms) due to constant right ventricular apical pacing (Fig. 1). Transthoracic echocardiography showed dilation of the heart (left ventricular end-diastolic diameter – LVEDD – was 7.4 cm), left systolic dysfunction and overt intra- and inter-ventricular asynchrony. The ejection fraction (EF), calculated by the Simpson and Teichholz method, was 25% (Fig. 2).
Figure 1.

Pre-implant ECG showing sinus rhythm with wide QRS interval due to constant right ventricular apical pacing.
Figure 2.
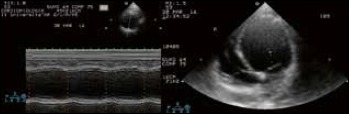
M-mode scan of the left ventricle derived from two-dimensional parasternal short axis view and apical view. A marked left ventricular dilation can be noted (end diastolic diameter = 74 mm). The interventricular septum is hypokinetic and motion of the left ventricular posterior wall is virtually absent.
The interrogation of ICD revealed absence of intrinsic spontaneous ventricular rhythm, not sustained paroxysmal episodes of atrial flutter/fibrillation and ventricular tachycardias and no episodes of malignant sustained ventricular arrhythmias requiring device intervention. According to the current guidelines (23), his medical therapy was adjusted and included aggressive loop diuretic therapy, β-blockers, spironolactone and ACE inhibitors. In order to rule out an ischemic aetiology of dilated cardiomyopathy and consequent heart failure, a diagnostic coronary angiography was performed showing normal coronary arteries. Despite the aggressive medical therapy the patient experienced two episodes of acute heart failure over one year period, posing the indication for cardiac resynchronization therapy by a biventricular ICD-CRT (24). Before the intervention, a right subclavian venogram was performed which revealed a long segment of occlusion; any attempt to recanalise the right subclavian vein percutaneously failed. Venous stenosis or occlusion due to thrombosis/fibrosis resulting from the presence of the lead is a frequent side-effect in patients implanted with devices. In these cases, an epicardial approach is planned.
Technical procedure
The procedure was performed after written informed consent. Left antero-lateral thoracotomy was performed along the fourth intercostal space under general anesthesia. The patient was placed in a 45° rotation to the right side. A 3- to 4- cm long left minithoracotomy was performed through the fourth intercostal space between the anterior and mid- axillary line.
The pericardium was opened longitudinally anterior to the phrenic nerve and suspended with traction sutures to better expose the lateral wall. The epicardial lead (bipolar) was fixed at the anterolateral wall of LV. Electrical parameters were measured to verify the correct positioning of the new leads. Once a site with satisfactory pacing threshold was identified (impedance > 200 Ω and < 2000 Ω, sensing > 5 mV and pacing threshold measured at 0.5 ms < 2.0 V), the lead was sewn with 5/0 polypropylene sutures. The connector of the lead was tunelled to the ICD-CRT device pocket in the right pre-pectoral region. The previous endocardial right ventricular defibrillation lead was connected to the ICD-CRT generator (Fig. 3). The patient was extubated in the operating room and observed in the cardiac surgery recovery unit for 24 hours.
Figure 3.

Chest X-ray postero-anterior (PA) view showing leads and ICD-CRT generator placement.
Patient’s follow-up
The post-operative follow up included the assessment of NYHA functional class, ECG with determination of QRS duration and echocardiography. Left ventricular ejection fraction, left ventricular end-diastolic dimension and severity of mitral regurgitation (MR) values were collected to analyse the effect of CRT via epicardial LV lead placement on reverse ventricular remodelling. One month later an optimization of the atrio-ventricular and inter-ventricular intervals during cardiac resynchronization was performed by both ECG and echocardiogram.
At six-months follow-up, no relief of symptoms was reported by the patient. In that occasion ECG revealed paced biventricular rhythm with a still wide QRS interval (150 ms, Fig. 4), though of reduced size compared to the previous one, and no changes in the repolarization dispersion time. Despite an adequate biventricular pacing the patient remains in NYHA class-III and experienced a further episode of acute heart failure requiring hospitalization. The echocardiogram didn’t show an improvement of EF and LV stroke volume (Fig. 5). The ICD analysis showed no significant modification of the electrical parameters, paroxysmal atipical atrial flutter/fibrillation and 98% biventricular pacing rhythm. The patient experienced one episode of slow sustained monomorphic ventricular tachycardia (140 bpm), recognized in monitoring zone of the device, which required external electrical cardioversion.
Figure 4.

Post-implant ECG showing sinus rhythm with QRS different morphology (compared to the pre-implant one), due to constant biventricular pacing.
Figure 5.

Echocardiographic findings after 6-month ICD-CRT implantation. Please note that both heart dimensions and ejection fraction are unchanged. A blood clot (thrombus) in the left apical ventricle can be observed (white arrow).
Discussion
Conduction abnormalities are the most frequent finding of cardiac involvement in patients with DM1 and minor conduction defects can be present in early stages of the disease (1, 2, 25-27). More severe conduction defects may be cause of shortness of breath, dizziness, fainting, syncope, and even of sudden death. Left ventricular dilatation with overt systolic dysfunction is not frequent; however when present they may be more prominent than the muscle complaint. Cardiac symptoms generally occur later compared to the skeletal muscle weakness, but sometimes they may be the initial manifestation of the disease (1, 2, 25). In patient here reported, the early onset of heart failure could be related to the electromechanical delay caused by both intra- and inter-ventricular asynchrony due to chronic right apical pacing; the latter leads to regional structural changes causing a uncoordinated heart contraction that in turn accelerates the progression of the heart failure (28). Beside advances in the optimal medical treatment, strategies for medically refractory symptomatic advanced heart failure have emerged, including cardiac resynchronization therapy. Patients with NYHA class III or IV, with EF 35% or less, sinus rhythm with a QRS duration ≥ 130 ms and left bundle branch block (LBBB) or a QRS duration ≥ 150 ms irrespective of the QRS morphology, are eligible to receive a cardiac resynchronization therapy, according to the current guidelines (23). Basing on the progression of LV dysfunction, AV conduction disturbances and the frequent occurrence of ventricular tachyarrhythmia, Said et al. (29) hypothesized a role for biventricular ICD in patients with DM1 who need a permanent pacemaker implantation. Two previous papers (7, 30) reported an improvement in symptoms of heart failure, LVEF and reverse remodelling in one patient with DM1 showing an early onset ventricular dysfunction secondary to a complete LBBB by this approach. However, a clear consensus about biventricular pacing or the usage of ICD does not exist for this kind of patients.
The “standard of care” of left lead implantation for CRT still remains the less invasive transvenous approach (31). However, several issues may result in failed transvenous implantation of the LV lead such as anatomical limitations due to occlusion of the subclavian vein or the superior vena cava, or an abnormal anatomy of the coronary sinus. Furthermore, lead-related issues such as lead instability with repeated dislodgement, phrenic nerve stimulation despite electrical or physical optimization, or systemic conditions such as endocarditis may contribute to failed transvenous LV lead implantation (31). In these cases, the surgical placement of an epicardial LV lead is required with satisfactory long-term results (32).
Conclusions
The case here reported is the first patient with DM1 in which an epicardial left ventricular lead implantation was used for cardiac resynchronization therapy after failure of percutaneous attempt. At six-months follow-up, based on this experience, the epicardial CRT did not induce either symptom relief, nor improvement of the ejection fraction or reduction of the arrhythmic risk. A possible explanation of the heart failure in this patient may be the prolonged apical pacing; further studies are in progress to determine the consequences of long-term constant apical pacing in patients affected by Myotonic Dystrophy type 1.
References
- 1.Nigro G, Comi LI, Politano L, et al. Cardiomyopathies associated with muscular dystrophies. Engel A, Franzini-Armstrong C, Eds. Myology. New York: Mac Graw-Hill; 2004, pp. 1239-56. [Google Scholar]
- 2.Dello Russo A, Mangiola F, Della Bella P, et al. Risk of arrhythmias in myotonic dystrophy: trial design of the RAMYD study. J Cardiovasc Med (Hagerstown) 2009;10:51-8. [DOI] [PubMed] [Google Scholar]
- 3.Cudia P, Bernasconi P, Chiodelli R, et al. Risk of arrhythmia in type I myotonic dystrophy: the role of clinical and genetic variables. J Neurol Neurosurg Psychiatry 2009;80:790-3. [DOI] [PubMed] [Google Scholar]
- 4.D’Andrea A, Salerno G, Scarafile R, et al. Right ventricular myocardial function in patients with either idiopathic or ischemic dilated cardiomyopathy without clinical sign of right heart failure: effects of cardiac resynchronization therapy. Pacing Clin Electrophysiol 2009;32:1017-29. [DOI] [PubMed] [Google Scholar]
- 5.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53. [DOI] [PubMed] [Google Scholar]
- 6.Russo V, Rago A, Papa A, et al. Cardiac resynchronization improves heart failure in one patient with myotonic dystrophy type 1. A case report. Acta Myol 2012;31:154-5. [PMC free article] [PubMed] [Google Scholar]
- 7.St John Sutton MG, Plappert T, Abraham WT; Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 2003;107:1985-90. [DOI] [PubMed] [Google Scholar]
- 8.Russo V, Rago A, Papa AA, et al. Does a high percentage of right ventricular pacing influence the incidence of paroxysmal atrial fibrillation in Myotonic Dystrophy type 1 patients? Kardiol Pol 2013;71:1147-53. [DOI] [PubMed] [Google Scholar]
- 9.Nigro G, Russo V, Politano L, et al. Does Bachmann’s bundle pacing prevent atrial fibrillation in Myotonic Dystrophy type 1 patients? A 12 months follow-up study. Europace 2010;12:1219-23. [DOI] [PubMed] [Google Scholar]
- 10.Russo V, Nigro G, Papa AA, et al. Far field R-wave sensing in Myotonic Dystrophy type 1: right atrial appendage versus Bachmann’s bundle region lead placement. Acta Myol 2014;33:94-9. [PMC free article] [PubMed] [Google Scholar]
- 11.Russo V, Rago A, Politano L, et al. The effect of atrial preference pacing on paroxysmal atrial fibrillation incidence in Myotonic Dystrophy type 1 patients: a prospective, randomized, single-bind cross over study. Europace 2012;14:486-9. [DOI] [PubMed] [Google Scholar]
- 12.Nigro G, Russo V, Rago A, et al. Right atrial preference pacing algorithm in the prevention of paroxysmal atrial fibrillation in Myotonic Dystrophy type 1 patients: a long term follow-up study. Acta Myol 2012;31:139-43. [PMC free article] [PubMed] [Google Scholar]
- 13.Russo V, Nigro G, Rago A, et al. Atrial fibrillation burden in Myotonic Dystrophy type 1 patients implanted with dual chamber pacemaker: the efficacy of the overdrive atrial algorithm at 2 year follow-up. Acta Myol 2013;32:142-7. [PMC free article] [PubMed] [Google Scholar]
- 14.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 Guideline update for implantation of cardiac pacemakers and antiarrhythmia devices – summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). J Am Coll Cardiol 2002;40:1703-19. [DOI] [PubMed] [Google Scholar]
- 15.Russo V, Nigro G, Meo F, et al. The effect of atrial preference pacing on atrial fibrillation electrophysiological substrate in Myotonic Dystrophy type 1 population. Acta Myol 2014;33:127-5. [PMC free article] [PubMed] [Google Scholar]
- 16.Russo V, Meo F, Rago A, et al. Paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: P wave duration and dispersion analysis. Eur Rev Med Pharmacol Sci 2015;19:1241-8. [PubMed] [Google Scholar]
- 17.Russo V, Rago A, Nigro G. Atrial electromechanical delay in Myotonic Dystrophy type 1 patients. Eur Rev Med Pharmacol Sci 2015;19:3991-2. [PubMed] [Google Scholar]
- 18.Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Europace 2013;15:1070-118. [DOI] [PubMed] [Google Scholar]
- 19.Russo V, Rago A, Nigro G. Sudden cardiac death in neuromuscolar disorders: time to establish shared protocols for cardiac pacing. Int J Cardiol 2016;207:284-5. [DOI] [PubMed] [Google Scholar]
- 20.Russo V, Nigro G, Papa AA, et al. Adenosine-induced sinus tachycardia in a patient with myotonic dystrophy type 1. Acta Myol 2014;33:104-6. [PMC free article] [PubMed] [Google Scholar]
- 21.Russo V, Rago A, Meo F, et al. Ventricular fibrillation induced by coagulating mode bipolar electrocautery during pacemaker implantation in myotonic Dystrophy type 1 patient. Acta Myol 2014;33:149-51. [PMC free article] [PubMed] [Google Scholar]
- 22.Russo V, Rago A, Meo F, et al. Atrial septal aneurysms and supraventricular arrhythmias: the role of atrial electromechanical delay. Echocardiography 2015;32:1504-14. [DOI] [PubMed] [Google Scholar]
- 23.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2129-200. [DOI] [PubMed] [Google Scholar]
- 24.Russo V, Rago A, D’Andrea A, et al. Early onset “electrical” heart failure in Myotonic Dystrophy type 1 patient: the role of ICD biventricular pacing. Anadolu Kardiyol Derg 2012;12:517-9. [DOI] [PubMed] [Google Scholar]
- 25.Nigro G, Papa AA, Politano L. The heart and cardiac pacing in Steinert disease. Acta Myol 2012;31:110-6. [PMC free article] [PubMed] [Google Scholar]
- 26.Nigro G, Russo V, Vergara P, et al. Optimal site for atrial lead implantation in myotonic dystrophy patients: the role of Bachmann’s Bundle stimulation. PACE 2008;31:1463-6. [DOI] [PubMed] [Google Scholar]
- 27.Nigro G, Russo V, Politano L, et al. Right atrial appendage versus Bachmann’s bundle stimulation: a two year comparative study of electrical parameters in myotonic dystrophy type 1 patients. PACE 2009;32:1192-7. [DOI] [PubMed] [Google Scholar]
- 28.Nigro G, Russo V, Chiara A, et al. Autonomic nervous system modulation before the onset of sustained atrioventricular nodal reentry tachycardia. Ann Noninvasive Electrocardiol 2010;15:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Said SAM, Baart JC, de Voogt WG. Pacing for conduction disturbances in Steinert’s disease: a new indication for biventricular ICD? Neth Heart J 2006;14:258-62. [PMC free article] [PubMed] [Google Scholar]
- 30.Kilic T, Vural A, Ural D, et al. Cardiac resynchronization therapy in a case of myotonic dystrophy (Steinert’s disease) and dilated cardiomyopathy. Pacing Clin Electrophysiol 2007;30:916-20. [DOI] [PubMed] [Google Scholar]
- 31.Valls-Bertault V, Mansourati J, Gilard M, et al. Adverse events with transvenous left ventricular pacing in patients with severe heart failure: early experience from a single centre. Europace 2001;3:60-3. [DOI] [PubMed] [Google Scholar]
- 32.Doll N, Piorkowski C, Czesla M, et al. Epicardial versus transvenous left ventricular lead placement in patients receiving cardiac resynchronization therapy: results from a randomized prospective study. Thorac Cardiovasc Surg 2008;56:256-61. [DOI] [PubMed] [Google Scholar]


