Abstract
Ganoderma lucidum has high commercial value because it produces many active compounds, such as ganoderic acids (GAs). Salicylic acid (SA) was previously reported to induce the biosynthesis of GA in G. lucidum. In this study, we found that SA induces GA biosynthesis by increasing ROS production, and further research found that NADPH oxidase-silenced strains exhibited a partial reduction in the response to SA, resulting in the induction of increased ROS production. Furthermore, the localization of ROS shows that mitochondria are sources of ROS production in response to SA treatment. An additional analysis focused on the relationship between SA-induced ROS production and mitochondrial functions, and the results showed that inhibitors of mitochondrial complexes I and II exert approximately 40–50% superimposed inhibitory effects on the respiration rate and H2O2 content when co-administered with SA. However, no obvious superimposed inhibition effects were observed in the sample co-treated with mitochondrial complex III inhibitor and SA, implying that the inhibitor of mitochondrial complex III and SA might act on the same site in mitochondria. Additional experiments revealed that complex III activity was decreased 51%, 62% and 75% after treatment with 100, 200, and 400 µM SA, respectively. Our results highlight the finding that SA inhibits mitochondrial complex III activity to increase ROS generation. In addition, inhibition of mitochondrial complex III caused ROS accumulation, which plays an essential role in SA-mediated GA biosynthesis in G. lucidum. This conclusion was also demonstrated in complex III-silenced strains. To the best of our knowledge, this study provides the first demonstration that SA inhibits complex III activity to increase the ROS levels and thereby regulate secondary metabolite biosynthesis.
Keywords: Salicylic acid, Reactive oxygen species, Mitochondrial complex III, Ganoderic acids
Highlights
-
•
Mitochondria as a source of salicylic acid (SA) induced reactive oxygen species (ROS) production in Ganoderma lucidum.
-
•
SA induces the accumulation of ganoderic acids in Ganoderma lucidum by mitochondria ROS overproduction.
-
•
SA inhibits mitochondrial complex III activity to increase ROS and thereby induces ganoderic acids biosynthesis.
1. Introduction
Ganoderma lucidum (G. lucidum) is a well-known medicinal basidiomycete widely used in Southeast Asia, because it containing a wide range of immuno-modulatory and bioactive compounds [14], [71]. The triterpenes and polysaccharides isolated from G. lucidum have numerous biological activities, including anticancer, antioxidant, and hypocholesterolemic effects as well as inhibitory effects on adipocyte differentiation [59], [65]. Ganoderic acids (GAs) are some of the major secondary metabolites found in G. lucidum, and these compounds belong to the class of triterpenoids [50]. Because of their significant biological activity, the potential of GAs as medicinal agents has been proposed [35], [36]. In addition, researches on the GAs also provide a valuable foundation for studying the secondary metabolic processes in fungi. Therefore, many studies have investigated the different regulatory effects of various fermentation conditions on the biosynthesis of GAs. For example, limiting the nitrogen sources in liquid culture leads to an increase in the GA content [77], aspirin treatment is a powerful approach for triggering GA production [75], and acetic acid acts as an inducer to significantly increase GA biosynthesis [54]. Based on these findings, it can be concluded that many factors can influence GA biosynthesis, but defects in basic biology studies have hindered further increases in the commercial value of G. lucidum. In recent years, the genomic sequence and genetic transformation system of G. lucidum have been established [45], [63], [7]. These discoveries provide opportunities for the development of basic biological tools for investigating G. lucidum, and the resulting developments have promoted the use of G. lucidum as a potential model system for studying the complicated mechanisms of secondary metabolic pathways in higher basidiomycetes. Therefore, research on the regulatory pathways of secondary metabolism in G. lucidum has become more important.
Salicylic acid (SA), which is considered a plant hormone, shows a broad distribution in plants [28], plays a key role in the regulation of plant growth and development, and is involved in disease resistance in plants in response to various pathogenic attacks [11], [31], [56]. In addition, SA has recently been the focus of intensive research efforts due to its function as a signaling molecule during the plant responses to abiotic stresses, such as heavy metal, salinity, drought and temperature stresses [26], [32], [69], and a few studies have investigated its role in enhancing the production of secondary metabolites in plants. In Salvia miltiorrhiza cells, SA treatment exerts an obvious effect on the accumulation of phenolic compounds [13], and in Ulmus minor, SA treatment induces the accumulation of sinapyl alcohol and enhances resistance to pathogens [40]. In addition, SA induces salvianolic acid B production in Salvia miltiorrhiza [21] and increases volatile oil biosynthesis in Atractylodes lancea plantlets [70]. However, the function of SA in microorganisms is still not well understood. In G. lucidum, it has been reported that SA treatment can enhance GA accumulation [5], and this interesting phenomenon indicates that plant hormones can induce the biosynthesis of secondary metabolites in fungi. However, the mechanism through which SA regulates the secondary metabolism of G. lucidum remains unclear.
To investigate the signaling events induced by SA that result in GA accumulation, the ROS level under SA treatment was analyzed, and our results showed that GA accumulation was observed due to an SA-induced burst of ROS. Additional experiments found that the source of ROS overproduction induced by SA was not only dependent NADPH oxidase (NOX) but also included the mitochondria. To determine the effect of SA treatment on the mitochondria, the ROS levels and respiratory rate after co-treatment with various inhibitors of the mitochondria complex and SA were measured, and the data showed that mitochondria complex III is involved in SA treatment-induced ROS generation.
2. Materials and methods
2.1. Materials and growth conditions
G. lucidum strain ACCC53264 (obtained from the Agricultural Culture Collection of China) was used as the wild-type (WT) strain and grown at 28 °C in potato dextrose agar medium for 7 days. Seed cultures were grown in potato dextrose broth (PDB) medium and placed on a rotary shaker incubator at 150 rpm and 28 °C for 7 days. The fermentation experiments were performed at 28 °C in CYM (1% w/v maltose, 2% w/v glucose, 0.2% yeast extract, 0.2% tryptone, 0.05% MgSO4·7H2O, and 0.46% KH2PO4, with an initial pH of 5.5) for 7 days after inoculation with 4% (v/v) seed culture. NOX-silenced strains were also established as previously described [46].
2.2. Extraction and quantification of SA
SAs were extracted from the fungal mycelia using a previously described method [53], [73]. The G. lucidum strain was grown as described above in CYM for 7 days with or without SA (Sigma, USA), and the mycelia were then frozen in liquid nitrogen for extraction of endogenous free salicylic acid. The levels of free SA were quantified by HPLC based on a previously described method [72]. All the data were corrected based on an internal salicylic acid standard, and the free SA was measured.
2.3. Detection and quantification of GA and intermediates
The total ganoderic acids (GA) and cellular squalene and lanosterol were extracted from fungal mycelia and measured according to a previously described method [62]. To detect GAs and its mesostates under SA treatment, the mycelia were treated with 100 μM SA dissolved in ethanol for 0.5 h according to a previously described method [5]. In the pretreatment experiments, 7-day-old G. lucidum strains were pretreated with ascorbic acid (VC, 2 mM), N-acetyl cysteine (NAC, 1 mM), diphenyleneiodonium chloride (DPI, 50 μM), rotenone (Rot, 5 μM), 4,4,4-trifluoro-1-(2-thienyl)− 1,3-butanedione (TTFA, 10 μM) or antimycin A (AA, 5 μM) for 2 h prior to treatment with 100 μM SA.
2.4. ROS detection assay
The production of ROS was assessed according to a previously described method [46] with slight modifications. For fluorescent detection of the ROS, the mycelia were stained with 2′, 7′-dichlorodihydrofluorescein diacetate (DCHF-DA) for 20 min, the fluorescence was detected using a Zeiss Axio Imager A1 fluorescence microscope, and the average fluorescence intensities of DCFH-DA in the mycelia were analyzed using ZEN lite software (Zeiss). The H2O2 content of the hyphae liquid was measured by monitoring the A415 of the titanium-peroxide complex according to the method described by [3].
2.5. Detection of mitochondrial ROS production
The mitochondrial ROS production was measured using samples that were double-stained with DCFH-DA and Mito-Tracker Red CMXRos, as described by [74]. The fluorescence was detected using a Zeiss Axio Imager A1 fluorescence microscope, and the average fluorescence intensities were analyzed using ZEN lite software (Zeiss).
2.6. Isolation of G. lucidum mitochondria and measurement of the respiratory rate
The mitochondria were isolated as previously described [15], [20], with some modifications. All the steps were performed at 4 °C. The mycelia were frozen and powdered under liquid nitrogen with a mortar and pestle and suspended in a three-fold volume of ice-cold extraction buffer containing 250 mM sucrose, 1 mM EDTA, 0.5% (w/v) polyvinylpyrrolidone-40, 10 mM β-mercaptoethanol, and 50 mM Tris-HCl (pH 7.2). The mixture was homogenized extensively for 30 min on ice, and the homogenate was then centrifuged for 15 min at 1200×g. The supernatants were decanted and centrifuged for 20 min at 17,000×g. The pellets were resuspended and washed twice with wash buffer (250 mM sucrose and 50 mM Tris-HCl, pH 7.2). The mitochondria were finally resuspended in a small volume of wash medium.
The oxygen consumption was measured as described previously [48], [8], with some modifications. The oxygen consumed by isolated mitochondria was measured at 25 °C with a Clark-type oxygen electrode (Hansatech Ltd., UK) in 1 mL of reaction medium (0.25 M sucrose, 10 mM KCl, 5 mM EDTA, 20 mM HEPES/Tris pH 7.2, and 0.15% (w/v) bovine serum albumin). The mitochondrial oxygen consumption was measured with 5 mM malate plus 10 mM glutamate (complex I substrate) or 10 mM succinate (complex II substrate). In the experiments requiring the addition of different concentrations of SA, 5 μM Rot, 10 μM TTFA or 5 μM AA was added to the pre-treated samples.
2.7. Assays of the activities of respiratory chain complexes I, II and III
The enzyme activity assays were performed using the Tissue Mitochondrial Complex I, II and III Assay Kit (Comin Biotechnology, China) according to the instructions provided in the kit's manual. After 5 min of incubation in assay buffer at 25 °C, the Complex I activity was measured based on the decrease in the oxidation of NADH to NAD+, which was assessed by the absorbance at 340 nm. The Complex II activity was measured indirectly by monitoring the reduction of 2,6-dichloroindophenol based on the absorbance at 605 nm. Under catalysis by cytochrome reductase (complex III), the reduced ubiquinone (50 μM decylubiquinol) or coenzyme Q substrates were converted into ubiquinone or coenzyme Q, and ferricytochrome c (ferricyt c3+) was reduced to ferrous cytochrome c (ferrocyt c2+). After 5 min of incubation at 25 °C, the absorbance at 550 nm was obtained to assess the reduction of ferricytochrome c and thus obtain a measure of the activity of the enzyme.
2.8. Construction of RNAi plasmids and strains
The construction of a fungal RNA interference (RNAi)-silencing vector, pAN7-dual, was performed as previously described [45]. The glyceraldehyde-3-phosphate dehydrogenase (gpd) promoter and 35 S promoter were used to suppress the expression of the complex I assembly factor NDUFAF1 (CIA30) [43], the Succinate Dehydrogenase Subunit b (sdhB of complex II) [68] and the ubiquinone binding protein of complex III [66]. The coding regions of the genes were amplified by PCR using G. lucidum cDNA as the template and the primers listed in Table S1 in the Supplemental Material. The RNAi-silencing vectors pAN7-dual-Com1i, pAN7-dual-Com2i and pAN7-dual-Com3i were transferred into G. lucidum by electroporation. Dozens of transformants were selected randomly, and qRT-PCR analyses were performed to determine the silencing efficiency of the transformations. Two independent silencing strains with the highest silencing efficiency were selected for further study.
2.9. Real-time PCR analysis of gene expression
The levels of gene-specific mRNA expressed by the WT and isolated RNAi-transformant strains were assessed by quantitative real-time PCR, in accordance to our previous study [46]. The gene expression levels were determined by calculating the difference between the threshold cycle (CT) value of the analyzed gene and the CT value of the 18 S rRNA housekeeping gene. Quantitative real-time reverse transcriptase PCR (qRT-PCR) calculations analyzing the relative gene expression levels were performed according to the 2—△△CT method described by [37]. The gene fragments were amplified by real-time PCR using the primers shown in Table S1 in the Supplemental Material.
2.10. Statistical analyses
All the experimental data shown in this manuscript were collected from three independent samples to ensure the reproducibility of the trends and relationships observed in the cultures. Each error bar indicates the standard deviation (SD) from the mean obtained from triplicate samples. The sample means were analyzed with Student's t-test. Differences in mean values between groups were analyzed by a one-way analysis of variance (ANOVA) followed by Duncan's multiple range test.
3. Results
3.1. ROS production induced by salicylic acid in Ganoderma lucidum
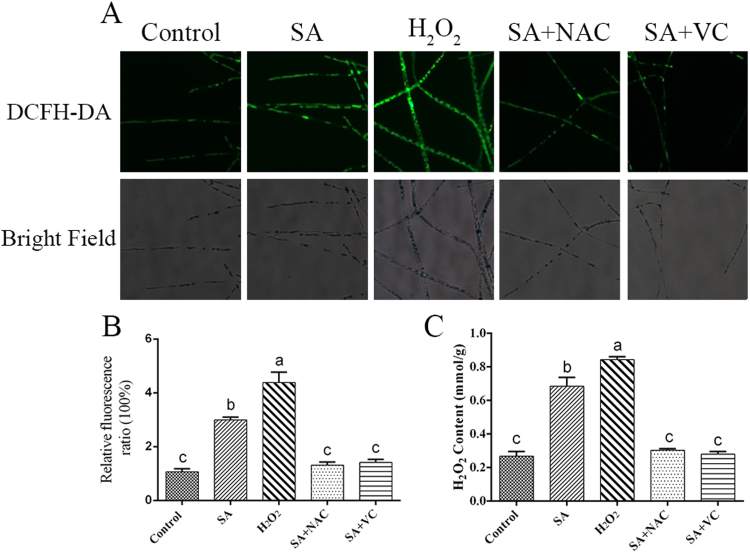
In our previous study, we found that SA can increase GA biosynthesis in G. lucidum [5], and the investigation of ROS signaling was related to GA biosynthesis in G. lucidum [33], [46]. Thus, ROS production in G. lucidum after treatment with 100 μM SA was analyzed by staining with a fluorescent probe (2′,7′-dichlorofluorescin diacetate, DCFH-DA). The fluorescence analysis showed that 100 μM SA induced ROS generation, and the ROS levels obtained after treatment with 100 μM SA were approximately 3-fold higher than those of the control. The SA-induced ROS accumulation was abolished by pre-treatment with the ROS scavengers N-acetyl-L-cysteine (NAC) and ascorbic acid (VC). The ROS content in the H2O2-treated sample, which was used as a positive control, was rapidly increased by approximately 4.3-fold compared with the control sample (Figs. 1A and 1B).
Fig. 1.
The ROS levels are increased in SA-treated strains. A. Change in ROS level detected by DCFH-DA staining in WT cells co-treated with 100 μM SA and ROS scavengers. B. Changes in the ROS fluorescence ratio in strains co-treated with ROS scavengers and 100 μM SA. C. H2O2 content after co-treatment with a ROS scavenger and 100 μM SA. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
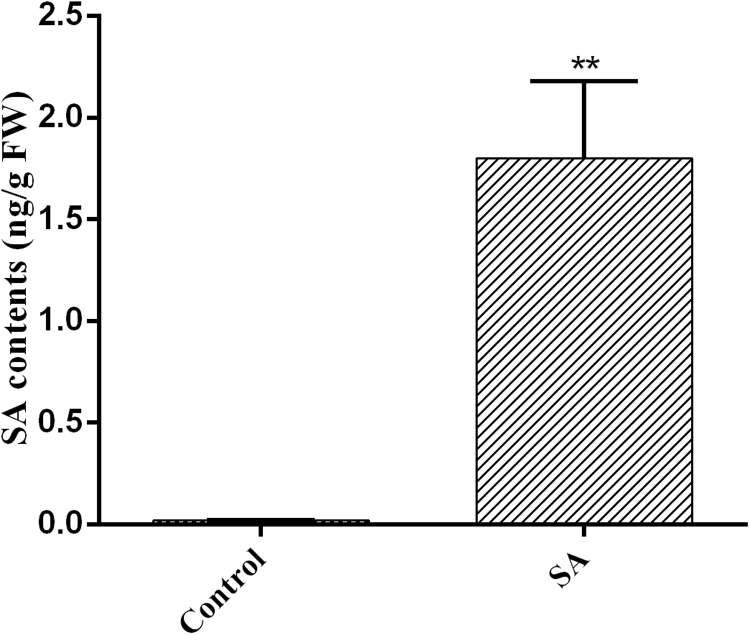
The intracellular H2O2 content was also quantitatively detected in the SA-treated samples, and the changes in the H2O2 content and the fluorescent observations showed a similar trend. The H2O2 content of the SA-treated sample was approximately 2.7-fold higher than that of the control, whereas exogenous NAC and VC pretreatment almost completely attenuated this effect. A sample treated with exogenous 10 mM H2O2, which was used as a positive control, showed approximately 3.2-fold increased levels of H2O2 compared with the control (Fig. 1C). The endogenous free SA level was also tested, and the free SA level in the SA-treated sample was increased by approximately 80-fold compared with that of the control sample (Fig. S1). Together, these results support the hypothesis that SA can increase the ROS levels in G. lucidum.
3.2. SA-regulated GA biosynthesis is dependent on ROS accumulation
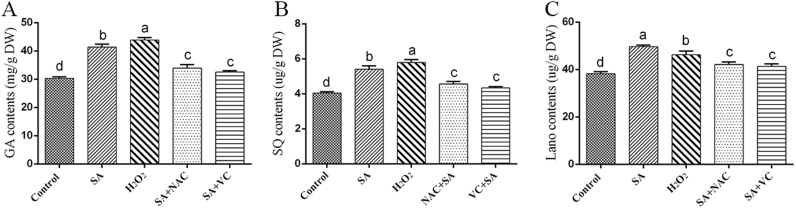
To clarify the role of ROS in SA-induced GA biosynthesis, the effect of NAC and VC pretreatment on the GA content induced by treatment with 100 μM SA was assessed. A sample treated with exogenous 10 mM H2O2 was used as the positive control, and the GA content in this sample was significantly increased by 36% after treatment with 100 μM SA. Pretreatment with NAC and VC reduced the SA-induced GA levels by approximately 28% and 31%, respectively, and the GA level obtained after treatment with 10 mM H2O2 was increased by 45% compared with that of the control (Fig. 2A). In addition, to assess the change in GAs from different perspectives, two important intermediate products, squalene (SQ) and lanosterol (Lano), were also analyzed. The changes in SQ and Lano showed a consistent trend with the changes in the GA level. Specifically, the SQ and Lano levels were significantly increased by approximately 33% and 29% in the SA-treated sample, respectively, compared with those in the control. In addition, the SQ and Lano contents of the sample co-treated with NAC and SA showed significant decreases of approximately 23% and 20%, respectively, compared with those of the SA-treated sample, and the SQ and Lano levels in the sample co-treated with VC and SA were approximately 24% and 22% lower than those of the SA-treated sample, respectively. The SQ and Lano contents were approximately 43% and 20% higher in the H2O2-treated sample relative to the control (Figs. 2B and 2C). These above-mentioned results demonstrated that the ROS overproduction induced by SA can improve GA biosynthesis in G. lucidum.
Fig. 2.
Effect of ROS scavengers on the GA biosynthesis in SA treatment strains. A. Total GA levels in WT cells co-treated with ROS scavengers and SA. B. SQ levels in WT cells co-treated with ROS scavengers and SA. C. Lano levels in WT cells co-treated with ROS scavengers and SA. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
3.3. NADPH oxidase silencing partially reduced the SA-induced increases in the ROS and GA contents
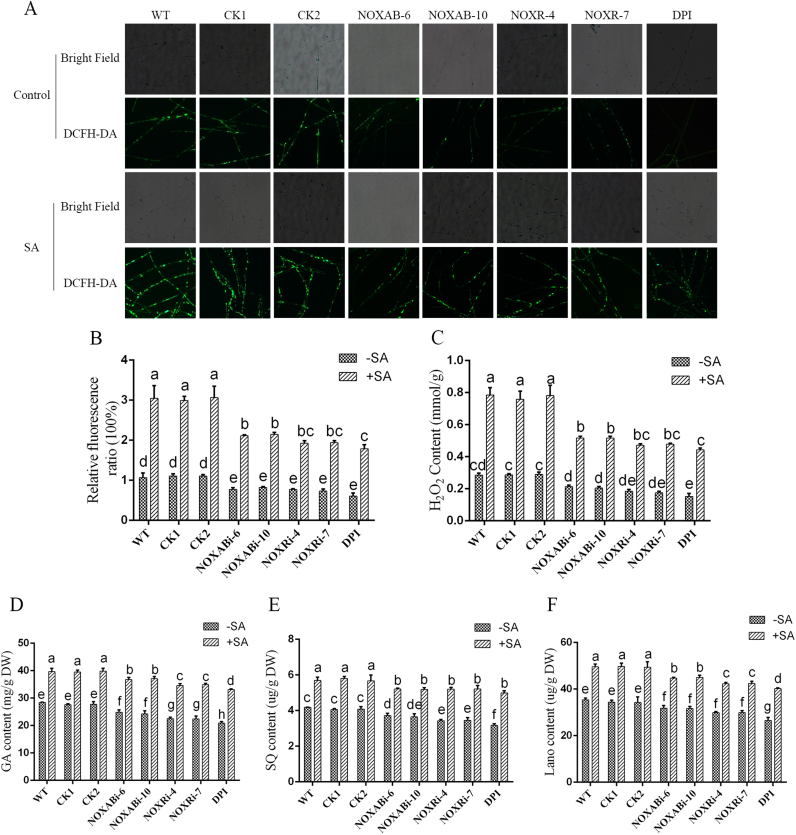
NADPH oxidase (NOX) is part of an enzyme system that catalyzes the generation of ROS [64] and was thus used in this study to analyze the sources of ROS overproduction induced by SA. Specifically, we used NOX-silenced strains (NOXABi-6, NOXABi-10 and NOXRi-4, and NOXRi-7) constructed in our laboratory [46], empty vector control strains (Si-control-1 and Si-control-2) and the wild-type strain treated with the NOX inhibitor DPI to examine the effect of 100 μM SA on the ROS content, and DCFH-DA fluorescent staining revealed that the NOXABi-6 and NOXABi-10 strains showed approximately 30% decreases in the ROS level compared with the wild-type and Si-control strains under SA treatment. The ROS levels of NOXRi-4 and NOXRi-7 were also approximately 35% lower than those of the wild-type and Si-control samples under SA treatment (Figs. 3A and 3B). Under SA treatment, the DPI-treated wild-type strain exhibited 30% lower ROS accumulation compared with the WT sample, as demonstrated through DCFH-DA detection (Figs. 3A and 3B). In addition, the H2O2 content in the WT and NOX-silenced strains after SA treatment was also quantitatively detected, and the results indicated that the H2O2 contents in the NOXABi and NOXRi strains were approximately 33% and 37% lower than those in the WT strain, respectively. Moreover, in the DPI-treated WT strain, the H2O2 content was 41% lower than that in the WT strain after treatment with both DPI and SA (Fig. 3C). The ROS and H2O2 contents in the DPI-treated WT strain followed the same trends observed in the NOX-silenced strains under SA treatment. The NOX-silenced and the DPI-treated wild-type strains showed SA-induced ROS and H2O2 levels that were significantly higher than those of the untreated wild-type sample. The results showed that inhibition of the function of NOX partly reduced the SA-induced increase in ROS. These findings suggested that NOX is involved in ROS production induced by SA in G. lucidum.
Fig. 3.
NOX inhibitor treatments and NOX silencing partially reduce the SA-induced increase in the ROS and GA contents. A. Change in ROS level detected by DCFH-DA staining in NOX inhibitor-treated WT strains and NOX-silenced strains under 100 μM SA treatment. B. Changes in the ROS fluorescence ratio in NOX inhibitor-treated WT strains and NOX-silenced strains under 100 μM SA treatment. C. H2O2 content in NOX inhibitor-treated WT strains and NOX-silenced strains under 100 μM SA treatment. D. Total GA levels in NOX inhibitor-treated WT strains and NOX-silenced strains under 100 μM SA treatment. E. SQ levels in NOX inhibitor-treated WT cells and NOX-silenced strains under 100 μM SA treatment. F. Levels of Lano in NOX inhibitor-treated WT strains and NOX-silenced strains under 100 μM SA treatment. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
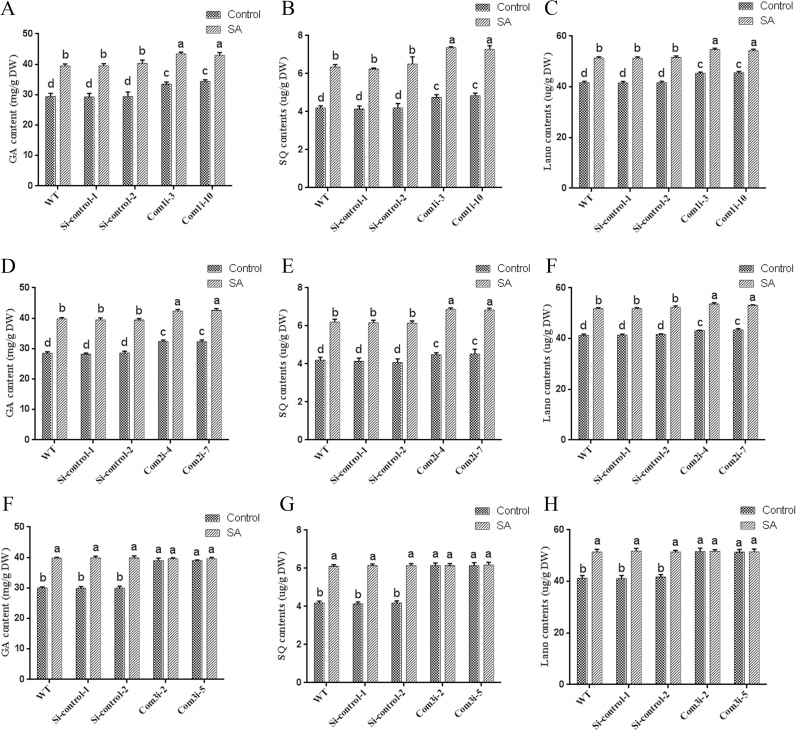
To examine the role of ROS generation by NOX in SA-induced GA biosynthesis, the GA contents of the wild-type and NOX-silenced strains after treatment with 100 μM SA were determined. As shown in Fig. 3D, under SA treatment, the GA level exhibited approximately 13% and 16% decreases in the NOXABi and NOXRi strains, respectively, compared with the WT sample. GA accumulation was stimulated by SA treatment, and approximately 19% of the observed inhibition of GA accumulation in the tested strains was recovered by DPI treatment. The contents of the intermediate metabolites SQ and Lano were also detected, and under SA treatment, the SQ contents of the NOXABi and NOXRi strains were approximately 11% and 13% lower than those observed in the WT sample. Similarly, the Lano levels showed decreases of 15% and 20% in the NOXABi and NOXRi strains, respectively, compared with the WT strain. Under SA treatment, the SQ and Lano levels in the DPI-treated WT strain were 20% and 24% lower than those in the WT strain, respectively. The SA-induced GA levels in the NOX-silenced and the DPI-treated wild-type strains were significantly higher than those of the untreated wild-type sample. Above all, these findings showed that the NOXi strains and DPI treatment can partly reduce the SA treatment-induced increase in the GA content. The results also verify that ROS produced by the NOX system participated in the SA-induced GA increase in G. lucidum and that the SA-mediated induction of ROS production might have other sources.
3.4. Subcellular localization of ROS accumulation induced by SA
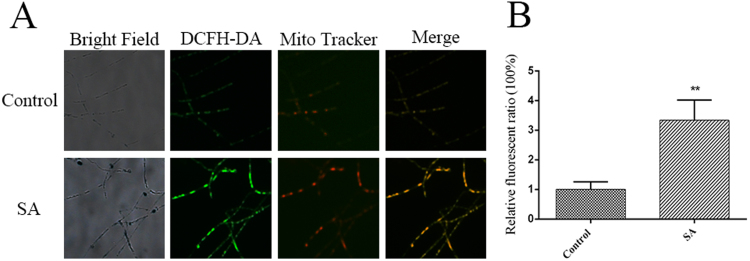
The mitochondria has been reported to be one of the primary sources of cellular ROS [64], and this finding provides a better understanding of the source of ROS overproduction induced by SA. Thus, the intracellular ROS localization was monitored by double-staining with the mitochondria-specific marker MitoTracker Red CMXRos and the ROS fluorescent probe DCFH-DA [18], [23]. As shown in Fig. 4A, under 100 μM SA treatment, the mycelium showed a strong increase in fluorescence overlap in the mitochondria and cytoplasm. An approximately 3-fold increase in mitochondrial ROS production was detected in the SA-treated sample compared with the control sample (Fig. 4B). These results showed that exogenous SA induced mitochondrial ROS generation and identified the mitochondria as sources of SA-induced ROS production in G. lucidum.
Fig. 4.
Subcellular localization of SA-induced ROS production. A. ROS production and localization in WT cells treated or not treated with SA were detected by double-staining with DCFH-DA and MitoTracker Red CMXRos. B. Levels of mitochondrial ROS in the WT strains treated or not treated with SA. The values are the means ± SDs of three independent experiments, and asterisks indicate significant differences compared with untreated strains (Student's t-test: **P < 0.01).
3.5. Effects of mitochondrial inhibitors on the respiration ratio and ROS under SA treatment
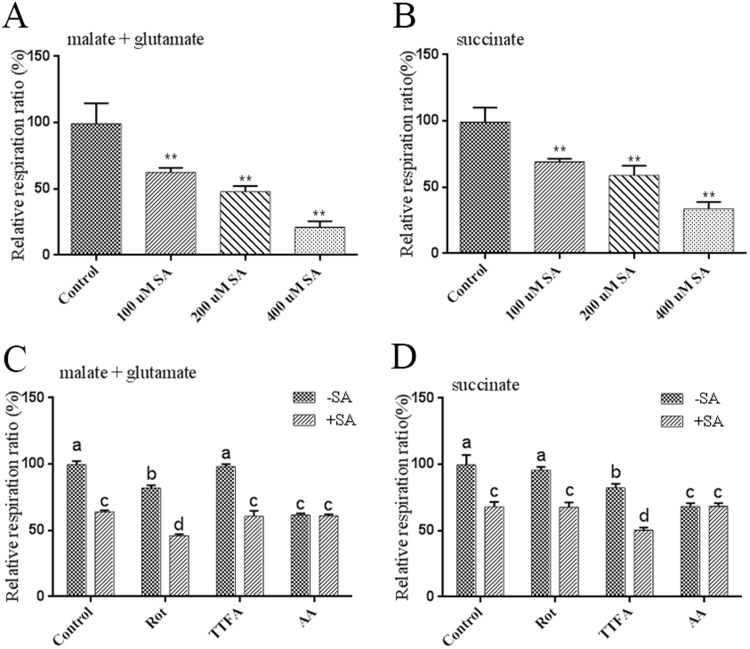
ROS are products of electron transport in the mitochondrial respiratory chain, and in this study, mitochondrial inhibitors were used to assess whether the SA-induced production of ROS is associated with respiratory functions. The respiratory rates were analyzed by measuring oxygen consumption in isolated mitochondria. The Clark-type oxygen electrode was used to determine the relative respiratory rate of mitochondria under different treatments in the presence of complex I (malate/glutamate) or II (succinate) substrates, which feed electrons into the electron transport chain at the complex I level via NADH or directly to complex II, respectively. As shown in Figs. 5A and 5B, using both malate/glutamate and succinate as the substrates, the exogenous application of SA gradually decreased the respiratory rate in a concentration-dependent manner. In the presence of malate/glutamate, the respiratory rates after treatment with 100 µM, 200 µM and 400 µM SA were decreased by 40%, 55% and 80%, respectively, compared with the control (Fig. 5A). In the presence of succinate, the respiratory rates obtained after treatment with 100 µM, 200 µM and 400 µM SA were 35%, 41% and 69% lower than that of the control, respectively (Fig. 5B). The data suggest that SA treatment could decrease the mitochondrial respiration rate in G. lucidum.
Fig. 5.
Effects of different mitochondrial inhibitors and different SA concentrations on the respiration rate in G. lucidum. A. Respiratory rate of isolated mitochondria in response to different SA concentrations in the presence of 5 mM malate plus 10 mM glutamate as the respiratory substrates. B. Respiratory rate of isolated mitochondria in response to different concentrations of SA in the presence of 10 mM succinate as the respiratory substrate. C. Respiratory rate of isolated mitochondria in response to treatment with different mitochondrial inhibitors and 100 μM SA in the presence of 5 mM malate plus 10 mM glutamate as the respiratory substrates. D. Respiratory rate of isolated mitochondria in response to treatment with different mitochondrial inhibitors and 100 μM SA in the presence of 10 mM succinate as the respiratory substrate. The values are the means ± SDs of three independent experiments, and asterisks indicate significant differences compared with untreated strains (Student's t-test: **P < 0.01). Different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
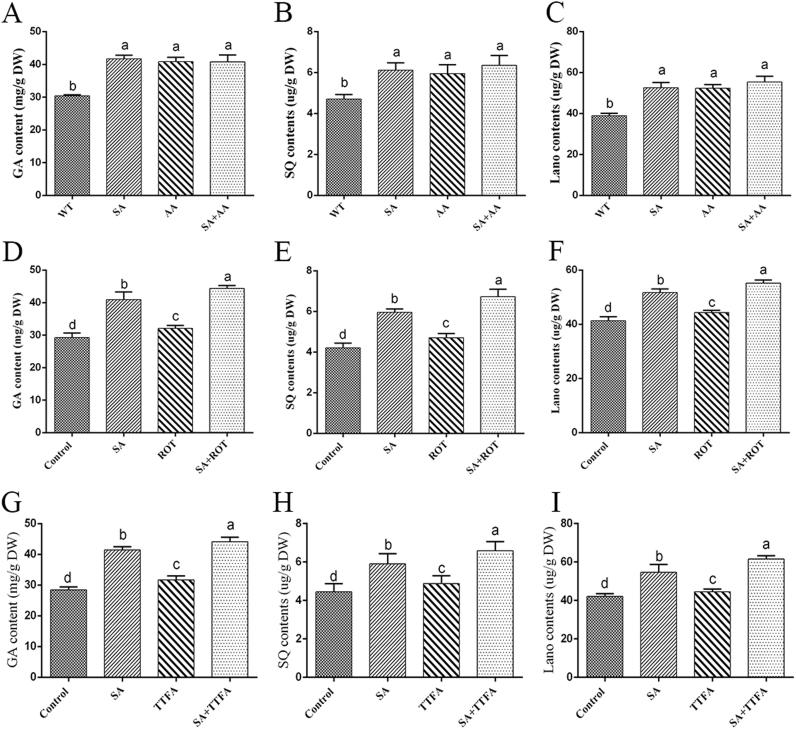
The mitochondrial complexes with important roles involving electron transmission in the mitochondrial respiratory chain can generate oxygen radicals [1], [6]. To determine the mechanism responsible for the observed inhibition of the respiration rate under SA treatment in G. lucidum, the respiration rates of samples pretreated with rotenone (ROT, inhibitor of complex I), thenoyltrifluoroacetone (TTFA, inhibitor of complex II), or antimycin A (AA, inhibitor of complex III) and then treated or not treated with 100 μM SA were measured. The results showed that 5 μM Rot decreased the mitochondrial respiration rate in the presence of malate/glutamate, but this decrease was lower than that observed with 5 μM AA. The sample treated with 10 μM TTFA exhibited no significant difference relative to the control when malate/glutamate was provided as the substrate (Fig. 5C). Furthermore, the sample co-treated with Rot and SA exhibited a lower respiration rate than the Rot-treated sample. A significant decrease in the respiration rate was observed in the sample co-treated with TTFA and SA compared with the TTFA-treated sample. However, no significant difference in the respiration rate was obtained between the sample co-treated with AA and SA and the AA- or SA-treated samples (Fig. 5C). Similar trends in the respiratory rate were obtained when succinate was provided as the substrate in the sample co-treated with AA and SA and the AA- or SA-treated samples (Fig. 5D). The results showed that the superimposed inhibitory effects on the respiration rate observed in samples co-treated with SA and either Rot or TTFA were greater than those of the individual treatments. This finding was likely obtained because Rot/TTFA and SA have different intracellular sites of action in the mitochondria, yielding different results in terms of the respiration rate. However, the samples co-treated with AA and SA produced no obvious superimposed inhibitory effects on the respiration rate relative to those subjected to a single treatment, which indicates that AA and SA might act on the same site in the mitochondria. These data suggest that complex III might be involved in SA-induced ROS generation.
3.6. Changes in mitochondrial respiratory complex activity under SA treatment
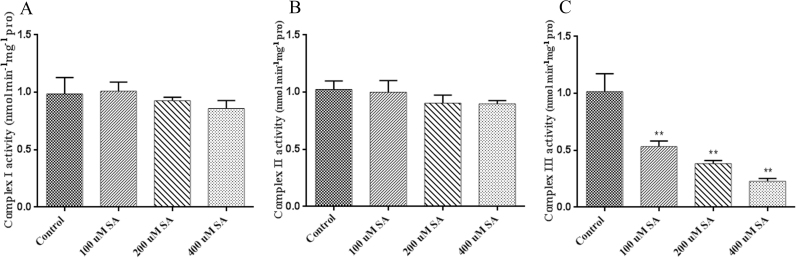
To determine whether SA can influence the function of the mitochondrial complex, we examined the effect of different SA concentrations on complex I, II and III activities in isolated mitochondria. Treatment with different concentrations of SA showed no obvious change on the activities of complex I and II (Figs. 6A and 6B), whereas complex III activity decreased with increases in the SA concentration. Specifically, compared with the control, the activity of complex III in the samples treated with 100 µM, 200 µM and 400 µM SA was significantly decreased by 51%, 62%, and 75%, respectively (Fig. 6C). These data suggested that SA treatment can inhibit the activity of complex III and influence the function of complex III in G. lucidum.
Fig. 6.
Changes in mitochondrial respiratory complex activity under SA treatment. A-C. Effects of different SA concentrations on complex I, II and III activity. The values are the means ± SDs of three independent experiments, and asterisks indicate significant differences compared with untreated strains (Student's t-test: **P < 0.01).
3.7. Analysis of the effects of SA on the respiration rate in WT and complex-silenced strains
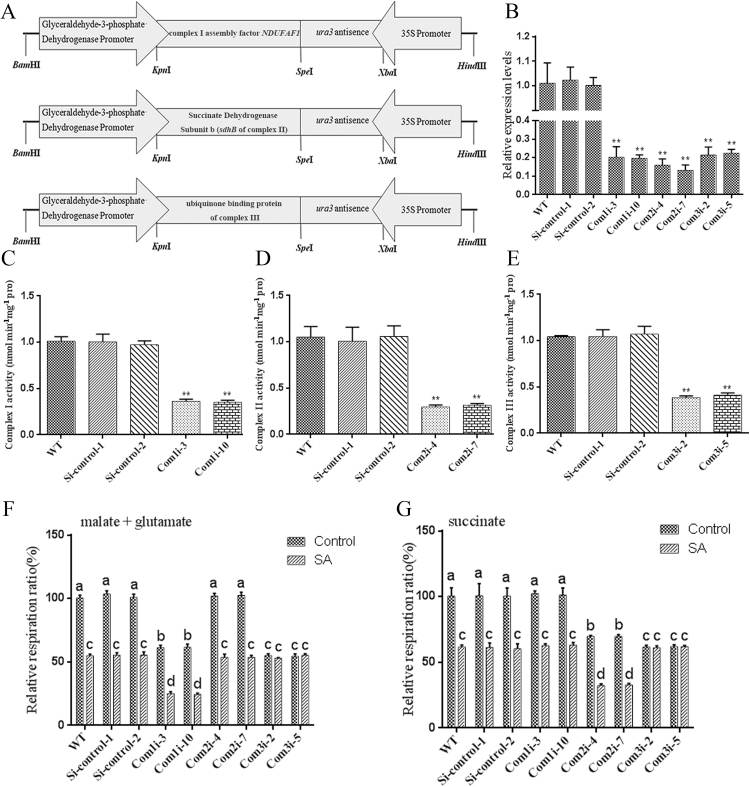
To validate the role of complex III under SA treatment, we constructed complex I assembly factor NDUFAF1 (CIA30) [43], the Succinate Dehydrogenase Subunit b (sdhB of complex II) [68] and the ubiquinone binding protein of complex III [66] gene-silenced strains by RNA interference using the RNAi expression cassette plasmids pAN7-dual-Com1i, pAN7-dual-Com2i, and pAN7-dual-Com3i (Fig. S3A), respectively, which carry the hygromycin B resistance gene as a selectable marker. The pAN7-ura3-dual vector was used as a Si-control, and qRT-PCR analyses were performed to determine the silencing efficiency of the transformation. The Com1i-3, Com1i-10, Com2i-4, Com2i-7, Com3i-2, and Com3i-5 strains were selected for further analyses (Fig. S3B), and their enzyme activities were detected. The complex I activity of the Com1i-3 and Com1i-10 strains were significantly reduced by approximately 65% compared with that of the WT strain (Fig. S3C), and the Com2i-4 and Com2i-7 strains showed a significant decrease of approximately 68% in complex II activity compared with the WT strain (Fig. S3D). In addition, complex III activity was significantly reduced by approximately 60% in the Com3i-2 and Com3i-5 strains, respectively, compared with the WT strain (Fig. S3E).
To detect the effect of SA on the mitochondrial complex, the respiratory rates of the Com1i-3, Com1i-10, Com2i-4, Com2i-7, Com3i-2, and Com3i-5 strains under 100 μM SA treatment were measured. In the presence of malate/glutamate, the respiration rates of the Com1i and Com3i strains were significantly decreased by approximately 38% and 44%, respectively, compared with the WT and Si-control strains, whereas the respiration rate showed no significant difference between the Com2i and WT strains when malate/glutamate was provided as the substrate (Fig. S3F). In the presence of succinate, the respiration rates of the Com2i and Com3i were significantly decreased by approximately 31% and 39%, respectively, compared with the WT and Si-control strains, and no significant difference in the respiration rate was found between the Com1i and WT strains (Fig. S3G). Obvious superimposed inhibitory effects on the respiration rate were observed in the Com1i and Com2i strains after SA treatment when malate/glutamate or succinate was provided as a substrate. Moreover, regardless of the substrate used, i.e., malate/glutamate or succinate, the respiration rate of the SA-treated Com3i strain was not significantly different from that of the untreated Com3i strains (Figs. S3F and S3G). The results showed that the effects of SA on the respiration rate might involve complex III.
3.8. ROS levels in AA (mitochondrial complex III inhibitor)-treated WT strain and complex-silenced strains under SA treatment
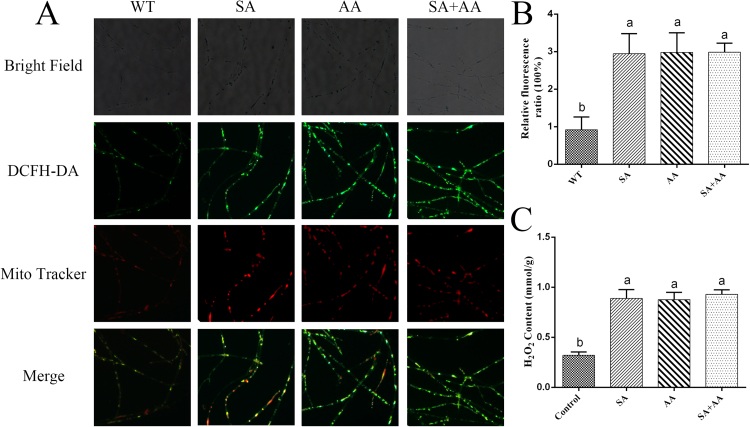
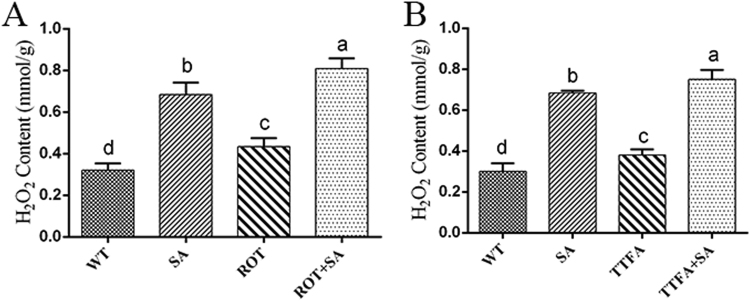
To understand the relationship between SA-induced ROS generation and mitochondrial complex III function, the ROS localization after treatment with 5 μM AA and 100 μM SA was detected by double-staining with MitoTracker Red CMXRos and DCFH-DA. Analysis of the obtained fluorescence images showed an obvious increase in the fluorescent overlap (by approximately 3.1-fold) in the AA-treated sample compared with the control. This trend was similar to that observed in the sample co-treated with AA and SA (Figs. 7A and 7B). H2O2 production was detected in the sample co-treated with AA and SA and in the AA-treated sample. The results showed that the H2O2 content showed a significant 2.2-fold increase in the 5 μM AA-treated sample compared with the control, and no significant difference in the H2O2 level was observed between the sample co-treated with AA and SA and the AA- or SA-treated samples (Fig. 7C). However, the H2O2 contents of the sample treated with 5 μM Rot and the 10 μM TTFA-treated sample exhibited 19% and 16% increases relative to the control, respectively. In addition, the sample co-treated with 5 μM Rot and SA showed approximately 83% and 16% higher H2O2 levels compared with the samples treated with only 5 μM Rot and the sample treated with only SA, respectively (Fig. S2A). The H2O2 level was approximately 88% and 10% higher in the sample co-treated with 10 μM TTFA and SA compared with that in the only TTFA- and the sample treated with only SA, respectively (Fig. S2B). The results showed that co-treatment with SA and either Rot or TTFA exhibited a superimposed effect on H2O2 production relative to that observed with the individual treatments. However, the co-treatment with AA and SA showed no obviously superimposed effect on the H2O2 content relative to the single treatments. Combined with the prior results (Fig. 5, Fig. 6, Fig. 7 and S2), these findings indicate that mitochondrial complex III plays an essential role in the signaling pathways involved in SA-induced ROS generation.
Fig. 7.
Effect of AA on ROS accumulation under SA treatment. A. ROS production and localization in WT cells treated with 5 μM AA and 100 μM SA were detected by double-staining with DCFH-DA and MitoTracker Red CMXRos. B. Levels of mitochondrial ROS in WT cells treated with 5 μM AA and 100 μM SA. C. H2O2 amounts in WT cells after treatment with 5 μM AA and 100 μM SA. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
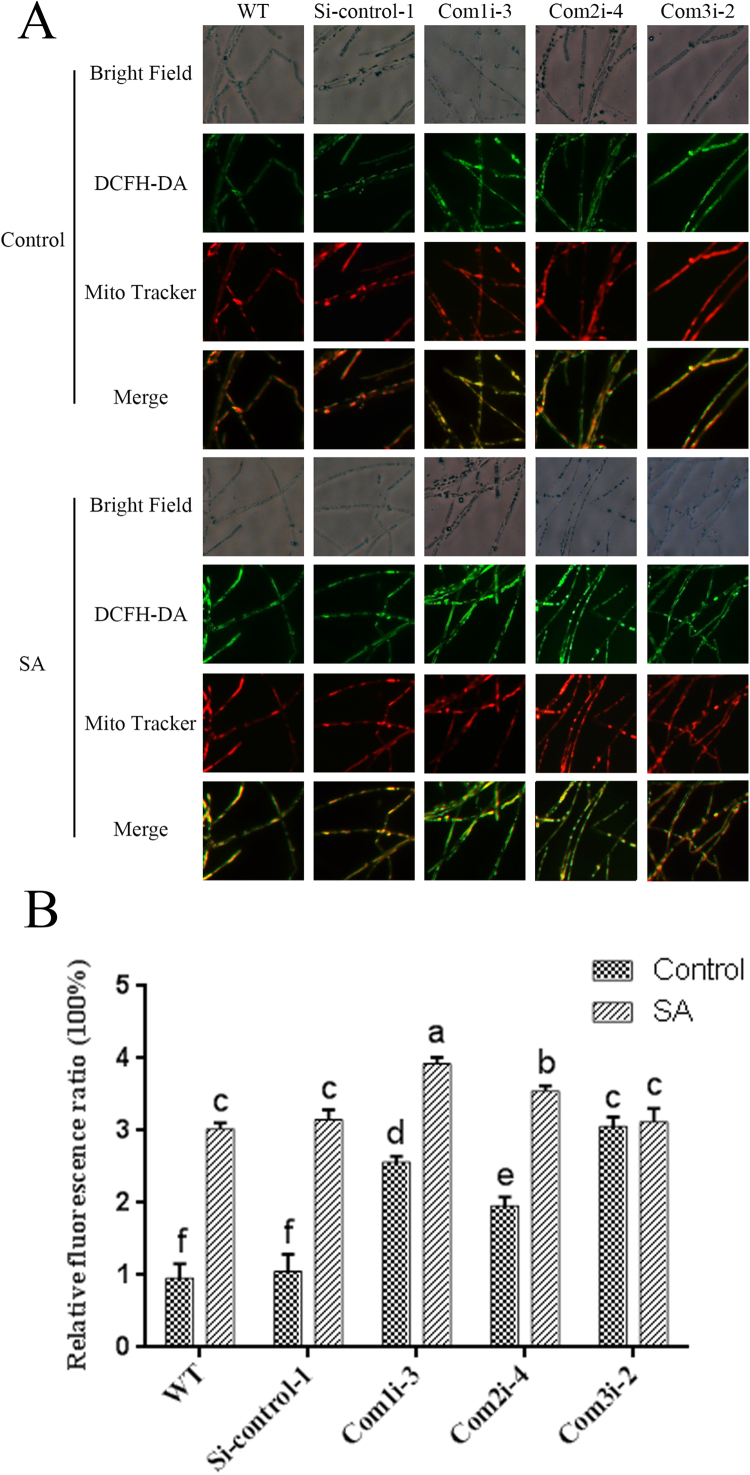
To further clarify the sites of ROS production in mitochondrial respiratory chain complexes, the mitochondrial ROS in the Com1i, Com2i and Com3i strains were detected, and consistent results were found for the two Com1i, Com2i and Com3i silenced strains, respectively. Furthermore, the results obtained for the Si-control-1 and Si-control-2 strains were consistent with those found for the WT strain. Thus, only the results for the WT, Si-control-1, and Com1i-3, Com2i-4 and Com3i-2 strains are included in the Fig. 8. The mitochondrial ROS levels in the Com1i-3 strain were 2.5-fold higher than those in the WT and Si-control strains (Fig. 8), and the mitochondrial ROS levels were significantly increased by 1.9-fold in the Com2i-4 strain compared with the WT and Si-control strains (Fig. 8). Moreover, the mitochondrial ROS contents of the Com3i-2 strain were significantly increased by approximately 3.1-fold compared with those in the WT and Si-control strains (Fig. 8). The mitochondrial ROS levels in the Com1i-3 and Com2i-4 strains were increased by 56% and 78% after treatment with 100 μM SA, but the SA treatment did not yield a significant difference in the mitochondrial ROS levels in the Com3i-2 strain (Fig. 8). The results showed that SA treatment exerted a superimposed effect on the level of mitochondrial ROS in the Com1i-3 and Com2i-4 strains but did not have an obvious superimposed effect on the mitochondrial ROS content in the Com3i-2 strains. These results support the idea that SA inhibits mitochondrial complex III activity to increase ROS generation.
Fig. 8.
ROS levels in complex I-, II- and III-silenced strains under SA treatment. A. ROS production and localization in the WT and complex I-, II- and III-silenced strains treated or not treated with SA were detected by double-staining with DCFH-DA and MitoTracker Red CMXRos. B. Levels of mitochondrial ROS in the WT and complex I-, II- and III-silenced strains treated or not treated with SA. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
3.9. GA content in WT strains treated with mitochondrial inhibitors and complex-silenced strains under SA treatment
To understand the effect of SA-induced increases in mitochondrial ROS on GA accumulation, the contents of GAs and two key intermediate metabolites in the presence of mitochondrial inhibitors and after treatment with 100 μM SA were measured. The individual treatments with 5 μM Rot or 10 μM TTFA exerted approximately 10% increases in the GA content compared with that of the control. However, a significant 41% increase in the GA content was observed in the 5 μM AA-treated sample compared with the control (Figs. 9A, 9D, and 9G). In addition, the GA content of the sample co-treated with Rot and SA was significantly increased by 37% and 13% compared with those observed with the individual treatments with Rot and SA, and the GA content of the sample co-treated with TTFA and SA was 40% and 9% higher than those obtained with the respective individual treatments. However, co-treatment with AA and SA did not significantly affect the GA content compared with that the individual treatments (Figs. 9A, 9D and 9G). The levels of the two key intermediate metabolites SQ and Lano showed the same trend as that obtained for the GA content (Figs. 9B, 9C, 9E, 9F, 9H and 9I). A comprehensive analysis of the accumulation of GAs and intermediate metabolites indicated that the SA-induced inhibition of complex III affected the biosynthesis of GAs (Figs. 9A-9I).
Fig. 9.
Effects of mitochondrial inhibitors on the GA content under SA treatment. A-C. Levels of total GAs, SQ and Lano in the 5 μM Rot-treated sample with or without SA. D-F. Levels of total GAs, SQ and Lano in the 10 μM TTFA-treated sample with or without SA. G-I. Levels of total GAs, SQ and Lano in the 5 μM AA-treated sample with or without SA. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
Assessment of the contents of GAs in the Com1i, Com2i and Com3i strains under 100 μM SA treatment revealed that the GA contents in the Com1i and Com2i strains were approximately 15% higher than that in the WT and Si-control strains (Figs. 10A and 10D). However, the GA content in the Com3i strain was significantly increased by 32% compared with that in the WT and Si-control strains (Fig. 10G). Moreover, treatment of the Com1i and Com2i strains with SA increased the GA content by approximately 30% (Figs. 10A, and 10D), but SA treatment did not significantly affect the GA content in the Com3i strain (Fig. 10G). An analysis of the levels of the two key intermediate metabolites SQ and Lano revealed the same trend as that obtained for the GA content (Figs. 10B, 10C, 10E, 10F, 10H and 10I). Similar results were observed in pharmacology experiments (Fig. 9), which showed that mitochondrial complex III caused ROS accumulation and that ROS accumulation played an essential role in SA-mediated GA biosynthesis in G. lucidum. The above-described results demonstrated that SA affects complex III activity to increase the ROS levels and that ROS are involved in the regulation of GA biosynthesis in G. lucidum.
Fig. 10.
GA content in complex-silenced strains under SA treatment. A-C. Levels of total GAs, SQ and Lano in the complex I-silenced strains treated or not treated with SA. D-F. Levels of total GAs, SQ and Lano in the complex II-silenced strains treated or not treated with SA. G-I. Levels of total GAs, SQ and Lano in the complex III-silenced strains treated or not treated with SA. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
4. Discussion
The phenolic compound salicylic acid (SA) is an important effector molecule in plants [28] and is considered a signal substance produced by plants in response to microbial invasion. The functions of SA in plants have been extensively researched, but to the best of our knowledge, the effects of SA on microorganisms have not been reported. Our studies first demonstrate the biological effects on microorganism exerted by SA and provides an effective approach to investigate SA. Previous studies found that SA participates in many important physiological processes in addition to disease resistance, such as improving the synthesis of secondary metabolites and responding to a series of environmental stress factors [13], [42]. However, the signal transduction mechanism induced by SA that is involved in regulating secondary metabolism is poorly understood. Different lines of evidence obtained from further research and development indicate that SA interplays with ROS in plants. In Arabidopsis, SA induces stomatal closure by increasing the endogenous concentration of ROS [27] and reduces the inhibitory effects of high salinity on germination by modulating ROS accumulation [32]. In addition, SA enhances artemisinin production by invoking a burst of endogenous ROS in Artemisia annua [22]. In the present study, SA significantly increased the levels of ROS in G. lucidum, and the observed excess production of ROS resulted in GA accumulation. The ROS measurement was performed with the fluorescent probe DCFH-DA. Although several limitations have been demonstrated on the DCF assays of intracellular ROS [58], the method is still widely accepted on SA treatment as the weak activity of SA to scavenge hydroxyl radical [19], [27], [76]. This SA-induced ROS accumulation was abolished by pretreatment with the ROS scavengers NAC and VC, which inhibited approximately 90% of the GA accumulation induced by SA but did not completely abolish it. This result was likely obtained because the promoter of HMGR (hydroxy-3-methylglutaryl-coenzyme A reductase), a key gene in the GA biosynthesis pathway, contains SA cis-acting elements. Additionally, exogenous SA could enhance the expression of the HMGR gene in G. lucidum [55]. Above all, these findings suggest that SA induces the generation of ROS, which are involved in regulating physiological processes in plants and microorganisms.
Secondary metabolism biosynthesis is a complex process which is regulated by many signals and physiological processes [57]. In this study, exogenous H2O2 can largely increase the level of both intracellular H2O2 and GA content while exogenous inhibitors of complex I or II had little effects on H2O2 and GA content. Silencing of complex I or II leads to dramatical increase of H2O2 concentration and little effects on GA production. These data suggested that ROS overproduction induced by SA can improve the biosynthesis of GA. However, the phenotype above has a slight difference. The phenomenon may be related to the silencing of mitochondrial complex which mainly affect the production of ROS, but the different influence on GA content might be because of the functional role in secondary metabolism biosynthesis of the silenced gene. In addition, the inhibitor of mitochondrial complex might have extra effects on the secondary metabolism biosynthesis besides of the active inhibition on mitochondrial complex. As a positive control, exogenous 10 mM H2O2 treatment may play profound effects on GA content as a stimulus directly. Our results demonstrate that the inhibition of complex activity will increase the ROS levels which are involved in GA biosynthesis regulation. Maybe, there are also some other regulated pathways unknown involved in this process.
Specifically, ROS act as mediators of intracellular signaling to regulate numerous physiological and biological responses [60], [61], and these species are generated in numerous cellular compartments and by multiple enzymes within a cell [16]. A key player in this network of ROS production acts through the mitochondrial respiratory and cytosolic NADPH oxidases [67]. Mitochondrial respiration results in the generation of ROS through electron reduction of O2 in the mitochondrial electron transport chain [51]. Mitochondrial complexes I and III are generally regarded as the primary sources of ROS production [2], [29], [44], [47], but mitochondrial complex II has also been reported to serve as a source of ROS [19], [24], [52]. The generated mitochondrial ROS can trigger muscle differentiation [39], influence plant growth and regulate plant stress-responsive genes [19]. The NAPDH oxidases (NOX) also play a prominent role in ROS generation·H2O2 accumulation is associated with the activation of NOX which is considered as the mainly superoxide generator [41], it might be the result of catalyzation from superoxide to H2O2 under the action of superoxide dismutase [25], [4]. In fact, NOX-dependent ROS production is required for cell wall damage-induced lignin production in Arabidopsis thaliana [12], and the ROS produced by NOXs function as signal molecules to regulate Na+/K+ homeostasis and thus improve the salt tolerance of Arabidopsis [38]. In our study, both NOX-silenced strains and DPI-treated samples showed partial reductions in their ROS content and GA accumulation after SA treatment. This finding suggests the existence of additional ROS sources (not just NOX) under SA treatment, and these results are consistent with our previous studies [46], [62]. Further study showed the involvement of mitochondrial ROS in the regulation of SA-induced GA biosynthesis. In Nicotiana tabacum, mitochondrial ROS bursts determine the cell fate during responses to pathogens [10], and the mitochondrial ROS regulate plant stress and defense responses in Arabidopsis [19]. In conclusion, the mitochondrial ROS might be broadly employed and could contribute to the physiological metabolism process.
The mitochondria play an important role in the signaling molecules generated by plant cells during cellular metabolism and amplify diverse signals, such those elicited by salicylic acid, nitric oxide, ROS and pathogens [17], [30]. The signals perceived by mitochondria usually impact their normal function, generating changes in respiration, membrane potential and ROS production [9]. A previous study have indicated that SA affects the mitochondrial electron transport chain and mitochondrial respiration, which play important roles in the basal defense against tobacco mosaic virus in tomato [34]. Another investigation found that SA is both an un-coupler and an inhibitor of mitochondrial electron transport that affects mitochondrial protein expression in Nicotiana tabacum [49]. The results presented here show that SA decreased complex III activity to influence the respiration rate and thereby regulate the ROS level in G. lucidum. In Arabidopsis thaliana, complex III is involved in H2O2 generation under SA treatment [48]. We can thus surmise that the functionary mechanisms of SA in microorganisms and plants are similar.
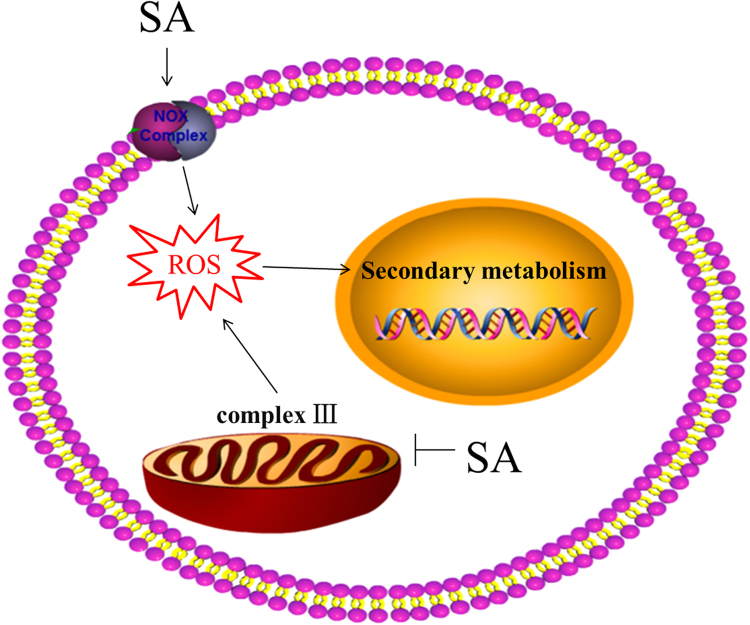
In conclusion, our data show that SA increases the ROS content and that both NOX and mitochondria participate in ROS generation. Further experiments found that the decrease in the mitochondrial respiratory rate induced by SA helps regulate ROS accumulation. In addition, mitochondrial complex III activity is inhibited by SA, and this effect plays an important role in decreasing the respiratory rate. In summary, in G. lucidum, SA inhibits complex III activity to increase the ROS levels, which are involved in regulating GA biosynthesis. Based on the findings, a potential cascade of the cellular events constituting the SA-mediated signaling pathway was proposed (Fig. 11). Our study contributes to a better understanding of the mitochondria-dependent mechanism of the SA-induced biological response in G. lucidum and might provide new insights into the cellular signaling cascade that regulates SA-mediated secondary metabolite biosynthesis.
Fig. 11.
Schematic representation of the potential cascade of cellular events constituting the SA-mediated ROS signaling pathway. SA treatment increases the production of ROS through different pathways in G. lucidum. On one hand, SA induces an ROS burst through the NOX complex (NOXAB and NOXR). On the other hand, SA inhibits mitochondria complex III activity to increase the ROS level. The ROS increased plays an essential role in the SA-mediated biosynthesis of GA in G. lucidum. The black solid arrows in the hypothetical model indicate data supported by experiments performed in the present study.
Acknowledgments
This work was supported by the earmarked fund for the China Agriculture Research System (CARS-20 to Mingwen Zhao), the National Natural Science Foundation of China (81773839 to Ang Ren and 31672212 to Liang Shi), the Natural Science Foundation of Jiangsu Province, China (BK20171377 to Ang Ren), the Science & Technology Pillar Program of Jiangsu Province and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYZZ16_0383 to Rui Liu).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.03.018.
Appendix A. Supplementary material
Fig. S1.
Effect of endogenous free SA levels in G. lucidum with or without SA treatment. The endogenous free SA levels in the control and SA-treated samples are shown. The values are the means ± SDs of three independent experiments, and asterisks indicate significant differences compared with the untreated strains (Student's t-test: **P < 0.01).
Fig. S2.
Effect of Rot and TTFA on the H2O2 content in G. lucidum under SA treatment. A. Amount of H2O2 in the WT strain after treatment with 5 μM Rot and 100 μM SA. B. H2O2 amount in WT cells after treatment with 10 μM TTFA and 100 μM SA. The values are the means ± SDs of three independent experiments, and different letters indicate significant differences between the lines (P < 0.05, according to Duncan's multiple range test).
Fig. S3.
Effects of SA on respiration rate in WT and complex-silenced strains. A. Construction of Com1, Com2 and Com3 RNAi expression cassette plasmids. The fragments were double-digested with the restriction enzymes KpnI and SpeI and inserted into the KpnI/SpeI restriction-digested pAN7-dual plasmid. In the plasmid, the transcriptions of ura3 and the complex I assembly factor NDUFAF1 (CIA30), the Succinate Dehydrogenase Subunit b (sdhB of complex II) or the ubiquinone binding protein of complex III are driven by the 35S promoter and the gpd promoter, respectively. B. Relative mRNA levels of the complex I assembly factor NDUFAF1 (CIA30), the Succinate Dehydrogenase Subunit b (sdhB of complex II) or the ubiquinone binding protein of complex III in G. lucidum. C. Complex I activity in the WT and Com1i strains. D. Complex II activity in the WT and Com2i strains. E. Complex III activity in the WT and Com3i strains. F. Respiratory ratio of isolated mitochondria from the WT and complex-silenced strains under 100 μM SA treatment in the presence of 5 mM malate plus 10 mM glutamate as the respiratory substrates. G. Respiratory rate of isolated mitochondria from the WT and complex-silenced strains under 100 μM SA treatment in the presence of 10 mM succinate as the respiratory substrate. The values are the means ± SDs of three independent experiments, and asterisks indicate significant differences compared with untreated strains (Student’s t-test: **P < 0.01). Different letters indicate significant differences between the lines (P < 0.05, according to Duncan’s multiple range test).
Supplementary material
References
- 1.Baldissera M.D., Souza C.F., Grings M., Parmeggiani B.S., Leipnitz G., Moreira K.L.S. Inhibition of the mitochondrial respiratory chain in gills of Rhamdia quelen experimentally infected by Pseudomonas aeruginosa: interplay with reactive oxygen species. Microb. Pathog. 2017;107:349–353. doi: 10.1016/j.micpath.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breton-Romero R., Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao P.F., Wu C.G., Dang Z.H., Shi L., Jiang A.L., Ren A., Zhao M.W. Effects of exogenous salicylic acid on ganoderic acid biosynthesis and the expression of key genes in the ganoderic acid biosynthesis pathway in the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes) Int J. Med Mushrooms. 2017;19:65–73. doi: 10.1615/IntJMedMushrooms.v19.i1.70. [DOI] [PubMed] [Google Scholar]
- 6.Castello P.R., Drechsel D.A., Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J. Biol. Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Xu J., Liu C., Zhu Y., Nelson D.R., Zhou S. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012;3:913. doi: 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevtzoff C., Yoboue E.D., Galinier A., Casteilla L., Daignan-Fornier B., Rigoulet M., Devin A. Reactive oxygen species-mediated regulation of mitochondrial biogenesis in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2010;285:1733–1742. doi: 10.1074/jbc.M109.019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombatti F., Gonzalez D.H., Welchen E. Plant mitochondria under pathogen attack: a sigh of relief or a last breath? Mitochondrion. 2014;19(Pt B):238–244. doi: 10.1016/j.mito.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Cvetkovska M., Vanlerberghe G.C. Coordination of a mitochondrial superoxide burst during the hypersensitive response to bacterial pathogen in Nicotiana tabacum. Plant Cell Environ. 2012;35:1121–1136. doi: 10.1111/j.1365-3040.2011.02477.x. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey D.A., Vlot A.C., Wildermuth M.C., Klessig D.F. Vol. 9. 2011. Salicylic Acid biosynthesis and Metabolism; p. e0156. (Arabidopsis Book). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denness L., McKenna J.F., Segonzac C., Wormit A., Madhou P., Bennett M. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong J., Wan G., Liang Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010;148:99–104. doi: 10.1016/j.jbiotec.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 14.El Enshasy H.A., Hatti-Kaul R. Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol. 2013;31:668–677. doi: 10.1016/j.tibtech.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Fecikova H., Turna J., Barath Z. Preparation of highly purified mitochondria of Neurospora crassa on a Percoll gradient. Folia Microbiol (Praha) 1983;28:409–413. doi: 10.1007/BF02879491. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadjev I., Stone J.M., Gechev T.S. Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 2008;270:87–144. doi: 10.1016/S1937-6448(08)01403-2. [DOI] [PubMed] [Google Scholar]
- 18.Gao C., Xing D., Li L., Zhang L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta. 2008;227:755–767. doi: 10.1007/s00425-007-0654-4. [DOI] [PubMed] [Google Scholar]
- 19.Gleason C., Huang S.B., Thatcher L.F., Foley R.C., Anderson C.R., Carroll A.J. Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proc. Natl. Acad. Sci. USA. 2011;108:10768–10773. doi: 10.1073/pnas.1016060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groebe K., Krause F., Kunstmann B., Unterluggauer H., Reifschneider N.H., Scheckhuber C.Q. Differential proteomic profiling of mitochondria from Podospora anserina, rat and human reveals distinct patterns of age-related oxidative changes. Exp. Gerontol. 2007;42:887–898. doi: 10.1016/j.exger.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Guo H., Dang X., Dong J. Hydrogen peroxide and nitric oxide are involved in salicylic acid-induced salvianolic acid B production in Salvia miltiorrhiza cell cultures. Molecules. 2014;19:5913–5924. doi: 10.3390/molecules19055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo X.-X., Yang X.-Q., Yang R.-Y., Zeng Q.-P. Salicylic acid and methyl jasmonate but not Rose Bengal enhance artemisinin production through invoking burst of endogenous singlet oxygen. Plant Sci. 2010;178:390–397. [Google Scholar]
- 23.Jakubowski W., Bartosz G. 2,7-dichlorofluorescin oxidation and reactive oxygen species: what does it measure? Cell Biol. Int. 2000;24:757–760. doi: 10.1006/cbir.2000.0556. [DOI] [PubMed] [Google Scholar]
- 24.Jardim-Messeder D., Caverzan A., Rauber R., de Souza Ferreira E., Margis-Pinheiro M., Galina A. Succinate dehydrogenase (mitochondrial complex II) is a source of reactive oxygen species in plants and regulates development and stress responses. New Phytol. 2015;208:776–789. doi: 10.1111/nph.13515. [DOI] [PubMed] [Google Scholar]
- 25.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang G., Li G., Xu W., Peng X., Han Q., Zhu Y., Guo T. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J. Proteome Res. 2012;11:6066–6079. doi: 10.1021/pr300728y. [DOI] [PubMed] [Google Scholar]
- 27.Khokon A.R., Okuma E., Hossain M.A., Munemasa S., Uraji M., Nakamura Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011;34:434–443. doi: 10.1111/j.1365-3040.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 28.Klessig D.F., Malamy J. The salicylic acid signal in plants. Plant Mol. Biol. 1994;26:1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- 29.Kowaltowski A.J., de Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Lam E., Kato N., Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- 31.Lee H.I., Leon J., Raskin I. Biosynthesis and metabolism of salicylic acid. Proc. Natl. Acad. Sci. USA. 1995;92:4076–4079. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S., Kim S.G., Park C.M. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol. 2010;188:626–637. doi: 10.1111/j.1469-8137.2010.03378.x. [DOI] [PubMed] [Google Scholar]
- 33.Li C.Y., Shi L., Chen D.D., Ren A., Gao T., Zhao M.W. Functional analysis of the role of glutathione peroxidase (GPx) in the ROS signaling pathway, hyphal branching and the regulation of ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet. Biol. 2015;82:168–180. doi: 10.1016/j.fgb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Liao Y., Tian M., Zhang H., Li X., Wang Y., Xia X. Salicylic acid binding of mitochondrial alpha-ketoglutarate dehydrogenase E2 affects mitochondrial oxidative phosphorylation and electron transport chain components and plays a role in basal defense against tobacco mosaic virus in tomato. New Phytol. 2015;205:1296–1307. doi: 10.1111/nph.13137. [DOI] [PubMed] [Google Scholar]
- 35.Lin S.B., Li C.H., Lee S.S., Kan L.S. Triterpene-enriched extracts from Ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen-activated protein kinases and G2-phase cell cycle arrest. Life Sci. 2003;72:2381–2390. doi: 10.1016/s0024-3205(03)00124-3. [DOI] [PubMed] [Google Scholar]
- 36.Liu C.D., Dunkin D., Lai J., Song Y., Ceballos C., Benkov K., Li X.M. Anti-inflammatory effects of Ganoderma lucidum triterpenoid in human Crohn's disease associated with downregulation of NF-kappa B signaling. Inflamm. Bowel Dis. 2015;21:1918–1925. doi: 10.1097/MIB.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Ma L., Zhang H., Sun L., Jiao Y., Zhang G., Miao C., Hao F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 2012;63:305–317. doi: 10.1093/jxb/err280. [DOI] [PubMed] [Google Scholar]
- 39.Malinska D., Kudin A.P., Bejtka M., Kunz W.S. Changes in mitochondrial reactive oxygen species synthesis during differentiation of skeletal muscle cells. Mitochondrion. 2012;12:144–148. doi: 10.1016/j.mito.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Martin J.A., Solla A., Garcia-Vallejo M.C., Gil L. Chemical changes in Ulmus minor xylem tissue after salicylic acid or carvacrol treatments are associated with enhanced resistance to Ophiostoma novo-ulmi. Phytochemistry. 2012;83:104–109. doi: 10.1016/j.phytochem.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Melillo M.T., Leonetti P., Bongiovanni M., Castagnone-Sereno P., Bleve-Zacheo T. Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol. 2006;170:501–512. doi: 10.1111/j.1469-8137.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 42.Miura K., Tada Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci. 2014;5:4. doi: 10.3389/fpls.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miwa S., Jow H., Baty K., Johnson A., Czapiewski R., Saretzki G. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat. Commun. 2014;5:3837. doi: 10.1038/ncomms4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moller I.M., Sweetlove L.J. ROS signalling-specificity is required. Trends Plant Sci. 2010;15:370–374. doi: 10.1016/j.tplants.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Mu D.S., Shi L., Ren A., Li M.J., Wu F.L., Jiang A.L., Zhao M.W. The development and application of a multiple gene co-silencing system using endogenous URA3 as a reporter gene in Ganoderma lucidum. Plos One. 2012;7:e43737. doi: 10.1371/journal.pone.0043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mu D.S., Li C.Y., Zhang X.C., Li X.B., Shi L., Ren A., Zhao M.W. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: an essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, and oxidative-stress resistance. Environ. Microbiol. 2014;16:1709–1728. doi: 10.1111/1462-2920.12326. [DOI] [PubMed] [Google Scholar]
- 47.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nie S., Yue H., Zhou J., Xing D. Mitochondrial-derived reactive oxygen species play a vital role in the salicylic acid signaling pathway in Arabidopsis thaliana. PLoS One. 2015;10:e0119853. doi: 10.1371/journal.pone.0119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norman C., Howell K.A., Millar A.H., Whelan J.M., Day D.A. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 2004;134:492–501. doi: 10.1104/pp.103.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paterson R.R. Ganoderma – a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Quinlan C.L., Treberg J.R., Perevoshchikova I.V., Orr A.L., Brand M.D. Native rates of superoxide production from multiple sites in isolated mitochondria measured using endogenous reporters. Free Radic. Biol. Med. 2012;53:1807–1817. doi: 10.1016/j.freeradbiomed.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinlan C.L., Orr A.L., Perevoshchikova I.V., Treberg J.R., Ackrell B.A., Brand M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012;287:27255–27264. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raskin I., Turner I.M., Melander W.R. Regulation of heat-production in the inflorescences of an Arum Lily by endogenous salicyclic acid. Proc. Natl. Acad. Sci. USA. 1989;86:2214–2218. doi: 10.1073/pnas.86.7.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren A., Li X.B., Miao Z.G., Shi L., Jaing A.L., Zhao M.W. Transcript and metabolite alterations increase ganoderic acid content in Ganoderma lucidum using acetic acid as an inducer. Biotechnol. Lett. 2014;36:2529–2536. doi: 10.1007/s10529-014-1636-9. [DOI] [PubMed] [Google Scholar]
- 55.Ren A., Ouyang X., Shi L., Jiang A.L., Mu D.S., Li M.J. Molecular characterization and expression analysis of GlHMGS, a gene encoding hydroxymethylglutaryl-CoA synthase from Ganoderma lucidum (Ling-zhi) in ganoderic acid biosynthesis pathway. World J. Microbiol. Biotechnol. 2013;29:523–531. doi: 10.1007/s11274-012-1206-z. [DOI] [PubMed] [Google Scholar]
- 56.Rivas-San Vicente M., Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- 57.Rohlfs M., Churchill A.C.L. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet. Biol. 2011;48:23–34. doi: 10.1016/j.fgb.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Salimi A., Roudkenar M.H., Sadeghi L., Mohseni A., Seydi E., Pirahmadi N., Pourahmad J. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox Biol. 2015;6:461–471. doi: 10.1016/j.redox.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanodiya B.S., Thakur G.S., Baghel R.K., Prasad G.B., Bisen P.S. Ganoderma lucidum: a potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 60.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi L., Gong L., Zhang X.Y., Ren A., Gao T., Zhao M.W. The regulation of methyl jasmonate on hyphal branching and GA biosynthesis in Ganoderma lucidum partly via ROS generated by NADPH oxidase. Fungal Genet. Biol. 2015;81:201–211. doi: 10.1016/j.fgb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Shi L., Fang X., Li M., Mu D., Ren A., Tan Q., Zhao M. Development of a simple and efficient transformation system for the basidiomycetous medicinal fungus Ganoderma lucidum. World J. Microbiol. Biotechnol. 2012;28:283–291. doi: 10.1007/s11274-011-0818-z. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki N., Miller G., Morales J., Shulaev V., Torres M.A., Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Thyagarajan-Sahu A., Lane B., Sliva D. ReishiMax, mushroom based dietary supplement, inhibits adipocyte differentiation, stimulates glucose uptake and activates AMPK. BMC Complement. Altern. Med. 2011;11:74. doi: 10.1186/1472-6882-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tormos K.V., Anso E., Hamanaka R.B., Eisenbart J., Joseph J., Kalyanaraman B., Chandel N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres M.A., Dangl J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Walker D.W., Hajek P., Muffat J., Knoepfle D., Cornelison S., Attardi G., Benzer S. Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant. Proc. Natl. Acad. Sci. USA. 2006;103:16382–16387. doi: 10.1073/pnas.0607918103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L.J., Fan L., Loescher W., Duan W., Liu G.J., Cheng J.S. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010;10:34. doi: 10.1186/1471-2229-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X.M., Yang B., Ren C.G., Wang H.W., Wang J.Y., Dai C.C. Involvement of abscisic acid and salicylic acid in signal cascade regulating bacterial endophyte-induced volatile oil biosynthesis in plantlets of Atractylodes lancea. Physiol. Plant. 2015;153:30–42. doi: 10.1111/ppl.12236. [DOI] [PubMed] [Google Scholar]
- 71.Wasser S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011;89:1323–1332. doi: 10.1007/s00253-010-3067-4. [DOI] [PubMed] [Google Scholar]
- 72.Yalpani N., Leon J., Lawton M.A., Raskin I. Pathway of salicylic-acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993;103:315–321. doi: 10.1104/pp.103.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yalpani N., Silverman P., Wilson T.M.A., Kleier D.A., Raskin I. Salicylic-acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991;3:809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao N., Greenberg J.T. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell. 2006;18:397–411. doi: 10.1105/tpc.105.036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.You B.J., Lee M.H., Tien N., Lee M.S., Hsieh H.C., Tseng L.H. A novel approach to enhancing ganoderic acid production by Ganoderma lucidum using apoptosis induction. Plos One. 2013;8:e53616. doi: 10.1371/journal.pone.0053616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao G.R., Zhang H.M., Ye T.X., Xiang Z.J., Yuan Y.J., Guo Z.X., Zhao L.B. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 2008;46:73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 77.Zhao W., Xu J.W., Zhong J.J. Enhanced production of ganoderic acids in static liquid culture of Ganoderma lucidum under nitrogen-limiting conditions. Bioresour. Technol. 2011;102:8185–8190. doi: 10.1016/j.biortech.2011.06.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material