Abstract
Here we present a successful intra-arterial thrombolysis performed in the second trimester of pregnancy (21 weeks). The intervention resulted in complete recanalization of the occluded right middle cerebral artery and favourable clinical and gestational outcome. Together with cases described in respective medical literature our report affirms that in pregnancy acute ischemic stroke could be treated effectively applying intra-arterial thrombolysis (using rt-PA). This therapy could provide opportunity to help in such desperate situations.
Keywords: ischemic stroke, pregnancy, intra-arterial thrombolysis
Introduction
In stroke, thrombolysis is the only approved therapy to establish reperfusion [1]. However, due to the concern of risk of hemorrhagic complications, especially intracranial bleeding, patients need to be carefully selected in randomized clinical trials based on inclusion and exclusion criteria [2,3,4,5,6].
Pregnancy due to its physiological changes is a procoagulant state [1]. A retrospective case-control-study of fifty thousands seven hundred and eleven births contained eight patients diagnosed with ischemic brain infarctions and one cerebral sinus thrombosis regarding to pregnant women; most events occurred during the first (3 patients) and the third trimester (4 patients) [7]. Another population based study showed lower incidence of ischemic brain infarction in pregnant women (0.03/1000 pregnancies, relative risk (RR) 0.7) than in age-matched controls, however, the incidence was increased (RR 2.5) during the immediate postpartum period [6].
There is inadequate and controversial information regarding the use of thrombolytics in pregnancy. Ones consider recombinant human tissue plasminogen activator (rt-PA) administration in pregnancy classified as category C drug (uncertain safety), others range it as category B drug (presumed safety based on animal studies) [8]. Intra-arterial thrombolysis has been reported as effective alternative for those patients with limited response to intravenous treatment (severe deficits, presentation between 3 and 6 hours after the event, and occlusion of a major vessel, cervical or intracranial arteries) [9]. Intra-arterial thrombolysis has been demonstrated to having a relatively high recanalization rate and a lower hemorrhagic rate compared with intravenous thrombolysis [10] and has the advantage of allowing delivery of thrombolytic agent to the site of the thrombus, in higher concentrations but using lower volumes, than intravenous infusion [11].
In this report we aim to present the case of a pregnant woman, with confirmed right middle cerebral artery occlusion resulting severe left sided hemiparesis treated by intra-arterial thrombolysis using rt-PA.
Case report
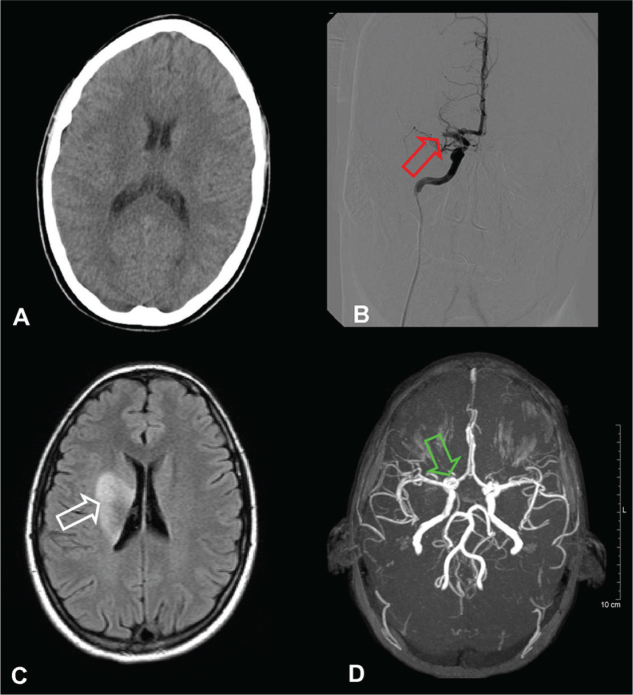
21-year-old right-handed woman, in the 21st week of gestation was admitted to Department of Neurology, city of Szolnok. The patient was a smoker, however, did not present with cardiovascular risk factors in her medical and family history. On admission she had a left central facial palsy, left upper limb plaegia, severe paresis in left lower extremity, Trömner, Hoffman and Babinski signs on her left side, the National Institute of Health Stroke Scale (NIHSS) score 8 points, blood pressure 100/70 mmHg and pulse-rate 76 beats/min. Non enhanced computed tomography (CT) brain-scan identified neither haemorrhage nor acute ischemic lesion (Figure 1).
Figure 1.
Cranial CT at 60 minutes after the stroke onset without any acute intracranial ischemic lesion or hemorrhage (image A); Angiography at 120 minutes after stroke onset showed complete occlusion of right middle cerebral artery (image B, red arrow); Cranial MRI at seven days after stroke onset and intra-arterial thrombolysis showing ischemic lesion of right sided basal ganglia (image C, white arrow); Cranial MR angiogram with intact Willis circle and complete recanalization of the right middle cerebral artery (image D, green arrow).
A CT angiogram of the cranial arteries revealed occlusion of right middle cerebral artery (MCA) at 1 cm distal from its origin. Intra-arterial thrombolysis was offered to the patient as the only effective therapy and the patient and her family were informed about the associated risk. After acceptance intra-arterial thrombolysis was administered by a neuroradiologist two hours and thirty minutes after the initial onset of symptoms. An angiographic Catheter HNBR 5.0-38-100 PNS H1 (COOK MEDICAL INC., Bloomington, Indiana, USA) was positioned via a trans-femoral approach into the distal region of right internal carotid artery. Twenty five milligrams of alteplase (rt-PA) was administered in fractions of 5 mg. Two hours after commencement of this treatment the patient had mild left sided central facial palsy and moderate left sided hemiparesis. After twenty four hours, a mild left-sided central facial palsy and hemiparesis were noticed. On the following day control skull CT confirmed a right sided para-ventricular ischemic lesion in the perfusion territory of right MCA. This area was of 3.0 × 1.5 cm in size, without causing midline shift or associated intracranial haemorrhage. The lesion affected the head of caudate nucleus. Seven days later, cranial MRI confirmed an incomplete lesion of the right nucleus lentiformis and small ischemic lesions in the temporooccipital border zone. A MR angiogram, taken at this time, showed intact circulation in the previously occluded right MCA (Figure 1).
Cardiological examination did not reveal any alteration which could have been the source of emboli.
Rehabilitation was started at the Department of Rehabilitation, MÁV Hospital, Szolnok. By the seventh day the patient had only minimal left-sided facial asymmetry, without any residual deficits. The NIHSS score was 1 point. Four months later, just prior to giving birth, presented with no change in her neurological status. During pregnancy, 0.6 mL enoxaparin was administered by subcutaneously injection, without any complication. A healthy baby was delivered by caesarean section weighing 3250 gram and 51 cm in length.
Following the successful and uncomplicated delivery and puerperium, the patient was advised to re-attend the hospital for routine haemostasis analysis. This recommendation was refused and the patient did not return.
Discussion
During pregnancy maternal physiological alterations result in haemostatic and haemodynamic changes accounting for a relative increased risk of thromboembolic complications, which occur in particular during the third trimester and the puerperium [12]. Cross et al. confirmed that majority of strokes associated with pregnancy were secondary to arterial occlusion [13]. The most frequent causes of arterial territory infarction include cardiac emboli or paradoxical emboli (valvular heart disease, coronary artery disease, patent foramen ovale), coagulopathy (deficiencies of protein C, protein S, and antithrombin III and activated protein C resistance) and arterial dissection [12]. Additionally, specific conditions related to pregnancy such as pre-eclampsia and eclampsia may lead to ischemic stroke, as well as more infrequent disorders such as choriocarcinoma, amniotic fluid embolism, peripartum cardiomyopathy and postpartum cerebral angiopathy [13].
Pregnancy has generally been excluded from clinical trials of thrombolysis due to the concern about risks of maternal or foetal haemorrhage, premature labour, placental abruption, foetal death or effects on the placenta [14]. A literature review showed that there are only a very few reported cases, and no large or controlled studies, which considered the use of intravenous (i.v.) or intra-arterial thrombolysis (alteplase or urokinase) during pregnancy for stroke.
In a previous case report of seven patients, where i.v. thrombolysis was used, one patient improved initially but subsequently there was a deterioration with upper extremity weakness; another patient died from a massive cerebral infarction after arterial dissection complicating angioplasty, though this was not shown to be clearly linked to the use of rt-PA. Five healthy full-term infants were born. However, in two cases medical termination of pregnancy was necessary, and one foetus died. I.V. thrombolysis had been administered in either first or the second trimester of pregnancy.
There are only four cases in which i.a. thrombolysis has been used in pregnancy. In three cases, rt-PA was used as a lytic agent, and in another case urokinase was used. Only one patient had intracranial bleeding (1.5-2.0 cm; right basal ganglia haematoma) resulting in worsening hemi-paresis and hemi-sensory loss. Her neurological status improved gradually, and by three months she had only minor symptoms. All of the patients delivered healthy infants. Half of i.a. thrombolysis was performed in first trimester, the other half in the third trimester [1,15,16,17,18,19,20].
Definite conclusions cannot be drawn from this small series of reports, however, it seems that thrombolytic therapy for acute ischemic stroke during pregnancy may result in favourable results. Complication rates were similar to those of non-pregnant patients and, at the same time, child outcome appeared not affected. Alteplase does not cross the placenta, and studies on rats and rabbits did not find any teratogenicity [21]. Nevertheless, the balance of risks and benefits to both mother and foetus, of using thrombolytic for ischemic stroke in pregnancy, should be considered in each clinical situation [15].
Footnotes
Conflict of interest:The authors declare that they have no conflict of interest.
References
- 1.Leonhardt G, Gaul C, Nietsch HH, Buerke M, Schleussner E.. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis. 2006;21:271–6. doi: 10.1007/s11239-006-5709-z. [DOI] [PubMed] [Google Scholar]
- 2.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S.. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–26. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 3.Clark WM, Albers GW, Madden KP, Hamilton S.. Thromblytic therapy in acute ischemic stroke study investigators. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Stroke. 2000;31:811–6. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Fieschi C. et al. Second European- Australasian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of Thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352:1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Fieschi C. et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–25. [PubMed] [Google Scholar]
- 6.The National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 7.Actilyse. European Agency for the Evaluation of Medicinal products. Available at web site. 20, 2007 Feb; http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documentid_308 [Google Scholar]
- 8.Kittner SJ, Stern BJ, Feeser BR.. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–84. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qureshi AI.. Endovascular treatment of cerebrovascular diseases and intracranial neoplasms. Lancet. 2004;363:804–13. doi: 10.1016/S0140-6736(04)15697-3. [DOI] [PubMed] [Google Scholar]
- 10.Ueda T, Sakaki S, Nochide I, Kumon Y, Kohno K, Ohta S.. Angioplasty after intra-arterial thrombolysis for acute occlusion of intracranial arteries. Stroke. 1998;29:2568–74. doi: 10.1161/01.str.29.12.2568. [DOI] [PubMed] [Google Scholar]
- 11.Ramee SR, Subramanian R, Felberg RA. et al. Catheter-based treatment for patients with acute ischemic stroke ineligible for intravenous thrombolysis. Stroke. 2004;35:e109–11. doi: 10.1161/01.STR.0000125711.94465.78. [DOI] [PubMed] [Google Scholar]
- 12.Jaigobin C, Silver FL.. Stroke and pregnancy. Stroke. 2000;31:2948–51. doi: 10.1161/01.str.31.12.2948. [DOI] [PubMed] [Google Scholar]
- 13.Cross JN, Castro PO, Jennett WB.. Cerebral strokes associated with pregnancy and the puerperium. BMJ. 1968;3:214–18. doi: 10.1136/bmj.3.5612.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Zotto E, Giossi A, Volonghi I, Costa P, Padovani A, Pezzini A.. Ischemic Stroke during Pregnancy and Puerperium. Stroke Res Res. 2011;2011:606780. doi: 10.4061/2011/606780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson DM, Kramer DC, Cohen E, Rochon M, Rosner M, Weinberger J.. Thrombolytic therapy for acute stroke in late pregnancy with intra-arterial recombinant tissue plasminogen activator. Stroke. 2005;36:e53–5. doi: 10.1161/01.str.0000166203.27135.27. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Margraf J, Kluck B, Jenny D, Castaldo J.. Thrombolytic therapy for ischemic stroke secondary to paradoxical embolism in pregnancy: a case report and literature review. Neurologist. 2012;18:44–8. doi: 10.1097/NRL.0b013e31823d7af0. [DOI] [PubMed] [Google Scholar]
- 17.Wiese KM, Talkad A, Mathews M, Wang D.. Intravenous recombinant tissue plasminogen activator in a pregnant woman with cardioembolic stroke. Stroke. 2006;37:2168–9. doi: 10.1161/01.STR.0000230286.95513.c2. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi Y, Kondo T, Ihara M, Kawamata J, Fukuyama H, Takahashi R.. Intravenous recombinant tissue plasminogen activator in an 18-week pregnant woman with embolic stroke. Abstract. Rinsho Shinkeigaku. 2010;50:315–9. doi: 10.5692/clinicalneurol.50.315. [DOI] [PubMed] [Google Scholar]
- 19.Tassi R, Acampa M, Marotta G. et al. Systemic thrombolysis for stroke in pregnancy. Am J Emerg Med. 2013;31(448):e1–3. doi: 10.1016/j.ajem.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Elford K, Leader A, Wee R, Stys PK.. Stroke in ovarian hyperstimulation syndrome in early pregnancy treated with intra-arterial rt-PA. Neurology. 2002;59:1270–2. doi: 10.1212/01.wnl.0000032492.77156.35. [DOI] [PubMed] [Google Scholar]
- 21.De Keyser J, Gdovinová Z, Uyttenboogaart M, Vroomen PC, Luijckx GJ.. Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke. 2007;38:2612–8. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]