Abstract
Introduction
Most children with fever without source will have a self limited viral infection though a small percent will develop a serious bacterial infection (SBI) like urinary tract infection, pneumonia, bacteraemia, meningitis or sepsis. The challenge facing practitioners is to distinguish between these two groups and currently biomarkers, like C-reactive protein (CRP) and Procalcitonin (PCT), are available for this purpose.
The aim of the current study was to identify SBI in infants with fever without an identifiable cause using the recently introduced “Lab-score” combining C-reactive protein, procalcitonin and urine dipstick results.
Methods
This survey is part of an observational study aimed at identifying children with fever without source at risk of SBI. Patients were recruited from the Emergency Department of Tîrgu Mures Emergency Clinical County Hospital, Romania, during 2013. SBI diagnosis was based on urine, blood and cerebrospinal fluid cultures and chest radiographs. For infants, aged 7 days to 12 months, CRP and PCT were determined and the “Lab-score” was calculated. Positive and negative likelihood ratios and post test probabilities were calculated for each parameter and score.
Results
Of the ninety infants included in the study, SBI was diagnosed in nineteen (21.11%). Ten had a urinary tract infection, seven had pneumonia, one had a urinary tract infection and bacteraemia, and one had sepsis. Positive and negative likelihood ratios for CRP (≥40.0 mg/L) and PCT (≥0.5 ng/mL) were 10.27/0.45 and 7.07/0.24 and post-test probabilities 73%/65%. For a “Lab-score” (≥3), positive and negative likelihood ratios were 10.43/0.28, and the posttest probability was 73%.
Conclusions
In our survey the “Lab-score” proved a strong predictor for the identification of febrile infants at risk of SBI, but showed no significant difference compared with CRP and PCT which both proved equally good predictors for SBI.
Keywords: fever, infants, serious bacterial infections, “Lab-score”
Introduction
Fever remains one of the main reasons why infants and young children are brought to an Emergency Departments (ED) with up to 20% not presenting with an apparent focus of infection even after a thorough history and clinical examination [1]. In clinical practice the challenge is to distinguish between the majority who will have a self limited viral infection and the 29% [2], who will develop a serious bacterial infection (SBI) such as a urinary tract infection (UTI), bacteraemia, pneumonia, meningitis or sepsis.
Previous studies demonstrated that clinical signs alone are not sufficiently reliable in identifying febrile children at risk of SBI [3,4]. The use of surrogate biomarkers like C-reactive protein (CRP) and Procalcitonin (PCT) have proved to be of valuable in diagnosing SBI [5,6] while traditional and previously standard markers such as the white blood cell count (WBC) and neutrophils, are becoming less used [7].
Though several studies have shown the value of CRP or PCT [8,9,10,11,12,13,14] neither was considered accurate enough to be used as a single predictor for diagnosing SBI [5,6,15]. This lead to a laboratory index score, named the “Lab-score”, being developed combining three predictive variables, CRP, PCT, and urinary dipstick, associated with SBI [16]. “Lab-score” showed promising results and has been validated and its accuracy tested in several studies [17,18,19,20].
The present study aimed to evaluate the performance of “Lab-score” in Romanian febrile infants with fever without source in order to identify those at risk of SBI.
The hypothesis was that the percentage at risk from SBI in Romania, may be larger than that observed in more developed countries where pneumococcal vaccination is routinely used.
Methods
This study is part of a larger prospective observational survey assessing the accuracy of WBC, CRP and PCT in identifying children with fever without source at risk of SBI.
This study was conducted in Tîrgu Mures Emergency Clinical County Hospital, Romania, and all patients included in the study were recruited from the hospital’s Division of Paediatrics emergency department (ED). All children aged 7 days to 12 months presenting at the hospital between January 2013 and September 2013, who had fever without clinical signs of infection, were included in the study. Fever was defined as the patient having a temperature ≥38°C, measured either in ED or at home using a thermometer. From 102 infants with fever without source, 90 were included in the study. Those with insufficient amount of serum for PCT determination and those without informed consent were excluded from the study. Children undergoing antibiotic treatment, with immunodeficiencies or fever lasting more than seven days were exclude from the study. Blood samples were taken from all children included in the study in order to perform WBC, CRP and PCT and a urine sample was collected for urinalysis.
Blood samples were mixed with ethylenediaminetetraacetic acid, and the WBC, erythrocytes and platelets counts were determined using an automated cell counter (Sysmex KX-21, Sysmex Corporation, Japan). High sensitive-CRP was measured on a PATHFAST rapid analyzer (Mitsubishi Chemical Medience Corporation) using a chemiluminescent enzyme immunoassay. Whenever a value above 30mg/L was present, samples were diluted 1:10 to obtain a quantitative result, in accordance with the manufacturer instructions. PCT was measured by a rapid semiquantitative immunochromatographic test (Brahms PCT-Q: Brahms Diagnostica Berlin) (range results: <0.5 ng/mL, ≥0.5 ng/mL, ≥2.0 ng/mL, ≥10.0 ng/mL). Urinalysis was determined using a dipstick analyser.
SBI diagnosis was determined by performing blood cultures, urine cultures, cerebrospinal fluid (CSF) culture, stool cultures and chest radiographs and finally established according to their positive or negative results.
Blood cultures were taken routinely in infants younger than three months and in older infants if red or amber features, according to NICE guidelines, were present [21]. When urinalysis was positive, urine cultures were taken for leukocyte esterase and/or nitrites. Urine was collected by catheter or by collection bags when catheterisation was not possible (e.g. phimosis) or when parents were reluctant to children having invasive procedures. Collection bags were changed every half hour in order to prevent contamination. Spinal tap and subsequent CSF culture was performed whenever suggestive clinical signs, like altered mental status, were present. Chest radiographs were taken whenever the clinician considered this to be necessary and were interpreted by a specialist radiologist.
After forty eight hours all children had a clinical follow up, or if discharged were contacted by telephone.
The final diagnosis was established after the follow up and results of cultures.
According to the “Lab-score” developed by Galetto- Lacour et al [16] 2 points are given for PCT ≥0.05 ng/ mL, 4 points for PCT ≥2.0 ng/mL; 2 points are given if the CRP value ranges between 40 mg/L and 99 mg/L and 4 points for a CRP ≥100 mg/L. One point is given for a positive leukocyte esterase and/or nitrates as indicated by positive urine dipstick result. The “Lab-score” values range from 0 to 9, and the cut-off of ≥3 points has been proposed as the most reliable for SBI prediction.
All data was collected in a Microsoft Excel Database. Sensitivity, specificity and positive and negative predictive values were calculated for each laboratory parameter and also for the “Lab-score” using MedCalc 9.5 software (MedCalc Software, Ostend, Belgium). For all variables, positive and negative likelihood ratios and post-test probabilities were calculated.
The study protocol was approved by the Ethics Committee of University of Medicine and Pharmacy Tîrgu Mures. Informed consent for all children was obtained from parents or tutors.
Results
Of the ninety infants analysed, forty were girls and fifty boys, with an average age of 6 month. The minimum value of temperature recorded at clinical examination was 38°C and the maximum value was 41.3°C, with an average value of 38.8°C. The minimum duration of fever from time of presentation was 1 hour and the maximum 72 hours, with an average value of 20 hours.
Nineteen children had SBI (21.1%), ten had UTI (11.1%), seven had pneumonia (7.7%), one had sepsis (1.1%), and one had both UTI and a bacteraemia (1.1%).
In the UTI group the most frequent isolated pathogen was E. Coli. Pseudomonas aeruginosa was isolated in one case of UTI and in the only case with a bacteraemia plus UTI, the pathogen isolated was Serratia marcescens. The patient diagnosed with sepsis according to Levy et al criteria [22] first presented with fever and admission to hospital was recommended at that time.
The parents refused admission and returned the following day when the child had overt clinical signs of severe sepsis. The infant was admitted to the paediatric intensive care unit but developed septic shock and died several days after.
In the non-SBI group the overwhelming majority had respiratory viral infections and viral gastroenteritis. One patient with aseptic meningitis, several patients had viral diseases with rash like rubella and roseola infantum and several patients had a fever without additional symptoms. In these cases, following negative cultures, a diagnosis of mild, unspecific and transitory viral disease was made.
The diagnostic characteristics of the “Lab-score” and the laboratory parameters for several commonly used cut-offs are shown in Table 1.
Table 1.
Sensitivity, specificity, positive and negative predictive values for the “Lab-score” and laboratory parameters for SBI
| Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|
| “Lab-score”∗(≥3) | 73.6 (48.7-90.8) | 92.9 (84.3-97.6) | 73.6 (48.7-90.8) | 92.9 (84.3-97.6) |
| WBC∗(≥15000/mm3) | 36.8 (16.2-61.6) | 84.5 (73.9-92.0) | 38.8 (17.3-64.2) | 83.3 (72.7-91.0) |
| CRP∗(≥20 mg/L) | 73.6 (48.7-90.8) | 88.7 (78.9-95.0) | 63.6 (40.6-82.7) | 92.6 (83.6-97.5) |
| CRP∗(≥40 mg/L) | 57.8 (33.5-79.7) | 94.3 (86.2-98.4) | 73.3 (44.9-92.2) | 89.5(80.0-95.2) |
| CRP∗(≥80 mg/L) | 47.3 (24.4-71.1) | 98.5 92.4-99.9) | 90.0 (55.5-99.7) | 87.5(78.2-93.8) |
| PCT∗(≥0.5 ng/mL) | 78.9 (54.4-93.9) | 88.7 (78.9-95.0) | 65.2 (42.7-83.6) | 94.0 (85.4-98.3) |
| PCT∗(≥2 ng/mL) | 47.3 (24.4-71.1) | 92.9 (84.3-97.6) | 64.2 (35.1-87.2) | 86.8 (77.1-93.5) |
| PCT∗(≥10 ng/mL) | 42.1 (20.2-66.5) | 97.1 (90.1-99.6) | 80.0 (44.4-97.4) | 86.2 (76.7-92.9) |
PPV indicates positive predictive value; NPV indicates negative predictive value WBC: white blood cells, CRP: C-reactive protein, PCT: Procalcitonin
cut-off value
The nomogram for the positive and negative likelihood ratios and the post-test probabilities for the “Lab-score” is shown in Figure 1. Figures 2 and 3 show the nomograms for the positive and negative likelihood ratios and the post-test probabilities for the best cut-off values found for CRP and PCT respectively.
Figure 1.
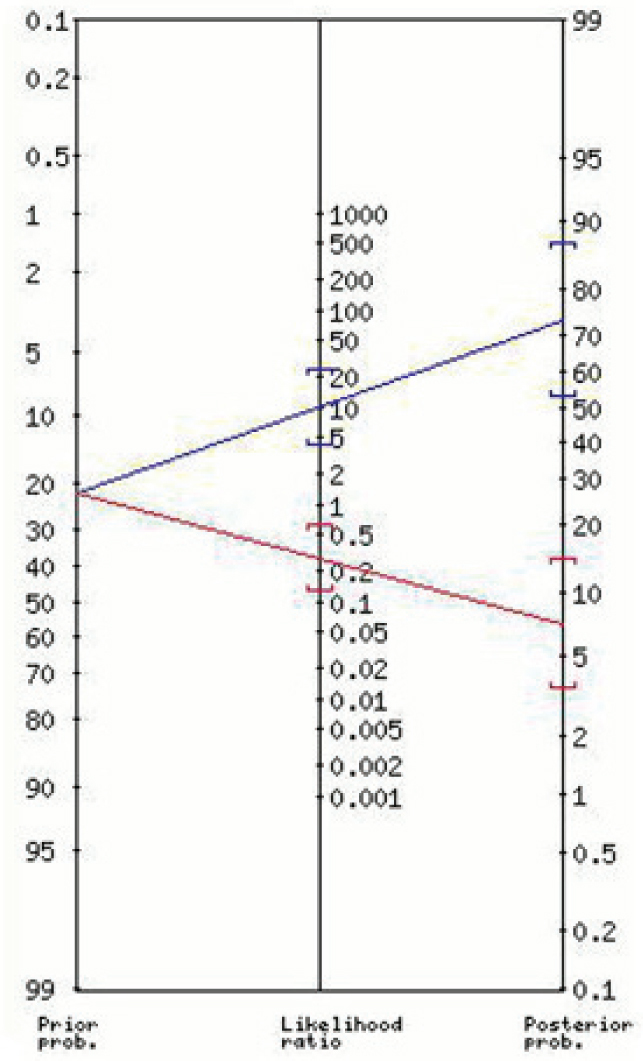
Positive and negative likelihood ratios and the post test probability for the “Lab-score” (≥3)
Figure 2.
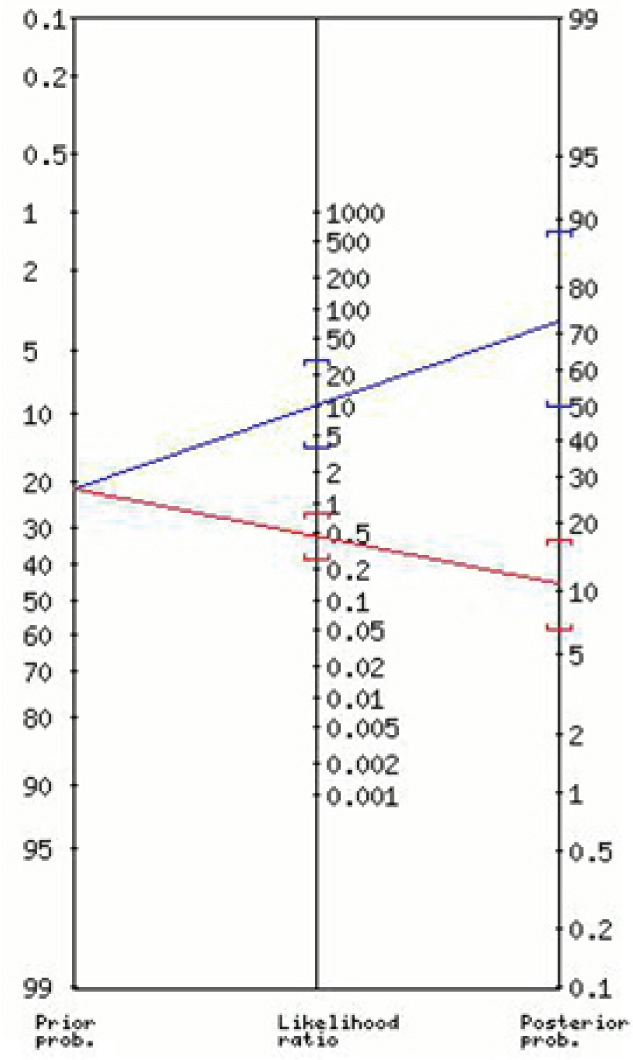
Positive and negative likelihood ratios and the post test probability for CRP (≥40 mg/L)
Figure 3.
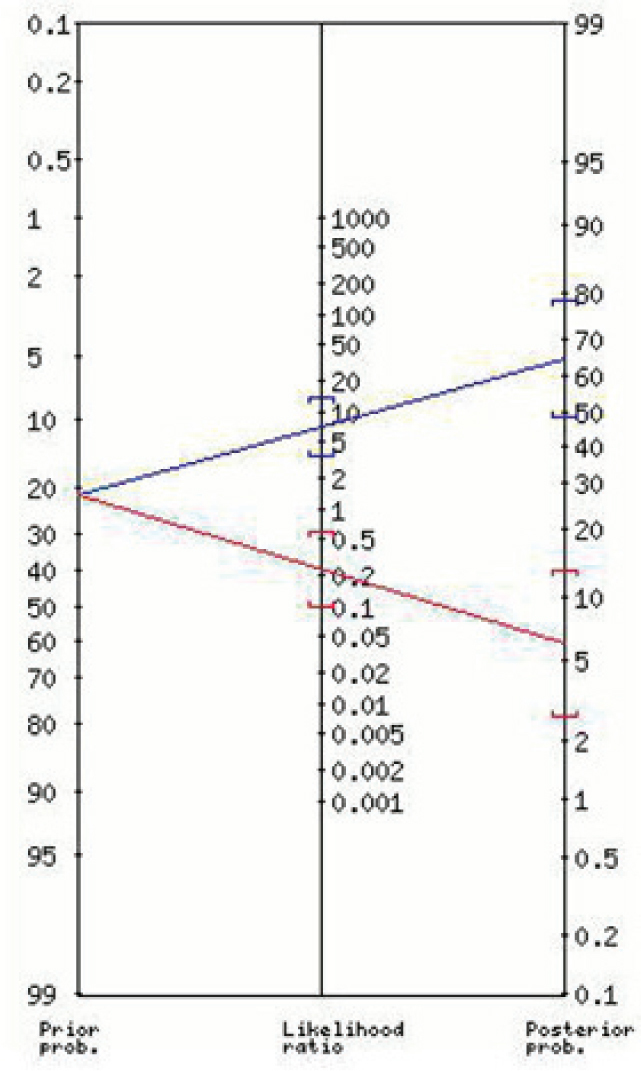
Positive and negative likelihood ratios and the post test probability for PCT (≥0.5 ng/mL)
The positive and negative likelihood ratios and posttest probabilities for the best cut-offs for CRP and PCT and for the “Lab-score” are given in Table 2. Additionally, LRs and PTP for the best cut-offs for CRP and PCT and for the Lab-score for predicting SBI are reported in Table 3.
Table 2.
Positive and negative likelihood ratios and post-test probabilities for the “Lab-score”. CRP and PCT
| LR + (95% CI) | PTP (95% CI) | LR – (95% CI) | PTP (95% CI) | |
|---|---|---|---|---|
| “Lab-score” ∗ (≥3) | 10.43 (4.39-25) | 73 (54-87) | 0.28 (0.13-0.6) | 7 (3-14) |
| CRP∗ (≥40 mg/L) | 10.27 (3.68-29) | 73 (50-89) | 0.45 (0.26-0.76) | 11 (7-17) |
| PCT∗ (≥0.5 ng/mL) | 7.01 (3.50-14) | 65 (48-79) | 0.24 (0.10-0.57) | 6(3-13) |
CRP: C-reactive protein. PCT: Procalcitonin. LR +: positive likelihood ratio. LR-: negative likelihood ratio. PTP: post-test probability
cut-off value
Table 3.
Positive and negative likelihood ratios and post test probabilities for the Lab-score, CRP and PCT
| LR + (95% CI) | PTP (95% CI) | LR – (95% CI) | PTP (95% CI) | |
|---|---|---|---|---|
| “Lab-score” ∗ (≥3) | 10.43 (4.39-25.0) | 73 (54-87) | 0.28 (0.13-0.6) | 7 (3-14) |
| CRP∗ (≥40 mg/L) | 10.27 (3.68-29.0) | 73 (50-89) | 0.45 (0.26-0.76) | 11 (7-17) |
| PCT∗ (≥0.5 ng/mL) | 7.01 (3.50-14.0) | 65 (48-79) | 0.24 (0.10-0.57) | 6 (3-13) |
CRP: C-reactive protein, PCT: Procalcitonin, LR +: positive likelihood ratio, LR-: negative likelihood ratio, PTP: post test probability, CI: confidence interval
cut-off value
Discussions
SBI prevalence in previous studies was shown to range between 11% [23] and 29% [2]. In the present study, 21% children with SBI were diagnosed. As expected from previous studies, UTI was the predominant SBI [24]. Pneumonia is the second most frequent SBI, and was only slightly higher than reported in previous studies conducted in countries where pneumococcal vaccination is carried out. Lack of pneumococcal vaccination in Romania may partly explain the fact that the incidence of pneumonia increases in children older than 12 month [25].
In accordance with previous studies [5,6,8,13], both CRP and PCT proved strong predictors of febrile children at risk of SBI. WBC failed both sensitivity [36.84% (95% CI: 16.28-61.64)] and specificity [84.51% (95% CI: 73.95-92.00)] as SBI predictors, supporting the results of a recent study [7].
Both CRP and PCT showed better specificity when higher cut-off values were used, but the compromise occasioned in a decrease in sensitivity and which may not be acceptable in clinical practice. Nonetheless, it was recently stated that the optimal cut-off value for CRP and PCT depends on whether the main clinical focus is “ruling in” or “ruling out” SBI [5]. In clinical decision making it is important not to miss an SBI, though equally important is to avoid “over-diagnose” which could lead to an overuse of antibiotics. It was concluded from the present study that the cut-off values of 40 mg/L for CRP and 0.5 ng/mL for PCT were best and that these are the cut-off values currently used in clinical practice [19].
The “Lab-score” was first derived from a population of one hundred and thirty five children and validated on a population of sixty seven children aged 7 days to 36 months recruited from a referral hospital in Geneva, Switzerland. All had been admitted to the emergency department presenting with fever without source with the same demographic and clinical characteristics. SBI was found in 27% of cases. The “Lab-score”, at a cut-off value of 3, best discriminated between children with and without SBI showing a sensitivity of 94% (95% CI: 82-99) and 94% (95% CI: 74-99) and a specificity of 81% (95% CI: 72-88) and 78% (95% CI: 64-87) in the derivation and validation set, respectively [16].
The “Lab-score” was further validated on a cohort of 408 children with FWS aged 7 days to 36 month recruited from the ED of a tertiary hospital in Padua, Italy. In this cohort, SBI was found in 22% and for a “Lab-score” cut-off value of 3, the sensitivity for the identification of SBI was 86% (95% CI: 77-92) and specificity was 83% (95% CI: 79-87) [17].
The “Lab-score” performance was also assessed by Bressan et al [18] to identify SBI in a cohort of 1012 patients and invasive bacterial infections (IBS) in a cohort of 1098. These patients were recruited from several EDs in Italy and Spain. SBI was found in 28%. In the identification of SBI, at a cut-off value of 3, a sensitivity of 52% (95% CI: 46-58) and specificity of 95% (95% CI: 93-96) were reported.
Recently, the “Lab-score” was validated and updated by Nijman et al [19], on a large cohort of children aged 1 month to 16 years attending with fever at the University Hospital Rotterdam, Netherlands. Of the 1084 children analysed, SBI was found in 16%. At a “Lab-score” cut-off value of 3, the sensitivity was 60% (95% CI: 52-67) and the specificity 86% (95% CI: 84-88).
The present study assessed the accuracy of the “Lab-score” on a smaller cohort of infants than the above studies. The sensitivity is lower than the originally reported by Galetto-Lacour et al and the first validation study, but higher than it was found in the other two. The specificity is higher than found in the original study and lower than in the mix Italian-Spanish study.
Both the positive and negative LRs for CRP (40 mg/L), PCT (0.5 ng/mL) were calculated, as was a “Lab-score (3)” resulting in strong prediction results for the “Lab-score” as well as for the CRP and PCT individually. A positive LR of 10.43 (95% CI: 4.39-25) for the “Lab-score” in the current study, gives a result corresponding to the one obtained in the mix Italian- Spanish study, the latter being the only study other than the current, which tested the “Lab-score” on infants. A similar result was obtained for CRP (40 mg/L): 10.27 (95% CI: 3.68-29), which gave better results than PCT [positive LR 7.01 (3.50-14)]. By calculating the LR to determine the post-test probability it was shows that for the “Lab-score” increases the probability of SBI from 21% to 73% (95% CI: 54-87), with similar data being obtained for CRP [73% (95% CI: 50-89)], but somewhat lower for PCT [65% (95% CI: 48-79)].
With a negative LR of 0.28 (0.13-0.6) the “Lab-score” is shown to be a good predictor of SBI at this cut-off value. PCT [(0.5 ng/mL) 0.24 (0.10-0.57)] performed slightly better than the “Lab-score”, both been better than CRP [0.45 (0.26-0.76)] which is partly in accordance with the views of the original study which reported a better result for the “Lab-score” [16] and with the group of infants from the study which first validated the “Lab-score” [17].
The strength of this present study is that all the laboratory tests required to establish a definitive diagnosis for each patient were available on site. The relatively small numbers included in the study is accepted as a possible weakness. Nevertheless, given the fact that the obtained results are comparable with those previously published from other countries, they allow valid inter-country comparisons to be drawn.
Even if the “Lab-score” best identify febrile infants at risk of SBI, the results were not significantly better than those given by currently and commonly used biomarkers on their own. This may have cost-benefit implications. It was recently stated that given the fact that there are no remarkable differences between CRP, PCT and the “Lab-score” in terms of performance, until larger studies are made would be wiser to determine only one biomarker to screen febrile children for SBI [19]. In a country like Romania, where resources are still limited, this might be a sensible clinical approach. To choose between CRP and PCT practitioners should take into consideration the biomarkers’ kinetics, and perform CRP in children with more than 12 hours of fever and PCT in children with less than 12 hours of fever, knowing that PCT has faster kinetics than CRP [26,27]. It was already shown that CRP performs better for SBI detection in children with more than 12 hours of fever [10] but there is also strong suggestion that PCT might perform better in the first 12 hours of fever [9,10,11,12,28,29].
Conclusion
The present study showed that the “Lab-score” to be a valuable tool for identifying Romanian febrile infants at risk of SBI, but only modestly outperforming CRP and PCT, both of which are good predictors of SBI.
Acknowledgments
We would like to acknowledge the nurses of the Emergency Department from Tîrgu Mures Emergency Clinical Hospital for the help provided in collecting the data for our survey.
Footnotes
Conflict of interest: None declared.
References
- 1.Baraff LJ.. Management of infants and young children with fever without source. Pediatr Ann. 2008;37:673–9. doi: 10.3928/00904481-20081001-01. [DOI] [PubMed] [Google Scholar]
- 2.Galetto Lacour A, Zamora SA, Gervaix A.. Bedside procalcitonin and C-reactive protein tests in children with fever without localizing signs of infection seen in a referralcenter. Paediatrics. 2003;112:1054–60. doi: 10.1542/peds.112.5.1054. [DOI] [PubMed] [Google Scholar]
- 3.Craig JC, Williams GJ, Jones M. et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594. doi: 10.1136/bmj.c1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D.. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet. 2010;375:834–45. doi: 10.1016/S0140-6736(09)62000-6. [DOI] [PubMed] [Google Scholar]
- 5.Van den Bruel A, Thompson MJ, Haj-Hassan T. et al. Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ. 2011;342:d3082. doi: 10.1136/bmj.d3082. [DOI] [PubMed] [Google Scholar]
- 6.Yo CH, Hsieh PS, Lee SH. et al. Comparison of the test characteristics of procalcitonin to C-reactive protein and leukocytosis for the detection of serious bacterial infections in children presenting with fever without source: a systematic review and meta-analysis. Ann Emerg Med. 2012;60:591–600. doi: 10.1016/j.annemergmed.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 7.De S, Williams GJ, Hayen A. et al. Value of white cell count in predicting serious bacterial infection in febrile children under 5 years of age. Arch Dis Child. 2014;99:493–9. doi: 10.1136/archdischild-2013-304754. [DOI] [PubMed] [Google Scholar]
- 8.Galetto Lacour A, Gervaix A, Zamora SA. et al. Procalcitonin, IL-6, IL-8, IL-1 receptor antagonist and C-reactive protein as identificators of serious bacterial infections in children with fever without localising signs. Eur J Pediatr. 2001;160:95–100. doi: 10.1007/s004310000681. [DOI] [PubMed] [Google Scholar]
- 9.Andreola B, Bressan S, Callegaro S, Liverani A, Plebani M, Da Dalt L.. Procalcitonin and C-reactive protein as diagnostic markers of severe bacterial infections in febrile infants and children in the emergency department. Pediatr Infect Dis J. 2007;26:672–7. doi: 10.1097/INF.0b013e31806215e3. [DOI] [PubMed] [Google Scholar]
- 10.Pratt A, Attia MW.. Duration of fever and markers of serious bacterial infections in young febrile children. Pediatr Int. 2007;49:31–5. doi: 10.1111/j.1442-200X.2007.02316.x. [DOI] [PubMed] [Google Scholar]
- 11.Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R.. Procalcitonin in young febrile infants for the detectin of serious bacterial infections. Pediatrics. 2008;122:701–10. doi: 10.1542/peds.2007-3503. [DOI] [PubMed] [Google Scholar]
- 12.Olaciregui I, Hernandez U, Munoz JA, Emparanza JI, Landa JJ.. Markers that predict serious bacterial infection in infants under 3 months of age presenting with fever of unknown origin. Arch Dis Dis. 2009;94:501–5. doi: 10.1136/adc.2008.146530. [DOI] [PubMed] [Google Scholar]
- 13.Manzano S, Bailey B, Gervaix A, Cousineau J, Delvin E, Girodias JB.. Markers for bacterial infection in children with fever without source. Arch Dis Dis. 2011;96:440–6. doi: 10.1136/adc.2010.203760. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan P, Grzybrowski M, Chen X. et al. Procalcitonin as a marker of serious bacterial infections in febrile children younger than 3 years old. Acad Emerg Emerg. 2014;21:171–9. doi: 10.1111/acem.12316. [DOI] [PubMed] [Google Scholar]
- 15.Nijman RG, Vergouwe Y, Thompson M. et al. Clinical prediction model to aid emergency doctors managing febrile children at risk of serious bacterial infections: diagnostic study. BMJ. 2013;346:f1706. doi: 10.1136/bmj.f1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galetto-Lacour A, Zamora SA, Gervaix A.. A score identifying serious bacterial infections in children with fever without source. Pediatr Infect Infect. 2008;27:654–6. doi: 10.1097/INF.0b013e318168d2b4. [DOI] [PubMed] [Google Scholar]
- 17.Galetto-Lacour A, Zamora SA, Andreola B. et al. Validation of a laboratory index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Dis. 2010;95:968–73. doi: 10.1136/adc.2009.176800. [DOI] [PubMed] [Google Scholar]
- 18.Bressan S, Gomez B, Mintegi S. et al. Diagnostic performance of the “Lab-score” in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239–44. doi: 10.1097/INF.0b013e318266a9aa. [DOI] [PubMed] [Google Scholar]
- 19.Nijman RG, Moll HA, Smit FJ. et al. C-reactive Protein, Procalcitonin and the “Lab-score” for Detecting Serious Bacterial Infections in Febrile Children at the Emergency Department: A Prospective Observational Study. Pediatr Infect Dis J. 2014;33:273–9. doi: 10.1097/INF.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 20.Herz AM, Greenhow TL, Alcantara J. et al. Changing epidemiology of outpatient bacteraemia in 3 – to 36 –months –old children after the introduction of the heptavalent-conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2006;25:293–300. doi: 10.1097/01.inf.0000207485.39112.bf. [DOI] [PubMed] [Google Scholar]
- 21.NICE Clinical Guideline. Feverish illness in children: assessment and initial management in children younger than 5 years. National Collaborating Center for Women’s and Child Health. 2007;47 [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC. et al. 2001 SCCM/ESICM/ACCP/ ATS/SIS International Sepsis Definitions Conference. Crit Care Care. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 23.Thayyil S, Shenoy M, Hamaluba M, Gupta A, Frater J, Verber IG.. Is procalcitonin useful in early diagnosis of serious bacterial infections in children? Acta Pediatrica. 2005;94:155–8. doi: 10.1111/j.1651-2227.2005.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 24.Shaw KN, Gorelick M, McGowan KL, Yakscoe NM, Schwartz JS.. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102:e16. doi: 10.1542/peds.102.2.e16. [DOI] [PubMed] [Google Scholar]
- 25.Mintegi S, Benito J, Pijoan JI. et al. Occult pneumonia in infants with high fever without source: a prospective multicenter study. Pediatr Emerg Care. 2010;26:470–4. doi: 10.1097/PEC.0b013e3181e582e4. [DOI] [PubMed] [Google Scholar]
- 26.Dandona P, Nix D, Wilson MF. et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–8. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- 27.Gendrel D, Bohuon C.. Procalcitonin as a marker of bacterial infection. Pediatr Infect Dis J. 2000;19:679–8. doi: 10.1097/00006454-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Gras-Le Guen C, Delmas C, Launay E. et al. Contribution of procalcitonin to occult bacteraemia detection in children. Scand J Infect Dis. 2007;39:1063–6. doi: 10.1080/00365540600904753. [DOI] [PubMed] [Google Scholar]
- 29.Bressan S, Andreola B, Cattelan F. et al. Predicting severe bacterial infections in well-appearing febrile neonates: laboratory markers accuracy and duration of fever. Pediatr Infect Dis J. 2008;27:227–32. doi: 10.1097/INF.0b013e3181b9a086. [DOI] [PubMed] [Google Scholar]


