Abstract
Objective
Impairments in emotion regulation, and more specifically in cognitive reappraisal, are thought to play a key role in the pathogenesis of anxiety disorders. However, the available evidence on such deficits is inconsistent. To further illustrate the neurobiological underpinnings of anxiety disorder, the present meta-analysis summarizes functional magnetic resonance imaging (fMRI) findings for cognitive reappraisal tasks and investigates related brain areas.
Methods
We performed a comprehensive series of meta-analyses of cognitive reappraisal fMRI studies contrasting patients with anxiety disorder with healthy control (HC) subjects, employing an anisotropic effect-size signed differential mapping approach. We also conducted a subgroup analysis of medication status, anxiety disorder subtype, data-processing software, and MRI field strengths. Meta-regression was used to explore the effects of demographics and clinical characteristics. Eight studies, with 11 datasets including 219 patients with anxiety disorder and 227 HC, were identified.
Results
Compared with HC, patients with anxiety disorder showed relatively decreased activation of the bilateral dorsomedial prefrontal cortex (dmPFC), bilateral dorsal anterior cingulate cortex (dACC), bilateral supplementary motor area (SMA), left ventromedial prefrontal cortex (vmPFC), bilateral parietal cortex, and left fusiform gyrus during cognitive reappraisal. The subgroup analysis, jackknife sensitivity analysis, heterogeneity analysis, and Egger’s tests further confirmed these findings.
Conclusions
Impaired cognitive reappraisal in anxiety disorder may be the consequence of hypo-activation of the prefrontoparietal network, consistent with insufficient top-down control. Our findings provide robust evidence that functional impairment in prefrontoparietal neuronal circuits may have a significant role in the pathogenesis of anxiety disorder.
Keywords: anxiety disorder, emotion regulation, cognitive reappraisal, fMRI, prefrontoparietal network, signed differential mapping
Introduction
Anxiety disorders are globally a very common and disabling group of mental disorders,1,2 the cause of particularly high economic burden and clinically significant personal distress.3 Many studies have sought to understand the neural basis of how pathological anxiety is triggered and maintained. Using emotion regulation questionnaires, psychological studies have indicated that anxiety disorders may entail impaired emotion regulation.4,5 Models of anxiety have highlighted that impaired regulation of negative affectivity plays a significant role in the pathogenesis of anxiety disorders,6,7 explaining the onset and maintenance of anxiety.7
Consequently, a clear understanding of the neural systems underlying emotion dysregulation in anxiety disorders is important for identifying biological targets to improve the specificity and efficacy of diagnostic and therapeutic interventions for anxiety disorder.
Emotion regulation has been conceptualized as the process by which individuals modify the expression, experience, and physiology of their emotions.8 According to models of emotion regulation, the most studied strategy is cognitive reappraisal, a type of antecedent-focused emotion regulation strategy that is achieved by altering one’s interpretation or appraisal of affective events.9,10 Cognitive reappraisal has been proven to be a high-efficiency way of regulating affect and physiological arousal,11 costing less in terms of cognitive resource12,13 compared with response-focused strategies (eg, expressive suppression) and having longer-lasting effects than attention-focused strategies (eg, distraction).14,15 First-line intervention approaches to mental disorder (eg, cognitive behavioral therapy) are closely related to cognitive reappraisal.16 Therefore, a better understanding of the brain mechanisms underlying cognitive reappraisal is crucial for translating basic and translational advances into clinical application.
In the past decades, the rapid growth of literature on fMRI neuroimaging studies has focused on neural correlates of cognitive reappraisal.3,10,17 A well-known behavior and fMRI study demonstrated that cognitive reappraisal resulted in prefrontal cortex responses and decreased negative emotion ratings in healthy control (HC).18 Recently, three meta-analyses showed that implementing cognitive reappraisal consistently activates a large regulatory network, including the high activated dorsolateral prefrontal cortex (dlPFC), dmPFC, dACC, vmPFC, SMA, and inferior/superior parietal cortex, as well as the hypo-activated amygdala and insula, during emotion down-regulation.10,15,17,19 This demonstrates that conscious cognitive reappraisal recruits a classic frontoparietal control network to modulate emotional responding in the amygdala.10,17,19 Although most previous cognitive reappraisal studies of healthy individuals describe a consistent neural regulatory network, whether and how these substrates differ in individuals with anxiety disorders remains poorly understood.
Recent investigations have begun to fill in these gaps, finding that impaired cognitive reappraisal of emotion is observable across anxiety disorders.3 Compared with healthy individuals, patients with social anxiety disorder (SAD) and generalized anxiety disorder (GAD) have been found to show less consistent activation of the dmPFC and dACC when down-regulating negative images using reappraisal.20–22 Elsewhere, a study of post-traumatic stress disorder (PTSD) found less dlPFC activation in patients compared with HC during cognitive reappraisal.23 Such findings show impaired top-down control of the prefrontal cortex during emotion regulation in patients with anxiety disorder. However, not all are entirely consistent. For example, one study using a similar task paradigm for SAD conversely showed increased activation of the dlPFC.20 Furthermore, another recent study found no significant differences in frontoparietal activity during reappraisal between groups of SAD patients and healthy participants.5 As this demonstrates, while there is some understanding of the neural mechanisms underlying anxiety disorders it has become increasingly difficult to aggregate and synthesize neuroimaging findings to reach conclusive agreement. Instead, further brain regions have been found to be associated with anxiety disorders (eg, the SMA and parietal cortex).3 One the one hand, anxiety disorder is often accompanied by multiple psychological and physical symptoms whose neural mechanisms cannot be explained by abnormalities in a single brain region.24 On the other, small and heterogeneous samples and substantial methodological differences between studies are likely to generate false positive results,25 while consistent results obtained by independent studies from different research groups are relatively robust. A comprehensive quantitative analysis (meta-analysis) of the results of multiple studies to obtain convergent experimental evidence could therefore be hugely beneficial.26 Hence, this study attempts to locate the core cognitive neuropsychology mechanisms that are specific to anxiety disorder, based on a meta-analysis of the extant literature.
To our knowledge, only one study has previously performed a meta-analysis of cognitive reappraisal fMRI studies in populations of patients with mood and anxiety disorders.27 However, in that study, only four studies of patients with anxiety disorder were investigated. Other subtypes of anxiety disorder, such as GAD or panic disorder (PD), were not included. Thus, the findings cannot be generalized to other anxiety disorders. Moreover, major depressive disorders and anxiety disorders were combined in the sample of Picó-Pérez et al.27 Two recent reviews have shown major depressive disorders and anxiety disorders to have different disorder-specific deficits in the neural mechanisms of emotion regulation28 involving cognitive reappraisal.3 For example, a malfunction of the dlPFC is more predictive of and specific to depression disorder (relative to anxiety disorders) with an inability to down-regulate emotion reactions,28 while reduced activity of the dACC is specific to anxiety disorders during down-regulation of negative emotion.3 Further, other potentially influential factors, such as anxiety disorder subtype, data-processing software, and MRI field strengths, were not considered in the prior meta-analysis. Therefore, the core neural mechanisms of cognitive reappraisal underlying anxiety disorders remain unclear. Since further original cognitive reappraisal studies of anxiety disorders have been published in recent years, it is worthwhile performing an updated fMRI meta-analysis to investigate the abnormal brain activity underlying reappraisal and to explore other clinical profiles of alterations in brain function.
Thus, the goals of this study were threefold. First, we conducted a pooled meta-analysis to identify the most prominent and replicable brain regions of impaired cognitive reappraisal in patients with anxiety disorders. To do this we used anisotropic effect-size signed differential mapping (AES-SDM) software – a newly developed meta-analytic method with increased specificity, sensitivity, and reliability of analyses – taking reported peak coordinates, statistical parametric maps, or both from original articles to reconstruct effect-size maps of the differences between patients and controls.29 With this method, unlike other coordinate-based meta-analytic approaches such as ALE,30 both positive and negative coordinates are recreated on the same map, which is a significant feature that prevents a particular voxel from erroneously appearing to be positive and negative at the same time.31 Second, subgroup meta-analyses were conducted to explore the heterogeneity and robustness of the main results. Specifically, we performed subgroup meta-analyses to compare treatment-naive/medicine-free anxiety disorder patients with HC. A stratified meta-analysis of the subtype of anxiety disorders was subsequently conducted. We also conducted subgroup meta-analyses on data-processing software (Analysis of Functional NeuroImages [AFNI]) and MRI field strengths (3.0 T). Third, meta-regression analyses assessed the effects of gender and other relevant clinical profiles, such as age and comorbidity.
Methods
Search strategies
A comprehensive systematic literature search was conducted using PubMed, the Cochrane Library, Web of Science, and Embase databases to identify functional magnetic resonance imaging literature on anxiety disorders, published before September 2017 and including “in press” articles. The search keywords were “panic disorder,” “agoraphobia,” “specific phobia,” “social phobia,” “social anxiety disorder,” “SAD,” “posttraumatic stress disorder,” “post-traumatic stress disorder,” “PTSD,” “obsessive compulsive disorder,” “obsessive-compulsive disorder,” “OCD,” “acute stress disorder,” “generalized anxiety disorder,” “generalized anxiety,” “GAD,” and “anxiety,” plus “functional magnetic resonance imaging,” “fMRI,” and “functional MRI,” as well as “reappraisal” and “cognitive reappraisal.” The reference lists of the identified trials and review articles were also manually checked to identify any other relevant papers.
Study eligibility criteria
We considered studies that compared the blood oxygen level dependent responses of patients with anxiety disorder with those of HC, when performing a reappraisal task. In this kind of task, participants undergo fMRI during a cognitive reappraisal task that requires them to reappraise (ie, reduce) or maintain emotional responses to affective pictures (eg, a negative picture). The affective pictures come from the International Affective Picture System (IAPS)32 or other databases. The contrasts of interest are the comparison of two conditions (reappraise and maintain) between clinical and healthy participants. Articles were included in the meta-analysis if they also met the following criteria: (1) patients in the studies had an anxiety disorder that met the criteria of the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV);33 (2) the articles were published in a peer-reviewed journal; (3) whole-brain analyses were applied to both patients and HC; (4) the results of whole-brain analyses were reported; (5) significant thresholds for data were used that were corrected for multiple comparisons. The corresponding authors of the studies qualifying for inclusion were contacted by email for additional information.
Articles were excluded if they met the following criteria: (1) anxiety disorder patients were not compared with HC; (2) they were conference abstracts or reviews; (3) they only reported region of interest results instead of whole-brain findings; (4) task activation analyses using the fMRI method were not used; (5) either the patient or HC group included ≤10 patients; and (6) where the same patient group was used in different publications, only the study with the largest sample was included.
Quality assessment and data extraction
We performed the literature searches, reviewed the retrieved articles, and extracted and cross-checked data. All the studies included were quality-assessed using a 12-point checklist (Table S1) that focused on both the clinical and demographic characteristics of the study samples and the imaging-specific methodology used. This checklist was based on a prior meta-analytic study34,35 that looked at structural MRI measures, but its critical variables are also significant for assessing task-based fMRI studies. Where inconsistent results were obtained, they were discussed and resolved by consensus review. For all studies entered into the meta-analysis, name of first author, publication year, basic demographic information (sample size, age, gender, and percentage of females), patient data (symptom severity, percentage of comorbidity, medication status), task stimulus material, data-processing method, MRI parameters, and three-dimensional coordinates, were extracted according to the AES-SDM method.36
Voxel-wise meta-analysis
Regional differences in activation between patients and HC during fMRI tasks were identified in the selected studies using AES-SDM software in a standard process. Systematic whole-brain jackknife sensitivity analysis was performed to examine the robustness (specifically the sensitivity) of the results. Using this method, where a previously significant brain region remains significant in all or most of the combinations of studies, it can be concluded that the finding is highly replicable.31 Recognizing the possible influence of medication status, subtype of anxiety disorder, and methodological differences between studies, we then performed several subgroup meta-analyses including medicine-naive patients, patients with SAD, patients with GAD and SAD, data processing software (AFNI), and MRI field strengths (3.0 T). Given the variability that exists in treatment status, we defined the sample of medicine-naive patients as participants who had been free of psychotropic medication for at least two months before the scan.37
All the above analytical processes were performed on the selected studies using AES-SDM software,38,39 which has previously been used with psychiatric patients31,40 and is described in detail elsewhere.29,31 Briefly, we used a relatively wide full-width at half-maximum (FWHM; 20 mm) to assign indicators of proximity to the reported three-dimensional coordinates, as this can optimally balance sensitivity and specificity in the AES-SDM.41 We employed the standard threshold (p<0.005 with peak Z>1, extent threshold of clusters >10 voxels) as recommended by the AES-SDM software developers. This is proposed to optimally balance the sensitivity and specificity of results and to be an approximate equivalent to corrected p-value=0.05 (indeed 0.025) in AES-SDM for the effect-size used. This method showed adequate sensitivity and excellent control of false positive results (~2.5%) compared with other meta-analytical procedures.29 Significant clusters obtained from AES-SDM analysis were overlaid on an MRIcron high-resolution brain template for display purposes.31 Publication bias was assessed using Egger’s test, as implemented in the AES-SDM software (version 5.141).
Heterogeneity and publication bias analysis
We examined the statistical (between-studies) heterogeneity of clusters using a random effects model with Q statistics (chi-square distribution converted to z values and tested with both permutations, p<0.005, cluster extent=10 voxels). Moreover, the possibility of publication bias associated with brain regions activated differently during reappraisal between patients and HC was examined using Egger’s test.
Meta-regression analysis
Some basic demographic and clinical information, such as the percentage of female patients, the percentage of comorbidity, sample size, mean age, race (white), and symptom severity (Liebowitz Social Anxiety Scale-Self Rated and Spielberger State-Trait Anxiety Inventory) were entered into the regression analysis to investigate their association with regional differences in activation between patients and HC during cognitive reappraisal. The main output for each variable above was the map of the regression slope. As described in previous meta-analytic studies, we set a more conservative probability threshold (p<0.0005) in order to reduce detection of spurious relationships as much as possible.
Results
Included studies and sample characteristics
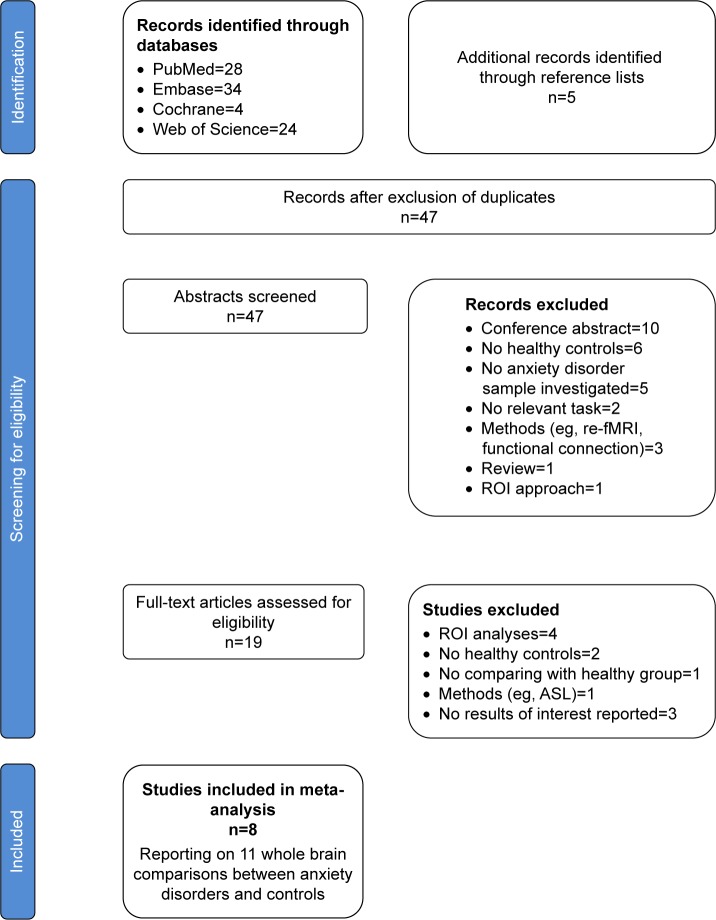
The search strategy described in the methods section yielded a total of 47 studies. Of these, eight studies,5,20–23,42–44 with 11 relevant patients vs HC comparisons, ultimately met the inclusion criteria. In total they included 219 patients with anxiety disorder (70 GAD, 36 PD, 14 PTSD, 82 SAD, 17 comorbid SAD/GAD), and 227 HC. In eight studies, one study contained a down-regulate negative and positive affect,22 other studies only included a down-regulate negative affect. One study5 that found no significant differences between SAD patients and HC during a cognitive reappraisal task was also included – in this case we used a textfile named “Gaebler.no_peaks” in order to report no activation peaks for a given contrast, as described in the meta-analysis tutorial.29,31,36 Patients with anxiety disorder and HC in the studies included were generally comparable in terms of age and gender. Regarding medication status, four studies with five relevant patients vs HC comparisons included patients undergoing treatment or who had undergone a “washout” less than two months before the scan. Figure 1 presents a flow diagram showing how we identified relevant studies. Table 1 summarizes the clinical and demographic data from all the enrolled studies.
Figure 1.
Search strategy used for the inclusion of the studies considered in the meta-analysis.
Abbreviations: ASL, arterial spin labeling; re-fMRI, resting-state functional magnetic resonance imaging; ROI, region of interest.
Table 1.
Demographic and clinical characteristics of the task-based fMRI studies included in the current meta-analysis
| Study | Anxiety disorder | Subjects, n
|
Mean age of patients (SD)
|
Females (%)
|
Medication status (subgroup) | Comorbidity (%) | Software (subgroup) | MRI (sub-group) | Statistical threshold | Quality score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | HC | Patient | HC | Patient | HC | ||||||||
| New et al23 | PTSD | 14 | 14 | 38.7 (11.2) | 31.7 (10.3) | 100 | 100 | Treatment-naive (unmedicated) | Pure PTSD | SPM 2 | 3.0 T |
p<0.05 Corrected |
11.5 |
| Gaebler et al5 | SAD | 21 | 23 | 30.5 (7.17) | 30.0 (7.99) | 76 | 78 | 17 patients treatment naive; 4 patients on medication (medicated) | 38 | SPM 8 | 3.0 T |
p<0.05 Corrected |
12 |
| Ziv et al21 | SAD | 27 | 27 | 31.1 (7.6) | 32.6 (9.5) | 44 | 48 | Treatment-naive for 3 months prior to scan (unmedicated) | 30 | AFNI | 3.0 T |
p<0.005 Corrected |
11.5 |
| Blair et al22 | SAD | 19 | 18 | 29.4 (8.70) | 33.4 (9.65) | 53 | 56 | Treatment-naive (unmedicated) | Pure SAD | AFNI | 1.5 T |
p<0.05 Corrected |
10.5 |
| Goldin et al20 | SAD | 15 | 17 | 31.6 (9.7) | 32.1 (9.3) | 60 | 53 | Treatment-naive (unmedicated) | NA | AFNI | 3.0 T |
p<0.005 Corrected |
11 |
| Blair et al22 | SAD/GAD | 17 | 18 | 35.7 (9.54) | 33.4 (9.65) | 71 | 56 | Treatment-naive (unmedicated) | 100 | AFNI | 1.5 T |
p<0.05 Corrected |
10.5 |
| Ball et al44 | PD | 18 | 22 | 29 (7) | 27 (9) | 83 | 50 | 6 week washout before scan (medicated) | 28 | AFNI | 3.0 T |
p<0.05 Corrected |
12 |
| Reinecke et al42 | PD | 18 | 18 | 36.5 (13.8) | 32.3 (12.1) | 78 | 78 | 3 patients had 48 hour washout before scan (medicated) | 17 | FSL | 3.0 T |
p<0.05 Corrected |
11 |
| Fitzgerald et al43 | GAD | 30 | 30 | 27.20 (7.57) | 25.43 (10.02) | 60 | 77 | 17 patients treatment-naive; 13 patients had 4 weeks washout before scan (medicated) | 60 | SPM 8 | 3.0 T |
p<0.05 Corrected |
12 |
| Ball et al44 | GAD | 23 | 22 | 35 (11) | 27 (9) | 74 | 50 | 6 week washout before scan (medicated) | 52 | AFNI | 3.0 T |
p<0.05 Corrected |
12 |
| Blair et al22 | GAD | 17 | 18 | 36.1 (11.75) | 33.4 (9.65) | 76 | 56 | Treatment-naive (unmedicated) | Pure SAD | AFNI | 1.5 T |
p<0.05 Corrected |
10.5 |
Abbreviations: AFNI, Analysis of Functional NeuroImages; fMRI, functional magnetic resonance imaging; FSL, Functional MRI of the brain Software Libraries package; GAD, generalized anxiety disorder; HC, healthy control; NA, not available; PD, panic disorder; PTSD, posttraumatic stress disorder; SAD, social anxiety disorder; SPM, statistical parametric mapping.
Meta-analysis of functional brain abnormalities during reappraisal in anxiety disorder
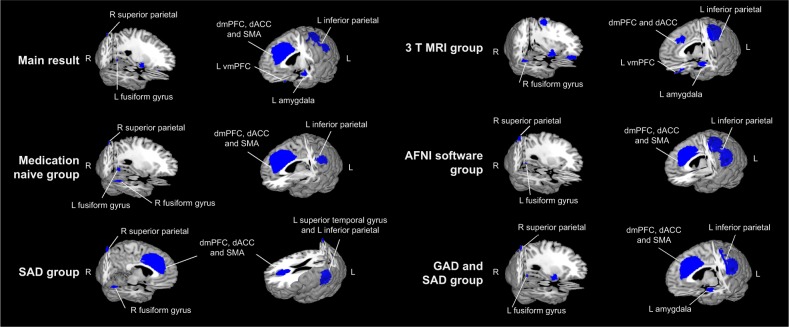
The results from the AES-SDM analysis are summarized in Figure 2 and Table 2. After correcting for multiple comparisons, compared with HC, patients with anxiety disorder showed significantly decreased activity during cognitive reappraisal in (1) the bilateral dmPFC (bilateral superior frontal gyrus, Brodmann area [BA] 9/32) and extending into the bilateral dACC (bilateral dorsal anterior cingulate, dorsal BA 24/32) and bilateral SMA (bilateral supplementary motor area, medial BA 6); (2) the left inferior parietal; (3) the right superior parietal gyrus; (4) the left amygdala; (5) the left fusiform gyrus; and (6) the left vmPFC (middle frontal gyrus, medial BA 11). Definitions of the dmPFC and dACC can be found in previous reviews.3,45 However, we detected no significantly enhanced activity during reappraisal in patients with anxiety disorder compared with HC.
Figure 2.
Regions of decreased (blue) activation in individuals with anxiety disorder compared with HC during cognitive reappraisal and subgroup analysis of medication naive group, 3 T MRI group, AFNI software group, SAD group and GAD and SAD group.
Abbreviations: AFNI, Analysis of Functional NeuroImages; dACC, dorsal anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; GAD, generalized anxiety disorder; HC, healthy control; L, left; R, right; SAD, social anxiety disorder; SMA, supplementary motor area; vmPFC, ventromedial prefrontal cortex.
Table 2.
Altered regional differences in activation during cognitive reappraisal in patients with anxiety disorder compared to healthy controls
| Brain regions
|
MNI coordinates
|
SDM z score | p-value | Voxels, n | Cluster breakdown | Heterogeneity | Jackknife sensitivity | ||
|---|---|---|---|---|---|---|---|---|---|
| AD < HC | X | Y | Z | ||||||
| Left superior frontal gyrus, medial, BA 32 | −2 | 28 | 38 | −2.667 | ~0 | 2,489 | Left superior frontal gyrus, medial, BA 8, 9, 24, 32 (464) Left anterior cingulate/paracingulate gyri, BA 32, 24 (473) Left median cingulate/paracingulate gyri, BA 24, 23, 32 (407) Left supplementary motor area, BA 6, 8, 24, 32 (212) Right median cingulate/paracingulate gyri, BA 24, 23, 32 (483) Right anterior cingulate/paracingulate gyri, BA 24, 32 (229) Right superior frontal gyrus, medial, BA 8, 9, 32 (114) Right supplementary motor area, BA 6, 8, 24, 32 (107) |
ns | 11 out of 11 |
| Left inferior parietal (excluding supramarginal and angular) gyri, BA 3 | −48 | −28 | 46 | −2.329 | 0.000002563 | 1,138 | Left inferior parietal gyri, BA 2, 3, 40 (290) Left postcentral gyrus, BA 1, 2, 3, 4, 6, 40 (561) Left supramarginal gyrus, BA 2, 3, 40, 48 (187) Left precentral gyrus, BA 3, 4, 6 (100) |
ns | 11 out of 11 |
| Left amygdala | −28 | −10 | −12 | −1.767 | 0.000503182 | 216 | Left amygdala, BA 20, 28, 34, 36 (139) Anterior commissure (41) Left inferior network (36) |
Significant | 9 out of 11 |
| Left fusiform gyrus, BA 18 | −26 | −72 | −12 | −1.483 | 0.002632022 | 69 | Left fusiform gyrus, BA 18, 19 (56) Left lingual gyrus, BA 18, 19 (13) |
Significant | 9 out of 11 |
| Right superior parietal gyrus, BA 7 | 26 | −74 | 56 | −1.696 | 0.000781834 | 39 | Right superior parietal gyrus, BA 7 (39) | ns | 8 out of 11 |
| Left middle frontal gyrus, orbital part, BA 11 | −24 | 34 | −16 | −1.478 | 0.002699077 | 27 | Left middle frontal gyrus, orbital part, BA 11 (27) | Significant | 9 out of 11 |
Abbreviations: AD, anxiety disorder; BA, Brodmann area; HC, healthy control; MNI, Montreal Neurological Institute; ns, non-significant; SDM Z, signed differential mapping Z-value.
Jackknife sensitivity analysis
A whole-brain jackknife sensitivity analysis was performed to examine the replicability of the findings of the pooled meta-analysis. As shown in Table 2, the results indicate that the decreased activation in the dmPFC, dACC, SMA and left inferior parietal was highly replicable, preserved as it was throughout all 11 combinations of studies. Results for the left amygdala, left fusiform gyrus, and left middle frontal gyrus remained significant in all but one combination, while those for the right superior parietal gyrus were significant in all but two combinations. Detailed results are listed in Table S2.
Subgroup meta-analyses
Given the possible influence of medication status, subtype of anxiety disorder, and methodological differences between studies, several subgroup meta-analyses were performed. As shown in Figure 2 and Table 3, these analyses suggest that the above results remained largely unchanged. In the subgroup of studies using 3.0 T MRI (eight datasets), all clusters in the combined analysis remained significant except for the right superior parietal gyrus. In the subgroup of studies using AFNI software (seven datasets), all clusters in the combined analysis remained significant except for the left amygdala and left middle frontal gyrus. In the subgroup of medication-naive patients (six datasets), the clusters of the dmPFC, dACC, SMA, left inferior parietal, right superior parietal gyrus, and left fusiform gyrus remained significant. In the subgroup of SAD (five datasets), the clusters of the dmPFC, dACC, SMA, left amygdala, and right superior parietal gyrus remained significant. In the subgroup of both GAD and SAD (eight datasets), the clusters of the dmPFC, dACC, SMA, left inferior parietal, left amygdala, left fusiform gyrus and right superior parietal gyrus remained significant.
Table 3.
Subgroup analyses of the included studies
| Subgroups | Bilateral superior frontal gyrus (dmPFC, dACC and SMA) | Left inferior parietal gyri | Left amygdala | Left fusiform gyrus | Left middle frontal gyrus (vmPFC) | Right superior parietal gyrus |
|---|---|---|---|---|---|---|
| Data sets using 3T MRI (n=8) | Y (except SMA) | Y | Y | Y | Y | N |
| The medication-naive patient (n=6) | Y | Y | N | Y | N | Y |
| Social anxiety disorder (n=5) | Y | N | Y | N | N | Y |
| Data sets using AFNI software (n=7) | Y | Y | N | Y | N | Y |
| Generalized anxiety disorder and social anxiety disorder (n=8) | Y | Y | Y | Y | N | Y |
Abbreviations: AFNI, Analysis of Functional NeuroImages; dACC, dorsal anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; N, no; SMA, supplementary motor area; vmPFC, ventromedial prefrontal cortex; Y, yes.
Heterogeneity analysis and publication bias
An analysis of heterogeneity revealed that a few regions with altered activation (left amygdala, left fusiform gyrus, and left middle frontal gyrus) showed significant statistical heterogeneity between studies (p<0.005). No strong evidence for publication bias was revealed by Egger’s test (p>0.05), except for the left amygdala (p=0.006).
As the left amygdala was absent in two of the five subgroup analyses, and showed a significant heterogeneity and publication bias, we excluded it from the core brain region contributing to impaired cognitive reappraisal in anxiety disorders.
Meta-regression analyses
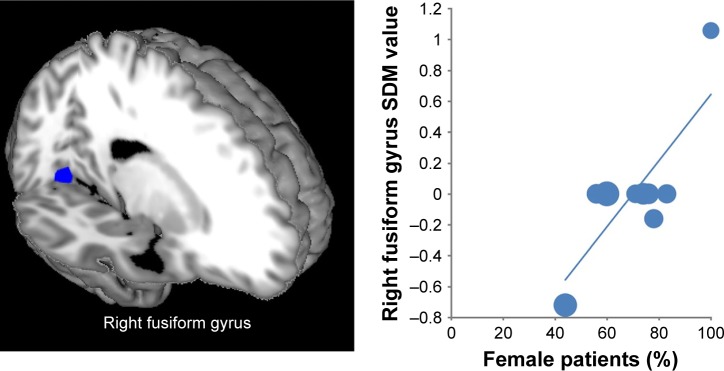
As shown in Figure 3, the percentage of female patients had a positive relationship with activation in the right fusiform gyrus (Montreal Neurological Institute coordinate: x=20, y=−64, z=−12; AES-SDM value=2.369, p=0.000125587; 77 voxels). However, this result should be interpreted with caution as it is based on only three studies. Percentage of comorbidity, sample size, and mean age of patients with anxiety disorder were not associated with decreased brain regions. Race (white) and symptom severity (Liebowitz Social Anxiety Scale-Self Rated and Spielberger State-Trait Anxiety Inventory) could not be explored because only six and five datasets respectively were available from the studies included.31
Figure 3.
Results of the meta-regression analysis showing the percentage of female patients with a positive relationship with activation in the right fusiform gyrus. In the graphs, AES-SDM values needed to create this plot were extracted from the peak of maximum slope significance, and each study is represented as a dot, whose size reflects sample size. The regression line (meta-regression signed differential mapping slope) is shown.
Abbreviations: AES, anisotropic effect-size; SDM, signed differential mapping.
Discussion
We believe that this is the first study to independently explore regional neurofunctional activation and simultaneously investigate subgroup meta-analyses and meta-regression analyses in patients with anxiety disorder (including most subtypes) during cognitive reappraisal. First, the pooled meta-analysis identified significantly decreased activity during cognitive reappraisal in the bilateral dmPFC, bilateral dACC, bilateral SMA, bilateral parietal cortex, left vmPFC, and left fusiform gyrus in patients with anxiety disorder compared with HC. Second, subgroup analyses indicated that the above findings remained largely unchanged. The robustness of the main findings was further demonstrated by the jackknife sensitivity analysis, heterogeneity analysis, and Egger tests. Finally, meta-regression analysis showed that activation in the right fusiform gyrus was positively correlated with percentage of female patients. These findings suggest that impaired cognitive reappraisal in anxiety disorder may be the consequence of hypo-activation in the prefrontal cortex, dACC, and parietal cortex during emotion regulation, consistent with insufficient top-down control.
The most common finding of the current study was decreased activation in the bilateral dmPFC of patients with anxiety disorder during cognitive reappraisal. A large number of cognitive reappraisal studies have shown that healthy individuals can activate the dmPFC to achieve successful emotion regulation,10 whereas here dmPFC activation was lower in patients with anxiety disorder during reappraisal. According to the results of cognitive emotion regulation questionnaire and behavioral research, anxiety disorder had impaired cognitive emotion regulation strategies involving cognitive reappraisal.44,46 Neuroimaging studies have found that decreased dmPFC activity in anxiety disorder might be associated with reduced emotion regulation capability.21 Furthermore, studies of healthy adults have found that activation in the dmPFC is associated with perceiving one’s affective states47,48 and self-related processing.49 A previous meta-analysis examining large samples of healthy subjects in cognitive reappraisal studies has suggested that the dmPFC may support semantic and self-reflective processes relevant to elaborating the meaning of affective stimuli or drawing inferences about one’s emotional state.10 According to the findings discussed above, one possible explanation for the decreased dmPFC is that patients are unable to recruit the dmPFC to assist in monitoring and reflecting on the meaning of altering their affective states during reappraisal, and that this contributes to a reduced ability to down-regulate negative reactivity. Thus, insufficient recruitment of dmPFC activity could be an important neural correlate of emotion regulation deficits in anxiety disorder.
Comparing patients with anxiety disorder and HC during reappraisal revealed clusters of decreased activity in the dmPFC and extending into the dACC and SMA. The dACC lies on the medial surface of the frontal lobes and plays a significant role in regulating top-down cognitive control and assigning appropriate control to other areas of the brain.50,51 The dACC has also been recognized as a key region of the emotion-regulation network,10,17,19 which is thought to mediate several functions including monitoring for conflict and detecting the likelihood of error commission, particularly involving attentional and executive control during the appraisal of negative emotion.50,52,53 Poor emotion regulation may involve impaired attention control. Theories based on cognitive and neuroscience research have indicated that deficient attentional control is associated with anxiety.54,55 A previous review also found that anxiety disorder shows impaired recruitment of the dACC during emotion regulation.3 Consequently, our findings suggest that anxiety disorder has a reduced ability to recruit the dACC implicated in top-down attention control to engage the emotion-regulation network. In addition, the SMA has also been consistently implicated as a core region of the emotion-regulation network in healthy people, and is associated with successful down-regulation of negative emotion.10,17 During reconceptualization (changing an appraisal) the pre-SMA may reflect the execution of this reconceptualization by reformulating mental representations through language and memory processing, with the anterior and posterior cluster of the SMA related to cognitive and executive aspects of motor behavior.17 Thus, an impaired SMA may reflect reduced recruitment of the executive and cognitive aspects at different stages, which contributed to emotion dysregulation in anxiety disorders.
The parietal cortex is one of the brain regions most commonly identified in fMRI studies of emotion regulation in anxiety disorders.20,22 This brain region is a part of the frontoparietal control region56 related to sensory information, with top-down response-related information to facilitate flexible, goal-directed behavior.57 Meta-analyses of neuroimaging studies have indicated that the parietal cortex is mainly involved in selective attention and might assist in holding reappraisals in mind.14,15 Compared with HC, patients with PTSD have a reduced ability to recruit the parietal cortex, implicated in top-down attentional control in the affective number Stroop task.58 In one structural neuroimaging study, the SAD patients showed increased cortical thickness in the frontoparietal network, which fitted with findings at a functional level showing frontoparietal networks to be associated with executive-controlling and attentional functions.59 Together, these results indicate that abnormalities in the parietal cortex may result in the dysfunction of top-down attentional control in anxiety disorder.
Another brain region showing abnormally reduced activity during appraisal was the left vmPFC. Because direct connections between the frontoparietal control region and amygdala associated with emotional response are relatively sparse compared with connections between vmPFC and the amygdala,60 frontoparietal areas are likely to influence the amygdala indirectly by modulating activity in the vmPFC.14,52 Based on animal and human research, vmPFC plays an important role in fear extinction and appraisal of negative emotion.10,52 A prominent neurobiological model of the vmPFC highlights top-down inhibition of the amygdala through the vmPFC as a crucial neural mechanism that may be defective in certain mood and anxiety disorders.61 Therefore, it might be speculated that patients with anxiety disorder cannot recruit the vmPFC through frontoparietal areas, in turn modulating the emotional response of the amygdala.
Besides cognitive and attentional regions, the left fusiform gyrus was found to have decreased functional activation in anxiety disorder during reappraisal. The fusiform gyrus is associated with the perception of affective stimuli.62 Commonly used emotion stimuli for probing neurobiological responses to cognitive reappraisal mainly come from the IAPS3 and involve face stimuli.32 During emotional face processing, healthy samples show greater neural activity in the fusiform gyrus,63 while highly anxious individuals show impaired ability to process emotional faces.64 Furthermore, SAD patients show significantly weaker activation in the left fusiform gyrus for emotional face perception, as compared with HC.65 Emotion perception is a significant part of emotion regulation; meta-analytic connectivity modeling analysis has shown the bilateral fusiform gyrus to be involved in cognitive emotion regulation.17 Thus, our findings suggest that an impaired fusiform gyrus may be an important neural correlate in anxiety disorders of cognitive emotion dysregulation. Notably, the meta-regression analysis identified a positive association between percentage of female patients and activation of the right fusiform gyrus. Female patients with anxiety disorder seem to have distinct neurobiological underpinnings compared with male patients.66 A previous neuroimaging study reported that the fusiform gyrus showed increased activity in females with stress compared with males with stress, when perceiving emotional faces.67 This result might reflect the fact that female patients with anxiety disorder have less difficulty recognizing and distinguishing emotions compared with male patients with anxiety disorder.
However, heterogeneity analysis showed that there was significant, unexplained between-study variability concerning the left vmPFC and left fusiform gyrus. In our meta-analytic study, besides heterogeneity analysis, we also attempted to control the quality of the studies included by applying strict selection criteria and performing jackknife sensitivity analysis, subgroup analyses, and meta-regression analyses in order to reduce heterogeneity as much as possible. Although the left vmPFC and left fusiform gyrus showed significant heterogeneity, a quantitative assessment, as measured by Egger’s test, was not significant. The jackknife sensitivity analysis further confirmed the robustness of the findings. Therefore, in our view, these two brain regions remain significant in our meta-analysis.
Interestingly, while our meta-analytic study found an abnormal neural network contributing to impaired cognitive emotion regulation, an altered dlPFC, typically found in cognitive reappraisal,10,17 was not found. The dlPFC has been proposed to play an important role in cognitive emotion regulation.15 This brain area was cognitive control center is related to action inhibition, working memory, reasoning, and social cognition.17 There are some explanations for the non-significant changes in the dlPFC in the present meta-analysis. First, although the dlPFC is a significant part of the frontoparietal control network, a flexible and superordinate system supporting adaptive behavioral control,57,68 different brain regions of the frontoparietal network have shared and unique functions across a broad range of cognitive demands.68 Normal activation in the dlPFC may not be affected because of help from other brain areas of the frontoparietal network. Behavior studies have shown that patients with anxiety disorder can successfully down-regulate their negative emotion,43,44 which may be in line with the above putative mechanism by which the activity of the dlPFC is not affected. Second, although in their review Zilverstand et al3 reported abnormal activity in the dlPFC in anxiety disorders, they did not conduct a meta-analysis to provide a comprehensive description of the neurobiological underpinnings of cognitive emotion dysregulation. Furthermore, some of the articles in their review did not have sufficient statistical power. For example, the article reported decreased activity in the dlPFC in anxiety disorder compared with HC during reappraisal on the basis of a comparison of group differences, and no task effect or group × task interactions were found in the prefrontal cortex.44
Compared with the study of Picó-Pérez et al,27 decreased activation in ventrolateral prefrontal cortex (vlPFC) and increased activation in insula, cerebellum, precentral and inferior occipital gyri were not observed in our study. These findings could be explained by two aspects. First, the vlPFC may support selection and inhibition of appropriate appraisals.10 As a significant part of the limbic network, the insula is critically involved in the perception and encoding of aversive stimuli.69 Previous systematic reviews suggested that dysfunction of lateral prefrontal cortex was predictive and specific to depression disorder28 and major depressive patients could not recruit lateral prefrontal cortical region (ie, vlPFC) to attenuate activity in the limbic network during cognitive reappraisal.45 In addition, relative to anxiety disorders, enhanced responses of limbic system during downregulation of emotion were specific findings to major depressive disorder.3 Second, enhanced responses of limbic system contributed to excessive emotional processing and emotional experience,3,45 the regions including cerebellum, precentral and inferior occipital gyri might be associated with the emotional experience.27 Therefore, these regions that were not observed in our study might reflect specific deficits of major depressive disorder in neural mechanisms of emotion regulation. These findings could be useful in distinguishing anxiety disorder from major depression.
Limitations
There are several limitations to our study that should be highlighted. First, the number of fMRI studies included was small; the literature search yielded only eight studies with 11 relevant patients vs controls comparisons. This could affect the generalizability of our results, particularly in the subgroup meta-analyses and meta-regressions analyses. Second, our meta-analysis was based on coordinates from published studies rather than raw statistical maps, which might reduce its accuracy.29 Third, the heterogeneity of the data acquisition and analysis techniques, including MRI field strengths, slice thickness, voxel size, and data-processing software, may reduce the accuracy of these results. Fourth, our meta-analysis included studies of medicine-naive patients who had undergone a medication washout period before scanning, so that longer-term influences of medication on brain function could not be completely excluded. Although we conducted a subgroup meta-analysis of the medicine-naive, these results should be interpreted with caution. Finally, some patients with anxiety disorder had co-morbid major depression. Anxiety and major depressive disorders may have different disorder-specific deficits in the neural mechanisms of cognitive reappraisal.3,28 Although the patients fulfilled our criteria for comorbid-major depression with anxiety disorder being the primary diagnosis, the influence of major depression cannot be completely ruled out.
Conclusion
In this meta-analysis, we identified the most robust functional neuroimaging findings on cognitive reappraisal in anxiety disorder. The results demonstrated that patients with anxiety disorder could not recruit the prefrontoparietal network, including the dmPFC, dACC, SMA, vmPFC, and parietal cortex, to down-regulate their emotion response. Our findings provide robust evidence that impairment of prefrontoparietal neuronal circuits may play an important role in the pathogenesis of anxiety disorder. This finding may provide novel targets for medical or cognitive-behavioral interventions and neuromodulation approaches (eg, transcranial magnetic stimulation). With longitudinal data, future investigations should further explore whether these functional abnormities are associated with structural changes or influenced by disease severity and medication status.
Supplementary materials
Table S1.
Imaging methodology quality assessment checklist (when criteria were partially met, 0.5 points were assigned)
| Category 1: subjects | Score (0/0.5/1) |
|---|---|
| 1. Patients were evaluated prospectively, specific diagnostic criteria were applied, and demographic data were reported | |
| 2. Healthy comparison subjects were evaluated prospectively, psychiatric and medical illnesses were excluded | |
| 3. Important variables (eg, age, gender, illness duration, onset time, medication status, comorbidity, severity of illness) were checked, either by stratification or statistically | |
| 4. Sample size per group >10 | |
|
| |
| Category 2: methods for image acquisition and analysis | |
|
| |
| 5. Magnet strength at least 1.5 T | |
| 6. MRI slice-thickness ≤3 mm | |
| 7. Whole brain analysis was automated with no a priori regional selection | |
| 8. Coordinates reported in a standard space | |
| 9. The imaging technique used was clearly described so that it could be reproduced | |
| 10. Measurements were clearly described so that they could be reproduced | |
|
| |
| Category 3: results and conclusions | |
|
| |
| 11. Statistical parameters for significant, and important non-significant, differences were provided | |
| 12. Conclusions were consistent with the results obtained and the limitations were discussed | |
| Total /12 | |
Table S2.
Sensitivity analyses of task-based fMRI studies of regional differences in activation in patients with anxiety disorder compared with healthy controls
| Study | Bilateral superior frontal gyrus (dmPFC, dACC and SMA) | Left inferior parietal gyri | Left amygdala | Left fusiform gyrus | Right superior parietal gyrus | Left vmPFC |
|---|---|---|---|---|---|---|
| New et al1 (PTSD) | Y | Y | Y | Y | Y | N |
| Gaebler et al2 (SAD) | Y | Y | Y | Y | Y | Y |
| Ziv et al3 (SAD) | Y | Y | N | N | Y | Y |
| Blair et al4 (SAD) | Y | Y | Y | Y | N | Y |
| Goldin et al5 (SAD) | Y | Y | Y | N | Y | Y |
| Blair et al4 (SAD/GAD) | Y | Y | Y | Y | N | Y |
| Ball et al6 (PD) | Y | Y | Y | Y | Y | Y |
| Reinecke et al7 (PD) | Y | Y | Y | Y | Y | Y |
| Fitzgerald et al8 (PD) | Y | Y | N | Y | Y | N |
| Ball et al6 (GAD) | Y | Y | Y | Y | Y | Y |
| Blair et al4 (GAD) | Y | Y | Y | Y | N | Y |
Abbreviations: dACC, dorsal anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; fMRI, functional magnetic resonance imaging; GAD, generalized anxiety disorder; N, no; PD, panic disorder; PTSD, post-traumatic stress disorder; SAD, social anxiety disorder; SMA, supplementary motor area; vmPFC, ventromedial prefrontal cortex; Y, yes.
References
- 1.New AS, Fan J, Murrough JW, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and post-traumatic stress disorder. Biol Psychiatry. 2009;66(7):656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Gaebler M, Daniels JK, Lamke JP, Fydrich T, Walter H. Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. J Psychiatry Neurosci. 2014;39(4):249–258. doi: 10.1503/jpn.130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 2013;3(1):20. doi: 10.1186/2045-5380-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair KS, Geraci M, Smith BW, et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72(6):476–482. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball TM, Ramsawh HJ, Campbellsills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med. 2013;43(7):1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinecke A, Filippini N, Berna C, et al. Effective emotion regulation strategies improve fMRI and ECG markers of psychopathology in panic disorder: implications for psychological treatment action. Transl Psychiatry. 2015;5:e673. doi: 10.1038/tp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald JM, Phan KL, Kennedy AE, Shankman SA, Langenecker SA, Klumpp H. Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. J Affect Disord. 2017;218:398–406. doi: 10.1016/j.jad.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
The authors wish to thank all investigators who have provided additional information regarding their studies upon our request. This work was supported by National Natural Science Foundation of China (grant no. 81401486) and the Natural Science Foundation of Shandong Province of China (ZR2015HL039).
Footnotes
Author contributions
Hai-Yang Wang was the main author of this study and participated in the conception and design of the study, literature search, methods, analysis and manuscript write-up; Xiao-Xia Zhang, Cui-Ping Si, Yang Xu, Qian Liu, He-Tao Bian, and Bing-Wei Zhang contributed to reviewing studies, extracting data and feedback on analysis; Xue-Lin Li and Zhong-Rui Yan, corresponding authors, participated in the design of the study, analysis and interpretation of data. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kessler RC, Aguilargaxiola S, Alonso J, et al. The global burden of mental disorders: An update from the WHO World Mental Health (WMH) Surveys. Epidemiol Psichiatr Soc. 2009;18(1):23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter AJ, Vos T, Scott KM, Ferrari AJ, Whiteford HA. The global burden of anxiety disorders in 2010. Psychol Med. 2014;44(11):2363–2374. doi: 10.1017/S0033291713003243. [DOI] [PubMed] [Google Scholar]
- 3.Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage. 2017;151:105–116. doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang BW, Xu J, Wang H, Yao H, Zhang L, Liu XW. Cognitive emotion regulation strategies in subjects with panic disorder. Chinese J Behav Med Brain Sci. 2014;23(6):484–486. Chinese. [Google Scholar]
- 5.Gaebler M, Daniels JK, Lamke JP, Fydrich T, Walter H. Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. J Psychiatry Neurosci. 2014;39(4):249–258. doi: 10.1503/jpn.130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders–republished article. Behav Ther. 2016;47(6):838–853. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion regulation and the anxiety disorders: an integrative review. J Psychopathol Behav Assess. 2010;32(1):68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998;2(3):271–299. [Google Scholar]
- 9.Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- 10.Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- 12.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 13.Yuan J, Long Q, Ding N, Lou Y, Liu Y, Yang J. Suppression dampens unpleasant emotion faster than reappraisal: Neural dynamics in a Chinese sample. Sci China Life Sci. 2015;58(5):480–491. doi: 10.1007/s11427-014-4739-6. [DOI] [PubMed] [Google Scholar]
- 14.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT. The Current State of Cognitive Therapy: A 40-Year Retrospective. Arch Gen Psychiatry. 2005;62(9):953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- 17.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation – an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87(Pt 2):345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldin PR, Mcrae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank DW, Dewitt M, Hudgenshaney M, et al. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biol Mood Anxiety Disord. 2013;3(1):20. doi: 10.1186/2045-5380-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair KS, Geraci M, Smith BW, et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72(6):476–482. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.New AS, Fan J, Murrough JW, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66(7):656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Turk-Browne NB. Functional interactions as big data in the human brain. Science. 2013;342(6158):580–584. doi: 10.1126/science.1238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2(2):150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Horn JD, Grafton ST, Rockmore D, Gazzaniga MS. Sharing neuroimaging studies of human cognition. Nat Neurosci. 2004;7(5):473–481. doi: 10.1038/nn1231. [DOI] [PubMed] [Google Scholar]
- 27.Picó-Pérez M, Radua J, Steward T, Menchón JM, Soriano-Mas C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:96–104. doi: 10.1016/j.pnpbp.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Liu YZ, Liu YZ, Lin WJ, et al. The core neural mechanisms underlying depression disorder: a meta-analysis of fMRI studies. Sci China. 2015;45(12):1214–1223. [Google Scholar]
- 29.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 31.Radua J, Mataixcols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Brit J Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 32.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. 2008. (Technical Report A-8). [Google Scholar]
- 33.American Psychiatric Association . DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 34.Du M, Liu J, Chen Z, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39(6):397–406. doi: 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, Yi G, Zhu H, et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: A voxel-based meta-analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:179–187. doi: 10.1016/j.pnpbp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Radua J, Rubia K, Canales-Rodríguez EJ, Pomarol-Clotet E, Fusar-Poli P, Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwabuchi SJ, Krishnadas R, Li C, Auer D, Radua J, Palaniyappan L. Localized connectivity in depression: A meta-analysis of resting state functional imaging studies. Neurosci Biobehav Rev. 2015;51(1):77–86. doi: 10.1016/j.neubiorev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Radua J, Grau M, Van Den Heuvel OA, et al. Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive–compulsive disorder. Neuropsychopharmacology. 2014;39(7):1547–1557. doi: 10.1038/npp.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76(8):616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norman LJ, Carlisi C, Lukito S, et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73(8):815–825. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- 41.Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127(1–3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Reinecke A, Filippini N, Berna C, et al. Effective emotion regulation strategies improve fMRI and ECG markers of psychopathology in panic disorder: implications for psychological treatment action. Transl Psychiatry. 2015;5:e673. doi: 10.1038/tp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitzgerald JM, Phan KL, Kennedy AE, Shankman SA, Langenecker SA, Klumpp H. Prefrontal and amygdala engagement during emotional reactivity and regulation in generalized anxiety disorder. J Affect Disord. 2017;218:398–406. doi: 10.1016/j.jad.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ball TM, Ramsawh HJ, Campbellsills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med. 2013;43(7):1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rive MM, Rooijen GV, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37(2):2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Zhang BW, Xu J, Chang Y, Wang H, Yao H, Tang D. Impaired cognitive reappraisal in panic disorder revealed by the late positive potential. Neuroreport. 2016;27(2):99–103. doi: 10.1097/WNR.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 47.Paradiso S, Johnson DL, Andreasen NC, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry. 1999;156(10):1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- 48.Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 49.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheth SA, Mian MK, Patel SR, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun L, Peräkylä J, Polvivaara M, et al. Human anterior thalamic nuclei are involved in emotion–attention interaction. Neuropsychologia. 2015;78(825):88–94. doi: 10.1016/j.neuropsychologia.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clemens B, Voß B, Pawliczek C, et al. Effect of MAOA genotype on resting-state networks in healthy participants. Cereb Cortex. 2015;25(7):1771–1781. doi: 10.1093/cercor/bht366. [DOI] [PubMed] [Google Scholar]
- 54.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 55.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 56.Wang HY, Xu GQ, Ni MF, et al. Neural mechanisms of implicit cognitive reappraisal: preceding descriptions alter emotional response to unpleasant images. Neuroscience. 2017;347:65–75. doi: 10.1016/j.neuroscience.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 57.Dodds CM, Sharon MZ, Robbins TW. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cerebral Cortex. 2011;21(5):1155–1165. doi: 10.1093/cercor/bhq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blair KS, Vythilingam M, Crowe SL, et al. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychol Med. 2013;43(1):85–95. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brühl AB, Hänggi J, Baur V, et al. Increased cortical thickness in a frontoparietal network in social anxiety disorder. Hum Brain Mapp. 2014;35(7):2966–2977. doi: 10.1002/hbm.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myersschulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry. 2012;17(2):132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geday J, Gjedde A, Boldsen AS, Kupers R. Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage. 2003;18(3):675–684. doi: 10.1016/s1053-8119(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 63.Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126(Pt 11):2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- 64.Rossion B, Dricot L, Devolder A, et al. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J Cogn Neurosci. 2000;12(5):793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- 65.Gentili C, Gobbini MI, Ricciardi E, et al. Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull. 2008;77(5):286–292. doi: 10.1016/j.brainresbull.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Kelimer LM, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mather M, Lighthall NR, Nga L, Gorlick MA. Sex differences in how stress affects brain activity during face viewing. Neuroreport. 2010;21(14):933–937. doi: 10.1097/WNR.0b013e32833ddd92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harding IH, Yücel M, Harrison BJ, Pantelis C, Breakspear M. Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. Neuroimage. 2015;106:144–153. doi: 10.1016/j.neuroimage.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 69.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Imaging methodology quality assessment checklist (when criteria were partially met, 0.5 points were assigned)
| Category 1: subjects | Score (0/0.5/1) |
|---|---|
| 1. Patients were evaluated prospectively, specific diagnostic criteria were applied, and demographic data were reported | |
| 2. Healthy comparison subjects were evaluated prospectively, psychiatric and medical illnesses were excluded | |
| 3. Important variables (eg, age, gender, illness duration, onset time, medication status, comorbidity, severity of illness) were checked, either by stratification or statistically | |
| 4. Sample size per group >10 | |
|
| |
| Category 2: methods for image acquisition and analysis | |
|
| |
| 5. Magnet strength at least 1.5 T | |
| 6. MRI slice-thickness ≤3 mm | |
| 7. Whole brain analysis was automated with no a priori regional selection | |
| 8. Coordinates reported in a standard space | |
| 9. The imaging technique used was clearly described so that it could be reproduced | |
| 10. Measurements were clearly described so that they could be reproduced | |
|
| |
| Category 3: results and conclusions | |
|
| |
| 11. Statistical parameters for significant, and important non-significant, differences were provided | |
| 12. Conclusions were consistent with the results obtained and the limitations were discussed | |
| Total /12 | |
Table S2.
Sensitivity analyses of task-based fMRI studies of regional differences in activation in patients with anxiety disorder compared with healthy controls
| Study | Bilateral superior frontal gyrus (dmPFC, dACC and SMA) | Left inferior parietal gyri | Left amygdala | Left fusiform gyrus | Right superior parietal gyrus | Left vmPFC |
|---|---|---|---|---|---|---|
| New et al1 (PTSD) | Y | Y | Y | Y | Y | N |
| Gaebler et al2 (SAD) | Y | Y | Y | Y | Y | Y |
| Ziv et al3 (SAD) | Y | Y | N | N | Y | Y |
| Blair et al4 (SAD) | Y | Y | Y | Y | N | Y |
| Goldin et al5 (SAD) | Y | Y | Y | N | Y | Y |
| Blair et al4 (SAD/GAD) | Y | Y | Y | Y | N | Y |
| Ball et al6 (PD) | Y | Y | Y | Y | Y | Y |
| Reinecke et al7 (PD) | Y | Y | Y | Y | Y | Y |
| Fitzgerald et al8 (PD) | Y | Y | N | Y | Y | N |
| Ball et al6 (GAD) | Y | Y | Y | Y | Y | Y |
| Blair et al4 (GAD) | Y | Y | Y | Y | N | Y |
Abbreviations: dACC, dorsal anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; fMRI, functional magnetic resonance imaging; GAD, generalized anxiety disorder; N, no; PD, panic disorder; PTSD, post-traumatic stress disorder; SAD, social anxiety disorder; SMA, supplementary motor area; vmPFC, ventromedial prefrontal cortex; Y, yes.