Abstract.
Urban malaria is an underestimated serious health concern in African countries. This study aimed to evaluate the risk of malaria transmission in an urban area by evaluating the level of human exposure to Anopheles bites using an Anopheles salivary biomarker (gambiae Salivary Gland Protein-6 peptide 1 [gSG6-P1] peptide). Two multidisciplinary cross-sectional studies were undertaken in five sites of Bouaké city (three urban districts and two surrounding villages, used as control; Côte d’Ivoire) during the rainy season and the dry season. Blood samples were obtained from children 6 months to 14 years of age for immunological tests. The level of anti-gSG6-P1 immunoglobulin G (IgG) antibodies was significantly higher in the rainy season than the dry season in both urban and rural sites (P < 0.0001). Interestingly, children with the highest anti-gSG6-P1 IgG responses in the rainy season were infected by Plasmodium falciparum. Surprisingly, no difference of anti-gSG6-P1 IgG level was observed between urban and rural areas, for either season. The current data suggest that children in the urban city of Bouaké could be as highly exposed to Anopheles bites as children living in surrounding villages. The immunological biomarker of human exposure to Anopheles bites may be used to accurately assess the potential risk of malaria transmission in African urban settings.
INTRODUCTION
The Roll Back Malaria partnership launched by the World Health Organization (WHO) in the early 2000s has significantly participated in reducing the burden of malaria worldwide. Indeed, between 2000 and 2015, malaria incidence globally fell by 37%. During the same period, the mortality rate fell by 60% in all age groups and by 65% in children aged less than 5 years.1
However, this considerable decrease in the malaria burden mainly occurred in rural areas where the major control strategies were widely implemented and appeared to be more efficient. In addition, the effectiveness of malaria control strategies and its pertinent evaluation appear to be more difficult to plan and to coordinate in urban areas.2 In sub-Saharan Africa, 39.1% of the population are presently living in urban cities.3 Urbanization in Africa is increasing at such a rate that it is estimated that 54% of the African population will live in urban areas by 2030.4 This urbanization and the mass migrations of human populations from the rural countryside are increasing so rapidly than most African cities that are struggling to cope with the pace and the extent of their urbanization. In malaria-endemic areas, large modern cities were previously conventionally considered low for malaria transmission because of the rarity of natural breeding as well as the level of potential breeding pollution. However, an increased risk of malaria in urban areas was highlighted after the anarchic occupation of urban space, which could favor the proliferation of breeding sites of malaria vectors5,6 and thus a local transmission of malaria.7 Indeed, an ecological adaptation of Anopheles mosquitoes is increasing in urban environments because of their genetic diversity and their considerable ecological plasticity.8 In addition, people living in urban areas could be at high risk of malaria morbidity and mortality because of their recent or late age-dependent acquisition or absence of protective immunity.7,9 Urban malaria is presently considered an emerging public health concern in Africa, and urban areas could be considered as high hot spots of malaria transmission.10
The present study focused on the risk of urban malaria in Bouaké, the second largest city in Côte d’Ivoire where malaria is transmitted on a perennial basis all year round, with seasonal upsurges.11 Malaria parasites, mainly Plasmodium falciparum, are transmitted by two Anopheles species: Anopheles gambiae s.l. and Anopheles funestus. This city suffered from an armed conflict and a sociopolitical crisis from 2002 to 2012. This situation led to a modification of the local environment and to an interruption of the implementation and/or maintenance of malaria-control initiatives.12
The evaluation of human exposure to Anopheles vectors, and consequently the risk of malaria, is presently based on entomological methods (sampling mosquito populations in households by human landing catches [HLCs], residual sprayings, etc.).13 However, these methods present several limitations when it comes to large-scale field studies and especially when exposure levels are low (dry season, altitude, local hot spots, and urban context).14 In addition, entomological methods are mainly applicable at the population level and do not allow the evaluation of the heterogeneity of Anopheles exposure between individuals. Indeed, attractiveness to mosquitoes as well as environmental and socioeconomic factors could induce substantial variations in individual exposure to Anopheles bites.15 Human landing catch using adult volunteers is presently the best method for evaluating individual human exposure; however, this method raises ethical questions and the results cannot be extrapolated to children.16
To improve the evaluation of malaria transmission/exposure according to the WHO recommendations, efforts are being made to develop new indicators at the individual level. Over the past decade, several studies have shown that the measurement of the antibody (Ab) response to arthropod vector saliva proteins in human populations is a pertinent biomarker for assessing the human exposure level to these arthropod bites and thus the risk of vector-borne diseases.17,18 Specifically for Anopheles exposure, the gambiae Salivary Gland Protein-6 peptide 1 (gSG6-P1) salivary peptide of An. gambiae was validated as a biomarker of human exposure to Anopheles bites.19 Indeed, this salivary peptide is specific to the Anopheles genus, antigenic, easily synthesized, and highly conserved between Anopheles species.19 The specific immunoglobulin G (IgG) response to gSG6-P1 peptide was especially relevant as a biomarker in a context of low exposure to Anopheles bites and in local hot spots of transmission.20 Recently, this biomarker was used for evaluating malaria risk in urban settings of Dakar city, Senegal. In this study, its use as an indicator has made it possible to 1) assess the heterogeneity of exposure to Anopheles bites and malaria risk according to urban districts20 and 2) evaluate the effectiveness of various vector-control measures.21
The present study aims to evaluate the human exposure level to Anopheles bites, and thus to the risk of malaria, using the Anopheles salivary biomarker, in children living in an endemic urban area (Bouaké). For this purpose, the evolution of anti-gSG6-P1 IgG responses to the salivary peptide of Anopheles was evaluated in children in this urban area between the rainy season and the dry season and was compared with nearby rural areas.
MATERIALS AND METHODS
Study area and population.
The study was carried out in Bouaké, the second most populous city in the country after Abidjan (the economic capital) with 542,000 inhabitants in 2014. It is located in a tropical wet climate zone with two main seasons: a dry season (November to April) and a rainy season (June to October).22,23 The city of Bouaké is composed of several districts and a large constellation of villages around it. Because of demographic factors, these villages are swallowed up by certain districts of the city. Bouaké lies on a flat relief and is covered with a wooded savannah and crossed by the river Kan. The city has a complete health care offering with health centers and pharmacies. It also has modern city infrastructures such as an airport, buildings, paved roads, schools, and industries. In this city, three urban districts (Kennedy, Dar-es-Salaam, and N’Gattakro), representative of these typical ecological situations, were selected for the present study. In parallel, two villages (Petessou and Allokokro) located close to Bouaké city (with a similar seasonal climate) were included as a rural control. In both urban and rural sites, the study population concerned children aged from 6 months to 14 years. Data were collected during two cross-sectional surveys: the first one in August 2014 (rainy season) and the second one in April 2015 (end of dry season). At each survey, households and children were randomly selected and sociological, geographical, entomological, and parasitological data were collected. For immunological assays, blood samples were collected in Microtainer tubes (Microvette 500 serum-Gel; Sarstedt, Nümbrecht, Germany). Once at the laboratory, the blood samples were centrifuged and serum were collected and stored at −20°C. For this study, in the immunological and parasitological analyses, we only included the same children (N = 89) who participated in both surveys (dry and rainy seasons) (Table 1).
Table 1.
Characteristics of the study population: entomological and parasitological results
| Rainy season | Dry season | |||||||
|---|---|---|---|---|---|---|---|---|
| N | n TBS | MPD | HBR | n TBS | MPD | HBR | ||
| Positive (%) | Geometric mean | An. (b/h/n) | Positive (%) | Geometric mean | An. (b/h/n) | |||
| Study area | Total | 89 | 79 (88.8) | 134 | 32 | 72 (80.9) | 12 | 15 |
| ≤ 5 years | 33 | 27 (81.8) | 90 | – | 29 (87.9) | 16 | – | |
| > 5 years | 56 | 52 (92.9) | 170 | – | 43 (76.8) | 11 | – | |
| Urban area | Dar-es-Salaam | 11 | 9 (81.8) | 13 | 2 | 6 (54.5) | 2 | 1 |
| Kennedy | 13 | 11 (84.6) | 302 | 35 | 13 (100) | 60 | 12 | |
| N’Gattakro | 14 | 13 (92.9) | 321 | 8 | 11 (78.6) | 20 | 2 | |
| Mean | 38 | 33 (86.8) | 111 | 15 | 30 (78.9) | 16 | 5 | |
| ≤ 5 years | 13 | 10 (76.9) | 28 | – | 11 (84.6) | 11 | – | |
| > 5 years | 25 | 23 (92) | 172 | – | 19 (76) | 18 | – | |
| Rural area | Pétéssou | 14 | 9 (64.3) | 8 | 78 | 12 (85.7) | 12 | 35 |
| Allokokro | 37 | 37 (100) | 453 | 38 | 30 (81.1) | 10 | 28 | |
| Mean | 51 | 46 (90.2) | 147 | 58 | 42 (82.4) | 10 | 32 | |
| ≤ 5 years | 20 | 17 (85) | 126 | – | 18 (90) | 18 | – | |
| > 5 years | 31 | 29 (93.6) | 165 | – | 24 (77.4) | 8 | – | |
An. = Anopheles; b/h/n = bite/human/night; HBR = human biting rate; MPD = mean parasite density; TBS = thick blood smear.
Entomological methods.
Anopheles adults were collected to evaluate the human-biting rate (HBR), which assesses the risk of malaria transmission. Adult mosquitoes were collected during two consecutive nights from 6:00 pm to 6:00 am by HLCs in September 2014 (rainy season) and March 2015 (dry season). The distribution of adult mosquito catchers was randomly made to avoid bias due to their particular attractiveness and their catching ability. Three houses were sampled at each site, and the same houses (in and out) were used for mosquito sampling during both dry and rainy seasons. Adult mosquito catchers gave prior informed consent and received yellow fever vaccination and antimalarial chemoprophylaxis as recommended by the National Malaria Control Program. The HBR, corresponding to the number of bites per human per night, was measured by dividing the number of mosquitoes caught by the total person/night used for the period. Captured Anopheles mosquitoes were arranged per hour and morphologically identified to species using the Gillies and Coetzee24 taxonomic keys.
Parasitological methods.
In each study site, the medical staff monitored children’s axillary temperature. At the same time as blood sampling, thick films and blood smears were performed in all the children studied to determine parasite density and Plasmodium species, respectively. Slides were fixed and stained with Giemsa and examined under microscope by two experienced technicians. The results are expressed in terms of Plasmodium prevalence and mean parasite density (MPD, geometric mean).
Salivary peptide gSG6-P1.
The gSG6-P1 peptide was synthesized and purified (> 95%) by Genepep SA (Saint Jean de Védas, France). The peptide was shipped in lyophilized form and then suspended in 0.22-μm ultrafiltered water and frozen at −20°C until use.
Evaluation of human IgG Ab level.
Enzyme-linked immunosorbent assays were carried out on sera to measure the IgG level to the gSG6-P1 peptide, as previously described.25 Briefly, Maxisorp plates (Nunc, Roskilde, Denmark) were coated with gSG6-P1 (20 μg/mL) in phosphate-buffered saline (PBS). After washing (demineralized water + Tween 0.1%), each serum sample was incubated in duplicate at 4°C overnight at a 1/320 dilution (in PBS with 1% Tween 20). Mouse biotinylated antihuman IgG (BD Pharmingen, San Diego, CA) was incubated at a 1/4,000 dilution in PBS with 1% Tween (1.5 hours at 37°C) and peroxidase conjugated Extravidin (Sigma, Saint-Louis, MO) was then added (1/20,000 1 hour at 37°C). Colorimetric development was carried out using 2.2-azino-bis (3 ethylbenzthiazoline 6-sulfonic acid) diammonium salt (Sigma) in 50 mM citrate buffer (pH = 4, containing 0.003% H2O2; Sigma) and absorbance (optical density [OD]) was measured at 405 nm. Individual results were expressed as the ΔOD value: ΔOD = ODx − ODn, where ODx represents the mean of the individual OD value in both wells with gSG6-P1 antigen and ODn the individual OD value in a blank well containing no gSG6-P1 antigen.
Statistical analysis.
Data were analyzed with GraphPad Prism® (GraphPad Software, San Diego, CA). Fisher’s exact test was used to compare P. falciparum prevalence between two age groups (≤ 5 years and > 5 years) and HBR between the dry season and the rainy season. After verifying that the values in each group did not assume a Gaussian distribution, the nonparametric Mann–Whitney U test was used to compare IgG levels between uninfected and infected children and also between IgG level to gSG6-P1 in urban areas and rural areas. The Wilcoxon matched pair test was used to compare paired samples between the rainy and dry seasons. Fisher’s exact test was used to compare the HBR between two groups. All differences were considered as significant at P < 0.05.
Ethics statements.
This study followed the ethical principles recommended by the Edinburgh revision of the Declaration of Helsinki. The present study was approved by the Ethics Committee of the Côte d’Ivoire Ministry of Health (June 2014; No. 41/MSLS/CNER-dkn). Written informed consent of all parents or guardians of children who participated in the study was obtained before inclusion.
RESULTS
Entomological and parasitological results.
The entomological (aggressiveness of Anopheles) and parasitological (prevalence, geometric mean of Plasmodium density) results in children are presented according to the areas and seasons studied (Table 1). In the rainy season, the main Plasmodium species was P. falciparum responsible for 79% and 75% of malaria infections in urban and rural areas, respectively (data not shown). Plasmodium malariae was the only other species found in the study. The prevalence of P. falciparum remained very high in both rainy (88.8%) and dry (80.9%) seasons and was similar between urban and rural areas and in both age groups (≤ 5 versus > 5 years old) (Table 1). In contrast, Plasmodium densities (MPD, geometric mean) were different depending on the season. MPD drastically decreased in the dry season (12 parasites/μL) compared with the rainy season (134 parasites/μL) in both urban and rural areas (P < 0.0001).
The entomological results showed that the vast majority (97%) of Anopheles species collected belonged to the An. gambiae complex (data not shown). Overall, the HBR ranged from 32 to 15 bite/human/night (b/h/n) in the rainy season and the dry season, respectively. However, this HBR varied depending on the area studied and was always higher in the rural areas than in the urban areas in both the rainy season (58 b/h/n versus 15 b/h/n) and the dry season (32 b/h/n versus 5 b/h/n). The HBR also seemed lower in the dry season than the rainy season, for all study areas considered (urban or rural), although no significant differences were obtained (all P > 0.05, Fischer exact test, Table 1).
IgG level to gSG6-P1 salivary peptide according to season.
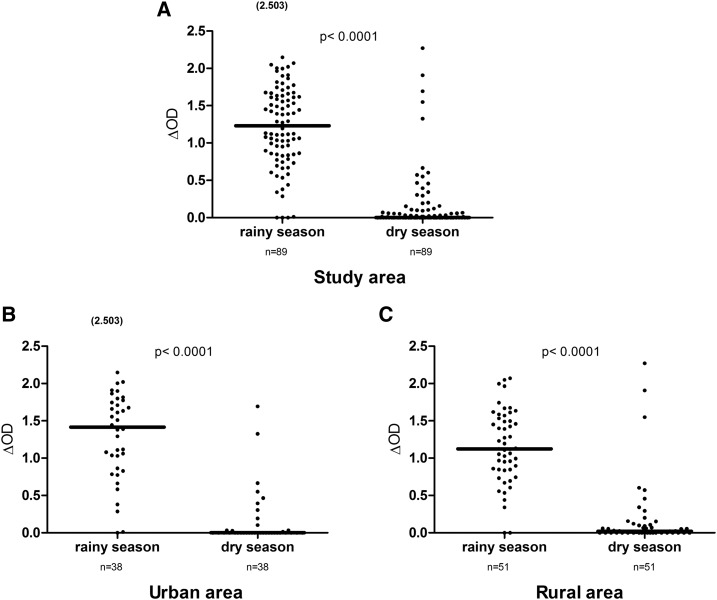
Specific IgG levels of the same children were assessed during the dry and rainy seasons in both urban and rural areas (Figure 1). Considerable variations were observed between the rainy season and dry seasons. Overall, specific IgG levels were significantly higher in the rainy season than in the dry season in the study area (Figure 1A; P < 0.0001, Wilcoxon test). A similar season-dependent pattern was observed when data were analyzed separately in urban and rural areas (Figure 1B and C; all P < 0.0001, Wilcoxon test). However, it should be noted that some individuals presented high IgG responses during the dry season in both urban and rural areas.
Figure 1.
Immunoglobulin G (IgG) levels to gambiae Salivary Gland Protein-6 peptide 1 (gSG6-P1) peptide according to season. Dot plots show the individual specific IgG level (optical density [OD] value) to gSG6-P1 between the rainy season and the dry season in the study area (N = 89; A) in the urban area (N = 38; B) and the rural area (N = 51; C) for the same children. Bars indicate the median value for each season. Number in parentheses on the figure above the dot plots indicates values greater than OD = 2.5. The difference was statistically significantly high in the rainy season (P < 0.0001, nonparametric Wilcoxon matched-pair test).
Furthermore, specific IgG levels were compared between the two age groups (≤ 5 and > 5 years old, data not shown). No significant difference was observed in the rainy season (P = 0.187) and the dry season (P = 0.137). As observed at the population level, IgG responses significantly decreased in the dry season compared with the rainy season in both age groups (P = 0.0014 for ≤ 5 years and P < 0.0001 for > 5 years).
IgG levels to gSG6-P1 peptide according to malaria status.
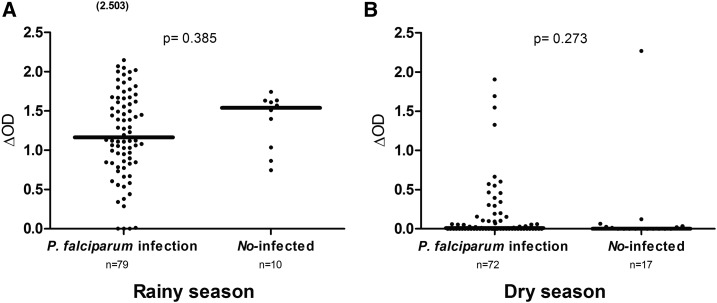
Specific IgG levels were also compared between uninfected children and Plasmodium-infected children (Figure 2). Anti-gSG6-P1 IgG levels were similar depending on the status of P. falciparum infection in the dry (P = 0.2730) and the rainy season (P = 0.3855, Mann–Whitney test). In both seasons, specific IgG responses to the gSG6-P1 peptide were mainly observed in malaria-infected children. However, it should be noted that some positive and relatively high specific IgG responses were also observed in uninfected children in the rainy season (Figure 2A). This result was not observed in the dry season (Figure 2B). The same results were observed in the urban and rural areas in the rainy season (data not shown).
Figure 2.
Immunoglobulin G (IgG) response levels to gambiae Salivary Gland Protein-6 peptide 1 (gSG6-P1) peptide from uninfected and infected children. Dot plots indicate the individual IgG level (Δ optical density [OD] value) to the gSG6-P1 peptide from uninfected individuals and children with Plasmodium falciparum infection in the rainy season (A) and in the dry season (B). The P value was determined according to the Mann–Whitney U test. Number in parentheses in (B) above the dot plots indicates values greater than OD = 2.5.
Comparison of IgG responses to gSG6-P1 peptide between urban and rural areas.
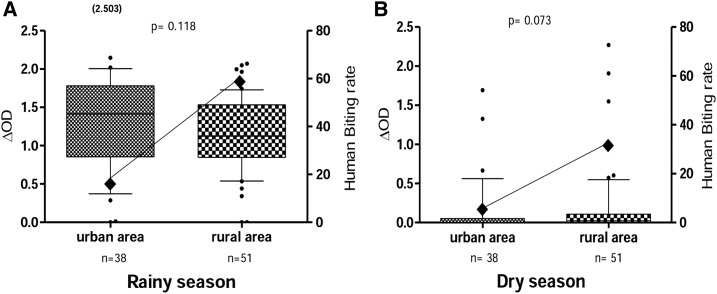
Specific IgG response levels and entomological data (HBR) in urban and rural areas were compared (Figure 3). Entomological data indicated that HBRs were higher in rural areas than in urban areas for both seasons. Surprisingly, similar specific IgG responses were observed between urban and rural areas during the rainy (Figure 3A) and dry (Figure 3B) seasons. The median of IgG levels appeared higher in the urban area (median value = 1.41) than the rural area (median value = 1.123) during the rainy season, although the difference was not significant.
Figure 3.
Immunoglobulin G (IgG) response to gambiae Salivary Gland Protein-6 peptide 1 (gSG6-P1) peptide and Anopheles biting rate in urban area and rural area according to season. Box plots show the anti-gSG6-P1 IgG level (Δ optical density [OD]) between the urban area and the rural area in the (A) rainy and (B) dry seasons. Boxes display the median ΔOD value, 25th and 75th percentiles. The whiskers show the 10th/95th percentiles and the dots indicate the outliers. Differences between the two groups were tested using the Mann–Whitney U test. The number in parentheses on the figure above the dot plots indicates values greater than OD = 2.5. The black rhombus represents the Anopheles gambiae s.l. human biting rate.
DISCUSSION
Malaria transmission is dogmatically considered to be higher in rural areas where environmental conditions are favorable for Anopheles larval development compared with urban contexts. Unexpectedly, our results show first that the prevalence of malaria infection in children was similar and high in both rural and urban areas. In contrast, entomological data clearly indicated a higher HBR in rural areas than the city, which could indicate the potential limitations of entomological methods in the urban context. In addition, the level of human exposure to Anopheles bites in the city of Bouaké, evaluated by IgG responses to the Anopheles gSG6-P1 salivary peptide, was as high as those observed in the rural control group and therefore similar to the trends observed for parasitological data. Indeed, the level of anti-gSG6-P1 IgG was significantly higher in the rainy season than in the dry season in both urban and rural sites and, surprisingly, no difference in the specific IgG level was observed between urban and rural areas.
In the Bouaké urban context, malaria transmission followed two different patterns. The first pattern was observed in the district where the shallows had been transformed for gardening. These shallows were an Anopheles-breeding site. This semirural context could be similar to the malaria transmission observed in rural areas, which is dependent on rainfall.26,27 The second pattern was observed in districts where stream flows were transformed for growing rice. In these districts, high HBRs were observed in the rice-growing period, and malaria transmission varied from one district to another. The size of the rice fields, the number of growing cycles in the year, the farming system, and the lack of synchronization of rice growing were key parameters whose combination could influence transmission in these districts. The HBR and malaria transmission were very heterogeneous in Bouaké. Each district constituted a particular case with its own influence, which may depend on human activities or natural characteristics of the environment.28 The diversity of situations observed in Bouaké illustrates the importance of local conditions on the HBR justifying our heterogeneous entomological results. This could explain why in urban areas children were more exposed to Anopheles bites in the rainy season (season with a high Anopheles biting rate) than in the dry season, as observed in the present study in the entomological and immunological results.
The evaluation of human exposure to Anopheles bites using a salivary biomarker showed here that the level of IgG responses to the gSG6-P1 peptide was significantly higher in the rainy season than in the dry season. The immunological results confirm that the population of young children received more Anopheles bites in the rainy season, consistent with the entomological data. It has previously been shown that the gSG6-P1 peptide sequence is strongly preserved between different malaria vector species, and specific IgG responses to this peptide were then the biomarker of Anopheles genus exposure.19 Thereafter, several studies also showed that the gSG6-P1 salivary peptide is a suitable immunological biomarker for assessing low-level human exposure to Anopheles bites.20,25,29,30 According to the entomological data, it was here observed that the HBR was 1) higher in the rural areas than in the urban areas during both seasons and 2) lower in the dry season than the rainy season for both urban and rural areas. However, the results with the salivary biomarker appeared to be contradictory. Indeed, no difference in specific IgG level was observed between the urban and rural areas in either the rainy season or the dry season. Although nonsignificant, the median IgG level appeared even higher in urban areas than rural areas in the rainy season. These results suggest that children could be highly exposed to Anopheles bites in urban areas, similar to rural areas. This similar exposure level between urban and rural areas, clearly observed during the rainy season, appeared to have logical consequences on malaria transmission. Indeed, parasitological data indicated that prevalence and Plasmodium densities were also similar between urban and rural areas in the rainy season, which seems to confirm immunological results but was contradictory to entomological data (higher in rural than urban areas). The very high Plasmodium density/prevalence reported in urban areas and comparable to those observed in rural areas strengthens immunological observations, indicating that individuals were exposed at the same level of vector bites in urban and rural areas.
Convergent results between parasitological and immunological data highlight the limits of such entomological methods in the particular context of urban malaria. Two hypotheses could be advanced to explain these discrepancies. First, it could be possible that people in urban areas use several vector control strategies (incense, mosquito coils, repellent plants, spray bombs, and curtains) in addition to insecticide-treated bed nets (ITNs), as previously indicated in Dakar city, Senegal.20 The second hypothesis postulates that classical entomological methods may not be adapted to the urban context because of the multitude of breeding sites and of sociological factors specific to the urban population (height and type of households, use of air conditioners, fans, and well-closed windows).
Immunological results also indicated no significant difference in specific IgG levels between malaria-infected children and uninfected children. Nevertheless, all malaria-infected children presented a high IgG level in the rainy season, suggesting that they were highly exposed to Anopheles bites, as previously shown in Senegal and the Americas.25,31 The absence of statistical significance could be due either to the very small sample of uninfected children (10 or 17 individuals depending on the season) or to the high exposure level to Anopheles bites. Future comparative studies are needed to compare malaria status and biomarker data in low-endemic urban areas versus rural areas. Interestingly, most children presenting high IgG responses to gSG6-P1 in the dry season were infected by P. falciparum. This suggests that children could still be bitten by malaria vectors during the dry season, which could indicate the ineffective use or lack of use of malaria control strategies by populations during the dry season when mosquito density is low. There is no special characteristic that distinguishes children with a high IgG response to the gSG6-P1 in the dry season from other children. However, several studies showed that lifestyle, individual attractiveness for Anopheles, as well as the distance between houses and larval sites could affect human exposure to mosquito bites.32–34 In addition, the Anopheles salivary biomarker was previously validated for assessing individual, spatial, and temporal heterogeneity of exposure to Anopheles bites, thus to the risk of malaria transmission in different settings.29,35,36 For these reasons, we can hypothesize that these children with a specific IgG level do not sleep under ITNs or slept under damaged ITNs, or do not use other personal vector control strategies.
The present study highlighted the considerable advantage of using this type of individual biomarker tool in the particular context of low exposure to malaria vectors during the dry season for monitoring the risk of malaria transmission. The biomarker of human exposure to Anopheles bites seems to be more sensitive than the entomological method for evaluating human–vector contact and thus the risk of malaria transmission in the particular context of urban malaria. This immunological biomarker of human exposure to Anopheles bites could be routinely used to accurately assess the increasing risk of malaria transmission in African urban settings and the implementation of vector control strategies in this particular epidemiological context.
Acknowledgments:
We gratefully acknowledge the populations of Bouaké health districts (Kennedy, Dar-es Salaam and N’Gattakro) and Pétéssou and Allokokro villages, especially householders and guardians of children for their kind support and collaboration. We thank Dr. Linda Northrup of “English Solutions” society for the English grammatical corrections of the manuscript.
REFERENCES
- 1.OMS , 2016. Paludisme Aide-mémoire 94. Available at: http://cdrwww.who.int/mediacentre/factsheets/fs094/fr/. Accessed July 15, 2016.
- 2.Pages F, Orlandi-Pradines E, Corbelc V, 2007. Vectors of malaria: biology, diversity, prevention, and individual protection. Med Mal Infect 37: 153–161. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Department of Economic and Social Affairs , 2008. World Urbanization Prospects: The 2007 Revision (Highlights), ESA/P/WP/205 (New York). Available at: http://www.un.org/esa/population/publications/wup2007/2007WUP_Highlights_web.pdf. Accessed July 15, 2016.
- 4.OMS , 2009. Bidonvilles, changement climatique et santé humaine en Afrique sub-saharienne. Bull World Health Organ 87: 886.20454473 [Google Scholar]
- 5.Martens P, Hall L, 2000. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis 6: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne N, 2007. Urban malaria risk in sub-Saharan Africa: where is the evidence? Travel Med Infect Dis 5: 135–137. [DOI] [PubMed] [Google Scholar]
- 7.Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC, 2003. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg 68: 169–176. [PubMed] [Google Scholar]
- 8.Coluzzi M, Sabatini A, Petrarca V, Deco MA, 1979. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg 73: 483–497. [DOI] [PubMed] [Google Scholar]
- 9.Carnevale P, Robert V, Le Goff G, Etienne F, Manga L, Agogbeto M, Chippaux JP, Mouchet J, 1993. Données entomologiques sur le paludisme urbain en Afrique tropicale. Cahier Santé 3: 239–245. [Google Scholar]
- 10.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW, 2005. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol 3: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adja AM, N’Goran KE, Kengne P, Koudou GB, Toure M, Koffi AA, Tia E, Fontenille D, Chandre F, 2006. Tranmission vectorielle du paludisme en savane arborée à Gansé en Côte d’Ivoire. Med Trop 66: 445–449. [PubMed] [Google Scholar]
- 12.Betsi NA, Koudou BG, Cissé G, Tschannen AB, Pignol AM, Ouattara Y, Madougou M, Tanner M, Utzinger J, 2007. Effect of an armed conflict on human resources and health systems in Côte d’Ivoire. Trop Med Int Health 8: 903–906. [DOI] [PubMed] [Google Scholar]
- 13.Chouaibou M, Simard F, Chandre F, Etang J, Darriet F, Hougard JM, 2006. Efficacy of bifenthrin-impregnated bednets against Anopheles funestus and pyrethroid-resistant Anopheles gambiae in north Cameroon. Malar J 5: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, Anderson RA, Killeen GF, 2009. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J 8: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mboera LE, 2005. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzan Health Res Bull 3: 117–124. [DOI] [PubMed] [Google Scholar]
- 16.Smith T, Killeen G, Lengeler C, Tanner M, 2004. Relationships between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am J Trop Med Hyg 71: 80–86. [PubMed] [Google Scholar]
- 17.Remoue F, Cornelie S, Ngom A, Boulanger D, Simondon F, 2005. Immune responses to arthropod bites during vector-borne diseases. Garraud O, ed. Update in Tropical Immunology Kerala, India: Transworld Research Network, 377–400. [Google Scholar]
- 18.Schwartz B, Ribeiro J, Goldstein M, 1990. Anti-tick antibodies: an epidemiologic tool in Lyme disease research. Am J Epidemiol 132: 58–66. [DOI] [PubMed] [Google Scholar]
- 19.Poinsignon A, et al. 2008. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One 3: e2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drame PM, et al. 2012. IgG responses to the gSG6-P1 salivary peptide for evaluating human exposure to Anopheles bites in urban areas of Dakar region, Senegal. Malar J 11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drame PM, Diallo A, Poinsignon A, Boussari O, Dos Santos S, Machault V, Lalou R, Cornelie S, LeHesran JY, Remoue F, 2013. Evaluation of the effectiveness of malaria vector control measures in urban settings of Dakar by a specific Anopheles salivary biomarker. PLoS One 8: e66354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dossou-Yovo J, Doannio JMC, Diarrassouba S, Chauvancy G, 1998. Impact d’aménagements de rizières sur la transmission du paludisme dans la ville de Bouaké, Côte d’Ivoire. Bull Soc Pathol Exot 91: 327–333. [PubMed] [Google Scholar]
- 23.Kouassi AM, Kouamé KF, Koffi YB, Dje BK, Paturel JE, Oulare S, 2010. Analysis Of Climate Variability And Its Influences On Seasonal Rainfall Patterns In West Africa: The Case Of The Watershed Of N’zi (Bandama) In Ivory Coast. Cybergeo: European Journal of Geography, Environment, Nature, Landscape, Document 513. Available at: https://cybergeo.revues.org/23388. Accessed February 9, 2017.
- 24.Gillies M, Coetzee M, 1987. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region), No. 55. Johannesburg, South Africa: The South African Institute for Medical Research.
- 25.Sagna AB, et al. 2013. Plasmodium falciparum infection during dry season: IgG responses to Anopheles gambiae salivary gSG6-P1 peptide as sensitive biomarker for malaria risk in northern Senegal. Malar J 12: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert V, Carnevale P, 1984. Les vecteurs des paludismes en Afrique subsaharienne. Etudes Médicales 2: 79–90. [Google Scholar]
- 27.Robert V, Carnevale P, Ouedraogo V, Petrarca V, Coluzzi M, 1988. La transmission du paludisme humain dans un village de savane du Sud-Ouest du Burkina Faso. Ann Soc Belg Med Trop 68: 107–121. [PubMed] [Google Scholar]
- 28.Dossou-Yovo J, Doannio JMC, Diarrassouba S, Chauvancy G, 1998. Impact d’aménagements de rizières sur la transmission du paludisme dans la ville de Bouaké, Côte d’Ivoire. Bull Soc Pathol Exot 91: 327–333. [PubMed] [Google Scholar]
- 29.Poinsignon A, Cornelie S, Ba F, Boulanger D, Sow C, Rossignol M, Sokhna C, Cisse B, Simondon F, Remoue F, 2009. Human IgG response to a salivary peptide, gSG6-P1, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malar J 8: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poinsignon A, et al. 2010. First attempt to validate the gSG6-P1 salivary peptide as an immuno-epidemiological tool for evaluating human exposure to Anopheles funestus bites. Trop Med Int Health 15: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 31.Londono-Renteria B, et al. 2015. An. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: a pilot study. Parasit Vectors 8: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dye C, Hasibeder G, 1986. Population dynamics of mosquito-borne disease: effects of flies which bite some people more frequently than others. Trans R Soc Trop Med Hyg 80: 69–77. [DOI] [PubMed] [Google Scholar]
- 33.Takken W, Knols BG, 1999. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol 44: 131–157. [DOI] [PubMed] [Google Scholar]
- 34.Qui YT, Smallegange RC, Van Lool JJA, Ter Braak CJF, Takken W, 2006. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s.s. Med Vet Entomol 20: 280–287. [DOI] [PubMed] [Google Scholar]
- 35.Sagna AB, et al. 2013. gSG6-P1 salivary biomarker discriminates micro-geographical heterogeneity of human exposure to Anopheles bites in low and seasonal malaria areas. Parasit Vectors 6: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drame PM, et al. 2010. Human antibody responses to the Anopheles salivary gSG6-P1 peptide: a novel tool for evaluating the efficacy of ITNs in malaria vector control. PLoS One 5: e15596. [DOI] [PMC free article] [PubMed] [Google Scholar]