Abstract.
Blood samples from 805 students attending 42 elementary schools in Mopti, Sikasso, and Koulikoro regions, and Bamako district in Mali participated in a school water, sanitation, and hygiene intervention. Immunoglobulin (Ig) G responses to several antigens/pathogens were assessed by a multiplex bead assay (MBA), and the recombinant Taenia solium T24H antigen was included. Of all students tested, 8.0% were positive to rT24H, but in some schools 25–30%. A cluster of 12 widespread school locations showed not only a relative risk of 3.23 for T. solium exposure and significantly higher IgG responses (P < 0.001) but also significantly lower elevation (P = 0.04) (m, above sea level) compared with schools outside the cluster. All schools at elevations < 425 m showed significantly higher IgG responses (P = 0.017) than schools at elevations ≥ 425 m. The MBA is an excellent serological platform that provides cost-effective opportunities to expand testing in serosurveys.
INTRODUCTION
Taenia solium, the pork tapeworm, is a parasite that has a two-host life cycle: one with humans as the only definitive host carrying the intestinal adult tapeworm (taeniosis) that sheds eggs into the feces, resulting in environmental contamination through open defecation in open areas, and the second with pigs, as the intermediate hosts, ingesting the eggs that develop into cysticerci (porcine cysticercosis), generally in the muscular tissue. In addition to pigs, humans may also act as an intermediate host for T. solium after fecal–oral ingestion of the tapeworm eggs or by poor personal hygiene (autoinfection).1,2 The disease often affects the brain, causing neurocysticercosis, which is the most common cause of adult-onset seizures in developing countries.3–5 Resources for surveillance and control are limited, and taeniosis/cysticercosis is included among the neglected tropical diseases defined by the World Health Organization.
A systematic review of T. solium cysticercosis reported overall seroprevalences by antigen or antibody detection of about 7% in Africa, 4% in Latin America, and 4% in Asia, but higher seroprevalences of 34–39% have been reported in countries such as Zambia where only about 1% of the population practice the Islamic religion that discourages ingestion of pork.6–9 There is a paucity of published information on cysticercosis in Mali in West Africa. However, a study in the adjacent country of Burkina Faso focused on three villages, one with free-roaming pigs, one with confined pigs, and one with limited numbers of pigs owned by Muslim farmers.10 In the three villages, the prevalence of T. solium cysticercosis antigen detected in blood was 10.3%, 1.4%, and 0%, respectively, indicating an association between lower prevalence of cysticercosis and the absence of pork ingestion by the Muslim population.10 In Mali, it has been estimated that 90% of the population are of Muslim faith; thus, cysticercosis prevalence might be expected to be low, especially compared with that in Zambia.7–9
By multiplex bead assay (MBA), immunoglobulin (Ig) G responses to many antigens/pathogens and vaccine-preventable diseases were assessed in students attending schools to determine the longitudinal impact evaluation of the Dubai Cares Initiative in Mali and Water, Sanitation, and Hygiene (DCIM WASH) in schools project. The DCIM WASH project was a comprehensive school-based WASH intervention in 900 schools in Bamako Capital District and the Koulikoro, Mopti, and Sikasso regions of Mali; detailed methods of the parent study are described elsewhere.11 The school WASH impact evaluation was not designed for a systematic survey on cysticerosis seroprevalence, and school-aged children were not ideal for determination of cysticercosis prevalence; however, the opportunity allowed us to include the recombinant cysticercosis antigen, rT24H, which has been used as a marker for T. solium metacestode (larval stage) exposure. By enzyme-linked immunoelectrotransfer blot, the rT24H antigen has shown a sensitivity and specificity of at least 96% and 99%, respectively, using serum specimens collected from confirmed neurocysticercosis patients and from persons not infected with the pathogen.12–15
MATERIALS AND METHODS
Study population.
Using stratified random sampling based on region, 21 of 100 schools with WASH benefits participated in the parent study along with 21 matched comparison schools without WASH benefits, for a total of 42 schools. Matched comparison schools were located within the same educational district and matched on baseline enrollment size and school WASH characteristics.11 At each school, an average of 19 students per school were selected from a list of all pupils enrolled in grade levels 1–6 (age range, 4–17 years) using stratified random sampling based on pupil gender and grade. This sample size was determined by the maximum number of samples that could be collected based on the study budget. School enrollments ranged from 71 to 651 students per school. For each school, a global positioning system acquired latitude and longitude coordinates and elevation above sea level in meters (m, range 320–542) (https://opendatakit.org/) was obtained, and ArcGIS (Esri, Redlands, CA) was used to plot coordinates.
The study was reviewed and approved by the Ethics Committee of the National Institute of Public Health Research (Comité d’Ethique de l’Institute Nationale de Recherché en Santé Publique) and the Institutional Review Board of Emory University (Atlanta, GA). Laboratory staff from the Centers for Disease Control and Prevention had no contact with children or access to personal identifiers. Before blood spot collection, signed/fingerprinted informed consent was provided by the parent or guardian of each randomly selected pupil, and each pupil provided informed verbal assent. The trial was registered at ClinicalTrials.gov (NTC01787058).
Blood spot collection.
Whole blood specimens were collected by finger prick onto extensions from a filter paper wheel (TropBio Pty Ltd., Townsville, Queensland, Australia) with each extension designed to absorb 10 μL. After collection and drying, the dried blood spots were stored at −20°C, as previously described.16 A single filter paper wheel per child was collected between January and June 2014, part of the Malian dry season.
Antigen bead coupling.
To prepare the beads for use in detecting anti-cysticercosis antibodies, carboxyl groups on the surface of specifically classified spectral magnetic polystyrene microspheres (MagPlex Beads, Luminex Corporation, Austin, TX) were converted to reactive esters using the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide method (Calbiochem, Woburn, MA). Glutathione-S-transferase (GST) was linked to rT24H (for purification purposes) (GST–rT24H) and was covalently coupled to 12.5 million activated beads by amide bonds in 50 mM 2-(N-morpholino) ethane sulfonic acid and 0.85% NaCl, pH 5.0, using 141 μg GST–rT24H.13 On a differently classified bead, 15 μg of GST was coupled to 12.5 million beads in the same buffer. Coupling efficiency was determined using sera and a reagent known to be highly reactive to GST–rT24H and GST by the MBA.
Immunoglobulin G elution and detection.
One dried blood spot extension from each child was placed in 0.5 mL of elution buffer consisting of phosphate buffered saline (PBS) with 0.5% bovine serum albumin, 0.3% Tween 20, 0.1% sodium azide, 0.5% polyvinyl alcohol, 0.8% polyvinylpyrrolidone, and 0.1% casein and allowed to elute overnight at 4°C. Afterward, the elution was further diluted 1:4 with the same elution buffer that contained sufficient amounts of crude Escherichia coli extract for a final concentration of 3 μg/mL. The E. coli extract absorbs E. coli antibodies that could react with minute and extraneous E. coli proteins (due to non-elimination in the purification process) coupled to the beads. After overnight storage at 4°C, the eluate was exposed to antigen-coupled beads for 1.5 hours at room temperature. Bound antigen-specific IgG was detected on the coupled beads as previously described.17 Between steps, the magnetic beads were washed three times with 0.05% Tween 20 in PBS, using a Bio-Plex Pro II Wash Station (Bio-Rad, Hercules, CA). A Bio-Plex 100 reader with Bio-Plex Manager 6.1 software (Bio-Rad) calculated the median fluorescence intensity (MFI, channels 1–32,766) from each bead classification from each well and determined the mean MFI from duplicate wells. Background (bg) fluorescence from a blank with no dried blood spot was subtracted (MFI-bg) and used as data.
Cutoff determination.
A serological cutoff for antibody detection was determined based on the mean plus 3 standard deviations of antibody levels detected in 86 serum specimens from North American adults who had not traveled outside the United States.
Cluster analysis.
The spatial scan statistic implemented in SaTScan was used to search for elliptically shaped, spatial clusters of schools with elevated prevalence of T. solium assessed by the relative risk to schools outside the cluster.18,19 The cluster of schools at the 5% level of significance is represented by a convex hull.
Statistics.
The Mann–Whitney rank sum test was used to determine any significant differences in IgG responses (MFI-bg) to the GST–rT24H between schools at elevations < 425 m and schools at elevations ≥ 425 m, any significant differences in IgG responses between schools inside and outside the cluster shown in Figure 1B, and any significant differences between elevations of schools inside and outside the cluster shown in Figure 1B. P < 0.05 was considered significant.
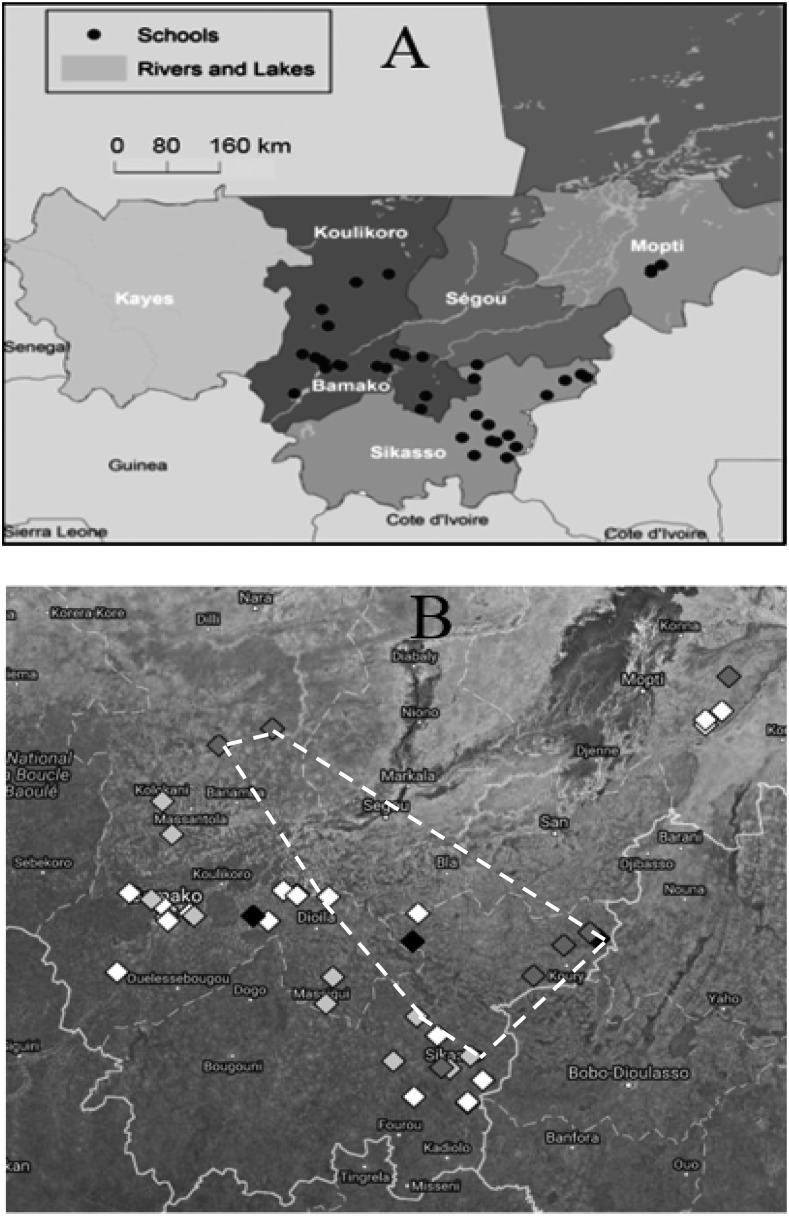
Figure 1.
Map of Mali in western Africa, showing political districts with schools and percentage of students tested positive for glutathione-S-transferase (GST)-rT24H per school. (A) Forty-two schools were located by the global positioning system and are indicated by black circles: Mopti (four schools), Sikasso (16), Koulikoro (20), and Bamako district capital (2). (B) The percentage of students per school who tested positive to the GST–rT24H antigen are represented by gray-scale diamonds: white, 0% (17/42 schools); light gray, 5–10% (14/42); medium gray, 15–20% (7/42); and black, 25–30% (4/42). An average of 19 students were randomly selected (range, 14–20) from each school. The cluster of 12 schools on or inside the white dashed line showed a relative risk of 3.23 for Taenia solium exposure compared with the 30 schools outside the cluster. Some symbols overlap.
RESULTS
Antigen coupling and cutoff.
Serum specimens known to be highly reactive to GST–rT24H and GST showed high MFI-bg, indicating sufficient antigen coupling. The cutoff for GST–rT24H used to define “positive” samples was 1,068 MFI-bg, and the inter-plate coefficient of variation for the positive control was < 14.5%. Beads with GST showed no appreciable reactivity: mean, 154 MFI-bg and range, −2 to 929 MFI-bg. The dried blood spots were in excellent condition and showed near-maximum MFI-bg (IgG responses) to various antigens other than GST–rT24H and GST.
Grouping of schools by percentage of GST–rT24H–positive students.
Table 1 shows the range of the percentage of students tested per school who were positive to GST–rT24H, which were arbitrarily grouped into 0%, 5–10%, 15–20%, and 25–30%. In addition, the number of students tested in each group is listed along with the number of schools per group, average number of students tested per school, the range of number of students enrolled per school, and the median elevation per school group.
Table 1.
Schools arbitrarily grouped by the percentage of students per school positive to GST–rT24H, the number of participating schools, the number of students tested, the average number of students tested per school, the range of number of students enrolled per school, and the median elevation of the grouped schools
| Percentage of students antibody positive/school | Gray-scale symbol (Figure 1B) | Number of schools | Number of students tested | Average number of students tested/school | Range of students enrolled/school | Median elevation (m) |
|---|---|---|---|---|---|---|
| 0 | White | 17 | 314 | 19 | 71–528 | 415 |
| 5–10 | Light gray | 14 | 273 | 20 | 73–596 | 378 |
| 15–20 | Dark gray | 7 | 138 | 20 | 77–422 | 410 |
| 25–30 | Black | 4 | 80 | 20 | 159–388 | 374 |
| Total | – | 42 | 805 | – | – | – |
GST = glutathione-S-transferase.
School locations by the global position system.
Figure 1A is a provincial map of southern Mali, with the geographical position system–located schools indicated by black circles: Mopti, four schools; Sikasso, 16; Koulikoro, 20; and Bamako district capital, two. Shown in Figure 1B are the same schools that are grouped by gray-scale diamonds, indicating the percentage of students who were tested positive to the GST–rT24H antigen per school: white, 0% (17/42 schools); light gray, 5–10% (14/42 schools); medium gray, 15–20% (7/42 schools); and black, 25–30% (4/42 schools). These groups are also listed in Table 1, which showed an insignificant but decreasing trend in elevation from 0% grouped schools to 25–30% grouped schools. Of 805 students tested, 64 (8.0%) tested positive to the GST–rT24H antigen. The median MFI-bg from all students for GST–rT24H was 238, and the MFI-bg range was 31–21,168.
Elevation and T. solium exposure.
In this study, there might be a relationship between elevation and T. solium exposure. For students attending schools at elevations < 425 m, the median MFI-bg was 364, which was significantly higher (P = 0.014) than the median MFI-bg of 297 from students attending schools at elevations ≥ 425 m. In Figure 1B, students attending a cluster of 12 schools, on or inside the dashed white lines, showed a relative risk of 3.23 of T. solium exposure (37 positive of 240 tested) by SaTScan compared with students attending schools outside this cluster (21 positive of 565 tested). Furthermore, the 12 schools inside the cluster showed a significantly lower median elevation of 365 m (P = 0.04) than the median elevation of 401 m of the schools outside the cluster. Also, the 12 schools showed a significantly higher median MFI-bg of 486 (P < 0.001) than the median MFI-bg of 302 for the 30 schools outside the cluster.
DISCUSSION
Our study has shown that 8.0% (64/805) of all students tested were IgG positive to the GST–rT24H antigen. As high as 25–30% of students tested in some schools (Figure 1B) were IgG positive to the antigen, suggesting hot spots, and these schools ranged in enrollments, 159–388 (Table 1), where only 5.1–12.5% of the student enrollment was tested. The relatively low fluctuations in the percent positive to GST–rT24H could be from a number of factors in Mali: 1) the predominately Muslim religion in many areas discourages pork ingestion, but possibly fewer Muslims live in areas such as that shown by the cluster of 12 schools in Figure 1B; 2) the number of pigs in Mali is < 10 per 1,000 persons,10 but some areas could have higher pig populations such as in the cluster of 12 schools shown in Figure 1B; and 3) the impact of annual mass drug administration against lymphatic filariasis that uses the drug albendazole which is known to be effective against cysticercosis.8,20 However, people can be infected despite low levels of pork consumption if sanitation is poor, and this may be the case in those schools where students tested positive for GST–rT24H. It is of interest that a patient with neurocysticercosis has been reported in Mali.21 Also of interest, the four schools within the cluster near the Burkina Faso border (Figure 1B, one black symbol and three medium gray symbols) showed a high percentage of GST–rT24H positives, and it is known that Burkina Faso is only about 60% Islamic, far less than the 90% in Mali.2,22 This information on T. solium seroprevalence may help to identify areas with potential T. solium exposures and help in the design of control strategies.
Determination of cycticercosis prevalence using children is not ideal, because prevalence in adults can be higher due to more exposures than in children.6 In this study, MFI-bg (IgG responses) showed an increasing trend with age from grade levels 1–6. In fact, students in grade levels 5 and 6 (median ages, 12 and 13 years, respectively) had significantly higher MFI-bg (P < 0.003) than students in grade level 1 (median age, 6.5 years) (data not shown).
We found no studies reporting a relationship between immune responses to GST–rT24H and elevation. Here, students in schools at elevations < 425 m showed significantly higher IgG responses (P = 0.014) than students in schools at elevations ≥ 425 m. Furthermore, those 12 schools in the cluster in Figure 1B showed significantly higher IgG responses (P < 0.001) and significantly lower elevations (P = 0.04) than those schools outside the cluster, suggesting, possibly, drainage of contaminated water to lower elevations. Flooding occurs almost annually in Mali as it did in August 2013, less than a year before these blood samples were collected.23 The elevation range for the schools studied was only 320–542 m; thus, the lower elevations were not obstructed by mountains. However, there could be unknown factors, such as the number of pigs and the number of cysticercosis carriers in the various elevations that could be influencing our elevation/immune results; thus, further investigation is required to confirm a relationship between elevation and immune responses.
In this study, data were acquired simultaneously on multiple antigens from multiple pathogens, thus conserving on specimens as well as cost and labor. The MBA is at least as sensitive as enzyme-linked immunosorbent assay.24 Identifying and maintaining funding for surveillance for a single pathogen can be difficult. In this study, GST–rT24H was one of 38 antigens from 22 different pathogens that were included and will be reported in the future. The MBA is notable in that it not only provided data from dried blood spots from a school surveillance study for evaluating effectiveness of WASH improvements11 but also provided serological information, in a non-designed study, on exposures of T. solium taeniosis/cysticercosis in the southern portion of Mali.
Acknowledgments:
We would like to thank the parents, students, and teachers who allowed us to conduct this work, and the Government of Mali. We would also like to thank the research team, including Abdoulaye Sow, Seydou Samaké, Salif Ismaïla Traoré, Fatoumata Habib Traoré, Karim Diamoutene, Yacouba Sogore, Alpha Oumar Haidara, and Niélé Hawa Diarra and Samba Diop from the University of Bamako. We also thank the UNICEF, WaterAid, CARE, Oxfam, and Save the Children teams for their support, specifically Jérémie Toubkiss, Yagouba Diallo, Seydou Niafo, Touréba Keïta, Assitan Sogoré, Salimata Togola, Fatoumata Haïdara, Mamadou Diallo, Zoumana Cissé, Ousmane Haïdara, and Thierno Bocoum.
Disclaimer: Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Service. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Garcia HH, Nash TE, Del Brutto OH, 2014. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13: 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash TE, Garcia HH, 2011. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol 7: 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno E, Bartoloni A, Zammarchi L, Strohmeyer M, Bartalesi F, Bustos JA, Santivanez S, Garcia HH, Nicoletti A, Group CPS, 2013. Epilepsy and neurocysticercosis in Latin America: a systematic review and meta-analysis. PLoS Negl Trop Dis 7: e2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, Stoner JA, Rainwater E, Dickey M, 2011. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis 5: e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler AS, 2012. Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathog Glob Health 106: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coral-Almeida M, Gabriel S, Abatih EN, Praet N, Benitez W, Dorny P, 2015. Taenia solium human cysticercosis: a systematic review of sero-epidemiological data from endemic zones around the world. PLoS Negl Trop Dis 9: e0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mwape KE, Phiri IK, Praet N, Speybroeck N, Muma JB, Dorny P, Gabriel S, 2013. The incidence of human cysticercosis in a rural community of eastern Zambia. PLoS Negl Trop Dis 7: e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braae UC, Saarnak CF, Mukaratirwa S, Devleesschauwer B, Magnussen P, Johansen MV, 2015. Taenia solium taeniosis/cysticercosis and the co-distribution with schistosomiasis in Africa. Parasit Vectors 8: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Department of State , 2010. Zambia International Religious Freedom Report 2010. Available at: https://www.state.gov/j/drl/rls/irf/2010/148728.htm. Accessed September 3, 2017.
- 10.Carabin H, Millogo A, Praet N, Hounton S, Tarnagda Z, Ganaba R, Dorny P, Nitiema P, Cowan LD; Evaluation du Fardeau Economique de la Cysticercose Au Burkina F , 2009. Seroprevalence to the antigens of Taenia solium cysticercosis among residents of three villages in Burkina Faso: a cross-sectional study. PLoS Negl Trop Dis 3: e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinies V, Garn JV, Chang HH, Freeman MC, 2016. The impact of a school-based water, sanitation, and hygiene program on absenteeism, diarrhea, and respiratory infection: a matched-control trial in Mali. Am J Trop Med Hyg 94: 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noh J, Rodriguez S, Lee YM, Handali S, Gonzalez AE, Gilman RH, Tsang VC, Garcia HH, Wilkins PP, 2014. Recombinant protein- and synthetic peptide-based immunoblot test for diagnosis of neurocysticercosis. J Clin Microbiol 52: 1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corstjens PL, de Dood CJ, Priest JW, Tanke HJ, Handali S; Cysticercosis Working Group in Peru , 2014. Feasibility of a lateral flow test for neurocysticercosis using novel up-converting nanomaterials and a lightweight strip analyzer. PLoS Negl Trop Dis 8: e2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handali S, et al. 2010. Development and evaluation of a magnetic immunochromatographic test to detect Taenia solium, which causes taeniasis and neurocysticercosis in humans. Clin Vaccine Immunol 17: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handali S, et al. 2010. Multiantigen print immunoassay for comparison of diagnostic antigens for Taenia solium cysticercosis and taeniasis. Clin Vaccine Immunol 17: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, Mkocha H, Martin DL, West SK, Gaydos C, Lammie PJ, 2012. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis 6: e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss DM, Chard AN, Trinies V, Doumbia S, Freeman MC, Lammie PJ, 2017. Serological responses to filarial antigens in Malian children attending elementary schools. Am J Trop Med Hyg 96: 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulldorf M, 1997. A spatial scan statistic. Commun Stat Theory 26: 1481–1496. [Google Scholar]
- 19.Kulldorf MaIMS, Inc. , 2015. SaTScanTM v9.4: Software for the Spatial and Space-Time Scan Statistics Available at: http://www.satscan.org/. Accessed June 12, 2017.
- 20.Coulibaly YI, et al. 2015. The impact of six annual rounds of mass drug administration on Wuchereria bancrofti infections in humans and in mosquitoes in Mali. Am J Trop Med Hyg 93: 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiga YDM, Bouteille B, Konate A, Diarra M, Maiga M, Marjolet M, 2009. A propos d’un cas autochtone de neurocysticercose au Mali (premier cas de la litterature?). Bull Soc Pathol Exot 102: 211–214. [PubMed] [Google Scholar]
- 22.Bureau of Democracy HR, and Labor , 2010. International Religious Freedom Report, 2010 Available at: https://www.state.gov/j/drl/rls/irf/2010/148665.htm. Accessed October 10, 2017.
- 23.(OCHA) UNOftCoHA , 2013. Mali: Complex Emergency, Situation Report No. 39 Available at: https://reliefweb.int/sites/reliefweb.int/files/resources/Sitrep39_EN.pdf. Accessed September 12, 2017.
- 24.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE, 2001. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry 45: 27–36. [DOI] [PubMed] [Google Scholar]