Abstract.
Acute febrile illness (AFI) is a major cause of morbidity and mortality in India and other resource-limited settings, yet systematic etiologic characterization of AFI has been limited. We prospectively enrolled adults (N = 970) and children (age 6 months to 12 years, N = 755) admitted with fever from the community to Sassoon General Hospital in Pune, India, from July 2013 to December 2015. We systematically obtained a standardized clinical history, basic laboratory testing, and microbiologic diagnostics on enrolled participants. Results from additional testing ordered by treating clinicians were also recorded. A microbiological diagnosis was found in 549 (32%) participants; 211 (12%) met standardized case definitions for pneumonia and meningitis without an identified organism; 559 (32%) were assigned a clinical diagnosis in the absence of a confirmed diagnosis; and 406 (24%) had no diagnosis. Vector-borne diseases were the most common cause of AFI in adults including dengue (N = 188, 19%), malaria (N = 74, 8%), chikungunya (N = 15, 2%), and concurrent mosquito-borne infections (N = 23, 2%) occurring most frequently in the 3 months after the monsoon. In children, pneumonia was the most common cause of AFI (N = 214, 28%) and death. Bacteremia was found in 68 (4%) participants. Central nervous system infections occurred in 58 (6%) adults and 64 (8%) children. Etiology of AFI in India is diverse, highly seasonal, and difficult to differentiate on clinical grounds alone. Diagnostic strategies adapted for season and age may reduce diagnostic uncertainty and identify causative organisms in treatable, fatal causes of AFI.
INTRODUCTION
India has the world’s largest absolute burden of infectious diseases, where fever is a commonly reported symptom.1,2 Acute febrile illness (AFI) is responsible for 1.9 million deaths annually in India.3 Despite the immense health-care burden AFI poses in India and beyond, the myriad possible etiologies of AFI presents a daunting challenge to clinicians in tropical low- and middle-income countries. Lack of accurate diagnosis for AFI drives inappropriate antimicrobial use,4 a key factor in the emergence of antimicrobial resistance.5 Antimicrobial resistance is a critical health threat in India, the world’s largest consumer of antibiotics.6 Public health measures, including vaccine deployment, vector control, and monitoring for emerging infectious diseases, require an accurate understanding of AFI epidemiology.
Small sample sizes, retrospective study design, and narrow participant age limit the generalizability of prior work to characterize AFI etiology in Indian settings.7,8 Lack of AFI etiology understanding in India significantly hinders establishment of optimal clinical guidelines, resource allocation, and intervention implementation.1 Studies in other tropical low- and middle-income countries demonstrate the feasibility of protocol-driven efforts to describe AFI etiology and have guided management policy.9,10 We prospectively enrolled adults and children with AFI to systematically characterize the etiology of AFI among hospitalized patients in Pune, India.
MATERIALS AND METHODS
Study site and participants.
Patients admitted to medicine and pediatric wards, including intensive care units, at Byramjee Jeejeebhoy Government Medical College-Sassoon General Hospital (BJGMC-SGH) in Pune, Maharashtra, India, from July 2013 through December 2015 were assessed during working hours, Monday–Saturday, for enrollment. Byramjee Jeejeebhoy Government Medical College-Sassoon General Hospital is a large, 1,400-bed urban tertiary public hospital serving the poor in Pune, India, a city of > 5 million people. Newly admitted patients > 6 months of age who had documented axillary temperature ≥ 38.0°C or complaint of fever reported by patient or caregiver lasting ≥ 24 hours were eligible and consented to participate. Inpatient transfers from other hospitals, minor-age orphans, and subjects of police investigation were excluded. Written informed consent was obtained from all participants ≥ 18 years of age and from the legal guardians of those < 18 years; assent was obtained from participants aged 12–18 years. Institutional review board approval was granted by BJGMC-SGH and Johns Hopkins School of Medicine.
Clinical data and specimen collection.
Within 24 hours of admission, a dedicated study nurse and physician measured axillary temperature, obtained a standardized clinical history, and collected nasopharyngeal and urine samples. The treating clinician or study laboratory technician collected peripheral blood of which 5 mL was directly inoculated into aerobic and anaerobic blood culture bottles (BD BACTEC). As part of routine care, additional clinical samples were collected and radiography performed per the discretion of the treating clinician.
Laboratory methods.
Blood cultures were performed on the Bactec Microbial Detection system (Becton Dickinson, Franklin Lakes, NJ) with subsequent speciation (BD Phoenix) as previously reported.11 Additional peripheral blood tests performed per study protocol included malaria antigen rapid diagnostic test (RDT) (SD Bioline, Gyeonggi-do, Republic of Korea),12 smear microscopy for malaria, chikungunya immunoglobulin M (IgM) combo RDT (CTK Onsite, San Diego, CA),13 dengue early enzyme-linked immunosorbent assay non-structural protein 1 (NS1) antigen capture (PanBio, Brisbane, Australia),14 clinical chemistry, and complete blood count. Human immunodeficiency virus (HIV)-1/2 rapid test (Alere, Waltham, MA)15 was performed for participants ≥ 18 years of age, pediatric participants with tuberculosis, and children of parents with HIV if their HIV status was unknown. The previously described procedures were performed in a laboratory that underwent external quality assurance programs by the College of American Pathologists and the Viral Quality Assurance program of the AIDS Clinical Trials Group.
Nasopharyngeal swabs were tested for influenza (BIONEXIA Influenza A+B rapid test; bioMérieux, Marcy l’Etoile, France) by the study clinician at the bedside. Additional clinician-directed tests included leptospirosis IgM (PanBio), dengue NS1 antigen, dengue IgM, chikungunya, hepatitis A IgM, hepatitis E IgM, cerebrospinal fluid (CSF) cryptococcal antigen, and culture of blood, urine, CSF, and sputum with speciation using traditional biochemical methods. Tuberculosis testing included sputum and CSF smear, culture, and Cepheid GeneXpert MTB assay.
Confirmed case definitions.
Cases of dengue, chikungunya, leptospirosis, hepatitis A and E, and influenza were defined by positive test results. Malaria was defined by positive smear microscopy or RDT. Bacterial cultures collected within the first 2 days of admission were considered for analysis. Bacteremia was defined by blood culture growth of a non-commensal organism.16 Urinary tract infection (UTI) was defined as a positive urine culture and dysuria or urine microscopy showing ≥ 10 white blood cells (WBC) cells/high power field. Positive stool culture confirmed gastroenteritis/enterocolitis. Pneumonia was confirmed if a radiograph identified a pulmonary infiltrate and the participant had cough, tachypnea, or hypoxia, or if there was a positive sputum culture or pneumococcal urine antigen. Tuberculosis was confirmed in participants with any positive tuberculosis microbiological test result (smear, Xpert, and culture). Meningitis was defined as the presence of headache or altered mental status, and CSF pleocytosis of > 5 WBC/μL or pathogen identification in CSF.17,18 Tuberculous meningitis was defined by the aforementioned meningitis criteria plus a positive tuberculosis test, modified from consensus definitions according to available clinical information.19 Biopsies showing malignancy or fungal infection were considered confirmed diagnoses.
Clinical diagnosis.
Admission diagnosis assigned by treating clinicians was recorded from the medical record on enrollment. Follow-up diagnosis was recorded at discharge or enrollment day 7, whichever came first. Nonspecific AFI etiologies such as “sepsis” were analyzed as “no diagnosis.” Vital status was determined at discharge. Participants meeting confirmed case definition criteria were assigned the confirmed diagnosis as the study diagnosis. Participants not meeting confirmed case criteria were assigned a clinical diagnosis if one was identified by treating clinicians. If more than one confirmed or clinical diagnosis was identified, a diagnosis was adjudicated per defined criteria (Supplemental Tables 1 and 2). Diagnoses were grouped by system affected or classified as systemic. If bacteremia was found in addition to UTI, pneumonia, or meningitis, the system-level diagnosis was assigned to genitourinary, respiratory, or central nervous system (CNS), respectively. Blood culture data results have been previously summarized.11
Data analysis.
Participants > 12 years of age were considered adults and participants ≤ 12 years of age were considered children. Demographic and clinical characteristics were analyzed for association with diagnoses using Fisher’s exact test, Wilcoxon rank sum test, and multivariable logistic regression. Variables were selected for multivariable model inclusion if they were available for > 90% of participants and demonstrated individual association with a diagnosis or were a key demographic factor. Data were analyzed in R.20 Using known test characteristics and observed study prevalence, we constructed Bayesian models to estimate missed diagnoses due to limited sensitivity tests.21,22
RESULTS
Study participants.
Of 57,177 patients admitted during the study period, 6,339 patients with possible AFI were screened. Among 3,161 patients meeting AFI criteria, 970 adults and 755 children were enrolled (Table 1). Reasons for non-enrollment include age < 6 months (N = 145), inter-facility transfer (N = 463), absconded or left against medical advice before enrollment (N = 148), death before enrollment (N = 77), orphans (N = 14), police investigation (N = 32), administratively unable to enroll (N = 214), refused consent (N = 99), mentally unable to consent (N = 22), and others (N = 222). There were 127 (13%) adults and 18 (2%) children with HIV among all patients; 106 (11%) adults and 666 (88%) children were not tested for HIV. A total of 243 (25%) adults and 136 (18%) children reported antibiotic use in the week before admission. Overlap of clinical syndromes was common—313 (18%) had both respiratory and CNS symptoms, 139 (8%) had both CNS and abdominal symptoms, and 187 (11%) had both respiratory and abdominal symptoms (Supplemental Figure 1).
Table 1.
Demographic and clinical features of 1,725 participants with acute febrile illness
| Demographic/clinical feature | Adults, N = 970 with feature, n (%) or median (IQR) | Children, N = 755 with feature, n (%) or median (IQR) | P value |
|---|---|---|---|
| Age, median (IQR) | 30 (21–45) | 2 (1–6) | – |
| Male | 557 (57) | 425 (56) | 0.66 |
| Household income < 5,000 ₹/month | 367 (38) | 312 (41) | 0.15 |
| Farmer or laborer | 326 (34) | 310 (41) | 0.0015 |
| Smoking* | 99 (10) | – | – |
| Alcoholism* | 95 (10) | – | – |
| HIV† | 127 (13) | 18 (2) | 0.17 |
| Hospitalized within the past 3 months‡ | 126 (13) | 72 (10) | 0.062 |
| Antibiotic use in week before admission | 243 (25) | 136 (18) | 0.0005 |
| Days of symptoms, median (IQR) | 5 (3–8) | 4 (3–8) | 0.002 |
| Cough | 336 (35) | 454 (60) | < 0.0001 |
| Diarrhea | 178 (18) | 125 (17) | 0.34 |
| Altered mental status | 126 (13) | 142 (19) | 0.0013 |
| Rigors | 815 (84) | 324 (43) | < 0.0001 |
| Headache | 456 (47) | 64 (8) | < 0.0001 |
| Respiratory sign or symptom | 439 (45) | 515 (68) | < 0.0001 |
| Severe malnutrition§ | – | 133 (18) | – |
| Leukopenia‖ | 226 (23) | 64 (8) | < 0.0001 |
| Leukocytosis‖ | 229 (24) | 303 (40) | < 0.0001 |
| Thrombocytopenia¶ | 517 (53) | 149 (20) | < 0.0001 |
| Anemia# | 98 (10) | 99 (13) | 0.056 |
HIV = human immunodeficiency virus; IQR = interquartile range; N = number; ₹ = Indian rupee.
Data collected for 816 (84%) adults.
HIV status known for 864 (89%) adults and 89 (12%) children.
Data collected for 758 (78%) adults and 560 (74%) children.
WBC lower limit of normal 4.1–6 × 109/L, upper limit of normal 8.9–11 × 109/L, variably defined by age and sex.
Platelets < 150 × 109/L.
Hemoglobin < 7 g/dL.
Confirmed diagnoses.
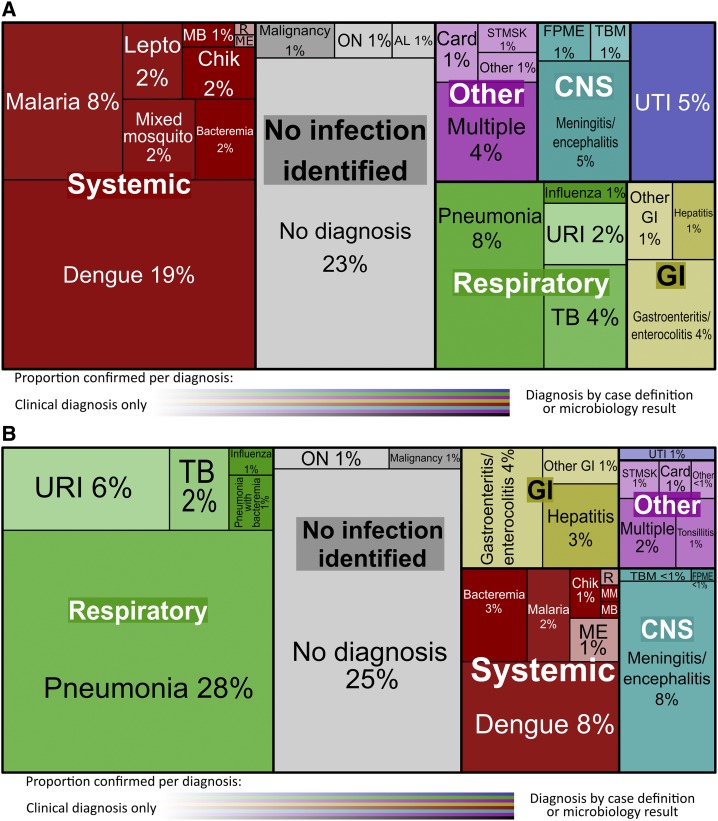
Confirmed diagnoses were determined in 760 (44%) participants; 99 (13%) had multiple confirmed diagnoses (Figure 1A and B). Dengue, the most common confirmed diagnosis, was found in 252 (15%) participants (Table 2); 102 (6%) participants tested positive for malaria, 35 (2%) for chikungunya, 18 (1%) for leptospirosis; 23 (1%) participants had multiple mosquito-borne diseases. Blood cultures were positive in 68 (4%) participants, among whom 29 had other confirmed diagnoses, including 10 with pneumonia, seven with mosquito-borne infections, five with UTI, three with CNS infections, two with enterocolitis, one with leptospirosis, and one with meningitis and a zoonotic infection. Two blood cultures grew Salmonella typhi.
Figure 1.
(A) Unique acute febrile illness (AFI) diagnoses in adults, N = 970. (B) Unique AFI diagnoses in children, N = 755. AL = alcoholic liver disease or withdrawal; Card = cardiac; Chik = chikungunya; CNS = central nervous system; FPME = fungal/parasitic meningitis/encephalitis; GI = gastrointestinal; Lepto = leptospirosis; MB = mosquito-borne illness with bacteremia < 1%; ME = measles/post-measles with respiratory infection < 1%; MM = mixed mosquito-borne illness < 1%; ON = other noninfectious < 1%; R = rickettsia < 1%; ST/MSK = soft tissue/musculoskeletal < 1%; T = tonsillitis < 1%; TB = tuberculosis; TBM = tuberculosis meningitis; URI = upper respiratory tract infection; UTI = urinary tract infection. Strength of confidence in the displayed diagnoses is expressed in tile shading density on a transparency scale from 40% to 100%, 40% corresponding to clinical diagnoses only, and 100% to all confirmed diagnoses. This figure appears in color at www.ajtmh.org.
Table 2.
Diagnostic tests performed and confirmed infections in 1,725 participants with acute febrile illness
| Adults, N = 970 | Children, N = 755 | P value | |
|---|---|---|---|
| Dengue (NS1 antigen ELISA or IgM) | 201/905 (22%) | 51/630 (8%) | < 0.0001 |
| Chikungunya (IgM) | 30/829 (4%) | 5/605 (1%) | 0.0004 |
| Malaria (blood smear or RDT) | 88/957 (9%) | 14/746 (2%) | < 0.0001 |
| Leptospirosis (IgM) | 18/155 (12%) | 0/9 (0%) | – |
| Pulmonary infiltrate (X-ray or CT) | 61/154 (40%) | 121/230 (53%) | 0.0164 |
| Respiratory culture | 12/62 (19%) | 4/7 (57%) | 0.0455 |
| Strep pneumoniae (urine antigen) | 5/228 (2%) | 0 | – |
| Influenza (RDT) | 8/672 (1%) | 5/503 (1%) | 1 |
| Tuberculosis (AFB smear, culture, or GeneXpert)* | 20/970 (2%) | 5/755 (1%) | 0.0152 |
| Meningitis (CSF WBC > 5 or organism identified) | 50/116 (43%) | 44/145 (30%) | 0.038 |
| CSF culture | 6/46 (13%) | 9/80 (11%) | 0.7807 |
| Cryptococcal meningitis (cryptococcal antigen) | 2/8 (25%) | 1/1 (100%) | 0.3333 |
| Blood culture before antibiotics | 8/281 (3%) | 19/383 (5%) | 0.2326 |
| Blood culture after antibiotics† | 29/661 (4%) | 11/357 (3%) | 0.3981 |
| Urine culture | 31/131 (24%) | 5/42 (12%) | 0.1277 |
| Stool culture | 4/42 (10%) | 3/18 (17%) | 0.4185 |
| Hepatitis A* | 2/970 (< 1%) | 14/755 (2%) | 0.0005 |
| Hepatitis E* | 4/970 (< 1%) | 0/755 (0%) | – |
CSF = cerebrospinal fluid; CT = computed tomography; ELISA = enzyme-linked immunosorbent assay; RDT = rapid diagnostic test; Strep = Streptococcus; WBC = white blood cell.
Number of tests performed is unknown, positive results only are reported.
Includes participants who received antibiotics in the week before admission.
Pneumonia was identified in 191 (11%) participants, among whom 10 also had bacteremia, five had positive pneumococcal urine antigen, four had pulmonary tuberculosis, three had meningitis, five had influenza, six had mosquito-borne illnesses, and three had other confirmed diagnoses. There were 16 (1%) participants with positive respiratory cultures and 13 (1%) with influenza. There were 25 (1%) participants with confirmed tuberculosis, most without X-ray showing pneumonia. Samples confirming tuberculosis were from extrapulmonary sources in nine participants.
Lumbar puncture was performed on 261 participants. Cerebrospinal fluid demonstrated meningitis in 94 (5%) participants and cultures were positive in 15 (1%) participants. Three had cryptococcal meningitis, three had biopsy-proven mucormycosis, and five had tuberculous meningitis. There were 200 participants with either dysuria or pyuria among whom 25 had positive urine cultures. There were 7 (< 1%) with positive stool cultures, two of which also had bacteremia. Hepatitis A was found in 16 (1%) participants and hepatitis E in 4 (< 1%) participants. There were 4 (< 1%) participants with streptococcal pharyngitis. Ten (1%) participants had biopsy-proven malignancy.
Clinician diagnoses.
There were 965 (56%) participants with no confirmed diagnosis. Treating clinicians assigned a working or discharge diagnosis to 559 (58%) participants without confirmed diagnoses; no specific diagnosis was reported for 406 (24%) participants. Pneumonia was the most common clinical diagnosis (N = 132), in whom chest radiography results were available for 30 (22%); 29 (97%) were abnormal but did not meet criteria for infiltrate. Other common clinical diagnoses included upper respiratory infection (N = 65, 4%), gastroenteritis/enterocolitis (N = 64, 4%), and UTI/pyelonephritis (N = 38, 2%). There were 33 (2%) participants with a clinical diagnosis of tuberculosis of which 20 (63%) cases were based on chest X-ray findings.
Final study diagnoses did not match admission diagnoses for 715 (51%) patients, including 334 (19%) who had no etiology identified on admission. Among patients with discordant diagnoses, more than one-third were eventually diagnosed with vector-borne diseases, including dengue (N = 163, 18%), malaria (N = 38, 5%), chikungunya (N = 20, 2%), and multiple mosquito-borne infections (N = 22, 2%). Other common discordant clinical diagnoses were pneumonia (N = 87, 10%), tuberculosis (N = 32, 4%), and meningitis/encephalitis (N = 40, 4%).
Association of clinical and demographic features and confirmed diagnoses.
Vector-borne disease was more common in adults (N = 296, 31%) than in children (N = 69, 9%) (P < 0.0001) but more common among children ≥ 5 years of age (N = 46, 18%) than children less than 5 years (N = 23, 5%) (P < 0.0001). Rigors occurred in 1,139 (66%) participants and more commonly in participants with dengue (89%, P < 0.0001) and malaria (92%, P < 0.0001) (Table 3, Supplemental Tables 3 and 4). Participants with dengue were less likely to have a low income compared with those without dengue (23% versus 41%, P < 0.0001); participants with bacteremia were more likely to have a low income compared with those without (53% versus 37%, P = 0.022). The occupation of 37% of participants or their parents was farmer or laborer, which was more common in participants with malaria (54%, P < 0.0001) and meningitis (52%, P < 0.0001). Alcoholism was associated with meningitis diagnosis (P < 0.0001).
Table 3.
Demographic and clinical features of 1,725 participants with acute febrile illness by confirmed, nonexclusive diagnosis
| Demographic/clinical feature | All participants, N = 1,725; n (%) | Dengue, N = 252 | Malaria, N = 102 | Bacteremia, N = 68 | Pneumonia, N = 191 | Meningitis, N = 94 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | P | n (%) | P | n (%) | P | n (%) | P | n (%) | P | ||
| Age, median (IQR) | 18 (3–33) | 22 (17–33) | < 0.01 | 24 (19–34) | < 0.01 | 18.5 (2–42.8) | 0.32 | 4 (1–27.5) | < 0.01 | 15 (5.25–35) | 0.94 |
| Male | 982 (57) | 174 (69) | < 0.01 | 81 (79) | < 0.01 | 37 (54) | 0.71 | 103 (54) | 0.39 | 48 (51) | 0.24 |
| Household income < 5,000 (₹/month) | 679 (39) | 58 (23) | < 0.01 | 40 (39) | 1 | 36 (53) | 0.02 | 76 (40) | 0.94 | 43 (46) | 0.23 |
| Farmer or laborer | 636 (37) | 78 (31) | 0.05 | 55 (54) | < 0.01 | 23 (34) | 0.61 | 83 (43) | 0.05 | 49 (52) | < 0.01 |
| Smoking* | 100 (7) | 18 (8) | 0.78 | 12 (13) | 0.03 | 1 (3) | 0.51 | 13 (7) | 1 | 6 (8) | 0.65 |
| Alcoholism* | 96 (7) | 5 (2) | < 0.01 | 9 (10) | 0.18 | 4 (11) | 0.29 | 12 (6) | 1 | 11 (14) | 0.01 |
| HIV† | 145 (15) | 6 (3) | < 0.01 | 3 (4) | < 0.01 | 5 (12) | 0.66 | 20 (28) | < 0.01 | 17 (31) | < 0.01 |
| Hospitalized within past 3 months‡ | 198 (15) | 12 (5) | < 0.01 | 9 (9) | 0.52 | 9 (26) | 0.09 | 24 (13) | 0.63 | 15 (16) | 0.18 |
| Antibiotic use in past week | 379 (22) | 61 (24) | 0.41 | 25 (25) | 0.54 | 15 (22) | 1 | 40 (21) | 0.78 | 24 (26) | 0.37 |
| Days of symptoms, median (IQR) | 5 (3–8) | 4 (3–6) | 0.01 | 5 (4–8) | 0.2 | 5 (3–10) | 0.49 | 5 (3–8) | 0.16 | 4.5 (3–8) | 0.75 |
| Cough | 790 (46) | 63 (25) | < 0.01 | 23 (23) | < 0.01 | 32 (47) | 0.9 | 174 (91) | < 0.01 | 25 (27) | < 0.01 |
| Diarrhea | 303 (18) | 27 (11) | < 0.01 | 8 (8) | < 0.01 | 15 (22) | 0.33 | 26 (14) | 0.13 | 11 (12) | 0.13 |
| Altered mental status | 268 (16) | 15 (6) | < 0.01 | 8 (8) | 0.02 | 13 (19) | 0.39 | 16 (8) | < 0.01 | 62 (66) | < 0.01 |
| Rigors | 1,139 (66) | 224 (89) | < 0.01 | 94 (92) | < 0.01 | 44 (65) | 0.8 | 98 (51) | < 0.01 | 69 (73) | 0.14 |
| Headache | 520 (30) | 119 (47) | < 0.01 | 52 (51) | < 0.01 | 17 (25) | 0.42 | 23 (12) | < 0.01 | 49 (52) | < 0.01 |
| Respiratory sign or symptom | 954 (55) | 76 (30) | < 0.01 | 33 (32) | < 0.01 | 39 (57) | 0.8 | 188 (98) | < 0.01 | 34 (36) | < 0.01 |
| Severe malnutrition | 133 (20) | 7 (14) | 0.57 | 2 (22) | 0.69 | 1 (4) | 0.04 | 26 (25) | 0.14 | 5 (13) | 0.31 |
| Leukopenia | 290 (17) | 95 (38) | < 0.01 | 19 (19) | 0.69 | 9 (14) | 0.61 | 18 (9) | < 0.01 | 10 (11) | 0.15 |
| Leukocytosis | 532 (31) | 23 (9) | < 0.01 | 14 (14) | < 0.01 | 30 (47) | < 0.01 | 84 (44) | < 0.01 | 41 (45) | < 0.01 |
| Thrombocytopenia | 666 (39) | 193 (77) | < 0.01 | 85 (83) | < 0.01 | 26 (39) | 1 | 36 (19) | < 0.01 | 25 (27) | 0.02 |
| Anemia | 197 (12) | 5 (2) | < 0.01 | 15 (15) | 0.26 | 10 (15) | 0.33 | 24 (13) | 0.63 | 6 (7) | 0.17 |
| eGFR < 60 mL/min/1.73 m2 | 278 (16) | 14 (6) | < 0.01 | 6 (6) | < 0.01 | 28 (42) | < 0.01 | 30 (16) | 0.92 | 8 (9) | 0.04 |
| Alanine transaminase > 60 units/L | 317 (20) | 95 (38) | < 0.01 | 24 (24) | 0.3 | 9 (14) | 0.41 | 19 (11) | < 0.01 | 17 (19) | 1 |
eGFR = estimated glomerular filtration rate; HIV = Human immunodeficiency virus; IQR = interquartile range; ₹ = Indian rupee.
Data collected for 816 (84%) adults.
HIV status known for 864 (89%) adults and 89 (12%) children.
Data collected for 758 (78%) adults and 560 (74%) children.
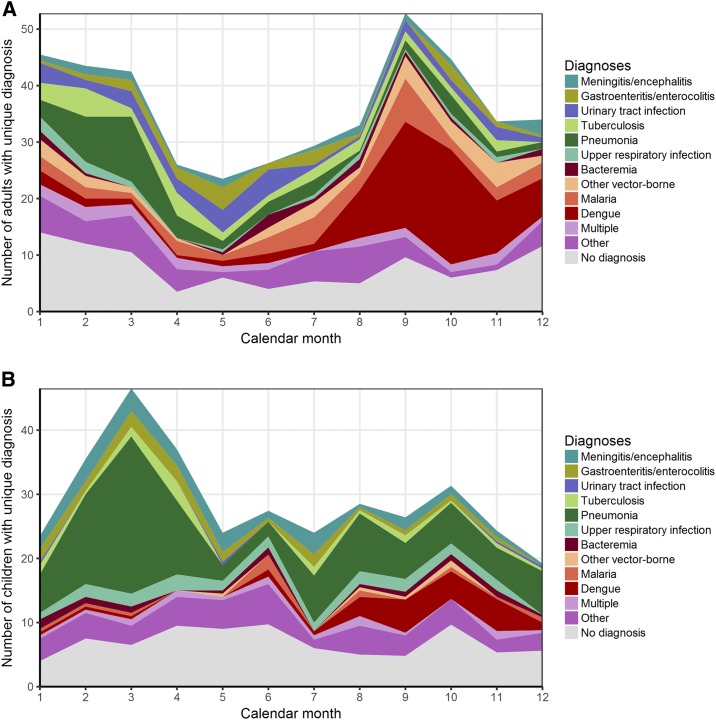
Leukopenia was found in 95 (38%) of the participants with dengue compared with 290 (17%) of all participants (P < 0.0001). Thrombocytopenia, occurring in 666 (39%) participants, was more common in those diagnosed with dengue (77%, P < 0.0001) and malaria (83%, P < 0.0001). Leukocytosis was associated with bacterial illnesses, including bacteremia (P = 0.0085), pneumonia (P < 0.0001), and meningitis (P < 0.0001). Vector-borne disease was more common among participants in August to December (Figure 2A and B). Pneumonia prevalence was highest in March. Peak AFI enrollment was during March and September for children and adults, respectively.
Figure 2.
(A) Unique diagnoses for 970 adults per calendar month. (B) Unique diagnoses for 755 children per calendar month. This figure appears in color at www.ajtmh.org.
All-cause mortality.
Among 68 (7%) adults and 61 (8%) children who died, a confirmed diagnosis was found in 36 (53%) of the adults and 23 (38%) of the children; a clinical diagnosis was determined in 16 (24%) adults and 18 (30%) children (Supplemental Table 5). Meningoencephalitis was confirmed in 13 (19%) adults and 3 (5%) children who died; five children were suspected on clinical grounds to have meningoencephalitis. Respiratory infections were suspected in 13 (19%) of the adults and 21 (34%) of the children who died and confirmed in eight adults and 12 children.
Bayesian model to estimate AFI based on published test characteristics.
We constructed a Bayesian model to estimate the true prevalence of AFI diagnoses based on published test characteristics of imperfect diagnostic tests. Based on the model, we likely underestimated dengue, malaria, and bacteremia, and to a lesser extent influenza, whereas we may have overestimated chikungunya and leptospirosis (Table 4).
Table 4.
Estimates of acute febrile illness diagnoses using a Bayesian model of published test characteristics
| Test | Sensitivity % | Specificity % | Adults | Children | ||
|---|---|---|---|---|---|---|
| Positive tests, n/N (%) | True prevalence estimate, n (%, 95% CI) | Positive tests, n/N (%) | True prevalence estimate, n (%, 95% CI) | |||
| Dengue22 | 66–72 | 97–98 | 201/905 (22) | 270 (30, 26–34) | 51/630 (8) | 55 (9, 6–12) |
| Chikungunya28 | 20 | 93 | 30/829 (4) | 15 (2, 0–6) | 5/605 (1) | 8 (1, 0–5) |
| Bacteremia after antibiotic exposure29 | 50–64 | 100 | 40/1,018 (4) | 64 (6, 4–9) | 11/357 (3) | 5 (2–9) |
| Leptospirosis30 | 31–52 | 66–91 | 18/155 (12) | 14 (9, 0–28) | 0/9 (0) | 3 (0–81) |
| Influenza31 | 36–70 | 96–100 | 8/672 (1) | 10 (2, 0–4) | 5/503 (1) | 7 (1, 0–4) |
| Malaria RDT32,33 | 24–89 | 81–99 | 86/929 (9) | 98 (8, 0–30) | 14/703 (2) | 12 (2, 0–6) |
| Malaria microscopy32,34 | 29–91 | 98–100 | 74/933 (8) | 142 (15, 7–27) | 11/733 (2) | 14 (2, 0–6) |
CI = confidence interval; RDT = rapid diagnostic test.
DISCUSSION
In a large prospective cohort of acutely febrile hospitalized adults and children in India, we were able to establish a confirmed diagnosis in 44% and a clinical diagnosis in 32% of participants and detailed several important findings. First, vector-borne disease was the most common cause of AFI found in adults, including dengue, which was more common than that was previously reported. Respiratory infections were the most common cause of AFI in children. Second, there was striking seasonal variation in AFI etiology. Finally, there was a significant overlap in clinical syndromes and basic laboratory findings among participants with disparate causes of AFI, limiting the ability to determine diagnosis without targeted testing.
Vector-borne diseases were the most common overall cause of AFI in this cohort. However, vector-borne diseases were more common in older children and younger adults, which may reflect their greater risk of exposure and higher risk for secondary dengue. The dengue prevalence—21% in adults and 7% in children—is higher than that previously reported in Indian and Southeast Asian AFI studies.8,9 Furthermore, our Bayesian model (Table 4) demonstrated that more sensitive diagnostics may have found dengue in 30% of adults and 9% of children. The second most common vector-borne infection was malaria. India’s National Framework for Malaria Elimination classifies Maharashtra state, with a population over 110 million, as being in a pre-elimination phase, reporting only 68 malaria deaths in 2014.23 As we report five malaria deaths in 2014 at one single site in Maharashtra, official figures likely underestimate malaria burden and, thus, the requisite effort for elimination. Leptospirosis was also frequently found as a cause of AFI, even though testing was only performed in a minority of patients. Vector-borne diseases represented one-third of eventually confirmed diagnoses not identified by clinicians on admission. Current vector-borne disease RDTs are often unavailable at the point-of-need in tropical low- and middle-income inpatient settings, in favor of centralized laboratory-based testing. Without rapid testing, delays may increase the length of stay, delay the targeted therapy, and increase the unnecessary antibiotic use.
There was extensive seasonal variability in AFI burden and etiology. In the 3 months after the monsoon, vector-borne diseases were found in more than half of adults, driving peak AFI admission. Respiratory infectious diseases were more common in February through April for adults and children, which was the primary driver of seasonal AFI admission variation for children. Tailored testing and treatment algorithms could exploit seasonal variability in AFI. Global warming and changing weather patterns may exacerbate future seasonal variability of AFI burden and etiology.
Our results suggest that clinical features of vector-borne disease are often too similar to be differentiated without pathogen-specific testing. Such similarity in clinical features may limit the interpretability of population-based studies using verbal autopsy to estimate the burden of diseases such as malaria.3 Proposed strategies to expand actionable diagnoses for AFI promote syndrome-based multiplex platforms.24 We found that clinical features typical for specific AFI diagnoses could be misleading. For example, 25% of patients with dengue had cough and 26% of patients with diarrhea had confirmed respiratory infection, meningitis, or vector-borne disease. In addition, less than half of participants with pneumonia, bacteremia, and meningitis had leukocytosis, providing concern that a syndrome-guided diagnostic approach may shunt patients down incorrect pathways and fail to identify correct diagnoses.
Our study has several limitations. As a single hospital–based study, we only report that the relative burden of AFI causes severe enough to warrant hospital admission, which may vary across geographically and socially diverse India. The public hospital setting catered to a predominantly poor urban population, but the 39% with household income < $73/month mirrors the proportion nationally.25 The hospital was also colocated with a large outpatient HIV clinic and, therefore, our proportion with HIV coinfection is impacted by that referral bias. We likely underestimated leptospirosis and tuberculosis burden because of selective testing. Testing for other potential causes of AFI such as scrub typhus was not available. Antibiotic exposure may have limited blood culture yield, although true prevalence estimates suggest that pre-culture antibiotic exposure was responsible for only 24 cases of false-negative bacteremia. Despite these limitations, we have prospectively and systematically evaluated AFI etiology in both adults and children in one of the largest cohorts in a tropical low- and middle-income setting.
In summary, we found that vector-borne disease is the most common cause of febrile illness in hospitalized patients in an Indian cohort, highly seasonal, difficult to differentiate clinically, and frequently missed on admission. Despite extensive testing, AFI cause remained unconfirmed in 32% of participants and a mystery in 24% of cases and deaths. Our results suggest that AFI diagnostics must provide faster, more sensitive, and pathogen-specific results. Higher sensitivity testing for common AFI causes such as dengue may reduce diagnostic uncertainty. Deployment of high-quality, low-cost point-of-care diagnostics may reduce admission diagnosis misclassification. Laboratory capacity to detect pathogens causing meningitis and respiratory infections, which together represent 42% of deaths in this cohort, is urgently needed to identify this sizable burden of treatable, fatal AFI. Determining AFI etiology is important for individual patients to choose correct lifesaving antimicrobials and to communities to prevent unnecessary antimicrobial use and to direct vaccine and vector-control efforts. Without regular AFI surveillance, emerging infectious diseases go unnoticed until they have surpassed the ability of public health measures to contain them. Future efforts must balance the priorities of providing diagnostics with improved test characteristics while ensuring that they are accessible, affordable, and sustainable in low- and middle-income settings.
Supplementary Material
Supplemental figure and tables
Acknowledgments:
We thank our study staff and participants. We also thank Becton Dickinson for training and discounted pricing on materials for the Phoenix and Bactec systems. We would also like to thank the clinicians and laboratory personnel at BJGMC-SGH who provided care for the study participants.
Note: Supplemental figure and tables appear at www.ajtmh.org.
REFERENCES
- 1.John TJ, Dandona L, Sharma VP, Kakkar M, 2011. Continuing challenge of infectious diseases in India. Lancet 377: 252–269. [DOI] [PubMed] [Google Scholar]
- 2.Salvi S, Apte K, Madas S, Barne M, Chhowala S, Sethi T, Aggarwal K, Agrawal A, Gogtay J, 2015. Symptoms and medical conditions in 204 912 patients visiting primary health-care practitioners in India: a 1-day point prevalence study (the POSEIDON study). Lancet Glob Health 3: e776–e784. [DOI] [PubMed] [Google Scholar]
- 3.Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, Bassani DG, Suraweera W, Laxminarayan R, Peto R, 2010. Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet 376: 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee D, Sen S, Begum SA, Adhikari A, Hazra A, Das AK, 2015. A questionnaire-based survey to ascertain the views of clinicians regarding rational use of antibiotics in teaching hospitals of Kolkata. Indian J Pharmacol 47: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, Guerin PJ, Piddock LJ, 2016. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387: 176–187. [DOI] [PubMed] [Google Scholar]
- 6.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R, 2014. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 14: 742–750. [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsen SK, Haugen CN, Rupali P, Mathai D, Langeland N, Eide GE, Morch K, 2013. Fever in the tropics: aetiology and case-fatality—a prospective observational study in a tertiary care hospital in South India. BMC Infect Dis 13: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi R, Colford JM, Jr., Reingold AL, Kalantri S, 2008. Nonmalarial acute undifferentiated fever in a rural hospital in central India: diagnostic uncertainty and overtreatment with antimalarial agents. Am J Trop Med Hyg 78: 393–399. [PubMed] [Google Scholar]
- 9.Mayxay M, et al. 2013. Causes of non-malarial fever in Laos: a prospective study. Lancet Glob Health 1: e46–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crump JA, et al. 2013. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis 7: e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mave V, et al. 2017. High burden of antimicrobial resistance and mortality among adults and children with community-onset bacterial infections in India. J Infect Dis 215: 1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratsimbasoa A, Fanazava L, Radrianjafy R, Ramilijaona J, Rafanomezantsoa H, Ménard D, 2008. Evaluation of two new immunochromatographic assays for diagnosis of malaria. Am J Trop Med Hyg 79: 670–672. [PubMed] [Google Scholar]
- 13.Yap G, Pok K-Y, Lai Y-L, Hapuarachchi H-C, Chow A, Leo Y-S, Tan L-K, Ng L-C, 2010. Evaluation of Chikungunya diagnostic assays: differences in sensitivity of serology assays in two independent outbreaks. PLoS Negl Trop Dis 4: e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aryati A, Trimarsanto H, Yohan B, Wardhani P, Fahri S, Sasmono RT, 2013. Performance of commercial dengue NS1 ELISA and molecular analysis of NS1 gene of dengue viruses obtained during surveillance in Indonesia. BMC Infect Dis 13: 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiwango RM, et al. 2013. Evaluation of current rapid HIV test algorithms in Rakai, Uganda. J Virol Methods 192: 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention , 2017. NHSN Organism List (All Organisms, Top Organisms, Common Commensals, MBI Organisms, and UTI Bacteria) January Available at: https://www.cdc.gov/nhsn/xls/master-organism-com-commensals-lists.xlsx. Accessed February 19, 2017.
- 17.Leber AL, et al. 2016. Multicenter evaluation of the BioFire FilmArray meningitis encephalitis panel for the detection of bacteria, ciruses and yeast in cerebrospinal fluid specimens. J Clin Microbiol 54: 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray P, Badarou-Acossi G, Viallon A, Boutoille D, Arthaud M, Trystram D, Riou B, 2007. Accuracy of the cerebrospinal fluid results to differentiate bacterial from non bacterial meningitis, in case of negative gram-stained smear. Am J Emerg Med 25: 179–184. [DOI] [PubMed] [Google Scholar]
- 19.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ, Marais BJ, 2010. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 10: 803–812. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team , 2015. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 21.Speybroeck N, et al. 2011. True versus apparent malaria infection prevalence: the contribution of a Bayesian approach. PLoS One 6: e16705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan X, Wang X, Yuan Q, Zheng Y, Zhang H, Wu Y, Yang J, 2015. Evaluation of the diagnostic accuracy of nonstructural protein 1 Ag-based tests for dengue virus in Asian population: a meta-analysis. BMC Infect Dis 15: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Directorate of National Vector Borne Disease Control Programme , 2016. National Framework for Malaria Elimination in India 2016–2030. Delhi, India: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. [Google Scholar]
- 24.Caliendo AM, et al. Infectious Diseases Society of A , 2013. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 57 (Suppl 3): S139–S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rukmini S, 2014. Steady Rise in Income But Services Still Inadequate: NCAER. New Delhi, India: The Hindu.
- 26.World Health Organization United Nations Children’s Fund , 2009. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 27.Khadilkar V, et al. 2015. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr 52: 47–55. [DOI] [PubMed] [Google Scholar]
- 28.Prat CM, Flusin O, Panella A, Tenebray B, Lanciotti R, Leparc-Goffart I, 2014. Evaluation of commercially available serologic diagnostic tests for chikungunya virus. Emerg Infect Dis 20: 2129–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodes J, et al. 2010. Antibiotic use in Thailand: quantifying impact on blood culture yield and estimates of pneumococcal bacteremia incidence. Am J Trop Med Hyg 83: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desakorn V, Wuthiekanun V, Thanachartwet V, Sahassananda D, Chierakul W, Apiwattanaporn A, Day NP, Limmathurotsakul D, Peacock SJ, 2012. Accuracy of a commercial IgM ELISA for the diagnosis of human leptospirosis in Thailand. Am J Trop Med Hyg 86: 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busson L, Hallin M, Thomas I, De Foor M, Vandenberg O, 2014. Evaluation of 3 rapid influenza diagnostic tests during the 2012–2013 epidemic: influences of subtype and viral load. Diagn Microbiol Infect Dis 80: 287–291. [DOI] [PubMed] [Google Scholar]
- 32.Haanshuus CG, et al. 2016. A high malaria prevalence identified by PCR among patients with acute undifferentiated fever in India. PLoS One 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanja EW, Kuya N, Moranga C, Hickman M, Johnson JD, Moseti C, Anova L, Ogutu B, Ohrt C, 2016. Field evaluation of diagnostic performance of malaria rapid diagnostic tests in western Kenya. Malar J 15: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahende C, Ngasala B, Lusingu J, Yong T-S, Lushino P, Lemnge M, Mmbando B, Premji Z, 2016. Performance of rapid diagnostic test, blood-film microscopy and PCR for the diagnosis of malaria infection among febrile children from Korogwe District, Tanzania. Malar J 15: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure and tables