Abstract.
Cryptosporidium is a leading cause of diarrhea among Kenyan infants. Ceramic water filters (CWFs) are used for household water treatment. We assessed the impact of CWFs on diarrhea, cryptosporidiosis prevention, and water quality in rural western Kenya. A randomized, controlled intervention trial was conducted in 240 households with infants 4–10 months old. Twenty-six weekly household surveys assessed infant diarrhea and health facility visits. Stool specimens from infants with diarrhea were examined for Cryptosporidium. Source water, filtered water, and filter retentate were tested for Cryptosporidium and/or microbial indicators. To estimate the effect of CWFs on health outcomes, logistic regression models using generalized estimating equations were performed; odds ratios (ORs) and 95% confidence intervals (CIs) are reported. Households reported using surface water (36%), public taps (29%), or rainwater (17%) as their primary drinking water sources, with no differences in treatment groups. Intervention households reported less diarrhea (7.6% versus 8.9%; OR: 0.86 [0.64–1.16]) and significantly fewer health facility visits for diarrhea (1.0% versus 1.9%; OR: 0.50 [0.30–0.83]). In total, 15% of intervention and 12% of control stools yielded Cryptosporidium (P = 0.26). Escherichia coli was detected in 93% of source water samples; 71% of filtered water samples met World Health Organization recommendations of < 1 E. coli/100 mL. Cryptosporidium was not detected in source water and was detected in just 2% of filter rinses following passage of large volumes of source water. Water quality was improved among CWF users; however, the short study duration and small sample size limited our ability to observe reductions in cryptosporidiosis.
INTRODUCTION
Unsafe drinking water consumption increases the risk of diarrheal illness, a major cause of morbidity and mortality among infants and young children in sub-Saharan Africa.1–3 Mortality rates are highest among those < 2 years old; in 2015, diarrhea killed an estimated 499,000 children worldwide.4,5 In rural western Kenya, infants and young children bear the greatest burden of diarrhea-related illness and death.6,7
Diarrhea is a common symptom of enteric infections and is clinically defined as ≥ 3 loose stools in 24 hours. Diarrheal episodes vary in severity and can last anywhere from several hours to many weeks. Extended or repeated episodes can lead to malnutrition, which in turn increases the risk of severe diarrheal infection and death.8–11 Improving access to safe drinking water can decrease the risk of diarrhea and malnutrition in young children.4,12,13
Cryptosporidium, a highly chlorine-tolerant protozoan parasite, is a leading cause of moderate-to-severe diarrhea among Kenyan children, particularly in those between 0 and 11 months.6,7 Symptoms of cryptosporidiosis generally begin 2–10 days after infection. The most common symptom is watery diarrhea.14 Cryptosporidiosis is associated with longer diarrheal duration (median of 5–10 days) than that seen with other causes and may relapse after initial symptoms have resolved.15–17 Among children in developing countries, diarrhea often lasts for 14 days or longer.15,18 Cryptosporidium is spread through direct or indirect contact with infected feces. Severe infections are more likely to occur among young children and persons with weakened immune systems.14
Although many species of Cryptosporidium can infect humans and a wide range of animals, most human infections are caused by Cryptosporidium hominis (primarily transmitted through person-to-person contact) and Cryptosporidium parvum (transmitted through both person-to-person and animal-to-person contact).19 Both C. hominis and C. parvum can be transmitted through the consumption of contaminated water.20 Effective water treatment methods to remove or inactivate Cryptosporidium reduce the risk of illness.21
Studies have demonstrated that ceramic water filters (CWFs), particularly when coated in colloidal silver, can be an effective method to improve the microbial quality of household water.22–25 Ceramic filters remove bacteria and parasites by physical removal (because of ceramic pore size) and have been reported to effectively reduce bacteria through inactivation when produced with colloidal silver, which is recognized as an effective microbial disinfectant.26,27 There is some evidence of virus removal,22 but data are variable.27 The effectiveness of CWFs in removing bacterial and protozoan pathogens depends heavily on the production quality of the filter.22,28–31 In laboratory studies where microsphere particles 1–10 μm in size were used (i.e., a typical size range for bacteria and protozoa), CWFs made according to the Potters for Peace guidelines for manufacture have reportedly reached removal rates of > 99%.22
Ceramic water filters are most appropriate in areas where there is a capacity for quality ceramic filter production. CeraMaji CWFs are manufactured in Kenya (Figure 1). Each filter is made by combining local clay and tempter materials and is dipped in colloidal silver after firing (see www.ceramaji.com). Filters hold about 8 L of water and are suspended inside a plastic receptacle fitted with a tap and a lid. Following filter production, the manufacturer conducts visual assessments and performs pressure, flow rate, microbiological, and pore size testing. Filters sell for a local commercial price of approximately $30 USD and, if correctly maintained, can last approximately 1–3 years for a family of up to five people. CeraMaji CWFs are manufactured according to the Potters for Peace guidelines as previously described.25
Figure 1.
Photo of ceramic filter with accompanying tap and plastic receptacle. This figure appears in color at www.ajtmh.org.
We conducted an intervention trial to assess the effectiveness of household CWFs to reduce the burden of diarrhea and cryptosporidiosis acquired by infants and young children through drinking water in rural Kenya.
METHODS
Study population and sampling.
The Kenya Medical Research Institute (KEMRI)/Centers for Disease Control and Prevention (CDC) Health and Demographic Surveillance System (HDSS),32 specifically the Asembo HDSS in Siaya County, western Kenya, was used as the sampling frame for this non-blinded, randomized, controlled intervention trial. This rural area borders Lake Victoria. Residents, predominantly of the Luo ethnic group,33 earn their living through small-scale business, farming, and fishing.34
Sample size calculations were computed to detect differences in diarrheal prevalence in the two study groups.35–37 Other health outcomes, including fever, cough, and difficulty in breathing in the last 48 hours, were also measured to assess reporting bias. Group sample sizes for the study were computed as 120 children 4–10 months old in the intervention group and 120 children 4–10 months old in the control group (240 enrolled children in total). These group sample sizes achieve 86% power to detect an odds ratio (OR) of 0.68 in a study design with 26 repeated measurements, or when comparing proportions of longitudinal prevalence of diarrheal disease, an OR of 0.07 in the intervention group compared with 0.10 in the control group. The sample size calculations take into account the correlation between repeated measurements with an autoaggressive covariance structure and the expected proportion of longitudinal prevalence of diarrheal disease of 10% in the control group.38 The correlation between observations within the same study subject was assumed as 0.3 and the alpha level is 0.05. The sample size takes into consideration a 20% possible attrition rate.
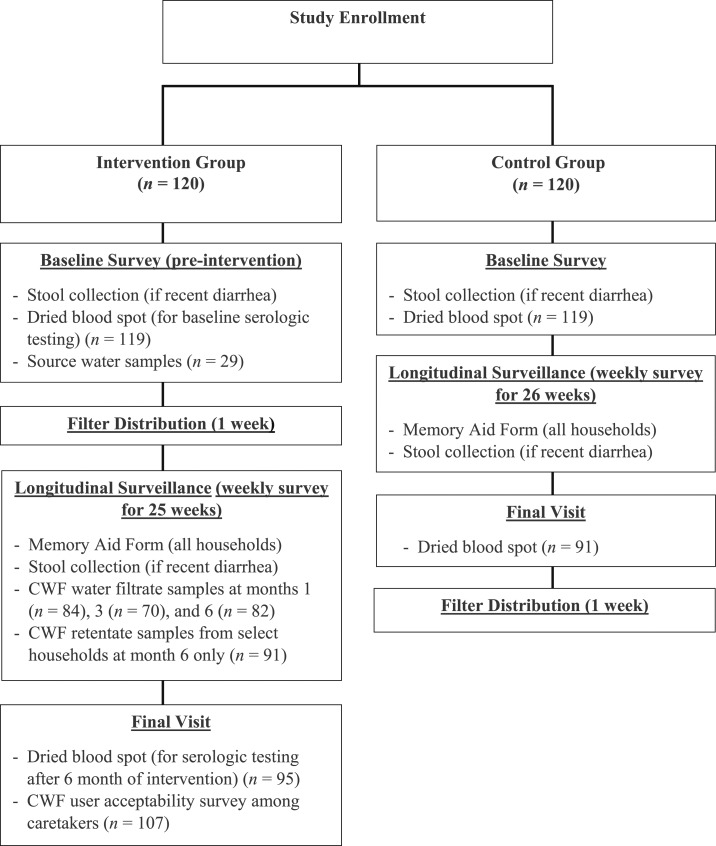
Only one child was enrolled per household. Each intervention household received a CWF (controls received a CWF when the study ended). Children living in households that already had a CWF or children outside of the age criteria were excluded. Study participants were selected at the individual level. Baseline, longitudinal surveillance, and user acceptability data (Figure 2) were collected between January and August 2013.
Figure 2.
Intervention trial structure and measures.
Epidemiologic methods.
Baseline.
Following enrollment, a baseline questionnaire was administered. The questionnaire collected information on drinking water sources, water treatment practices, infant feeding practices, water, sanitation, and hygiene related behaviors, infant water exposures, any recent illness in the enrolled infant (e.g., diarrhea, fever, and cough), and any recent health facility visits for illness.
Longitudinal surveillance.
Following CWF distribution, weekly questionnaires were administered to both the intervention (25 weeks; because of 1 week rollout of CWFs) and the control group (26 weeks). The weekly surveys captured the primary outcomes of any diarrhea since the previous visit and any recent health facility visits for illness. To assess reporting bias in the treatment group, we also captured any recent health facility visit, fever, cough, and difficulty breathing in the last 48 hours in the enrolled infants. In addition, surveys assessed drinking water sources, infant water exposures, and water treatment practices. Diarrhea memory aids were provided to all caretakers to capture the number of diarrhea episodes and number of days of diarrhea per diarrheal episode among enrolled children. After baseline, CWFs were distributed to intervention homes. Any reported cracked or broken filters were replaced within 7 days. Self-reported health facility visits were confirmed via chart abstraction.
User acceptability.
After weekly visits were completed, a user acceptability questionnaire was administered to all intervention households. Caretakers were asked about the taste of filtered water, patterns of use, child water consumption, perceptions of health impact, and preferred water treatment method.
Laboratory methods.
Stool testing.
Stool samples were collected at baseline and at weekly visits if caretakers reported that the child had diarrhea in the previous 7 days. Commercial immunoassays were used to test stools for Crytosporidium (Quik Chek™; TechLab, Inc., Blacksburg, VA). Molecular detection of Cryptosporidium using 18S and GP60-based polymerase chain reaction (PCR) assays was conducted, followed by species determination and subtyping through restriction fragment length polymorphism and sequence analyses of positive specimens.39
Seroincidence of Cryptosporidium.
A finger or heel-prick dried blood spot (DBS) specimen was collected at baseline and final weekly visit to test for the presence of Cryptosporidium antibodies.40 Each DBS contained approximately 10 μL of dried blood. Dried blood spot elutions were performed overnight at 4°C using 500 μL of elution buffer containing 10 mM Na2PO4 at pH 7.2, 0.85% NaCl, 0.3% Tween-20, 0.02% sodium azide, 0.5% casein, 0.5% polyvinyl alcohol, and 0.8% polyvinylpyrrolidone. Eluates (100 μL) were diluted with an additional 300 μL of buffer and Escherichia coli extract was added for a final concentration of 3 μg/mL. Multiplex assays were performed, as previously described,40 using Cp17 and Cp23 antigens for C. parvum.41 Cutoffs were determined by receiver operating characteristic analysis of multiplex results from abbreviated panels of sera (17 Western blot positive and 20 Western blot negative sera for antibodies to Cryptosporidium). To be classified as positive for Cryptosporidium-specific antibodies, a serum sample had to have responses > 428 and > 1,380 median fluorescent intensity minus background units for Cp17 and Cp23, respectively.
Water quality testing.
Water samples from a random subsample of intervention households (N = 29) were collected at the water source identified by study participants. A grab sample was collected at each source for on-site measurement of select water quality parameters, including temperature, pH (Pocket pH Tester; Hanna Instruments, Woonsocket, RI), turbidity (2020e turbidity meter; LaMotte Co., Chestertown, MD), and free chlorine residual (Color Disc Test Kit; Hach Co., Loveland, CO). Grab samples were transported to a laboratory at 4°C; and E. coli (an indicator of fecal contamination) were quantified using IDEXX® Colilert®-18 (IDEXX Laboratories, Inc., Westbrook, ME) methodology on a 1:10 dilution in sterile distilled water within 6 hours of collection. Paired, large volume (7–50 L, depending on turbidity of water) water samples were collected using dead-end ultrafiltration (DEUF). Filters were transported to a laboratory at 4°C and backflushed. The backflush was concentrated via centrifugation within 6 hours of collection.42,43 Nucleic acids were then extracted from each concentrate and shipped to CDC where immunomagnetic separation/immunofluorescence microscopy and real-time PCR were used for detection of Cryptosporidium.44,45
Two filtrate samples (i.e., water that had passed through the CWF) were collected from all intervention households at 1, 3, and 6 months. Samples designated for microbiological analysis were collected in sterile sample bottles containing sodium thiosulfate. Free chlorine residual was tested on-site to determine whether households had performed additional treatment on filtered water. Water quality parameters, including pH and turbidity, were measured in the laboratory and E. coli were quantified, as described previously. At 6 months, retentate samples (i.e., a rinse of a filter’s interior) were collected from randomly selected intervention filters (N = 91) to assess retention of Cryptosporidium oocysts within filter walls. The bottom and side walls of the CWF were rinsed with 500 mL of sterile distilled water and scrubbed with a sterile brush. The sample was then pipetted into a sterile sample container. Turbidity of a subsample was measured, followed by concentration of the remaining volume and molecular testing using real-time PCR.22,45
Statistical analysis.
Data were analyzed using SAS software version 9.3 (SAS Institute, Inc., Cary, NC). Missed rounds, those where caretakers were not present or were unable to complete the survey, were not included in analyses.
Epidemiologic data.
Generalized estimating equations (GEEs) were used to estimate the effect of having a CWF on weekly diarrheal, febrile, and respiratory illness outcomes.46 Odds ratios and 95% confidence intervals (CIs) were derived from the robust model estimates using an autoregressive correlation structure to account for repeated measures.47,48
For analysis of episodes and duration of diarrhea, respondents with more than 20% of their Memory Aid information missing were excluded. The cessation of a diarrheal episode was signified by at least two consecutive days of normal stool. Subsequent diarrhea was considered a new episode. The number of episodes per child were calculated. To compare the number of diarrhea episodes per child between intervention and comparison groups, a negative binomial model was performed. To estimate differences in duration of episodes, a Poisson model with a variance component to account for repeated episodes within a child, was performed. Incident Rate Ratios (IRR) and 95% Confidence Intervals for overall counts of diarrheal episodes and duration of episodes.
Laboratory data.
Generalized estimating equations were used to estimate the effect of having a CWF on a child having a stool sample positive for Cryptosporidium. Odds ratios and 95% CIs are derived from the robust model estimates using an autoregressive correlation structure to account for the repeated measures.47,48
A χ2 test for independence was used to compare serologic data between treatment groups collected at baseline and final visit. For comparison purposes, serologic data were subset to only children who were negative for Cryptosporidium-specific antibodies at baseline.
Ethical review.
The study protocol was approved by an Institutional Review Board of the CDC, Atlanta, GA (Protocol no. 6369) and KEMRI (Protocol no. 2439). Written informed consent was obtained. This study was registered as a randomized, controlled intervention trial at ClinicalTrials.gov, with study identifier number: NCT01695304.
RESULTS
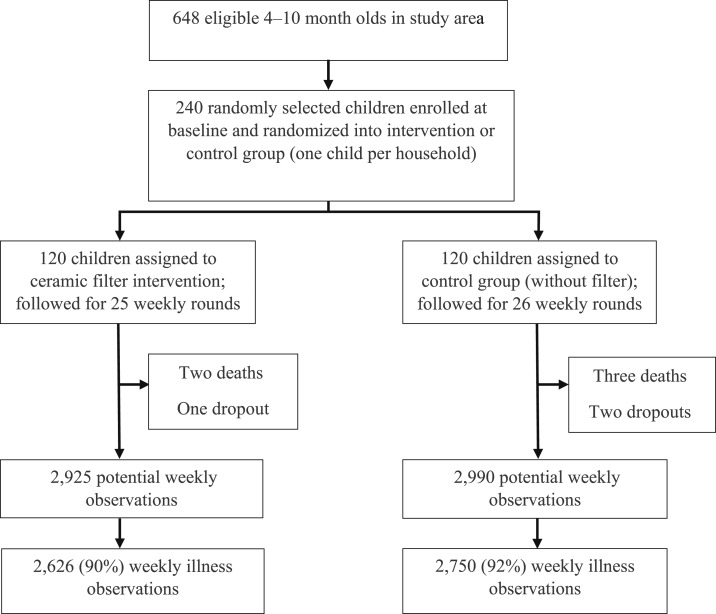
Two hundred and forty of 648 eligible infants 4–10 months old in the HDSS were randomly selected and enrolled at baseline. Enrollees were randomized into an intervention or control group, each consisting of 120 children (Figure 3). During the study period, 102 (85%) intervention and 106 (88%) control group children had at least 20 weeks of observational data.
Figure 3.
Trial profile.
Baseline.
Baseline demographic characteristics were similarly distributed. Infants 5–10 months old were overrepresented in the sample because of the timing of HDSS census rounds. At baseline, most households in both groups reported drinking surface water or using public taps as their primary drinking water source. A total of 60.8% of intervention and 58.3% of control households reported using a chlorine product to treat their household drinking water. However, of those currently using chlorine treatment, fewer than 8% of households in both groups reported treating their drinking water within the last 24 hours. Of 40 stored household drinking water samples tested, only 10% had a positive free chlorine residual. No households reported filter use (Table 1).
Table 1.
Baseline characteristics by treatment group
| Intervention N = 120 (%) | Control N = 120 (%) | P value* | ||
|---|---|---|---|---|
| Sex | Female | 66 (55.0) | 62 (51.8) | 0.60 |
| Male | 54 (45.0) | 58 (48.2) | – | |
| Median age at enrollment, months (range)† | 7 (4–10) | 8 (4–10) | 0.52 | |
| Primary caretaker | Mother | 118 (98.3) | 116 (96.7) | 0.68‡ |
| Other | 2 (1.7) | 4 (3.3) | – | |
| Caretaker education | Less than primary school education | 42 (35.0) | 47 (39.2) | 0.50 |
| Completed primary school education or above | 78 (65.0) | 73 (60.8) | – | |
| Home ownership | Own | 111 (92.5) | 114 (95.0) | 0.42 |
| Rent | 9 (7.5) | 6 (5.0) | – | |
| Number of individuals per household, mean (range)§ | 5.8 (2–13) | 5.7 (2–12) | 0.77 | |
| Number of children under 60 months in household, median (range)† | 2 (1–7) | 2 (1–5) | 0.59 | |
| Primary water source | Surface water | 47 (39.2) | 40 (33.3) | 0.32 |
| Public tap | 35 (29.2) | 35 (29.2) | – | |
| Rainwater | 15 (12.5) | 26 (21.7) | – | |
| Borehole | 5 (4.2) | 9 (7.5) | – | |
| Others‖ | 14 (11.7) | 14 (11.7) | – | |
| Water treatment¶ | Any chlorine treatment# | 73 (60.8) | 70 (58.3) | 0.69 |
| Boil | 22 (18.3) | 23 (19.2) | 0.87 | |
| Strain through cloth | 11 (9.2) | 19 (15.8) | 0.12 | |
| Others | 7 (5.8) | 6 (5.0) | 0.78 | |
| Reported water treatment (≤ 24 hours) | 9 (7.6) | 8 (6.8) | 0.80 | |
P value based on χ2 test.
Median and range reported, P value based on Wilcoxon rank sum test.
Fisher’s exact test.
Mean and range reported, P value based on two-sample t test.
Combined variable represents primary water sources reported by < 5% of either treatment group.
Represents what caretakers reported as usually used to make water safer to drink.
Reported use of chlorine-based treatment including WaterGuard, PUR water purification sachet, chlorine tablet, or bleach.
At baseline, the majority of caretakers in both groups reported giving their child drinking water (90% and 87.5%, P = 0.5). Caretakers reported introducing children to water at as early as 14 days old, with a median age of 4 months for both groups. Almost all children (93.9%) were exposed to drinking water before the age of 7 months. Furthermore, approximately half of the children in each group (53.7% and 44.8%) were given untreated drinking water. Nearly one-fifth of the children in both groups were exposed to recreational water sources (Table 2).
Table 2.
Baseline infant water consumption and exposures among those who reported giving their child drinking water
| Intervention N = 108 (%) | Control N = 105 (%) | P value* | ||
|---|---|---|---|---|
| Mean age at initial consumption, months† | 4 (0.03–8) | 4 (0.1–9) | 0.89 | |
| Age at initial water consumption, months | < 1 | 12 (11.1) | 10 (9.5) | 0.75 |
| 1–3 | 33 (30.6) | 30 (28.6) | – | |
| 4–6 | 55 (50.9) | 60 (57.1) | – | |
| 7–10 | 8 (7.4) | 5 (4.8) | – | |
| Infant consumption of untreated water | 58 (53.7) | 47 (44.8) | 0.15 | |
| Recreational water exposure via bathing or swimming‡ | 21 (17.7) | 20 (16.8) | 0.86 | |
P value based on χ2 test.
Mean and range reported, P value based on two sample t test.
Reported recreational water sources included river, lake, dam, and pond.
Weekly surveillance.
Health outcomes.
Over 90% of intervention households reported CWF use since the previous visit. Among households participating in ≥ 20 (77%) weekly rounds, 91.2% reported using the filter since the previous weekly visit. Households reported filling the CWF between 1 and 3 times per week. During the study, approximately 12 of 120 filters were replaced within 7 days of incident because of breakage, cracking, or slow flow. Self-reported diarrhea was lower in the intervention group than that in the control group; however, not statistically significant. Differences in fever, cough, and difficulty breathing were similar between groups (Table 3). Children in the intervention group were significantly less likely to visit a health facility for diarrhea compared with those in the control group (OR: 0.50; 95% CI: 0.30–0.83). An attempt was made to verify all 507 reported health facility visits for diarrhea, with 70% confirmed via direct chart abstraction from the child’s medical records at the listed facility.
Table 3.
Weekly reported illnesses and reported health facility visits for illness in the intervention and control groups
| Illness | Intervention (N = 2,625) | Control (N = 2,746) | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Illness | ||||
| Diarrhea | 200 (7.6) | 244 (8.9) | 0.86 (0.64–1.16) | 0.33 |
| Fever | 375 (14.3) | 396 (14.4) | 1.00 (0.80–1.26) | 0.97 |
| Cough | 418 (15.9) | 447 (16.3) | 0.97 (0.74–1.25) | 0.80 |
| Difficulty breathing | 116 (4.4) | 107 (3.9) | 1.16 (0.77–1.75) | 0.48 |
| Any illness | 412 (15.7) | 507 (18.5) | 0.83 (0.68–1.01) | 0.06 |
| Health facility visits | ||||
| Diarrhea | 25 (1.0) | 53 (1.9) | 0.50 (0.30–0.83) | 0.01 |
| Fever | 145 (5.5) | 188 (6.6) | 0.80 (0.61–1.05) | 0.11 |
| Respiratory illness | 90 (3.4) | 79 (2.9) | 1.20 (0.83–1.73) | 0.34 |
| Any illness | 234 (8.9) | 278 (10.1) | 0.88 (0.71–1.09) | 0.23 |
CI = confidence interval.
Diarrheal duration.
Of the 175 observation days per child for the memory aid, 90 (76%) intervention and 94 (80%) control group children had at least 140 (80%) days of data recorded. The median number of diarrheal episodes was two (range 0–13) for the intervention group and one (range 0–12) for the control group; this difference was not statistically significant (IRR: 1.18; 95% CI: 0.87–1.59). In total, 69 (77%) of 90 children from the intervention group and 75 (80%) of 94 children from the control group had at least 1 episode of diarrhea over the study period. Among the 144 children with at least one diarrheal episode, the median duration of diarrhea per episode was significantly lower in the intervention group (2 days; range 1–16) than that in the control group (3 days; range 1–28) (IRR: 0.81; 95% CI: 0.67–0.98). Overall, the median number of total reported diarrheal days, regardless of episodes, for all children was not significantly different between treatment groups.
User acceptability.
In total, 107 caretakers in the intervention group completed the user acceptability survey. All caretakers reported that water produced by CWF had an acceptable taste and that filters were easy to use and were an improvement over previous water treatment methods used. All reported that filtered water was used for drinking and that enough water was filtered to meet their family’s needs. All caretakers reported using filtered water for drinking; 87% reported using filtered water for preparing food. Almost half (N = 52) of the caretakers reported filling the CWF at least once per day and 41.1% ≥ 2 times per day. Three-fourths of intervention caretakers (74.5%) noted an improvement in the child’s health. Most caretakers (98.1%) reported that it was easy to give their child filtered water; however, 43% of caretakers reported that in addition to filtered water, they also continued to give their child unfiltered water.
Laboratory findings.
Stool testing.
In total, 110 stool samples were collected from 66 intervention children and 106 samples were collected from 67 control children. Samples from 15% (N = 10) of intervention children and 12% (N = 8) of control children were positive for Cryptosporidium by either the enzyme immunoassay or the PCR method (OR: 1.18; 95% CI: 0.44–3.13) (Table 4). Seven intervention children and one control child tested positive using both methods.
Table 4.
Number of intervention and control children with Cryptosporidium-positive stool specimens
| Test result per child | Intervention children (N = 66 [%]) | Control children (N = 67 [%]) | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Cryptosporidium positive by EIA or PCR | 10 (15.2)* | 8 (11.9)† | 1.18 (0.44–3.13) | 0.74 |
| Cryptosporidium positive by EIA‡ | 8 (12.1) | 4 (6.0) | 2.02 (0.60–6.76) | 0.26 |
| Cryptosporidium positive by PCR§ | 9 (13.6) | 5 (7.5) | 1.63 (0.51–5.22) | 0.41 |
CI = confidence interval.
Seven children were positive by both EIA and PCR.
One child was positive by both EIA and PCR.
EIA = enzyme immunoassay.
PCR = polymerase chain reaction.
Of the nine intervention children who were PCR positive for Cryptosporidium, four children were positive for C. hominis and four children were positive for C. parvum; one child was positive for both C. hominis and C. parvum and one child tested positive for C. hominis on two separate occasions. Of the 10 Cryptosporidium-positive samples, four were C. hominis subtype leA11G3T3 (including both samples from the child who tested positive on two separate occasions), three were C. parvum subtype llcA5G3b, and one was C. parvum subtype lleA7G1; two C. hominis–positive samples were unable to be subtyped. Of all intervention children with Cryptosporidium-positive stool specimens, only one household reported a slow-flowing CWF. No households with Cryptosporidium-positive children reported having a broken CWF.
Of the five control children who were PCR positive for Cryptosporidium, two children were positive for C. hominis and three children were positive for C. parvum; one child tested positive for C. hominis on two separate occasions. Of the six Cryptosporidium-positive samples, two were C. hominis subtype leA11G3T3 (both samples from the child who tested positive on two separate occasions), one was C. hominis subtype laA26R3, two were C. parvum subtype llcA5G3b, and one was C. parvum subtype llaA11G3R1; two C. hominis–positive samples were unable to be subtyped.
Seroincidence of Cryptosporidium.
At baseline, 23 (9.7%) children were seropositive for Cryptosporidium, 10 (8.4%) of 119 children in the intervention group and 13 (10.9%) of 119 children in the control group; this difference was not statistically significant. Among children who tested negative for Cryptosporidium antibodies at baseline, 27 (28.4%) of 95 intervention children and 25 (27.5%) of 91 control children had seroconverted at the final visit (P = 0.89).
Water quality.
Source water samples tested (N = 29) included surface water (55%), rainwater (24%), unprotected dug wells (10%), protected dug wells (7%), and tube wells/boreholes (4%). The median temperature was 27°C (range 23–33°C), the median pH was 7.2 (range 6.2–8.4), and the median turbidity was 71.1 nephelometric turbidity units (range 0.1–1,158.0). Free chlorine was not detected in any source water sample. Nearly all samples (N = 28; 93%) were positive for E. coli, with a median concentration of 469 most probable number (MPN)/100 mL (range < 10–2.4 × 104 MPN/100 mL). Cryptosporidium was not detected in any source water tested.
After 1 month of filter use, 71% (N = 80/112) filtrate samples contained < 1 MPN E. coli/100 mL. At 3 and 6 months, percentages were not statistically different from month 1, with 73% (N = 78/107) and 70% (N = 75/107) of samples, respectively, containing < 1 MPN E. coli/100 mL. Percentages of samples in high (101–1,000 MPN E. coli/100 mL) and very high risk (> 1,000 MPN E. coli/100 mL) categories were low (1–5%) and did not change considerably over time. Over the 6-month study period, the median turbidity of filtered water ranged from 0.5 to 1.0 NTU and the median pH ranged from 7.5 to 7.8. Free chlorine was not detected in any filtrate samples. At 6 months, retentate from 91 filters was analyzed. The median turbidity of retentate was 113.0 NTU (range 2.6–1,591.0). At the study end, two retentate samples (2%) were PCR positive for Cryptosporidium; no oocysts were detected by microscopy.
DISCUSSION
Globally, diarrhea killed approximately one-half million children in 2015.4 Cryptosporidium is a leading cause of diarrhea in Kenya, where infants and young children are at greatest risk.6,16 We conducted a non-blinded, randomized, controlled intervention trial in Siaya County, western Kenya, to determine the effectiveness of household CWFs for reducing the burden of diarrhea and cryptosporidiosis acquired by infants and young children.
The early introduction of untreated drinking water from unsafe sources places young children at high risk for diarrheal illness and death; this practice was common among study households. More than a third of study households relied on surface water for drinking and less than 20% reported using a treatment method (boiling) capable of inactivating Cryptosporidium; 87% of all study children reportedly received household drinking water in infancy. In addition to drinking water consumption, caretakers also reported supplementing the child’s diet with food items that required water for mixing, including porridge and cow’s milk. Given potential inconstancies in water treatment and possible recontamination during storage, the percentage of study children at risk for exposure to diarrhea-causing waterborne pathogens was likely even higher than reported figures suggest.
During the course of the study, filters with decreased performance or those reported as cracked or broken were replaced, representing 10% of the filters distributed to intervention households. Similar studies do not always provide information on filter performance in the field, making it difficult to broadly compare the performance of the filters used.49,50 In a single study where performance information was provided, more than 30% of the CWFs distributed to intervention households were broken or temporarily out of service.51 Although the replacement rate in the current study was considerably less, additional consideration of CWF durability and handling guidance is warranted.
Most intervention households (75.7%) reported using the filter ≥ 3 times per week. This was confirmed through direct observation of water in the filter at the time of most of the weekly household visits. A similar study in Bolivia also reported high levels of consistent use.51
Despite high levels of reported filter use among intervention households, only moderate decreases in illness rates were observed. The proportion of children with at least one diarrheal episode over the study period was slightly lower in the intervention group (77% versus 80% in the intervention and control groups respectively) as was the median number of diarrheal days (4.0 versus 4.5). Also, among children with at least one diarrheal episode, the median duration of diarrheal episodes was significantly reduced in the intervention group (2 days versus 3 days in control group). Perhaps most importantly, intervention children had a significantly lower incidence rate for health facility visits for diarrhea, most of which were confirmed via chart abstraction at health facilities. It is unclear if the remainder was unconfirmed because of poor record keeping at the health facility level, errors in the reported facilities, reporting bias, or other reasons because we did not have a specific process, such as a form at individual health facilities, for prospectively capturing this information during the trial. Ultimately, intervention children visited a facility for diarrhea only half as frequently as children in the control group. These findings suggest that CWF use in intervention households may have had an impact in decreasing severe diarrhea from certain enteric pathogens that cause a longer illness and may have required a health facility visit.
Over the course of the study, stool samples from 18 children with diarrhea (approximately 9% of all stool samples from children with diarrhea) tested positive for Cryptosporidium spp., comparable with rates from an earlier study in Guinea-Bissau.52 Cryptosporidium positivity rates did not differ significantly by the study group. At baseline, 9.7% of children had serologic evidence of previous Cryptosporidium infection. By study end, 52 (28%) of 186 children had seroconverted to positive, with no statistically significant difference in seroconversion rates by group. Together, these findings suggest that Cryptosporidium infection was common even in children 10 months old and younger and that not all Cryptosporidium infections were captured by stool sample collection and testing. The equal rates of seroconversion in both study groups suggest that the CWF either failed to remove or inactivate Cryptosporidium in household drinking water, or that study participants in the intervention arm acquired Cryptosporidium from untreated drinking water or from other sources (e.g., person-to-person transmission, consumption of cow’s milk or other contaminated food items, or direct/indirect animal contact) in much the same way that participants in the control group may have been exposed. Future Cryptosporidium research should address other transmission routes and risk factors.
No source water sample tested positive for Cryptosporidium at baseline. However, this does not necessarily indicate an absence of Cryptosporidium contamination. Elevated turbidity in surface waters can impact the ability to recover microbes; laboratory studies indicate that the DEUF method has an average recovery efficiency of 43% for C. parvum in turbid surface water.4,44 However, source water sampling at baseline did indicate high levels of fecal contamination. Nearly all (93%) source water samples collected at baseline were positive for E. coli.
No E. coli were detected in more than 70% of filtrate samples in each of three sampling rounds, representing a substantial reduction in microbial contamination with filter use and safe storage of treated water. There was a substantial reduction in turbidity in filtered water as compared with source water, which likely improved user acceptance and uptake of the treatment method. Filtered water was not tested for Cryptosporidium presence or viability; these data would provide more direct evidence of CWF efficacy against Cryptosporidium in a household setting. The low level of detection of Cryptosporidium in just 2% of retentate samples may be related to one of more factors, including 1) low concentration of Cryptosporidium in source waters, 2) methodological constraints (e.g., low recovery because of the elution procedure or high turbidity of retentate samples), or 3) poor filter performance. Additional field studies of Cryptosporidium, including studies assessing oocyst viability, in filters and in filtered household drinking water are warranted.
Although caretakers in intervention households reported high levels of filter use, 43% noted that they continued to give their infant or young child unfiltered water. The inconsistency in exclusive reliance on filtered drinking water could account for the relatively small impact of the CFW on diarrhea and lack of impact on cryptosporidiosis. Failure to rely exclusively on household water treatment interventions for drinking water can greatly diminish their impact.53,54 Furthermore, because the filter is not portable and because filtered water has no residual chemical protection against recontamination, intervention children whose caretaker regularly used the filter at home may still have been exposed to unsafe water outside the home.
This study had several important limitations. This was a short-duration pilot study powered primarily to detect difference is reported diarrhea rates between study groups. This limited our ability to detect difference in all included measures between the treatment and control groups. Furthermore, primary water sources may have changed from baseline over the course of the study, and different water sources may pose different levels of risk and thereby result in reduced rates of exposure and illness from diarrheal pathogens, including Cryptosporidium.55 In addition, as funding was limited, costs associated with more comprehensive source water sampling were prohibitive. Expanding water sampling to consider variations in source water contamination and stored water in control households would have provided additional insights into the potential benefits of CWF use. Lastly, stool specimen collection posed unique logistical challenges. When diarrhea was reported and the infant did not produce a stool during the visit, caretakers were asked to collect a sample of the next stool for testing. However, returning to the home to collect the child’s stool before the next weekly visit was logistically challenging.
Ceramic water filters did improve water quality, and despite the limitations of this study, it suggests that they reduced the severity and duration of diarrheal episodes, even if their impact on cryptosporidiosis was not directly observed. A larger sample size, longer study duration, and increased emphasis on exclusive use of filtered water by study participants may demonstrate more accurately the potential for CWF to prevent diarrhea and specific infections such as cryptosporidiosis. Continued research on the key exposures that influence rates of cryptosporidiosis among young children is needed to better define more comprehensive interventions.
Acknowledgments:
This work was conducted under the collaboration between CDC and the Kenya Medical Research Institute (KEMRI). This study used data generated by the KEMRI/CDC HDSS, which is a member of the International Network for the Demographic Evaluation of Populations and their Health (INDEPTH). We acknowledge the contributions of and thank the KEMRI staff for supporting the data collection and processing including Alex Awuor Ondeng, Alfred Ogol, Jacob Okal, Hillary Koech, Jairus Abuom, Lillian Nyamulo, Carolyne Ouma, Caren Oreso, Caleb Omedi, Rhoda Wendo, Fanuel Ochola, Willis N’Ginja, Nelson Apollo, Judith Owino, George Otiende, Vincent Ogweno, Lilian Arita, Evans Apondi, and Jillay Apiyo; the KEMRI/CDC HDSS team; Kayla Laserson, Robert Breiman, Barry Fields, Michele Parsons, Robert Quick, Benjamin Nygren, and Michael Arrowood of the Centers for Disease Control and Prevention, Atlanta, GA; and Miranda Delahoy, Emory University, Atlanta, GA. We are grateful to the caretakers in the community who participated in this work. This article is published with the approval of the director, KEMRI.
Disclaimer: The findings and conclusions in this report are the findings and conclusions of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
REFERENCES
- 1.Boschi-Pinto C, Young M, Black RE, 2010. The Child Health Epidemiology Reference Group reviews of the effectiveness of interventions to reduce maternal, neonatal and child mortality. Int J Epidemiol 39: i3–i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNICEF/WHO , 2009. Diarrhea: Why Children Are Still Dying and What Can Be Done. Geneva, Switzerland: WHO/UNICEF. [Google Scholar]
- 3.Black RE, et al. Child Health Epidemiology Reference Group of WHO and UNICEF , 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375: 1969–1987. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2015 Mortality and Causes of Death Collaborators , 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui L, et al. Child Health Epidemiology Reference Group of WHO and UNICEF , 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 6.Kotloff KL, et al. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet 382: 209–222. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly CE, et al. 2012. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005–2007: a cohort study. PLoS Med 9: e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima AA, Guerrant RL, 1992. Persistent diarrhea in children: epidemiology, risk factors, pathophysiology, nutritional impact, and management. Epidemiol Rev 14: 222–242. [DOI] [PubMed] [Google Scholar]
- 9.Bhutta ZA, Nelson EA, Lee WS, Tarr PI, Zablah R, Phua KB, Lindley D, Bass D, Phillips A, 2008. Recent advances and evidence gaps in persistent diarrhea. J Pediatr Gastroenterol Nutr 47: 260–265. [DOI] [PubMed] [Google Scholar]
- 10.Lima AA, et al. 2000. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis 181: 1643–1651. [DOI] [PubMed] [Google Scholar]
- 11.Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, Guerrant RL, 2002. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg 66: 590–593. [DOI] [PubMed] [Google Scholar]
- 12.Cairncross S, Hunt C, Boisson S, Bostoen K, Curtis V, Fung IC, Schmidt WP, 2010. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol 39: i193–i205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, Cavill S, Allen E, Uauy R, 2013. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev 8: CD009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Public Health Association , 2015. Cryptosporidiosis. Heyman D, ed. Control of Communicable Diseases Manual, 20th edition. Washington, DC: American Public Health Association. [Google Scholar]
- 15.Schilling KA, et al. 2017. Factors associated with the duration of moderate-to-severe diarrhea among children in rural western Kenya enrolled in the global enteric multicenter study, 2008–2012. Am J Trop Med Hyg 97: 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delahoy MJ, et al. 2012. Cryptosporidium infection in children less than five years old with moderate-to-severe diarrhea in rural western Kenya, 2008–2011. Am J Trop Med Hyg 87: 154–155. [Google Scholar]
- 17.Shirley DAT, Moonah SN, Kotloff KL, 2012. Burden of disease from cryptosporidiosis. Curr Opin Infect Dis 25: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochoa TJ, Salazar-Lindo E, Cleary TG, 2004. Management of children with infection-associated persistent diarrhea. Semin Pediatr Infect Dis 15: 229–236. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization , 2009. Risk Assessment of Cryptosporidium in Drinking Water. Geneva, Switzerland: WHO. [Google Scholar]
- 20.Carey CM, Lee H, Trevors JT, 2004. Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocyst. Water Res 38: 818–862. [DOI] [PubMed] [Google Scholar]
- 21.Speich B, Croll D, Fürst T, Utzinger J, Keiser J, 2016. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect Dis 16: 87–99. [DOI] [PubMed] [Google Scholar]
- 22.Bielefeldt AR, Kowalski K, Schilling C, Schreier S, Kohler A, Summers RS, 2010. Removal of virus to protozoan sized particles in point-of-use ceramic water filters. Water Res 44: 1482–1488. [DOI] [PubMed] [Google Scholar]
- 23.Sobsey MD, Stauber CE, Casanova LM, Brown JM, Elliott MA, 2008. Point-of-use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ Sci Technol 42: 4261–4267. [DOI] [PubMed] [Google Scholar]
- 24.Brown J, Sobsey MD, Loomis D, 2008. Local drinking water filters reduce diarrheal disease in Cambodia: a randomized, controlled trial of the ceramic water purifier. Am J Trop Med Hyg 79: 394–400. [PubMed] [Google Scholar]
- 25.Lantagne DS, 2001. Investigation of the Potters for Peace Colloidal Silver Impregnated Ceramic Filter Report 1: Intrinsic Effectiveness. Allston, MA: Alethia Environmental. [Google Scholar]
- 26.Abebe LS, Su Y, Guerrant RL, Swami NS, Smith JA, 2015. Point-of-use removal of Cryptosporidium parvum from water: independent effects of disinfection by silver nanoparticles and silver ions and by physical filtration in ceramic porous media. Environ Sci Technol 49: 12958–12967. [DOI] [PubMed] [Google Scholar]
- 27.Oyanedel-Craver VA, Smith JA, 2008. Sustainable colloidal-silver-impregnated ceramic filter for point-of-use water treatment. Environ Sci Technol 42: 927–933. [DOI] [PubMed] [Google Scholar]
- 28.Brown J, Chai R, Wang A, Sobsey MD, 2012. Microbiological effectiveness of mineral pot filters in Cambodia. Environ Sci Technol 46: 12055–12061. [DOI] [PubMed] [Google Scholar]
- 29.Mittelman AM, Lantagne DS, Rayner J, Pennell KD, 2015. Silver dissolution and release from ceramic water filters. Environ Sci Technol 49: 8515–8522. [DOI] [PubMed] [Google Scholar]
- 30.Lemons A, Branz A, Kimirei M, Hawkins T, Lantagne D, 2016. Assessment of the quality, effectiveness, and acceptability of ceramic water filters in Tanzania. J Water Sanit Hyg Dev 6: 195–204. [Google Scholar]
- 31.Rayner J, Skinner B, Lantagne D, 2013. Current practices in manufacturing locally-made ceramic pot filters for water treatment in developing countries. J Water Sanit Hyg Dev 3: 252–261. [Google Scholar]
- 32.Adazu K, et al. 2005. Health and demographic surveillance in rural western Kenya: a platform for evaluating interventions to reduce morbidity and mortality from infectious diseases. Am J Trop Med Hyg 73: 1151–1158. [PubMed] [Google Scholar]
- 33.Hawley WA, et al. 2003. Implications of the western Kenya permethrin-treated bed net study for policy, program implementation, and future research. Am J Trop Med Hyg 68: 168–173. [PubMed] [Google Scholar]
- 34.Lindblade KA, et al. 2004. Sustainability of reductions in malaria transmission and infant mortality in western Kenya with use of insecticide-treated bednets: 4 to 6 years of follow-up. JAMA 291: 2571–2580. [DOI] [PubMed] [Google Scholar]
- 35.Guanghan L, Liang KY, 1997. Sample size calculations for studies with correlated observations. Biometrics 53: 937–947. [PubMed] [Google Scholar]
- 36.Rochon J, 1998. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med 17: 1643–1658. [DOI] [PubMed] [Google Scholar]
- 37.Guo Y, Logan HL, Glueck DH, Muller KE, 2013. Selecting a sample size for studies with repeated measures. BMC Med Res Methodol 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omore R, et al. 2013. Health care-seeking behavior during childhood diarrheal illness: results of health care utilization and attitudes surveys of caretakers in western Kenya, 2007–2010. Am J Trop Med Hyg 89 (Suppl 1): 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao L, et al. 2009. Subtype analysis of Cryptosporidium specimens from sporadic cases in Colorado, Idaho, New Mexico, and Iowa in 2007: widespread occurrence of one Cryptosporidium hominis subtype and case history of an infection with the Cryptosporidium horse genotype. J Clin Microbiol 47: 3017–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, Mkocha H, Martin DL, West SK, Gaydos C, Lammie PJ, 2012. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis 6: e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Priest JW, Moss DW, Visvervara GS, Jones CC, Li A, Isaac-Renton JL, 2010. Multiplex assay detection of immunoglobulin G antibodies that recognize Giardia intestinalis and Cryptosporidium parvum antigens. Clin Vaccine Immunol 17: 1695–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith CM, Hill VR, 2009. Dead-end hollow-fiber ultrafiltration for recovery of diverse microbes from water. Appl Environ Microbiol 75: 5284–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mull B, Hill VR, 2012. Recovery of diverse microbes in high turbidity surface water samples using dead-end ultrafiltration. J Microbiol Methods 91: 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill VR, Narayanan J, Gallen RR, Ferdinand KL, Cromeans T, Vinje J, 2015. Development of a nucleic acid extraction procedure for simultaneous recovery of DNA and RNA from diverse microbes in water. Pathogens 4: 335–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jothikumar N, da Silva AJ, Moura I, Qvarnstrom Y, Hill VR, 2008. Detection and differentiation of Cryptosporidium hominis and Cryptosporidium parvum by dual TaqMan assays. J Med Microbiol 57: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 46.Liang KY, Zeger SL, 1986. Longitudinal data analysis using generalized linear models. Biometrika 73: 13–22. [Google Scholar]
- 47.Zeger SL, Liang KY, 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130. [PubMed] [Google Scholar]
- 48.Zeger SL, Liang KY, Albert PS, 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44: 1049–1060. [PubMed] [Google Scholar]
- 49.Abebe L, Smith J, Narkiewicz S, Oyanedel-Craver V, Conaway M, Alukhethi S, Samie A, Brant J, Dillingham R, 2013. Ceramic water filters impregnated with silver nanoparticles as a point-of-use water-treatment intervention for HIV-positive individuals in Limpopo province, South Africa: a pilot study of technological performance and human health benefits. J Water Health 12: 288–300. [DOI] [PubMed] [Google Scholar]
- 50.Clasen TF, Parra GG, Boisson S, Collin S, 2005. Household-based ceramic water filters for the prevention of diarrhea: a randomized, controlled trial of a pilot program in Columbia. Am J Trop Med Hyg 73: 790–795. [PubMed] [Google Scholar]
- 51.Clasen TF, Brown J, Collin S, Suntura O, Cairncross S, 2004. Reducing diarrhea through the use of household-based ceramic water filters: a randomized, controlled trial in rural Bolivia. Am J Trop Med Hyg 70: 651–657. [PubMed] [Google Scholar]
- 52.Mølbak K, Højlyng N, Gottschau A, Sá JC, Ingholt L, da Silva AP, Aaby P, 1993. Cryptosporidiosis in infancy and childhood mortality in Guinea Bissau, West Africa. BMJ 307: 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosa G, Kelly P, Clasen T, 2016. Consistency of use and effectiveness of household water treatment practices among urban and rural populations claiming to treat their drinking water at home: a case study in Zambia. Am J Trop Med Hyg 94: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosa G, Huaylinos ML, Gil A, Lanata C, Clasen T, 2014. Assessing the consistency and microbiological effectiveness of household water treatment practices by urban and rural populations claiming to treat their water at home: a case study in Peru. PLoS One 9: e114997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fawell J, Nieuwenhuijsen MJ, 2003. Contaminants in drinking water: environmental pollution and health. Br Med Bull 68: 199–208. [DOI] [PubMed] [Google Scholar]