Abstract.
There is evidence that elderly patients with cutaneous leishmaniasis (CL) have more mucosal and disseminated diseases than young patients and their cells produce less antigen-induced interferon (IFN)-γ. Herein, we compared the roles of interleukin (IL)-10 and IL-15 as modulators of antigen-induced immune responses and the incidence of adverse reaction and response to therapy in young versus elderly patients with CL. Study participants included 35 senior (60–85 years) and 35 young (18–40 years) patients who had a diagnosis of CL documented by typical cutaneous lesions containing Leishmania braziliensis DNA. Elderly patients had less lymph node enlargement. Antigen-induced blood cell cytokine responses were studied in the absence or presence of IL-10 antibody or exogenously added recombinant IL-15. The ratio of IFN-γ/IL-10 was lower in elderly patients, and IFN-γ production was enhanced by either neutralization of IL-10 or exogenous recombinant IL-15 in blood cells from elderly but not young patients. Patients were treated three times weekly with antimony at 20 mg/kg/day for 20 doses. Although there was no difference in response to therapy between the two groups, two young patients needed rescue therapy with amphotericin B. Ventricular arrhythmias and ventricular overload were more frequent in elderly patients. We conclude that elderly patients have alterations in the immune response that may influence clinical manifestations, but we did not find that they had a higher failure rate than young subjects to antimony therapy. However, because of the high rate of electrocardiographic abnormalities during therapy, antimony should not be used in elderly patients with CL.
INTRODUCTION
Cutaneous leishmaniasis (CL) is the most common clinical form of tegumentary leishmaniasis. Cutaneous leishmaniasis is caused by a large number of Leishmania species and is most common in Africa, Asia, the Middle East, and South and Central America.1 Brazil has the highest number of cases of American tegumentary leishmaniasis (ATL). The species responsible for ATL in Brazil are Leishmania Viannia braziliensis, Leishmania Viannia guyanensis, Leishmania Viannia shawi, Leishmania Viannia lainsoni, and Leishmania mexicana.2,3 The village of Corte de Pedra, Tancredo Neves, Bahia, is one of the more important endemic areas of L. V. braziliensis transmission in Brazil. Cutaneous leishmaniasis is characterized by one or more well-demarcated ulcers with raised borders. In CL caused by L. V. braziliensis, one or several enlarged lymph nodes are usually observed preceding or concomitant with the cutaneous lesions.4 In addition, patients with CL due to L. V. braziliensis may develop disseminated manifestations such as mucosal leishmaniasis (ML) or disseminated leishmaniasis (DL).5–7 Because of the possibility of metastatic lesions, topical therapy is not recommended for treatment of CL in Brazil.8 Disseminated manifestations occur predominantly in young adult males, but 11.2% of patients with CL are older than 50 years and elderly patients have a higher incidence of ML and DL than young subjects.9
Most data related to the disease in the elderly, defined as older than 65 years for men and 60 years for women, come from epidemiologic and clinical studies with the participation of few elderly patients.10 In a retrospective study comparing CL in young versus senior patients, we found that patients older than 60 years were more likely to have a previous history of CL, less lymph node enlargement, and higher frequencies of ML and DL. In this study, we also documented that older patients produced less interferon (IFN)-γ and more interleukin (IL)-10 than young patients.11 Because the pathogenesis of CL is dependent on the host immune response,12–15 it is likely that alterations in the immune response may change not only the clinical presentation of the disease but also the response to therapy in the elderly. It is known that IFN-γ plays an important role in the control of Leishmania braziliensis infection, although an exaggerated and inappropriately modulated type 1 inflammatory response may cause tissue damage as observed in ML or DL. There is evidence that activation and subsequent cytokine production by CD4+ and CD8+ T cells and monocytes are associated with pathology in CL.14,16–18 It is also known that either impairment of the immunologic response as observed in diffuse CL19 or the exaggerated inflammatory reaction observed in ML or DL is associated with a poor response to therapy.4,20
In Brazil, meglumine antimoniate is the drug recommended by the Ministry of Health to treat CL. The response to therapy is quite variable, ranging from 28% to 100% depending on the location where the study was performed and the duration of illness.21–23 In CL patients diagnosed early, before the appearance of an ulcer, a high failure rate of antimony therapy (up to 72%) has been observed.23 In the endemic area where this study was performed, the antimony failure rate was less than 10% in 1994,24 whereas the failure rate has risen to 45–50% in CL patients over the past 10 years.25–27 Although there is a lack of evidence that age is associated with a poor response to therapy for CL, a few studies point out that adverse reactions are more frequent in the senior population.10 The aim of the present study was to determine if there are differences in the response to therapy and in the frequency and severity of adverse reactions among elderly and young patients and to better characterize abnormalities in the immune response of the elderly with CL and correlate immunologic findings with clinical outcomes.
MATERIALS AND METHODS
Ethical considerations.
Human studies protocols were reviewed and approved by the Institutional Review Board of the Federal University of Bahia. All subjects provided signed consent for participation in the study.
Study design.
This was a prospective observational study aimed at evaluating clinical manifestations, response, and adverse reactions to antimony therapy in senior and young patients with CL. Moreover, in a cross-sectional study, we compared the immune responses in these two groups of patients with an emphasis on establishing the roles of IL-10 and IL-15 in the modulation of the immune response and correlating immunologic features with clinical presentation of disease.
The study was performed between January 2014 and September 2016 at the Corte de Pedra health post, located in the municipality of Presidente Tancredo Neves, Bahia, Brazil. Inclusion criteria for elderly subjects were age between 60 and 85 years and illness duration between 30 and 60 days. Control subjects aged 18–40 years were matched to elderly subjects based on duration of illness (±5 days) and gender. During this period, 84 elderly patients sought medical attention for a cutaneous ulcer and had a diagnosis of leishmaniasis. Forty-nine patients were excluded from the study, among whom 13 had other clinical forms of ATL or an additional chronic disease. Moreover, 13 had an illness duration less than 30 or higher than 60 days, 11 were older than the age of inclusion, and 12 lived too far from the health post to be able to adhere to the scheduled visits during and after therapy. A total of 35 elderly and 35 young matched control patients were recruited. After cleaning and application of a local anesthetic, each participant was submitted to a 4-mm diagnostic punch biopsy of a border of the ulcer. The biopsy material was used to confirm the diagnosis of leishmaniasis by a quantitative polymerase chain reaction test for detection of L. braziliensis DNA.28
Sample size calculation was established based on an estimated difference of 30% between the groups in the response to therapy. To attain a power of 80% at a significance of 0.05, 35 patients in each group for a total of 70 patients were necessary. Patient clinical evaluations were conducted on days 0, 30, 60, and 90 days and a final evaluation was performed 6 months after entry in the study. All patients were treated with 20 doses of Glucantime (Sanofi-Aventis, São Pauo, Brazil), 20 mg/kg/weight/day applied on Monday, Wednesday, and Friday starting on day 0. They were asked about the type and severity of adverse reactions and whether therapy was altered or stopped because of side effects. Laboratory tests and electrocardiogram (ECG) were performed on days 0 and 30.
Soluble Leishmania antigen (SLA) and cell separation and cultivation.
Soluble Leishmania antigen was prepared from an isolate of L. braziliensis as previously described.28 Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Ficoll–Hypaque (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient centrifugation. After washing three times in 0.9% NaCl, PBMCs were adjusted to 3 × 106 cells in 1 mL of Roswell Park Memorial Institute medium-1640 (Gibco Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco Laboratories) and gentamicin (0.5 mg/mL) (Gibco Laboratories). The cells were placed on 24-well plates and incubated for 72 hours at 37°C and 5% carbon dioxide in the presence or absence of SLA (5 μg/mL), recombinant IL-15 (10 ng/mL) (PeproTech, Rocky Hill, NJ), or anti-IL-10 (10 μg/mL) (R&D Systems, Minneapolis, MN). After 72 hours, supernatants from PBMCs were collected and stored at −70°C.
Cytokine determination.
Levels of IFN-γ, tumor necrosis factor (TNF), chemokine ligand 9, IL-1β, and IL-10 were determined by the enzyme-linked immunosorbent assay (ELISA) sandwich method using reagents from R&D Systems. The results are expressed as pg/mL.
The levels of IFN-γ, IL-10, IL-1β, TNF, and CXCL9 (R&D Systems) were measured in supernatants of cultures by ELISA according to the manufacturer’s instructions and the results are expressed in pg/mL.
Clinical laboratory tests.
Red and white blood cell counts; plasma levels of sodium, potassium, creatinine, urea, and transaminases; and ECG were performed on days 0 and 30 of therapy.
Statistical analyses.
As the sample results were not normally distributed, statistical analyses were performed with nonparametric tests. Age data are presented as the mean ± standard deviation and the other continuous variables are presented as median and coefficient interval (CI). Categorical data were compared using the Fisher exact test and for continuous variables, the Mann–Whitney U test was used. The Spearman r test was used in the correlation analysis. GraphPad Prism 5 (San Diego, CA) was used to carry out the statistical evaluations using a P value of < 0.05 for statistical significance.
RESULTS
The clinical features of the 35 elderly and 35 young patients with CL who participated in the study are shown in Table 1. There were no significant differences between the group in gender, illness duration, or number, size, and localization of lesions. The frequency of satellite lymphadenopathy was lower in the elderly, as was the size of the affected lymph node. The median size of the largest lymph node in the elderly was 0 mm (CI 6.3–18), in contrast to 36.6 mm in young patients (CI 27.3–41.7 mm, P < 0.001).
Table 1.
Demographic and clinical features of cutaneous leishmaniasis in young and elderly patients
| Demographic/clinical features | Elderly (N = 35) | Young (N = 35) | P value |
|---|---|---|---|
| Age (mean ± SD) | 67 ± 5.2 | 31 ± 7.5 | P < 0.0001* |
| Frequency of males | 20 (57%) | 22 (63%) | NS |
| Illness duration (days) | 45 (38.92–51.43) | 35 (32.08–40.25) | NS |
| Total no. of lesions (mean ± SD) | 42 (1.29 ± 0.67) | 43 (1.14 ± 0.35) | NS |
| Size of the major lesion (mm)† | 20 (17.77–24) | 19 (18.19–23.36) | NS |
| Frequency of patients with lymph node enlargement | 15 (43%) | 30 (86%) | P < 0.01‡ |
| Size of the lymph node (mm)† | 0 (6.26–18) | 36.6 (27.3–41.74) | P < 0.0001§ |
| Leishmania skin test (mm) | 15 (14.90–17.44) | 15 (15.19–18.26) | NS |
NS = not significant; SD = standard deviation.
Student t test.
Median and range.
Fisher’s exact test.
Mann–Whitney test.
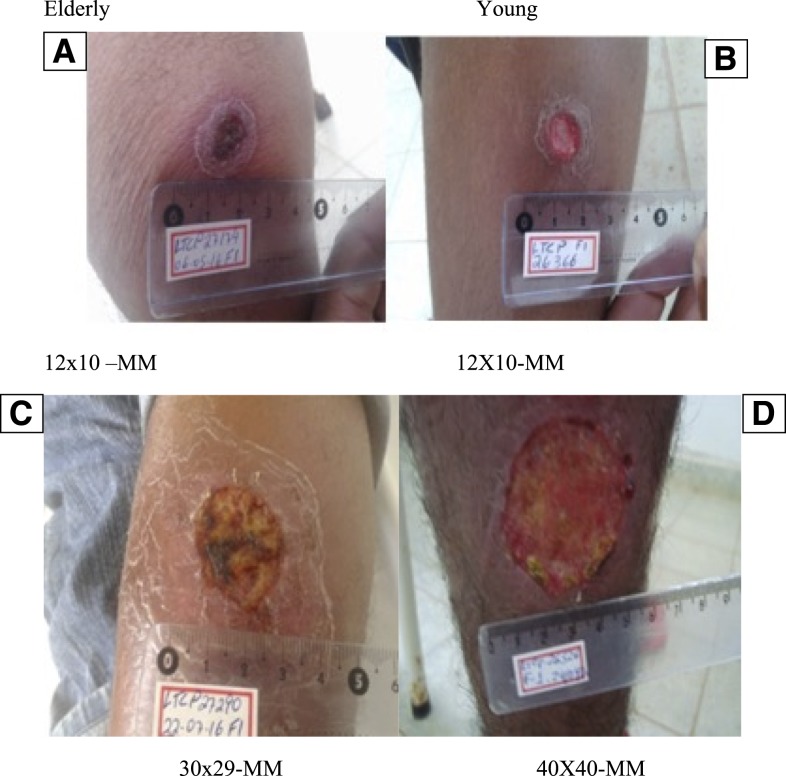
Photographs of ulcerated lesions from two elderly and two young patients are shown in Figure 1. Both patients and participants of the study presented with clinical ulcerated lesions with raised borders as shown in this figure. The sizes of the ulcers were similar between the two elderly and young patients as were the characteristics of their lesions.
Figure 1.
Clinical features of cutaneous ulcers of two elderly and two young participants of the study. Pictures taken on day 0 of two elderly patients (A and C) and two young patients (B and D). All four lesions were located on the inferior limbs. The elderly patients (A and B) had ages of 64 and 24 years, respectively, and illness duration of 30 days. Patients in figures C and D had ages of 64 and 30 years, respectively, and illness duration of 40 days. This figure appears in color at www.ajtmh.org.
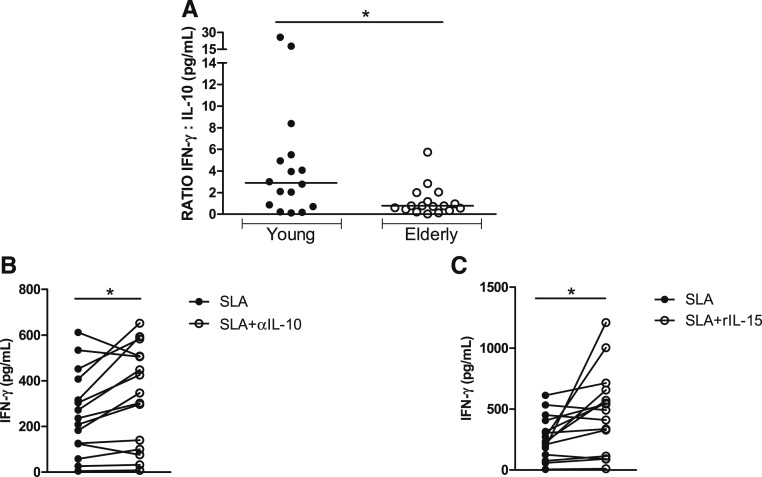
We have previously shown that PBMCs from elderly patients with CL produce less antigen-induced IFN-γ and more IL-10 than PBMCs from young patients.11 Here, we extended the characterization of the immune response by measuring IL-1β, CXCL9, and TNF levels in the two groups of patients. No significant difference was observed between the groups regarding the production of these cytokines (data not shown). The antigen-induced IFN-γ/IL-10 ratio was higher in young patients than in the elderly (Figure 2). Moreover, to better understand if IL-10 played a role in down-modulating IFN-γ production, we neutralized IL-10 in PBMC cultures stimulated with SLA with anti-IL-10 monoclonal antibodies (10 μg/mL). Whereas neutralization of IL-10 enhanced IFN-γ levels in the elderly subjects from 236 (5–612 pg/mL) to 347 (8–652 pg/mL) (P < 0.05), there was no significant change in the production of IFN-γ in the supernatants of PBMCs from young subjects after neutralization of IL-10 from 259 (26–1,223 pg/mL) to 264 (16–1,236 pg/mL), P > 0.05. We also evaluated if exogenous addition of IL-15 was able to enhance IFN-γ production. Whereas recombinant interleukin-15 enhanced IFN-γ production by PBMCs from elderly subjects from 236 (5–612 pg/mL) to 492 (10–1,210 pg/mL), P < 0.05 as shown in Figure 2, exogenous addition of IL-15 did not change IFN-γ levels in young patients from 385 (26–1,223 pg/mL) to 502 (66–1,148 pg/mL), P > 0.05.
Figure 2.
Ratio of interferon (IFN)/interleukin (IL)-10 and ability of anti-IL-10 and recombinant IL-15 to enhance IFN-γ production in elderly patients with cutaneous leishmaniasis (CL). Peripheral blood mononuclear cells from CL patients were stimulated with soluble Leishmania antigen (SLA) (5 μg/mL), anti-IL-10 (10 μg/mL), and rIL-15 (10 ng/mL) for 72 hours. Interferon-γ and IL-10 levels were determined in culture supernatants by enzyme-linked immunosorbent assay. (A) Interferon-γ/interleukin-10 ratio in elderly (N = 17) and young (N = 16) patients. (B) Interferon-γ levels after neutralization of IL-10 in the elderly (N = 15). (C) Interferon-γ levels after addition of rIL-15 in 15 patients for the elderly group. Statistical analyses were performed using the Mann–Whitney U test (A) and Wilcoxon rank test (B and C), *P < 0.05.
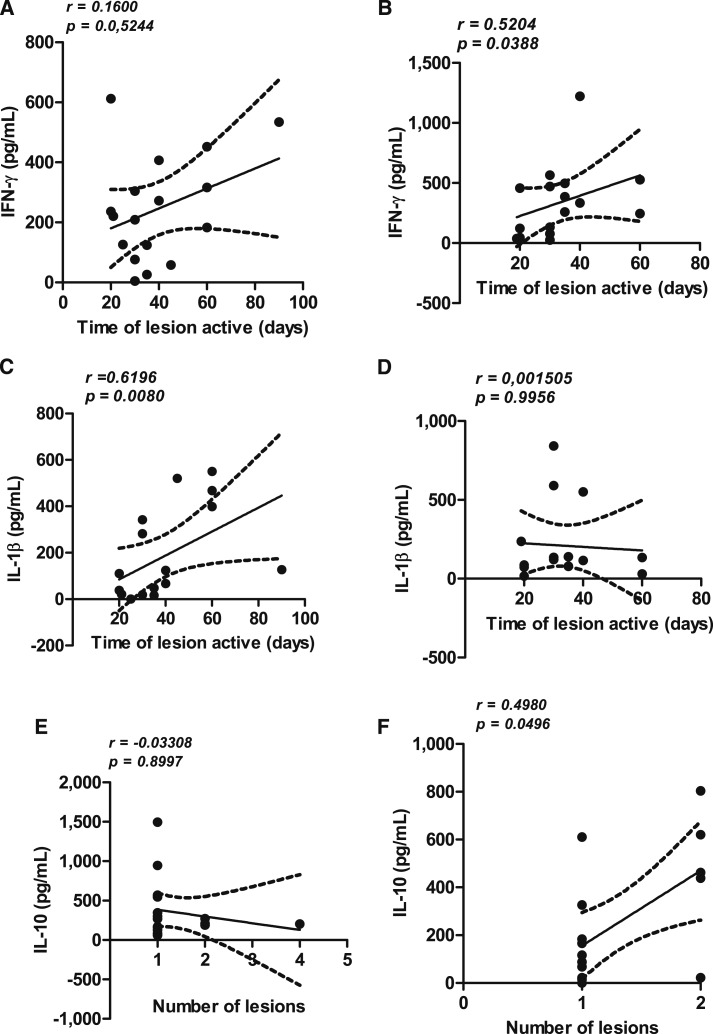
Correlations between cytokine levels, illness duration, and number of cutaneous lesions are shown in Figure 3. There was a direct correlation between illness duration and IFN-γ production in young patients (R = 0.52, P = 0.03) and IL-1β in the elderly (R = 0.61, P < 0.05). There was a direct correlation between IL-10 levels and number of lesions in young patients but not in the elderly (Figure 3). There was no difference in IFN-γ, IL-1β, TNF, and IL-10 levels (P > 0.05) in patients who cured after or failed the antimony therapy. There was also no correlation between the number of lesions with IFN-γ, IL-1β, and TNF levels.
Figure 3.
Correlation between cytokine levels and clinical findings in cutaneous leishmaniasis (CL). Cytokine levels in supernatants of peripheral blood mononuclear cells stimulated with soluble Leishmania antigen (5 μg/mL) in elderly (N = 17) and young (N = 16) patients with CL were correlated with illness duration in the elderly (A and C) and the young (B and D). The correlation between interleukin (IL)-10 and number of lesions in elderly patients is expressed in E and in young patients in F.
The therapeutic response to meglumine antimoniate in elderly and young patients with CL is shown in Table 2. Although the total number of patients cured at the 90-day time point was higher and the healing time was lower in the elderly compared with young patients, there was no statistically significant difference between these outcomes in the two groups. Patients who failed therapy were treated with one or two additional series of antimony and all were cured, with the exception of two young women who experienced relapses after three series of antimony and were eventually treated and cured with amphotericin B.
Table 2.
Therapeutic response to pentavalent antimony in elderly and young patients with cutaneous leishmaniasis from Leishmania braziliensis
| Elderly (N = 35) | Young (N = 35) | P value | |
|---|---|---|---|
| Cure in 60 days (%) | 12 (34%) | 13 (37%) | NS |
| Cure in 90 days (%) | 07 (20%) | 04 (11%) | NS |
| Total cured (%) | 22 (63%) | 17 (49%) | NS |
| Antimony therapeutic failure (%) | 13 (37%) | 18 (51%) | NS |
| Healing time (days, [range]) | 91 (28–246) | 121 (47–477) | NS |
Statistical differences were compared by Fisher’s exact test.
The main adverse reactions observed in the two groups of patients are listed in Table 3. Data are shown before treatment on day 0 and during the first course of treatment on day 30, at which time 2/3 of total antimony doses had been administered. There was no difference between the two groups in the frequency of arthralgia, myalgia, vomiting, or fever at either time point. There were also no differences in sodium, potassium, transaminase, urea, or creatinine levels on day 0 or on day 30 between the groups. At that time, 2/3 of the total dose of antimony had been administered. One patient from the elderly group had a mildly elevated creatinine (1.4) on day 0, but this did not increase by 30 days. Among the young group members, one patient had a mildly elevated creatinine (1.3) after therapy. An abnormal urea level was detected in one elderly patient during therapy.
Table 3.
Main adverse reactions and laboratory values in patients with cutaneous leishmaniasis treated with meglumine antimoniate
| Adverse reactions | Elderly (N = 35) | Young (N = 35) | P value | ||
|---|---|---|---|---|---|
| Day 0 | Day 30 | Day 0 | Day 30 | ||
| Arthralgia | 9 (26%) | 17 (49%) | 13 (37%) | 13 (37%) | NS |
| Fever | 7 (20%) | 1 (3%) | 13 (37%) | 6 (17%) | NS |
| Vomiting | 0 (0%) | 4 (11%) | 0 (0%) | 4 (11%) | NS |
| Sodium (D-0, D-30) | 140 (126–146) | 142 (137–147) | 140 (133–147) | 141 (135–146) | NS |
| Potassium (D-0, D-30) | 5.7 (3.6–9) | 5.8 (3.7–9.1) | 5.4 (3.4–10) | 5.3 (3.4–9.2) | NS |
| Alanine transaminase (D-0, D-30) | 21 (6–51) | 20 (9–42) | 25 (6–60) | 23.5 (8–93) | NS |
| Creatinine (D-0, D-30) | 1 (0.1–1.4) | 1 (0.1–1.4) | 0.9 (0.5–1.2) | 0.9 (0.5–1.3) | NS |
| Urea (D-0, D-30) | 33.5 (18–42) | 31 (21–82) | 28 (14.8–45) | 26 (17–45) | NS |
Data are the results of evaluations before therapy (day 0) and 2/3 of the way through the first course of antimony treatment on day 30.
Because conduction abnormalities can occur as a complication of antimony therapy, we cataloged the number of patients with ECG abnormalities in the elderly or young groups on day 30 after therapy. These data are shown in Table 4. As expected, before therapy, there were more abnormalities in the ECG in the elderly than in the young. However, on day 30, the newly acquired changes in ECGs were more frequent and more severe in the elderly group. Death due to myocardial infarction occurred in one elderly patient 16 days after completion of antimony therapy.
Table 4.
Major electrocardiographic abnormalities in elderly or young patients with cutaneous leishmaniasis before and during treatment with pentavalent antimony
| Abnormality | Elderly (N = 28) | Young (N = 34) | P value | ||
|---|---|---|---|---|---|
| Before therapy | After therapy | Before therapy | After therapy | ||
| Ventricular repolarization | 3 (10.7%) | 6 (21.5%) | 3 (8.8%) | 4 (11.8%) | NS |
| Ventricular overload | 0 (0%) | 4 (21.5%) | 0 (0%) | 0 (0%) | P < 0.05* |
| Right bundle branch block | 1 (3.6%) | 4 (14.5%) | 0 (0%) | 1 (2.9%) | P < 0.05* |
| Prolongation of the corrected QT interval | 0 (0%) | 2 (7.2%) | 0 (0%) | 0 (0%) | NS |
| Premature ventricular contraction | 1 (3.6%) | 4 (14.5%) | 1 (2.9%) | 1 (2.9%) | P < 0.05* |
| Sinus bradycardia | 1 (3.6%) | 1 (3.6%) | 1 (2.9%) | 1 (2.9%) | NS |
Comparison between the frequency of abnormalities before and after therapy in the elderly and also between the elderly and young patients after therapy.
DISCUSSION
Parasitic diseases occur predominantly in children and in young adults, but the number of patients older than 60 years with ATL has doubled in the last 20 years.9 Despite this increase, there have been few studies examining any differences in leishmaniasis between this age group and younger subjects. In this study, we showed that elderly patients had lower frequencies of satellite lymph node enlargement and lower IFN-γ production than young patients, confirming our previous study. Whereas enhancement of IFN-γ production in seniors was achieved by neutralization of IL-10 or by addition of recombinant IL-15 to peripheral blood cell cultures, manipulation of these cytokines did not change IFN-γ levels in young patients. We also show that the relationship between cytokine production and clinical findings differs between elderly and young patients. Despite these differences in immune response, elderly and young patients had similar cure rates with antimony therapy and similar chemistries. However, there was a higher rate of adverse electrocardiographic changes in elderly subjects, leading us to suggest that other forms of therapy should be used in this population.
We found no difference in the number, localization, or size of lesions between elderly and young subjects. However, in two previous retrospective studies, ulcer size was higher in patients older than 60 years than in young patients.9,11 One possible explanation for these discordant findings could be the differences in the illness duration between the two groups, which was longer in the elderly than in young patients in previous studies.9,11 Here, because the inclusion criteria limited the illness duration to between 30 and 60 days and because one of the matching criteria for control selection was a similar time of lesion duration, we did not detect any difference in ulcer size between the two groups. We confirmed the observation that there is a lower frequency and smaller size of satellite lymph nodes in elderly than young subjects.
Lymph node enlargement may be the first sign of CL due to L. braziliensis4 and an extremely large lymph node size is a characteristic of L. braziliensis infection.26,29 The importance of the large lymph node in the pathogenesis of L. braziliensis infection has not been determined. In BALB/c mice infected in the footpad with Leishmania amazonensis, the removal of the popliteal lymph nodes leads to parasite dissemination.30 However, we did not find any association between the presence or size of enlarged lymph nodes and clinical manifestations of the disease, production of cytokines, or response to therapy. In fact, although the frequency of lymph node enlargement was lower in the elderly at the time of entry into the study, virtually all patients informed us that they had lymph node enlargement early in the disease, suggesting that lymph node enlargement might be shorter in duration in elderly patients than in younger patients.
It has been shown that elderly patients with CL report a past history of CL and that their PBMCs produce less antigen-induced IFN-γ and more IL-10 than those of younger patients with CL.11 As cured CL is thought to provide protective immunity against reinfection, these observations suggest that there might be a progressive impairment of immunologic memory and/or a less efficient type 1 immune response with age. Because IL-10 is the most important down-regulatory cytokine in leishmaniasis and IL-15 is an important cytokine in the induction of memory cells, we evaluated the influence of neutralization of IL-10 and exogenous addition of IL-15 in our patients. We observed that neutralization of IL-10 and addition of IL-15 had no effect on the immune responses of young patients with CL, but both interventions enhanced IFN-γ production in the elderly group. The effect of rIL-15 may explain why the elderly are less protected than young subjects from subsequent L. braziliensis infection. The different responses to IL-10 neutralization also suggest that the ability of IL-10 to downregulate IFN-γ production in the elderly may in part explain the lower lymphadenopathy. As IFN-γ production is decreased among the elderly but not completely absent, it is possible that the more modulated immune response observed in this group can attenuate the pathology induced by high levels of pro-inflammatory cytokines and chemokines, although it may not impair the host’s ability to control Leishmania proliferation.
Previous studies have shown a direct correlation between the frequency of T cells expressing TNF and IFN-γ and lesion size.16 Here, we evaluate the correlation of immunologic response in elderly and young patients with illness duration, number of lesions, and response to therapy. There was a direct correlation between IFN-γ and illness duration in young patients and a negative correlation between IL-10 production and the number of lesions in elderly patients. Illness duration was directly correlated with IL-1β in elderly subjects. Furthermore, an effect of IL-10 neutralization was only observed in elderly subjects. These findings suggest that cytokines may influence the expression of disease in the elderly compared with the young. Whereas IL-10 may have a negative impact in young patients by increasing the number of lesions, in elderly patients, this cytokine might attenuate the inflammatory response and reduce pathology by diminishing pro-inflammatory cytokines. Differences between the correlations between IFN-γ and illness duration might be related to the lower amounts of IFN-γ produced in older subjects. The differential involvement of IL-1β is intriguing and deserves future studies of potential inflammasome activity between the groups.
Meglumine antimoniate is the drug recommended for therapy of leishmaniasis in Brazil and is the most used drug for treatment of ATL in Latin America. In the present study, the response to antimony therapy did not differ between groups, although we observed lower response rates than other studies in Brazil and South America, in which the cure rates range from 78% to 91.4%.31,32 The genome of L. braziliensis is polymorphic, and genetic differences have been found between isolates of L. braziliensis from different regions of Brazil and even within the same endemic area.33 We previously showed that genetic polymorphisms correlate with different clinical outcomes of L. braziliensis infection in Corte de Pedra.33,34 We hypothesize that genetic differences between L. braziliensis isolates might also be associated with different responses to antimony therapy. Consistent with this hypothesis, the response to therapy observed in the present study was similar to others performed in the same endemic area.25–27 In Peru, a country endemic for Leishmania peruviana, L. braziliensis, and Leishmania guyanensis, it was shown that the elderly progressively lose specific immunity against Leishmania antigens and become susceptible again.35 However, the second episode is less severe and heals faster.35 Despite the fact that we did not find differences in cure rates between the two age groups, there was a trend toward an accelerated healing time in the elderly subject group. In addition, the only two patients who needed to be treated with amphotericin B because of failure of at least two courses of antimony were among the young patients. Thus, the attenuated type 1 immune response might have provided a clinical benefit to the elderly subjects.
High rates of arthralgia and myalgia have been observed in patients undergoing antimony therapy10,36 as well as increases in creatinine and transaminases.37,38 In addition, there are case reports of sudden death occurring in CL patients during antimony therapy.39,40 In an attempt to decrease the risks of adverse events associated with antimony therapy in leishmaniasis, patients in this study were treated with the same total dose of antimony, but injections were applied on Mondays, Wednesdays, and Fridays rather than daily. The frequencies of arthralgia and myalgia were similar in young and elderly patients and these manifestations were mild. Furthermore, only one patients in each group had an elevation of creatinine, and abnormal urea and trasaminases. As the cure rate was similar to that in other studies performed in the same endemic area,25–27 we conclude that increasing the interval time between the injections did not influence the response to therapy but may have attenuated myalgia and arthralgia and prevented common laboratory abnormalities observed during antimony therapy.
The ability of antimony to induce electrocardiographic changes has been well documented in the treatment of visceral and tegumentary leishmaniasis.36,41 In visceral leishmaniasis (VL), it has been clearly shown that electrocardiographic changes are dose dependent as they occurred in 22% of VL patients who received 10 mg of Sb5/kg/day, in 52% of those treated with 20–30 mg, and in 100% of patients treated with 30 mg of Sb5/kg/day.41 Herein, elderly patients demonstrated not only more electrocardiographic changes but also that the changes that were observed were more severe than those in young subjects. Specifically deserving of attention is the appearance of ventricular overload and ventricular arrhythmia in this group of patients. Because abnormalities were not associated with clinical complaints, antimony was not stopped in these patients. However, these findings indicate the high risk of the occurrence of heart failure and a potential higher risk of sudden death among the elderly subjects during antimony treatment.
We recognize that the number of participants in the study and the lack of histopathologic and in situ immunologic studies may have prevented the detection of additional implications of age in the pathogenesis and response to therapy among elderly patients. However, the observation that the changes in the immune response in the elderly did not influence the response to antimony therapy in CL, and the apparently greater suppressive effects of IL-10 are highly relevant findings. In addition, our observation that the rates of ventricular arrhythmia and ventricular overload in response to antimony therapy were higher in elderly than in young subjects may underscore a need to implement alternative and less toxic therapeutic approaches to elderly patients with CL.
Acknowledgments:
We would like to acknowledge the health-care professionals who provide outstanding medical care at the Corte de Pedra field site. We thank Cristiano Franco for the secretarial assistance.
REFERENCES
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M; WHO Leishmaniasis Control Team , 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: 35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimaldi G, Jr., Tesh RB, 1993. Leishmaniasis of the New World: current concepts and implications for future research. Clin Microbiol Rev 6: 230–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silveira FT, Lainson R, Corbett CE, 2004. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz 99: 239–251. [DOI] [PubMed] [Google Scholar]
- 4.Barral A, Guerreiro J, Bomfim G, Correia D, Barral-Netto M, Carvalho EM, 1995. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. Am J Trop Med Hyg 53: 256–259. [DOI] [PubMed] [Google Scholar]
- 5.Marsden PD, 1986. Mucosal leishmaniasis (“espundia” Escomel, 1911). Trans R Soc Trop Med Hyg 80: 859–876. [DOI] [PubMed] [Google Scholar]
- 6.Lessa MM, Lessa HA, Castro TW, Oliveira A, Scherifer A, Machado P, Carvalho EM, 2007. Mucosal leishmaniasis: epidemiological and clinical aspects. Rev Bras Otorrinolaringol (Engl Ed) 73: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, Mobashery N, Johnson WD, Jr., Carvalho EM, 2002. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J Infect Dis 186: 1829–1834. [DOI] [PubMed] [Google Scholar]
- 8.Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, Carvalho EM, Ephros M, Jeronimo S, Magill A, 2017. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 96: 24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jirmanus L, Glesby MJ, Guimarães LH, Lago E, Rosa ME, Machado PR, Carvalho EM, 2012. Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am J Trop Med Hyg 86: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diniz DS, Costa AS, Escalda PM, 2012. The effect of age on the frequency of adverse reactions caused by antimony in the treatment of American tegumentary leishmaniasis in Governador Valadares, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop 45: 597–600. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho AM, Amorim CF, Barbosa JL, Lago AS, Carvalho EM, 2015. Age modifies the immunologic response and clinical presentation of American tegumentary leishmaniasis. Am J Trop Med Hyg 92: 1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM, 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun 70: 6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado P, Araújo C, Da Silva AT, Almeida RP, D’Oliveira A, Jr., Bittencourt A, Carvalho EM, 2002. Failure of early treatment of cutaneous leishmaniasis in preventing the development of an ulcer. Clin Infect Dis 15: E69–E73. [DOI] [PubMed] [Google Scholar]
- 14.da Silva Santos C, Brodskyn CI, 2014. The role of CD4 and CD8 T cells in human cutaneous leishmaniasis. Front Public Health 2: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novais FO, Carvalho AM, Clark ML, Carvalho LP, Beiting DP, Brodsky IE, Carvalho EM, Scott P, 2017. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production. PLoS Pathog 13: e1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ, 2005. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett 15: 226–230. [DOI] [PubMed] [Google Scholar]
- 17.Giudice A, Vendrame C, Bezerra C, Carvalho LP, Delavechia T, Carvalho EM, Bacellar O, 2012. Macrophages participate in host protection and the disease pathology associated with Leishmania braziliensis infection. BMC Infect Dis 12: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso TM, Machado Á, Costa DL, Carvalho LP, Queiroz A, Machado P, Scott P, Carvalho EM, Bacellar O, 2014. Protective and pathological functions of CD8+ T cells in Leishmania braziliensis infection. Infect Immun 83: 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barral A, Costa JM, Bittencourt AL, Barral-Netto M, Carvalho EM, 1995. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. Int J Dermatol 34: 474–479. [DOI] [PubMed] [Google Scholar]
- 20.Machado PR, Lessa H, Lessa M, Guimarães LH, Bang H, Ho JL, Carvalho EM, 2007. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis 44: 788–793. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira-Neto MP, Schubach A, Mattos M, Goncalves-Costa SC, Pirmez C, 1997. A low-dose antimony treatment in 159 patients with American cutaneous leishmaniasis: extensive follow-up studies (up to 10 years). Am J Trop Med Hyg 57: 651–655. [DOI] [PubMed] [Google Scholar]
- 22.Arevalo J, et al. 2007. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis 15: 1846–1851. [DOI] [PubMed] [Google Scholar]
- 23.Unger A, O’Neal S, Machado PR, Guimarães LH, Morgan DJ, Schriefer A, Bacellar O, Glesby MJ, Carvalho EM, 2009. Association of treatment of American cutaneous leishmaniasis prior to ulcer development with high rate of failure in northeastern Brazil. Am J Trop Med Hyg 80: 574–579. [PMC free article] [PubMed] [Google Scholar]
- 24.Correia D, Macêdo VO, Carvalho EM, Barral A, Magalhães AV, de Abreu MV, Orge ML, Marsden P, 1996. Comparative study of meglumine antimoniate, pentamidine isethionate and aminosidine sulfate in the treatment of primary skin lesions caused by Leishmania (Viannia) braziliensis. Rev Soc Bras Med Trop 29: 447–453. [DOI] [PubMed] [Google Scholar]
- 25.O’Neal SE, Guimarães LH, Machado PR, Alcântara L, Morgan DJ, Passos S, Glesby MJ, Carvalho EM, 2007. Influence of helminth infections on the clinical course of and immune response to Leishmania braziliensis cutaneous leishmaniasis. J Infect Dis 1: 142–148. [DOI] [PubMed] [Google Scholar]
- 26.Machado PR, Ampuero J, Guimarães LH, Villasboas L, Rocha AT, Schriefer A, Sousa RS, Talhari A, Penna G, Carvalho EM, 2010. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis 4: e912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prates FV, Dourado ME, Silva SC, Schriefer A, Guimarães LH, Brito MD, Almeida J, Carvalho EM, Machado PR, 2017. Fluconazole in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis: a randomized controlled trial. Clin Infect Dis 64: 67–71. [DOI] [PubMed] [Google Scholar]
- 28.Weirather JL, et al. 2011. Serial quantitative PCR assay for detection, species discrimination, and quantification of Leishmania spp. in human samples. J Clin Microbiol 49: 3892–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sousa Ade Q, Parise ME, Pompeu MM, Coehlo Filho JM, Vasconcelos IA, Lima JW, Oliveira EG, Vasconcelos AW, David JR, Maguire JH, 1995. Bubonic leishmaniasis: a common manifestation of Leishmania (Viannia) braziliensis infection in Ceara, Brazil. Am J Trop Med Hyg 53: 380–385. [DOI] [PubMed] [Google Scholar]
- 30.Reed SG, Andrade ZA, Roters SB, Inverso JA, Sadigursky M, 1986. Leishmania mexicana amazonensis em camundongos endogâmicos “resistentes” após a remoção do linfonodo drenante. Clin Exp Immunol 64: 8–13. [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira-Neto MP, Mattos MS, Perez MA, Da-Cruz AM, Fernandes O, Moreira J, Gonçalves-Costa SC, Brahin LR, Menezes CR, Pirmez C, 2000. American tegumentary leishmaniasis (ATL) in Rio de Janeiro State, Brazil: main clinical and epidemiologic characteristics. Int J Dermatol 39: 506–514. [DOI] [PubMed] [Google Scholar]
- 32.Andersen EM, Cruz-Saldarriaga M, Llanos-Cuentas A, Luz-Cjuno M, Echevarria J, Miranda-Verastegui C, Colina O, Berman JD, 2005. Comparison of meglumine antimoniate and pentamidine for peruvian cutaneous leishmaniasis. Am J Trop Med Hyg 72: 133–137. [PubMed] [Google Scholar]
- 33.Queiroz A, Sousa R, Heine C, Cardoso M, Guimarães LH, Machado PR, Carvalho EM, Riley LW, Wilson ME, Schriefer A, 2012. Association between an emerging disseminated form of leishmaniasis and Leishmania (Viannia) braziliensis strain polymorphisms. J Clin Microbiol 50: 4028–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guimarães LH, et al. 2016. Atypical manifestations of cutaneous leishmaniasis in a region endemic for Leishmania braziliensis: clinical, immunological and parasitological aspects. PLoS Negl Trop Dis 10: e0005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies CR, Llanos-Cuentas EA, Pyke SD, Dye C, 1995. Cutaneous leishmaniasis in the Peruvian Andes: an epidemiological study of infection and immunity. Epidemiol Infect 114: 297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gontijo B, de Carvalho Mde L, 2003. American cutaneous leishmaniasis. Rev Soc Bras Med Trop 36: 71–80. [DOI] [PubMed] [Google Scholar]
- 37.Saldanha AC, Romero GA, Guerra C, Merchan-Hamann E, Macedo Vde O, 2000. Comparative study between sodium stibogluconate BP 88 and meglumine antimoniate in cutaneous leishmaniasis treatment. II. Biochemical and cardiac toxicity. Rev Soc Bras Med Trop 33: 383–388. [DOI] [PubMed] [Google Scholar]
- 38.De Paula CD, Sampaio JH, Cardoso DR, Sampaio RN, 2003. A comparative study between the efficacy of pentamidine isothionate given in three doses for one week and N-methil-glucamine in a dose of 20 mgSbV/day for 20 days to treat cutaneous leishmaniasis. Rev Soc Bras Med Trop 36: 365–371. [PubMed] [Google Scholar]
- 39.Costa JM, Garcia AM, Rêbelo JM, Guimarães KM, Guimarães RM, Nunes PM, 2003. Fatal case during treatment of American tegumentary leishmaniasis with sodium stibogluconate bp 88® (Shandong Xinhua). Rev Soc Bras Med Trop 36: 295–298. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira MC, Amorim RF, Freitas Rde A, Costa Ade L, 2005. American cutaneous leishmaniasis with fatal outcome during pentavalent antimoniate treatment. Rev Soc Bras Med Trop 38: 258–260. [DOI] [PubMed] [Google Scholar]
- 41.Chulay JD, Spencer HC, Mugambi M, 1985. Electrocardiographic changes during treatment of leishmaniasis with pentavalent antimony (sodium stibogluconate). Am J Trop Med Hyg 34: 702–709. [DOI] [PubMed] [Google Scholar]