Abstract.
This case report highlights the risk of severe cutaneous leishmaniasis (CL) by Leishmania infantum in patients undergoing immunosuppressant therapy who either live in an endemic area or are visiting in the transmission season. The case patient, resident in Majorca (Balearic Islands), presented 12 disseminated erythematous skin lesions, 1–6 cm in diameter, located on the scalp, cheek, umbilical region, and lower extremities 8 years after undergoing anti–tumor necrosis factor (TNF) therapy. Parasite presence in peripheral blood and high levels of specific antibodies were also observed, indicating a possible risk of CL shifting toward a visceral infection. However, once CL was diagnosed, anti-TNF therapy was discontinued and liposomal amphotericin B was administered, resulting in a complete healing of lesions, no Leishmania DNA detection in blood, and an important serological decrease in antibodies. The lack of data on the supposed epidemiological association between leishmaniasis and immunosuppressive therapy highlights the importance of implementing surveillance systems in endemic areas. No obvious relationship was found based on the data provided by the Balearic Islands Epidemiological System, in contrast with data reported in nearby endemic areas. This indicates that if the suspected association is to be clarified, greater efforts are needed to report information about concomitant diseases and therapies in leishmaniasis patients.
INTRODUCTION
Although immunosuppressive therapy can be highly effective for several immune-mediated diseases, tumor necrosis factor (TNF) inhibitors are associated with an increased risk of opportunistic infections,1 including histoplasmosis and tuberculosis as well as clinical forms of cutaneous leishmaniasis (CL) and visceral infection (VL) and even a mucocutaneous form (ML).2–6 Leishmaniasis, classed among the world’s most neglected diseases, is a group of parasitic diseases caused by protozoa of the genus Leishmania. Development of cutaneous, mucocutaneous, or visceral forms depends on the Leishmania species involved and the immune response of the host. The disease caused by Leishmania infantum is endemic in Mediterranean countries. In Spain, the two main clinical forms in humans are CL and VL, with some cases of ML being reported.7 In immunocompetent patients, the normal course of CL by L. infantum is spontaneous healing, whereas the clinical characteristics in cases of immunosuppression are more severe.8
CASE REPORT
We report the case of a 55-year-old male patient resident in Majorca in the Balearic Islands, who was diagnosed with psoriasis and rheumatoid arthritis 27 years ago and developed multiple disseminated erythematous skin lesions 8 years after undergoing anti-TNF therapy.
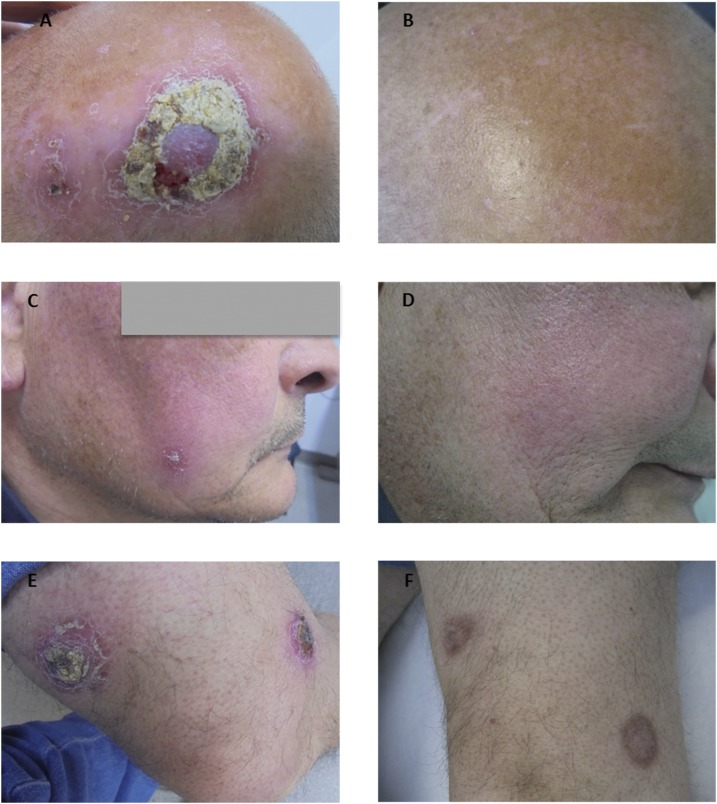
In December 2015, when visited in the Dermatology Department of the Hospital de Manacor, Majorca, the patient had 12 disseminated erythematous cutaneous lesions, seven of which had a crusty surface with a raised border and produced a serous exudate when the scab was removed. The dimensions of these lesions ranged from 1 to 6 cm in diameter in all locations (scalp, cheek, umbilical region, and lower extremities) (Figure 1). A skin biopsy specimen was collected from the lesion located in the upper right knee. A histology analysis by hematoxylin and eosin staining revealed forms compatible with Leishmania amastigotes, and an L. infantum strain was isolated after culture using Novy–MacNeal–Nicolle and Schneider media, molecularly typed by polymerase chain reaction (PCR)–restriction fragment length polymorphism.9 A scab sample was collected from the same lesion on Whatman filter paper no. 3 for a real time polymerase chain reaction (qPCR),10 which gave a positive result for Leishmania, also identified molecularly as L. infantum.9 At the same time, peripheral blood was extracted from the patient for serological and molecular studies. A serological study by Western blot (WB)11 identified the specific band pattern for Leishmania (14, 16, 24, 28, 30, 46, 65, and 70 kDa). In an in-house enzyme-linked immunosorbent assay (ELISA),11 high levels of antibodies were observed with a serological titter of 170 U (cut-off ELISA >20 U). The qPCR of the buffy coat gave a positive result with a low parasitic level detected (threshold cycle 39), although the in vitro culture was negative because of its lower sensitivity. These results may be explained by a low level of circulating parasites, which would be sufficient to render a positive PCR but not a positive culture.12 In the clinical examination and the analytical tests, no parameters suggestive of visceral involvement were observed, but because of the extensive cutaneous involvement, a regimen similar to that for VL affection was initiated. Treatment of diffuse CL began with intravenous Ambisome® (liposomal amphotericin B) (i.v.): 1–5, 10, 17, 24, 31, and 38 days with treatment, 400 mg daily (×10 days/total dose 40 g). The first four doses were administered to the patient when hospitalized and the remaining six were given weekly in the Internal Medicine Department without incident. The patient discontinued biological treatment, taking only corticoids. After 6 months, the lesions had totally disappeared and some presented hyperpigmentation (Figure 1). In the patient posttreatment follow-up in December 2016, when anti-TNF treatment had not yet been resumed, no Leishmania DNA in blood was detected by qPCR of the buffy coat, and a serological decrease in the ELISA values and band intensity and number was observed (Table 1).
Figure 1.
Clinical evolution of four of 12 leishmania skin lesions of the patient after 1 year of treatment with liposomal amphotericin B (i.v.). (A and B) Evolution of skin lesion on the scalp. (C and D) Evolution of skin lesion in the cheek. (E and F) Evolution of two skin lesions in the lower extremities.
Table 1.
Diagnostic techniques and samples studied pre- and posttreatment with Ambisome (liposomal amphotericin B)
| Biopsy | FPLI | Buffy coat | Blood serum | |||
|---|---|---|---|---|---|---|
| Pre-T | Pre-T | Pre-T | Post-T | Pre-T | Post-T | |
| Culture* | Positive | – | Growth not detected | – | – | – |
| PCR (Ct) | – | Positive (Ct 24) | Positive (Ct 39) | Negative (Ct 45) | – | – |
| WB (kDa) | – | – | – | – | 14, 16, 24, 28, 30, 46, 65, 70 | 14, 16, 30, 46, 65, 70 |
| ELISA (units) | – | – | – | – | 170 U | 35 U |
Ct = threshold cycle; ELISA = enzyme-linked immunosorbent assay; FPLI = filter paper lesion impression; Post-T = posttreatment; Pre-T = pretreatment; WB = Western blot.
Three months monitoring.
DISCUSSION
In cases of human CL, immunosuppressive treatment can modulate the clinical expression of leishmaniasis, as indicated in other studies,2 which describe CL cases with more severe symptoms than in immunocompetent patients. In patients with a background of rheumatoid arthritis, the risk of serious infection by Mycobacteria spp., Staphylococcus aureus, Listeria monocytogenes, Varicella zoster virus, and Leishmania spp. appears to be approximately 2-fold higher than in non–rheumatoid arthritis patients before any biological therapy.13 Taking into account the existence of this risk, Marcoval et al.2 recommend that in endemic leishmaniasis areas, the patient’s immune status against Leishmania be evaluated before the onset of and during anti-TNF treatment.
The current case, which exemplifies the severity of clinical manifestations of CL, was diagnosed during the course of anti-TNF treatment in a patient living in an endemic area of L. infantum. After being diagnosed with arthritis and psoriasis 27 years ago, he was initially treated with low doses of corticosteroids and began anti-TNF therapy in 2007, 8 years before developing CL symptoms. As there is no information about the patient’s immune status before the appearance of the symptoms, it is difficult to determine whether the CL is due to reactivation of a cryptic infection or a new infection.
Although most reports suggest that anti-TNF treatment favors the onset of atypical lesions in CL patients,2,14 a strong link has also been established between the development of leishmaniasis, corticosteroids, and immune status.4,15 Other studies also indicate that in CL patients with severe lesions caused by Leishmania braziliensis, anti-TNF treatment can help the healing process and enhance ulcer cicatrization,16 although no consensus has been established.17 In the present case, it is difficult to determine whether the severity of the CL symptoms was due to the concomitant disease, the administration of anti-TNF drugs or cortisone, or a combination of factors.
The presence of multiple lesions due to L. infantum, high levels of specific antibodies, and the detection of Leishmania DNA in peripheral blood all indicate that this type of patient should be treated with a regimen similar to that for VL. Subsequent monitoring is also advised to ensure a good prognosis and complete healing of the disease, avoiding the risk of a shift toward VL. In the posttreatment follow-up, the lesions had totally disappeared, no Leishmania DNA was detected in blood, and a significant serological decrease in the intensity and number of bands and ELISA values was observed. The loss of intermediate bands in the WB agrees with observations in other studies that bands of 12–30 kDa are the first to disappear when clinical improvement is observed and the disease is self-limited.11 The patient was discharged and the reestablishment of immunosuppressant therapy is being considered.
To clarify the suspected epidemiological association between leishmaniasis and the use of immunosuppressant therapy, risk assessment was carried out by compiling and analyzing data provided by the Balearic Islands Epidemiological System and from other geographical areas of Spain. Leishmaniasis is of public health significance and a notifiable disease in Spain, but the reported cases of CL between 1989 and 2008 represent only a third of the real incidence, because many CL patients are treated by dermatologists outside hospitals and go unreported.18 The zones of Spain with the highest incidence rate of leishmaniasis are Catalonia, the Balearic Islands, Madrid, and the Mediterranean areas of Andalusia, being between 0.45 and 2.83/100,000 inhabitants for 2009–2014.19 According to the official data for 2005–2015,20 the overall HL rate in the Balearic Islands in this period was 0.7–3.7 cases per year/100,000 inhabitants, with a 3:1 ratio of CL and VL cases. This proportion of CL cases is high compared with those reported in France (1:1), according to a review by Bañuls et al.21
When evaluating the incidence and epidemiological data of leishmaniasis, the only variables considered significant by the Balearic Islands epidemiological service are pediatric age groups and human immunodeficiency virus patients. Information on other types of immunosuppression, such as those caused by anti-TNF treatments, is not given a significance status. Among the 161 cases of human leishmaniasis declared in the period between 2010 and 2016 (23.4% VL, 77.6% CL), the involvement of immunosuppressive disease (not related to HIV or in children) is unknown in only 10.5% and linked in 6.8%.
According to our epidemiological review, patients treated with anti-TNF drugs in Mediterranean endemic areas such as Majorca do not appear to have a much higher risk of developing leishmaniasis compared with immunocompetent individuals. However, in the autonomous community of Valencia, where immunosuppressive diseases and immunosuppressive treatment have been classed as risk factors for leishmaniasis since 2007,22 21.15% of reported cases of leishmaniasis in 2015 also notify concomitant immunosuppressive disease and 19.23% treatment with an immunosuppressant.22 This discrepancy among data from different geographical areas of Spain suggests a need to raise awareness about the importance of reporting concomitant diseases and therapies in leishmaniasis patients. Providing more comprehensive information in the leishmaniasis notification forms and a more systematic reporting of cases would help to clarify the suspected epidemiological association between leishmaniasis and immunosuppressant therapy.
ETHICS STATEMENT
This research was approved by the Institutional Review Board of the Hospital de Manacor. The study was conducted according to the tenets of the Declaration of Helsinki. The adult subject provided written informed consent.
Acknowledgments:
We gratefully acknowledge A. Nicolau (Cap de Servei d’Epidemiologia de les Illes Balears) for providing data on the cases of human leishmaniasis declared in the Balearic Islands, thank Lucy Brzoska for her advice on the English preparation of the manuscript, and extend special thanks to the patient.
REFERENCES
- 1.Muñoz-Oca JE, Villarreal Morales ML, Nieves-Rodriguez A, Martínez-Bonilla L, 2017. Concomitant disseminated histoplasmosis and disseminated tuberculosis after tumor necrosis factor inhibitor treatment: a case report. BMC Infect Dis 17: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcoval J, Penín RM, Sabé N, Valentín-Medina F, Bonfill-Ortí M, Martínez-Molina L, 2017. Cutaneous leishmaniasis associated with anti-tumour necrosis factor-α drugs: an emerging disease. Clin Exp Dermatol 42: 331–334. [DOI] [PubMed] [Google Scholar]
- 3.Català A, Roé E, Dalmau J, Pomar V, Muñoz C, Yelamos O, Puig L, 2015. Anti-tumour necrosis factor-induced visceral and cutaneous leishmaniasis: case report and review of the literature. Dermatology 230: 204–207. [DOI] [PubMed] [Google Scholar]
- 4.Mueller MC, Fleischmann E, Grunke M, Schewe S, Bogner JR, Löscher T, 2009. Case report: relapsing cutaneous leishmaniasis in a patient with ankylosing spondylitis treated with infliximab. Am J Trop Med Hyg 81: 52–54. [PubMed] [Google Scholar]
- 5.Romaní-Costa V, Sánchez C, Moyá F, Estany C, 2004. Visceral leishmaniasis related to infliximab administration. Enferm Infecc Microbiol Clin 22: 310. [DOI] [PubMed] [Google Scholar]
- 6.Neumayr ALC, et al. 2013. Clinical aspects and management of cutaneous leishmaniasis in rheumatoid patients treated with TNF-α antagonists. Travel Med Infect Dis 11: 412–420. [DOI] [PubMed] [Google Scholar]
- 7.Alvar J, Cañavate C, Gutiérrez-Solar B, Jiménez M, Laguna F, López-Vélez R, Molina R, Moreno J, 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev 10: 298–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvar J, Aparicio P, Aseffa A, Den Boer M, Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J, 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21: 334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomás-Pérez M, Fisa R, Riera C, 2013. The use of fluorescent fragment length analysis (PCR-FFL) in the direct diagnosis and identification of cutaneous Leishmania species. Am J Trop Med Hyg 88: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisa R, Riera C, Ribera E, Gállego M, Portús M, 2002. A nested polymerase chain reaction for diagnosis and follow-up of human visceral leishmaniasis patients using blood samples. Trans R Soc Trop Med Hyg 96 (Suppl 1): S191–S194. [DOI] [PubMed] [Google Scholar]
- 11.Riera C, Valladares JE, Gállego M, Aisa MJ, Castillejo S, Fisa R, Ribas N, Carrió J, Alberola J, Arboix M, 1999. Serological and parasitological follow-up in dogs experimentally infected with Leishmania infantum and treated with meglumine antimoniate. Vet Parasitol 84: 33–47. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez-Marco T, Riera C, Fisa R, Girona-Llobera E, Sedeño M, Goodrich RP, Pujol A, Guillen C, Muncunill J, 2012. The utility of pathogen inactivation technology: a real-life example of Leishmania infantum inactivation in platelets from a donor with an asymptomatic infection. Blood Transfus 10: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downey C, 2016. Serious infection during etanercept, infliximab and adalimumab therapy for rheumatoid arthritis: a literature review. Int J Rheum Dis 19: 536–550. [DOI] [PubMed] [Google Scholar]
- 14.Schneider P, Bouaziz JD, Foulet F, Doung TA, Valeyrie Allanorea L, Bagot M, 2009. Leishmaniose cutanée multifocale à Leishmania infantum sous traitment par adalimumab. Ann Dermatol Venereol 136: 815–820. [DOI] [PubMed] [Google Scholar]
- 15.Roberts RM, Mukherjee J, Phillips D, 2016. Laryngeal leishmaniasis in a patient taking inhaled corticosteroids. BMJ Case Rep 2016: pii: bcr2016215444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira F, Bafica A, Rosato AB, Favali CB, Costa JM, 2011. Short report: lesion size correlates with Leishmania antigen-stimulated TNF-levels in human cutaneous leishmaniasis. Am J Trop Med Hyg 85: 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito G, Dourado M, Guimarães LH, Meireles E, Schriefer A, de Carvalho EM, Lima Machado PR, 2017. Oral pentoxifylline associated with pentavalent antimony: a randomized trial for cutaneous leishmaniasis. Am J Trop Med Hyg 96: 1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M; WHO Leishmaniasis Control Team , 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centro Nacional de Epidemiología, CIBER Epidemiología y Salud Pública (CIBERESP), Instituto de Salud Carlos III, 2016. Resultados de la vigilancia epidemiológica de las enfermedades transmisibles. Informe anual. Año 2014. Available at: http://gesdoc.isciii.es/gesdoccontroller?action=download&id=14/10/2016-3a996e69f2. Accessed September 28, 2017.
- 20.Govern de les Illes Balears , 2016. Xarxa de Vigilància Epidemiològica de les Illes Balears. Informes anuales 2005–2015. Available at: http://www.caib.es/sacmicrofront/noticias.do?idsite=337&tipo=12245&mcont=84507. Accessed September 29, 2017.
- 21.Bañuls AL, Bastien P, Pomares C, Arevalo J, Fisa R, Hide M, 2011. Clinical pleiomorphism in human leishmaniases, with special mention of asymptomatic infection. Clin Microbiol Infect 17: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 22.Generalitat Valenciana , 2016. Conselleria de Sanitat Universal i Salut Pública de la Generalitat Valenciana. Informes anuales 2005–2015. Available at: https://www.sp.san.gva.es/indexPortal.jsp?menuRaizPortal=SANMS50000&Portal=EPIDEMIOLOGIA&perfil=inst. Accessed September 29, 2017.