Abstract.
Burkholderia pseudomallei, the etiologic agent of melioidosis, is an important but under-recognized cause of disease in the tropics. Although first described over a century ago as a septicemic illness associated with morphine addicts in Rangoon, Burma, there is little information regarding the incidence of melioidosis in present-day Myanmar. To address this issue, we used two recently developed and validated serological assays to detect B. pseudomallei–specific antibodies in 124 serum samples obtained from febrile patients in the delta region of Myanmar. Using cutoff values derived from culture-confirmed melioidosis cases in neighboring Thailand, 3.2% of the samples exhibited reactivity profiles consistent with active B. pseudomallei infections. Collectively, these findings indicate that melioidosis likely represents a significant cause of morbidity and mortality in Myanmar and support the need for further studies to assess the true burden of disease in this country.
Burkholderia pseudomallei is a gram-negative, facultative intracellular bacterium that causes melioidosis in humans and animals. This organism is an environmental saprophyte and is highly endemic in Southeast Asia (SEA) and northern Australia, where it can be readily isolated from moist soils and surface waters. Typical routes of infection are percutaneous inoculation, ingestion, and inhalation.1 Certain underlying conditions, most notably diabetes mellitus, are risk factors for melioidosis, but seemingly healthy individuals can also be susceptible to the disease.1,2 The clinical manifestations of melioidosis are protean ranging from localized abscess formation to acute pneumonias and septicemias. Asymptomatic infections that can reactivate after many years have also been described. Without early diagnosis and administration of effective antibiotic therapy, melioidosis can be rapidly fatal. At present, no licensed vaccines exist.
Melioidosis is considered an emerging infectious disease in many developing tropical countries. A recent report estimates that the annual incidence of melioidosis is substantial with 165,000 cases and 89,000 deaths worldwide.3 The majority of cases (∼84%) are predicted to occur in South Asia (73,000 cases/year) and in the East Asia and Pacific region (65,000 cases/year). In northeast Thailand, there are at least 2,000 culture-confirmed cases per year with a mortality rate of 40%.4 In other parts of SEA, the prevalence of melioidosis is less well defined. Because the clinical symptoms of melioidosis are similar to those of several other infectious diseases, melioidosis may go unrecognized in endemic areas. Underdiagnosis of B. pseudomallei infections in many resource-poor regions is likely also due to limited microbiological facilities, lack of clinical, and laboratory expertise, awareness or both, and poor reporting systems.5 In Thailand, for example, many patients present at community hospitals that have limited diagnostic capabilities, which in the case of severe infections, often results in death before laboratory results can be obtained from secondary hospitals.
Burkholderia pseudomallei was first described in 1912 at Rangoon (Yangon) General Hospital in Burma (Myanmar) by pathologist Whitmore and his assistant Krishnaswami.6 In reports documented between 1915 and 1917, B. pseudomallei was isolated from 5% of all autopsies and accounted for more than 100 cases of the disease now known as melioidosis.7,8 Since then, however, there have been a limited number of reports in the literature describing evidence of melioidosis in Myanmar. In 2006, a study by Wuthiekanun et al.,9 demonstrated that 78% of new migrant workers from Myanmar to Thailand were seropositive for antibodies to B. pseudomallei. In 2008, Aung and Mar,10 found that ∼2% of pus specimens obtained from abscesses, from 133 patients admitted to 21 hospitals in Yangon, were positive for B. pseudomallei. Most recently, Chu et al.11 reported two fatal cases of culture-confirmed melioidosis that occurred on the Thai–Myanmar border. These studies along with the predicted incidence (6,247 cases/year) of melioidosis in Myanmar suggest that the disease is likely underreported and that further studies are warranted.3
Definitive diagnosis of melioidosis depends on the isolation of B. pseudomallei from clinical specimens. Identification of the organism by culture is time consuming (2–7 days), has low sensitivity (60%), and requires both experience and stringent laboratory biosafety practices. In addition, laboratories unfamiliar with B. pseudomallei can easily misidentify the bacterium. To overcome these problems, reliable, rapid serological assays represent an attractive complementary approach. We have recently developed and validated rapid enzyme-linked immunosorbent assays (ELISAs) using different polysaccharide and protein antigens as simple serological screening tools for melioidosis. Based on the results of these ELISAs, we have shown that B. pseudomallei type A O-polysaccharide (OPS) and the virulence-associated type 6 secretion system hemolysin co-regulated protein (Hcp1) are promising target antigens for serodiagnosis in different groups of melioidosis patients. Using serum from melioidosis patients and healthy individuals from endemic areas in northeast Thailand, the sensitivity and specificity of the OPS-ELISA was 71.7% and 95.7%, respectively.12 The sensitivity and specificity of the Hcp1-ELISA was 83.0% and 96.3%, respectively.13 Our evaluation demonstrated that these two ELISAs outperformed the indirect hemagglutination assay, a widely used serological test which has 69.5% sensitivity and 67.6% specificity in Thailand, suggesting that the ELISAs may be useful for serodiagnosis of melioidosis in endemic areas.
The objective of this study was to use the rapid ELISAs to survey melioidosis cases in febrile patients in Myanmar. This group was selected as the target population for our study because current recommendations suggest that melioidosis should be considered in febrile patients residing in endemic areas.14 Analysis of individual antibody titers to OPS and Hcp1 in serum samples from 103 melioidosis patients in Thailand showed the correlation (rho value) of these two ELISAs was only 0.46, suggesting that the patients with acute infection have independent responses to these antigens.13 To increase serodiagnostic confidence for screening serum samples in this study, we used a “duplex” ELISA approach to assess the antibody responses to OPS and Hcp1.
The study was approved by Ethical Review Board of Defense Services Medical Research Centre (DSMRC) (approval number DSMRC IRB/2017/75). A total of 124 febrile patients (123 male and one female) ranging from 20 to 50 years of age were recruited randomly from those visiting mobile clinics based in the delta region of Myanmar during the rainy season in 2016. A written informed consent was obtained from each of the participants enrolled in the study (no information regarding the occupations of the patients was obtained at the time). Three milliliters of whole blood was collected aseptically from each of the participants. The serum samples were stored at −80°C until required for use. The samples were tested by ELISA using OPS and Hcp1 as previously described.12,13 In brief, a 96-well U-bottom immunoplates (Nunc MaxiSorp U-bottom 96-Well plates; Thermo Scientific, Copenhagen, Denmark) were pre-coated with 1 μg/mL of OPS or 2.5 μg/mL of Hcp1 and incubated overnight at 4°C. Between each step, the ELISA plates were washed four times with phosphate buffered saline with 0.05% tween 20. After blocking at 37°C for 2 hours with 5% skim milk in phosphate buffered saline, the diluted patient serum samples (1:2,000) were added to plates in duplicate and then incubated at room temperature for 30 minutes. After washing, the plates were incubated for 30 minutes with horseradish peroxidase-conjugated antihuman immunoglobulin G (DAKO) diluted 1:2,000. Enzyme-linked immunosorbent assays were developed using 3,3’,5,5’-tetramethylbenzidine substrate. Results were determined as absorbance value (optical density 450 nm). Pooled serum from five Thai melioidosis patients and five Thai healthy donors were used as positive and negative controls, respectively.
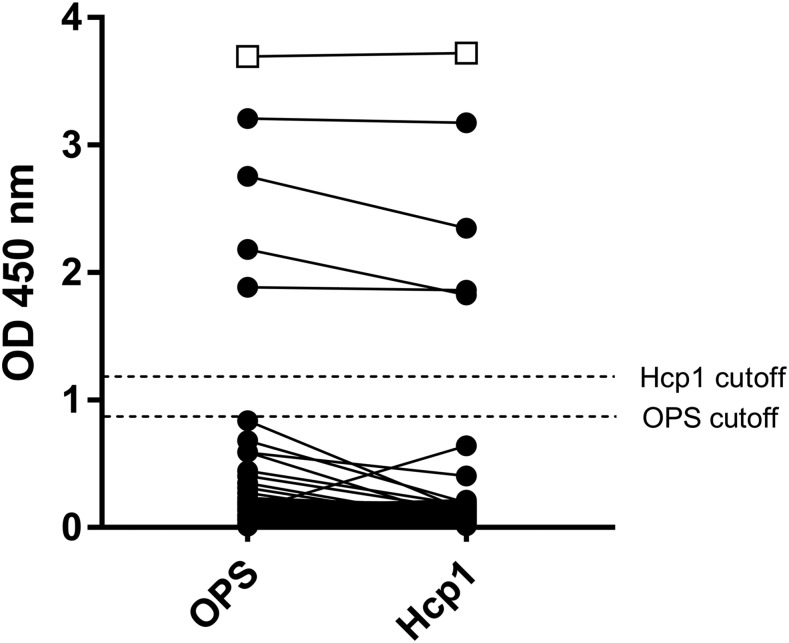
The optical density values of OPS-ELISA and Hcp1-ELISA varied significantly (OPS-ELISA, range = 0.011–3.208, median = 0.157, interquartile range [IQR] = 0.069–0.181; Hcp1-ELISA, range = 0.014–3.176, median = 0.153, IQR = 0.082–0.173). Using cutoff values derived from Thai healthy donors (0.875 for OPS-ELISA and 1.165 for Hcp1), the two ELISAs detected positive antibody responses in the same four Myanmar febrile patient serum samples (Figure 1). The results strongly suggest that these four patients (one female, aged 43 years and three males, aged 23, 31, and 49 years) have been exposed to B. pseudomallei and may have active infections. We were unable, however, to follow up with these individuals to confirm this.
Figure 1.
Serum-immune responses to purified O-polysaccharide (OPS) and hemolysin co-regulated protein (Hcp1) antigens. Serum samples were assayed for reactivity with the target antigens using single-dilution enzyme-linked immunosorbent assays. Connecting lines indicate identical serum samples. Closed circles represent serum from 124 Myanmar febrile patients. Open boxes represent pooled serum from five culture-confirmed Thai melioidosis patients (positive control). Closed boxes represent pooled serum from five healthy Thai donors (negative control).
Based on these preliminary findings, we believe that the OPS and Hcp1 “duplex” ELISAs will be suitable, simple, and affordable assays for conducting seroprevalence studies in Myanmar. We recognize, however, that larger studies using both healthy donor and culture-confirmed melioidosis patient serum will be necessary to determine country-specific cutoff values for these assays. Once a sufficient number of samples are collected and analyzed, we can then evaluate whether these ELISAs may be useful for serodiagnosing disease in Myanmar. We would predict that the Hcp1-ELISA, in particular, would be most useful because this antigen is only expressed after infection of a host and is specific to B. pseudomallei (and closely related Burkholderia mallei).15,16 Notably, B. pseudomallei Hcp1 differs significantly from the Hcp1 homologue in Burkholderia thailandensis, and thus, would exclude seroconversion that may have occurred because of exposure of individuals to nonpathogenic near-neighbor species.
Because the serum samples collected in this study were obtained from patients residing in the delta region and this area is predominated by farming and fishing communities, it is reasonable to predict that these people may have had an increased risk of exposure to B. pseudomallei, due to occupational activities, compared with people living in other regions of the country. Further studies will be required to determine if this might be the case and, if similar to Thailand, there are specific melioidosis “hot spots” within the country. In addition, it will be important to collect more comprehensive patient histories to assess whether there is a strong correlation between known predisposing conditions and seroconversion to OPS and Hcp1. For example, if a febrile, diabetic patient was seropositive for these antigens, there should be a heightened suspicion of an active B. pseudomallei infection. Studies are ongoing to address these important issues.
REFERENCES
- 1.Limmathurotsakul D, Kanoksil M, Wuthiekanun V, Kitphati R, deStavola B, Day NP, Peacock SJ, 2013. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: a matched case-control study. PLoS Negl Trop Dis 7: e2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currie BJ, Jacups SP, Cheng AC, Fisher DA, Anstey NM, Huffam SE, Krause VL, 2004. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop Med Int Health 9: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, et al. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1: 15008. [DOI] [PubMed] [Google Scholar]
- 4.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ, 2010. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg 82: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmaster AR, et al. 2015. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis 21, doi:10.3201/eid2102.141045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitmore A, Krishnaswami CS, 1912. A hitherto undescribed infective disease in Rangoon. Ind Med Gaz 47: 262–267. [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnaswami CS, 1917. Morphia injectors septicaemia. Ind Med Gaz 52: 296–299.29007776 [Google Scholar]
- 8.Stanton AT, Fletcher W, 1921. Melioidosis, a New Disease of the Tropics. Weltevreden, Batavia, (Dutch East Indies): Far Eastern Association of Tropical Medicine. Transactions of the Fourth Congress. Vol. 2, 196–198. [Google Scholar]
- 9.Wuthiekanun V, Langa S, Swaddiwudhipong W, Jedsadapanpong W, Kaengnet Y, Chierakul W, Day NP, Peacock SJ, 2006. Short report: melioidosis in Myanmar: forgotten but not gone? Am J Trop Med Hyg 75: 945–946. [PubMed] [Google Scholar]
- 10.Aung MK, Mar TT, 2008. Re-emergence of melioidosis in Myanmar. Trans R Soc Trop Med Hyg 102 (Suppl 1): S10–S11. [DOI] [PubMed] [Google Scholar]
- 11.Chu CS, Winearls S, Ling C, Torchinsky MB, Phyo AP, Haohankunnathum W, Turner P, Wuthiekanun V, Nosten F, 2014. Two fatal cases of melioidosis on the Thai-Myanmar border. F1000 Res 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suttisunhakul V, Wuthiekanun V, Brett PJ, Khusmith S, Day NP, Burtnick MN, Limmathurotsakul D, Chantratita N, 2016. Development of rapid enzyme-linked immunosorbent assays for detection of antibodies to Burkholderia pseudomallei. J Clin Microbiol 54: 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pumpuang A, Dunachie SJ, Phokrai P, Jenjaroen K, Sintiprungrat K, Boonsilp S, Brett PJ, Burtnick MN, Chantratita N, 2017. Comparison of O-polysaccharide and hemolysin co-regulated protein as target antigens for serodiagnosis of melioidosis. PLoS Negl Trop Dis 11: e0005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie BJ, 2015. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med 36: 111–125. [DOI] [PubMed] [Google Scholar]
- 15.Burtnick MN, Brett PJ, 2013. Burkholderia mallei and Burkholderia pseudomallei cluster 1 type VI secretion system gene expression is negatively regulated by iron and zinc. PLoS One 8: e76767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burtnick MN, et al. 2011. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun 79: 1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]