Abstract.
More than 80% of the global burden of the Plasmodium vivax is contributed by mainly three countries (India, Indonesia, and Pakistan). Reports from last decades have highlighted the occurrence of severe P. vivax malaria which was earlier considered to be benign. The recent trends of increasing P. vivax–associated morbidity and mortality emphasizes the need for early and accurate diagnosis of P. vivax malaria for the timely management of patients. Microscopy is considered a gold standard but needs experienced laboratory technologists. Over the last few years, Polymerase chain reaction (PCR) is being used as a highly sensitive and specific test but it requires expensive equipment which limits its use in the field. Therefore, in the present study, utility of visually improved loop-mediated isothermal amplification (LAMP) for the detection of P. vivax was evaluated targeting 18SrRNA gene in 145 microscopically confirmed P. vivax and 20 P. vivax negative patients. Sensitivity and specificity of LAMP was assessed with respect to microscopy and multiplex nested PCR (nPCR). Results of the LAMP assay was also correlated with rapid diagnostic test, multiplex nPCR and real-time PCR results. Overall, sensitivity and specificity of P. vivax–specific LAMP compared with microscopy were found to be 100% and 85%, respectively. Furthermore, detection limit for LAMP was found to be 0.8 copies/μL and it was also able to detect three complicated cases of P. vivax which were missed by microscopy. This study showed a LAMP assay to be a rapid and very sensitive method for the early diagnosis of both complicated and uncomplicated P. vivax malaria.
INTRODUCTION
Malaria, a major cause of morbidity and mortality, is the most widespread febrile illness with recent estimates of 216 million new cases and approximately 0.44 million deaths in the year 2016.1 Of the total malaria cases reported in 2016, 4% of them were caused by Plasmodium vivax alone. Most of the cases of P. vivax malaria have been reported from the World Health Organization (WHO) South-East Asia Region (58%), followed by the WHO Eastern Mediterranean Region (21%), and the WHO African Region (10%).1 India is one of the three countries known to contribute more than 80% to global P. vivax malaria burden. Classically severe malaria is known to be associated with P. falciparum infection. However, in recent years there has been an increase in the reported cases of severe malaria due to P. vivax.2 A number of studies have reported the presence of severe life threatening symptoms in P. vivax patients from Asia, South America, and Africa.3–6 Larger series of studies have clearly associated P. vivax infections with severe and fatal disease in both children and adults.7–9 These case series include a variety of severe manifestations associated with P. vivax infection, including severe anemia, thrombocytopenia, jaundice, respiratory distress and acute lung injury, acute kidney injury (AKI), splenic rupture, jaundice, coma, multi-organ dysfunction, and shock. In North India, where the present study was carried out, P. vivax is the predominant human malaria species. Thus, it is very important to closely observe the P. vivax associated complicated malaria cases so that timely action can be taken to manage these cases.
Microscopic examination of both thick and thin smears remains the gold standard for the diagnosis of malaria. Although it is inexpensive, specific, and reliable, it requires highly skilled personnel as compared with the most recent molecular techniques.10 In addition, in case of mixed infections and low parasitemia, microscopy often leads to misdiagnosis of Plasmodium species.11 Although rapid diagnostic tests (RDTs) are quick, convenient, and do not require expertise to analyze the results, they are expensive, have less sensitivity with lower parasitemia, and also give false positive results.12
Nucleic acid amplification testings are the most sensitive diagnostic modalities for malaria.13 Presently, several DNA amplification techniques are available for the prompt diagnosis of malaria such as polymerase chain reaction (PCR), nested PCR (nPCR), and real-time PCR (RT-PCR). Real-time PCR has been used for the qualitative and quantitative determination of human malaria parasites. The major advantage of RT-PCR over nPCR is that it results in continuous monitoring of the accumulating PCR products omitting the need for the post-amplification visualization step.14 These assays are highly sensitive and specific, and are capable of detecting malaria at the species level even at low parasite burden and even asymptomatic infections. But these tests require sophisticated instruments and skilled personnel for the accurate diagnosis of malaria.15 Moreover, it is inefficient in field setups because of relatively expensive equipment and advanced training.16,17
Loop mediated isothermal amplification (LAMP) uses a very simple technology for the amplification of DNA, making it field adaptable. Loop-mediated isothermal amplification thus has the potential to be used as a point-of-care testing in the resource limited setups. The generation of magnesium pyrophosphate leads to an increase in the turbidity as DNA gets amplified making end point visualizations of the results. Because of the intra-observer variations, the analysis of results becomes difficult. However, this reduces the utility of LAMP for developing countries. Currently, in India, only few studies have reported the utility of LAMP in malaria diagnosis18–20 but not in the prompt diagnosis of complicated P. vivax cases. In this study, the visually improved P. vivax–specific LAMP was developed for rapid and accurate diagnosis of complicated and uncomplicated P. vivax clinical isolates. The performance of developed P. vivax–specific LAMP was compared with the gold standard method.
MATERIAL AND METHODS
Ethics statement.
The present study was carried out as per the institutional guidelines. The research proposal and informed consents were thoroughly reviewed and approved by the ethical committee of the Postgraduate Institute of Medical Education and Research (PGIMER) (PGI/IEC/2014/88), Chandigarh, India.
Study site, participants and sample collection.
The study was carried out at PGIMER, North India. Approximately, 2–3 mL of blood was drawn from the patients who showed malaria-like symptoms at the time of admission in the outpatient department and inpatient department of PGIMER between May 2013 and October 2016. All subjects and/or significant others provided written informed consent before their participation. The patient’s clinical details and demographic profile including age and gender were recorded at the time of sample collection.
Laboratory analyses.
Microscopy and rapid antigen detection test.
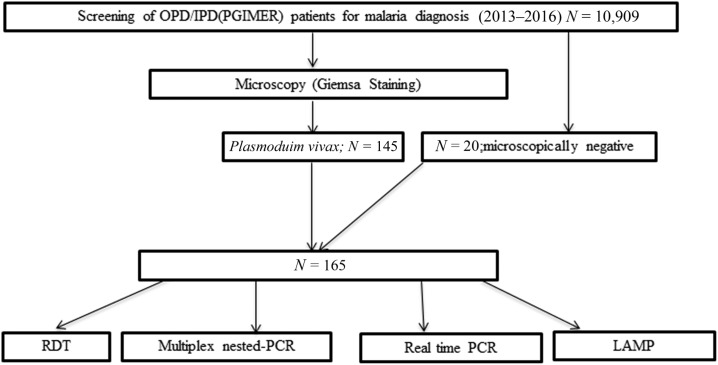
The collected samples were first subjected to the detection of malaria parasites using Giemsa microscopy and an expert microscopist examined all the slides. The samples tested positive for P. vivax and 20 microscopically negative samples selected randomly were then subjected to RDT using standard diagnostics BIOLINE rapid antigen detection kit which tests for the detection of histidine-rich protein II (HRP-II) antigen of Plasmodium falciparum and Plasmodium lactate dehydrogenase (pLDH) of P. vivax in human whole blood (Cat. No. 05FK80I40), as per manufacturer’s instructions (Alere Medical Pvt. Ltd., Gurgaon, India) (Figure 1). Patients positive for P. vivax were classified as uncomplicated and complicated, based on their clinical presentation.21
Figure 1.
Schematic representation of work performed in the present study.
DNA extraction.
DNA was extracted from all the P. vivax isolates and 20 microscopically negative samples using QIAamp DNA extraction kit, according to manufacturer’s instructions (Qiagen, Hilden, Germany). DNA obtained were stored at −20°C for further molecular work.
Multiplex nPCR.
Multiplex species-specific nPCR was performed by targeting the 18SrRNA gene to detect P. falciparum and P. vivax malaria parasites, as described earlier.22 In the genus-specific (primary PCR) master mix, 2 μL extracted genomic DNA was combined with 1× of Taq buffer (10×), 0.4 μM of both forward and reverse genus specific primers (rPLU1 and rPLU5), 0.6U of Taq Polymerase (Genei, Bangalore, India), and the mixture was made up to 25 μL with nuclease-free water. The amplification was initiated at 94°C for 4 minutes and followed by 35 cycles of 94°C for 30 seconds, 55°C for 1 minute, 72°C for 1 minute, and final extension at 72°C for 7 minutes. The product of primary PCR was diluted at 1:10 and used as a template for the species-specific multiplex nPCR. The PCR reaction mixture and conditions were same except annealing at 58°C for 1 minute. A negative control was included in each amplification reaction. The products of the secondary reactions were analyzed on 1.5% agarose gel stained with ethidium bromide and visualized under UV light.
Real-time PCR.
Real-time PCR was performed using the species-specific primers targeting 18SrRNA gene.22 The RT-PCR for P. vivax malaria diagnosis was carried out in ABI 7500 (Applied Biosystems, Carlsbad, CA). The PCR reaction mixture consisted of 1× (Powerup SYBR green master mix [Applied Biosystems]) master mix, 250 nM of each forward and reverse primers and 2 μL of genomic DNA template. Negative control was included in each reaction. The annealing was performed at 58°C for 30 seconds and fluorescent data was acquired at 72°C.
Loop-mediated isothermal amplification assay.
Loop-mediated isothermal amplification reaction was carried out using the Ampligene LAMP kit as per the manufacturer’s instruction with minor modifications (AmpliGene India Biotech Pvt. Ltd., Gujarat, India). The reaction mixture for LAMP consisted of three sets of primers as described earlier,23 1.6 μM of each forward and backward inner primer, 0.4 μM of each outer primer (F3 and B3), 1.2 μM each loop primers (loop primer forward and loop primer backward), isothermal master mix, 2 μL of DNA as template, and volume was made up to 12 μL with nuclease-free water. The reaction was carried out at 60.8°C for 30 minutes, followed by the enzyme inactivation step at 80°C for 2 minutes. Negative controls were also included in each test reaction.
Standardization of visually improved LAMP.
Loop-mediated isothermal amplification products were viewed on 3% agarose gel stained with ethidium bromide. For naked eye visual assessment, the concentration of SYBR Green I dye (Sigma, St. Louis, MO) was standardized using different dilutions. Positive samples presented a peculiar green fluorescent color whereas the negatives remained orange in color.
Statistical analysis.
Sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) of RDT, multiplex nPCR, P. vivax–specific RT-PCR, and LAMP were calculated using microscopy and nPCR as gold standards. Sensitivity was calculated at 95% confidence intervals (CIs) with MedCalc24 (MedCalc statistical software version 15.6.1; MedCalc, Ostend, Belgium). Cohen’s kappa of agreement between various techniques with respect to gold standard was calculated using IBM SPSS statistics 20 (IBM Corp, Armonk, NY). For determining the analytical sensitivity of nPCR and LAMP, the 10-fold serial dilutions of purified DNA were prepared and tests were then performed. The specificity was calculated using DNA of P. falciparum, Toxoplasma gondii, and Leishmania donovani clinical isolates; and Plasmodium bergehi DNA from culture to eliminate the probability of cross-reactivity.
RESULTS
Subject recruitment and microscopy.
A total of 10,909 malaria suspected patients were screened by microscopy, and of those, 1.8% (N = 196) patients were found to be positive for the malaria parasites. The majority of them were infected with P. vivax (N = 145, 74%). All the P. vivax confirmed patients were treated as per the National treatment guidelines.
Demographic and clinical presentation.
All the uncomplicated P. vivax patients showed symptoms such as high-grade fever with chills and rigors and general body weakness. Out of 148 P. vivax cases (positive by molecular techniques), 37.2% (N = 55) were presented with one or more than one complications with a male/female ratio of 1:0.6. The major complications found in the patients involved jaundice 18.2% (N = 10), seizures with altered sensorium 16.4% (N = 9), AKI 10.9% (N = 6), bleeding 9.1% (N = 5), severe anemia 7.3% (N = 4), and acute respiratory distress syndrome 3.6% (N = 2). Of 55 complicated P. vivax cases, individual coinfection of scrub typhus, Burkholderia cepacia sepsis, dengue, and typhoid were observed in four patients. The three cases which were reported to be negative by microscopy were of complicated type, and are of less than 12 years of age; and had seizure with altered sensorium, febrile encephalopathy, and AKI as the major clinical complication. Of these three patients, a death of a child (10Y/M) with a history of 3 days of illness was observed because of the complications associated with malaria infection such as seizures, hypotensive shock, nasal bleeding, febrile encephalopathy, and AKI with multiple organ dysfunction syndrome. Severe anemia was not observed in this complicated P. vivax malaria patient and also no concurrent infection with other pathogens was reported. However, coinfection with scrub typhus was observed in one of the microscopically negative patient. The male/female ratio was found to be 2:1 and median (interquartile range ) age of patients was 11 (5–26) years. Most of the patients were from the state of Haryana whereas others were from Punjab and Uttar Pradesh.
Rapid diagnostic test.
One hundred and forty five P. vivax microscopically positive and 20 microscopically negative isolates were examined by RDT. Majority of patients (86.7%, N = 143) were found to be positive for P. vivax by SD BIOLINE diagnostic test. The 3.03% (N = 5/165) cases were negative by RDT but were found to be positive for P. vivax by microscopy, LAMP, and multiplex nPCR.
Comparison of molecular methods with microscopy.
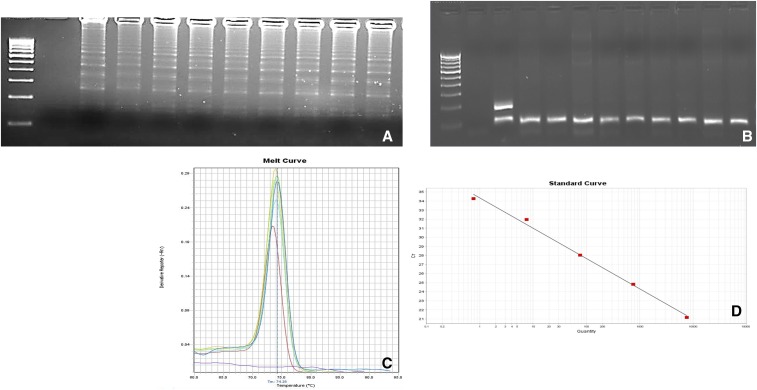
Molecular methods such as multiplex nPCR, RT-PCR, and visually improved P. vivax–specific LAMP assay were compared with microscopy. The visually improved P. vivax–specific LAMP (Figure 2A), multiplex nPCR (Figure 2B), and RT-PCR (Figure 2C and D) (linear regression coefficient R2 = 0.994) was found to be positive in a total of 90% (148/165) samples. Three additional cases which were negative by microscopy, turned out to be positive by multiplex nPCR, RT-PCR, and LAMP.
Figure 2.
Gel electrophoresis analysis of Plasmodium vivax–specific (A) loop-mediated isothermal amplification products. L = 100-bp molecular marker, NC = negative control (nuclease-free water), and lanes 1–9 = P. vivax–positive clinical isolates. (B) 18SrRNA-specific multiplex nested polymerase chain reaction (nPCR) products. Lane L = 100-bp DNA ladder, NC = negative control (nuclease-free water), PC = positive control (for P. vivax patient sample, 117 bp and for P. falciparum-3D7, 205 bp), and lanes 1–9 = P. vivax–positive species-specific multiplex nPCR products. (C) Real-time polymerase chain reaction results showing melting curve analysis for P. vivax clinical isolates. (D) Standard curve (y = −3.337x + 34.331), where y axis shows the cycle threshold. This figure appears in color at www.ajtmh.org.
Visual detection and turnaround time for LAMP assay.
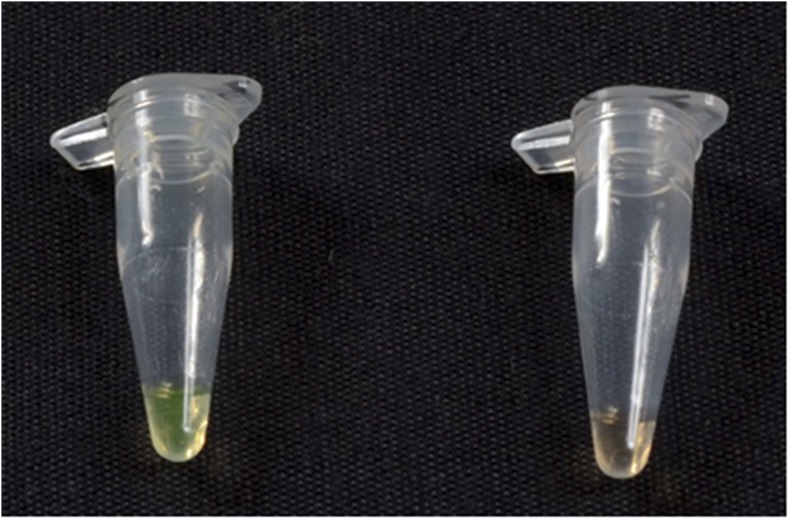
Visually improved LAMP assay was developed with the use of SYBR Green I dye (Sigma) at a dilution of 1:500. All the positive reactions showed a green fluorescent color, whereas the negative samples remained orange in color and the results were viewed with the naked eye (Figure 3). The performance of the LAMP assay with the visualization of results took around 40 minutes.
Figure 3.
Visual assessment immediately following loop-mediated isothermal amplification assay. Positive samples—Plasmodium vivax in green color and negative control (nuclease-free water) in orange color. This figure appears in color at www.ajtmh.org.
Estimation of clinical sensitivity and specificity of RDT, multiplex nPCR, RT-PCR, and P. vivax–specific LAMP compared with microscopy.
The clinical sensitivity and specificity was measured by taking gold standard microscopy positive and positive by any one of the four techniques (RDT, multiplex nPCR, RT-PCR, and LAMP) as true positive. The RDT was able to detect 86.7% (143/165) true P. vivax positive cases. The RDT however, failed to diagnose P. vivax in five cases those were positive by microcopy. The sensitivity and specificity of RDT was found to be 96.6% (95% CI: 92.14–98.87%) and 85% (95% CI: 62.11–96.79%), respectively. The PPV and NPV were found to be 98% and 77.4%, respectively. Multiplex nPCR, RT-PCR and P. vivax–specific LAMP was able to diagnose P. vivax in a total of 100% (148/148) clinical isolates but true positive remained 145 positive. Twenty microscopically negative samples were taken to check the clinical sensitivity and specificity of all the three molecular techniques. Multiplex nPCR, RT-PCR, and P. vivax–specific LAMP picked up three additional cases, positive for P. vivax earlier missed by microscopy.
The clinical sensitivity and specificity of all three nucleic acid based techniques remained the same when compared with microscopy and were found to be 100% (95% CI: 97.49–100.00%) and 85% (95% CI: 62.11–96.79%), respectively, and the PPV and NPV were found to be 98% and 100%, respectively (Table 1). The sensitivity and specificity of RT-PCR and LAMP were found to be 100% (95% CI: 97.5–100%) and 100% (95% CI: 83.2–100%), respectively when multiplex nested PCR was taken as gold standard (Table 2). The PPV and NPV of LAMP were also 100%. However, when RDT was compared with multiplex nPCR (taken as gold standard), the sensitivity and specificity were found to be 96.6% (95% CI: 92.3–98.9%) and 100% (95% CI: 80.5–100%), respectively.
Table 1.
Clinical sensitivity, specificity, PPV, and NPV of LAMP, multiplex nPCR, RT-PCR, and RDT; and agreement (Cohen’s kappa) of these techniques vs. microscopy
| Methods | Positive | Negative | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | Cohen’s kappa (κ) of agreement |
|---|---|---|---|---|---|---|---|
| Microscopy | |||||||
| LAMP | – | – | 100 | 85 | 98 | 100 | 0.909* |
| Positive | 145 | 3 | – | – | – | – | – |
| Negative | 0 | 17 | – | – | – | – | – |
| Multiplex nPCR | – | – | 100 | 85 | 98 | 100 | 0.909* |
| Positive | 145 | 3 | – | – | – | – | – |
| Negative | 0 | 17 | – | – | – | – | – |
| RT-PCR | – | – | 100 | 85 | 98 | 100 | 0.909* |
| Positive | 145 | 3 | – | – | – | – | – |
| Negative | 0 | 17 | – | – | – | – | – |
| RDT | – | – | 96.6 | 85 | 98 | 77.4 | 0.752* |
| Positive | 140 | 3 | – | – | – | – | – |
| Negative | 5 | 17 | – | – | – | – | – |
CI = confidence interval; LAMP = loop-mediated isothermal amplification; nPCR = nested PCR; NPV = negative predictive value; PCR = polymerase chain reaction; PPV = positive predictive value; RDT = rapid diagnostic test; RT-PCR = real-time PCR.
P value < 0.0001.
Table 2.
Clinical sensitivity, specificity, PPV, and NPV of LAMP, RT-PCR, microscopy, and RDT; and agreement (Cohen’s kappa) of these techniques vs. multiplex nPCR taken as reference method
| Methods | Positive | Negative | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | Cohen’s kappa (κ) of agreement |
|---|---|---|---|---|---|---|---|
| nPCR | |||||||
| LAMP | – | – | 100 | 100 | 100 | 100 | 1.000* |
| Positive | 148 | 0 | – | – | – | – | – |
| Negative | 0 | 17 | – | – | – | – | – |
| RT-PCR | – | – | 100 | 100 | 100 | 100 | 1.000* |
| Positive | 148 | 0 | – | – | – | – | – |
| Negative | 0 | 17 | – | – | – | – | – |
| Microscopy | – | – | 97.97 | 100 | 100 | 85 | 0.909* |
| Positive | 145 | 3 | – | – | – | – | – |
| Negative | 0 | 17 | – | – | – | – | – |
| RDT | – | – | 96.6 | 100 | 100 | 77.3 | 0.855* |
| Positive | 143 | 0 | – | – | – | – | – |
| Negative | 5 | 17 | – | – | – | – | – |
CI = confidence interval; LAMP = loop-mediated isothermal amplification; nPCR = nested PCR; NPV = negative predictive value; PCR = polymerase chain reaction; PPV = positive predictive value; RDT = rapid diagnostic test; RT-PCR = real-time PCR.
P value < 0.0001.
Analytical sensitivity and specificity.
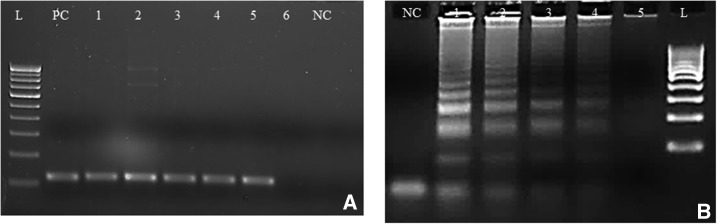
Plasmodium vivax–specific multiplex nPCR, RT-PCR, and LAMP were found to be highly sensitive. The analytical sensitivity of multiplex nPCR (Figure 4A), real time, and LAMP (Figure 4B) were found to be 8 copies/μL, 0.8 copies/μL, and 0.8 copies/μL, respectively. DNA of confirmed P. vivax, P. falciparum, T. gondii, and L. donovani clinical isolates; and P. bergehi from culture was used to check the specificity of P. vivax–specific LAMP assay. The specific step ladder pattern was observed only in case of P. vivax positive samples, showing the specificity, and others didn’t produce any such bands (data not shown).
Figure 4.
Representative images of nested polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) sensitivity targeting 18SrRNA gene. (A) Nested PCR sensitivity. L = 100-bp molecular marker, PC = microscopy confirmed clinical sample taken as positive control 8 × 105 copies/μL, NC = negative control (nuclease-free water), and lanes 1–6 = DNA concentrations of 8 × 104 copies/μL, 8 × 103 copies/μL, 8 × 102 copies/μL, 8 × 101 copies/μL, 8 copies/μL, and 0.8 copies/μL. (B) Plasmodium vivax–specific LAMP sensitivity. NC = negative control (nuclease-free water), lanes 1–5 = DNA concentrations of 8 × 102 copies/μL, 8 × 101 copies/μL, 8 copies/μL, 0.8 copies/μL, and 0.08 copies/μL, and L = 100-bp molecular marker.
DISCUSSION
Prompt and accurate identification and treatment of asymptomatic and symptomatic malaria cases is necessary to achieve the WHO goal of 90% reduction in global malaria incidence and mortality rates by 2030.25 For the diagnosis of malaria, microscopy has been considered as a gold standard but its sensitivity is affected by the level of experienced technician which further decreases in patients with low parasitemia. It has been well described that microscopy often misdiagnoses during mixed infections, and is also time consuming.11 In addition, misdiagnosis underestimates the prevalence of malaria infections in endemic region settings; which is responsible for approximately 20–50% human to mosquito transmission in very low transmission settings.26 Currently, a lot of strategies for control and elimination of malaria have been implicated which heavily depend on the accurate and prompt diagnosis of the disease.27 Furthermore, accurate and rapid diagnosis also determines the species-specific treatment initiation that ultimately leads to the management of the disease at early stages.
In this study, we have validated the P. vivax–specific visually improved LAMP assay on the clinical isolates. Overall, 87.9% (N = 145/165) samples were positive for P. vivax using microscopy whereas RDT showed positivity of 86.7% (N = 143/165) among P. vivax cases. The multiplex nPCR, RT-PCR, and LAMP reported a positivity of 90% (148/165) for P. vivax cases. The cataloged slide of the three complicated P. vivax malaria cases, which were reported to be positive by all the three molecular methods were then rescreened by microscopy and found to be negative. Among these three cases, a case of mortality was also reported. This further confirms the importance of molecular tests for the early diagnosis of complicated and uncomplicated malaria.
The sensitivity and specificity of P. vivax–specific visually improved LAMP assay compared with microscopy was found to be 100% and 85%, respectively. Our data is in concordance with previous studies where sensitivities and specificities was reported as 94–98.5% and upto 100% respectively, using microscopy as a reference standard.20,23,28,29 The low specificity of LAMP found in the present study was because of the three positive cases which came to be positive by all molecular techniques and negative by microscopy. Remarkably, three patients, negative by microscopy, were complicated P. vivax and one of them died during study period. Recently, a number of studies have been carried out using nPCR as gold standard to evaluate sensitivity and specificity of the newer diagnostic techniques of malaria. Ghayour Najafabadi et al.30 have used nPCR of blood as standard to compare the sensitivity and specificity of LAMP performed in blood, urine and saliva samples and was found to be 95.8% and 100%. Another study by Singh et al.18 has reported the sensitivity and specificity of 95.16% and 96.7%. In the present study, sensitivity and specificity of LAMP was found to be 100% when multiplex nPCR was taken as gold standard.
Severe vivax malaria has been reported in case series from different parts of the world including India.31–34 However, a large number of clinical studies present a clear proof of light microscopy being incapable of re-presenting the total parasite biomass in patients with severe malaria35–37 thus making microscopy less sensitive for malaria diagnosis. Also, pretreated cases before hospitalization may affect the diagnostic utility of malaria by light microscopy. The important challenges associated with the use of microscopy for the diagnosis of malaria cases have led to the development of alternative diagnostic methods.38
Most sensitive RDTs have similar limitations that of microscopy. The RDTs mainly rely on the detection of parasitic antigens such as LDH and HRP-II in a lateral flow format. These available RDTs are able to identify only the P. falciparum- and P. vivax-specific antigens and panmalarial antigens. The sensitivity and specificity of these RDTs are known to be lower for Plasmodium species other than P. falciparum.39 Because of the low parasitemia present in P. vivax patients and volatility of LDH at higher temperatures the RDT dependent P. vivax poses difficulties.40 In the endemic settings where both the P. falciparum and P. vivax are known to cause the severe form of malaria a combined PfHRP2/aldolase RDT test performed better regardless of any clinical manifestation. In the present study, detection was based on HRP-II antigen of P. falciparum and pLDH of P. vivax in whole blood and the sensitivity and specificity of RDT was found to be 96.6% and 85% for P. vivax. In various studies from India, sensitivity and specificity of RDTs have been reported from 84.2% to 98.70% and 96.5% to 98.9%41–43 which corroborates with present study. In a country such as India where P. vivax is responsible for causing uncomplicated and severe infection, the limited sensitivity of current used RDTs in detection of P. vivax is considered to be the major obstacle to long-term disease control programs and should be considered an urgent development goal in this field.44
Loop-mediated isothermal amplification has been used for the diagnosis of a wide variety of parasitic diseases.45–48 Isothermal amplification methods such as LAMP is a rapid and cheaper molecular method that allows the amplification of DNA by making the use of Bst polymerase with built in strand displacement capabilities allowing to work at isothermal conditions and not requiring higher temperatures as required in PCR. Also, nPCR is time consuming process which usually takes 6–7 hours for the complete amplification followed by the visualization of the results. Because of the intra-observer variations of the interpretation of the results, various modifications have been performed by implementing the use of real-time turbidometers49,50 and RT-PCR machines51 to read the endpoint results.52 Various colorometric methods for direct visualization of results have been tried in various parasitic diseases. Of the many dyes, such as Malachite green, hydroxy napthol blue, and Calcein; SYBR green has been used for the diagnosis of a large number of parasitic diseases.53–56 In the present study, the detection limit of P. vivax–specific LAMP was found to be 0.8 copies/μL which is in concordance with the previous report.11 The detection limit of nPCR and RT-PCR have been reported up to 1–5 P/μL of blood which is much better than the sensitivity of microscopy and RDTs.40 In the present study, modified LAMP assay was developed which took approximately 40 minutes to finish with visualization of results using SYBR Green I dye at dilutions of 1:500. This makes LAMP a rapid test with higher sensitivity and can be used in the detection of malaria, especially for complicated and asymptomatic patients where other detection methods such as RDT and microscopy may fail. Further modification of LAMP may lead to its use in the field and can also be used to detect patients with low parasitemia which is required for control and elimination of malaria.
CONCLUSION
In the present study, modified, fast, visually improved LAMP was developed which is capable of prompt diagnosis of complicated and uncomplicated P. vivax malaria cases in our setup. The developed assay reported to have a high sensitivity and specificity as compared with microscopy when multiplex nPCR is taken as gold standard. This method holds a good potential to be considered as a valuable tool for malaria control and elimination program.
Acknowledgment:
We thank Indian Council of Medical Research for providing Senior Research Fellowship to Hargobinder Kaur (No. 80/883/2014-ECD-1). The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.World Health Organization , 2017. World Malaria Report 2017. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Sharma S, Aggarwal KC, Deswal S, Raut D, Roy N, Kapoor R, 2013. The unusual presentation of a usual organism—the changing spectrum of the clinical manifestations of Plasmodium vivax malaria in children: a retrospective study. J Clin Diagn Res 7: 1964–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN, 2008. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, Kochar A, Khatri MP, Gupta V, 2009. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 80: 194–198. [PubMed] [Google Scholar]
- 5.Fabbri C, de Cássia Mascarenhas-Netto R, Lalwani P, Melo GC, Magalhães BML, Alexandre MAA, Lacerda MVG, Lima ES, 2013. Lipid peroxidation and antioxidant enzymes activity in Plasmodium vivax malaria patients evolving with cholestatic jaundice. Malar J 12: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ketema T, Bacha K, 2013. Plasmodium vivax associated severe malaria complications among children in some malaria endemic areas of Ethiopia. BMC Public Health 13: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushik JS, Gomber S, Dewan P, 2012. Clinical and epidemiological profiles of severe malaria in children from Delhi, India. J Health Popul Nutr 30: 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma R, Gohain S, Chandra J, Kumar V, Chopra A, Chatterjee S, Aneja S, Dutta AK, 2012. Plasmodium vivax malaria admissions and risk of mortality in a tertiary-care children’s hospital in north India. Paediatr Int Child Health 32: 152–157. [DOI] [PubMed] [Google Scholar]
- 9.Naha K, Dasari S, Prabhu M, 2012. Spectrum of complications associated with Plasmodium vivax infection in a tertiary hospital in south-western India. Asian Pac J Trop Med 5: 79–82. [DOI] [PubMed] [Google Scholar]
- 10.Chotivanich K, Silamut K, Day NPJ, 2007. Laboratory diagnosis of malaria infection—a short review of methods. NZJ Med Lab Sci 61: 4–7. [Google Scholar]
- 11.Lau Y-L, Lai M-Y, Fong M-Y, Jelip J, Mahmud R, 2016. Loop-mediated isothermal amplification assay for identification of five human Plasmodium species in Malaysia. Am J Trop Med Hyg 94: 336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO , 2017. Malaria Rapid Diagnostic Test Performance, Vol. 7. Results of WHO product testing of malaria RDTs: round 7 (2015–2016). Available at: http://www.who.int/malaria/publications/atoz/978924151268/en/. [Google Scholar]
- 13.Britton S, Cheng Q, McCarthy JS, 2016. Novel molecular diagnostic tools for malaria elimination: a review of options from the point of view of high-throughput and applicability in resource limited settings. Malar J 15: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perandin F, et al. 2004. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J Clin Microbiol 42: 1214–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piera KA, Aziz A, William T, Bell D, González IJ, Barber BE, Anstey NM, Grigg MJ, 2017. Detection of Plasmodium knowlesi, Plasmodium falciparum and Plasmodium vivax using loop-mediated isothermal amplification (LAMP) in a co-endemic area in Malaysia. Malar J 16: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN, 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61: 315–320. [DOI] [PubMed] [Google Scholar]
- 17.Hänscheid T, Grobusch MP, 2002. How useful is PCR in the diagnosis of malaria? Trends Parasitol 18: 395–398. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Singh DP, Savargaonkar D, Singh OP, Bhatt RM, Valecha N, 2017. Evaluation of SYBR green I based visual loop-mediated isothermal amplification (LAMP) assay for genus and species-specific diagnosis of malaria in P. vivax and P. falciparum endemic regions. J Vector Borne Dis 54: 54–60. [PubMed] [Google Scholar]
- 19.Patel JC, et al. 2014. Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, RealAmp, for the diagnosis of malaria in Thailand and India. J Infect Dis 210: 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surabattula R, Vejandla MP, Mallepaddi PC, Faulstich K, Polavarapu R, 2013. Simple, rapid, inexpensive platform for the diagnosis of malaria by loop mediated isothermal amplification (LAMP). Exp Parasitol 134: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization , 2012. Management of Severe Malaria, 3rd edition. Geneva, Switzerland: WHO [Google Scholar]
- 22.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA, 1999. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 60: 687–692. [DOI] [PubMed] [Google Scholar]
- 23.Han E-T, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T, 2007. Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J Clin Microbiol 45: 2521–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MedCalc , 2015. MedCalc Statistical Software. Version 15.6.1 Ostend, Belgium: MedCalc Software bvba. Available at: https://www.medcalc.org/. Accessed October 5, 2017.
- 25.World Health Organization , 2017. World Malaria Report 2017. Geneva, Switzerland: WHO. [Google Scholar]
- 26.Lucchi NW, et al. 2016. Evaluation of the illumigene malaria LAMP: a robust molecular diagnostic tool for malaria parasites. Sci Rep 6: 36808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera RS, Ding XC, Tully F, Oliver J, Bright N, Bell D, Chiodini PL, Gonzalez IJ, Polley SD, 2017. Development and clinical performance of high throughput loop-mediated isothermal amplification for detection of malaria. PLoS One 12: e0171126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao Z-Y, et al. 2011. Adaptation of a visualized loop-mediated isothermal amplification technique for field detection of Plasmodium vivax infection. Parasit Vectors 4: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh R, Savargaonkar D, Bhatt R, Valecha N, 2013. Rapid detection of Plasmodium vivax in saliva and blood using loop mediated isothermal amplification (LAMP) assay. J Infect 67: 245–247. [DOI] [PubMed] [Google Scholar]
- 30.Ghayour Najafabadi Z, Oormazdi H, Akhlaghi L, Meamar AR, Nateghpour M, Farivar L, Razmjou E, 2014. Detection of Plasmodium vivax and Plasmodium falciparum DNA in human saliva and urine: loop-mediated isothermal amplification for malaria diagnosis. Acta Trop 136: 44–49. [DOI] [PubMed] [Google Scholar]
- 31.Valecha N, et al. 2009. Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg 81: 758–762. [DOI] [PubMed] [Google Scholar]
- 32.Mehndiratta S, Rajeshwari K, Dubey AP, 2013. Multiple-organ dysfunction in a case of Plasmodium vivax malaria. J Vector Borne Dis 50: 71–73. [PubMed] [Google Scholar]
- 33.Lee H-J, Baek J-H, Chae M-H, Joo H, Lee J-S, Chung M-H, Park Y-K, Kim J-T, 2013. A case of vivax malaria complicated by adult respiratory distress syndrome and successful management with extracorporeal membrane oxygenation. Korean J Parasitol 51: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leal-Santos FA, Silva SBR, Crepaldi NP, Nery AF, Martin TOG, Alves-Junior ER, Fontes CJF, 2013. Altered platelet indices as potential markers of severe and complicated malaria caused by Plasmodium vivax: a cross-sectional descriptive study. Malar J 12: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dondorp AM, et al. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2: e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendriksen ICE, et al. 2012. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 measurement. PLoS Med 9: e1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber BE, William T, Grigg MJ, Parameswaran U, Piera KA, Price RN, Yeo TW, Anstey NM, 2015. Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog 11: e1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochola LB, Vounatsou P, Smith T, Mabaso ML, Newton CR, 2006. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis 6: 582–588. [DOI] [PubMed] [Google Scholar]
- 39.Murray CK, Gasser RA, Magill AJ, Miller RS, 2008. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev 21: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sattabongkot J, Tsuboi T, Han E-T, Bantuchai S, Buates S, 2014. Loop-mediated isothermal amplification assay for rapid diagnosis of malaria infections in an area of endemicity in Thailand. J Clin Microbiol 52: 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh N, Shukla MM, Shukla MK, Mehra RK, Sharma S, Bharti PK, Singh MP, Singh A, Gunasekar A, 2010. Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India. Malar J 9: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranjan P, Ghoshal U, 2016. Utility of nested polymerase chain reaction over the microscopy and immuno-chromatographic test in the detection of Plasmodium species and their clinical spectrum. Parasitol Res 115: 3375–3385. [DOI] [PubMed] [Google Scholar]
- 43.Elahi R, Mohon AN, Khan WA, Haque R, Alam MS, 2013. Performance of a HRP-2/pLDH based rapid diagnostic test at the Bangladesh-India-Myanmar border areas for diagnosis of clinical malaria. Malar J 12: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kochar DK, et al. 2010. Clinical features of children hospitalized with malaria—a study from Bikaner, northwest India. Am J Trop Med Hyg 83: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poon LLM, et al. 2006. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem 52: 303–306. [DOI] [PubMed] [Google Scholar]
- 46.Sriworarat C, Phumee A, Mungthin M, Leelayoova S, Siriyasatien P, 2015. Development of loop-mediated isothermal amplification (LAMP) for simple detection of Leishmania infection. Parasit Vectors 8: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang H-W, et al. 2013. Loop-mediated isothermal amplification targeting 18S ribosomal DNA for rapid detection of Acanthamoeba. Korean J Parasitol 51: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mewara A, Khurana S, Yoonus S, Megha K, Tanwar P, Gupta A, Sehgal R, 2017. Evaluation of loop-mediated isothermal amplification assay for rapid diagnosis of Acanthamoeba keratitis. Indian J Med Microbiol 35: 90–94. [DOI] [PubMed] [Google Scholar]
- 49.Hopkins H, et al. 2013. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 208: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polley SD, Mori Y, Watson J, Perkins MD, González IJ, Notomi T, Chiodini PL, Sutherland CJ, 2010. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J Clin Microbiol 48: 2866–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamura M, Makimura K, Ota Y, 2009. Evaluation of a new rapid molecular diagnostic system for Plasmodium falciparum combined with DNA filter paper, loop-mediated isothermal amplification, and melting curve analysis. Jpn J Infect Dis 62: 20–25. [PubMed] [Google Scholar]
- 52.Lucchi NW, Ljolje D, Silva-Flannery L, Udhayakumar V, 2016. Use of malachite green-loop mediated isothermal amplification for detection of Plasmodium spp. parasites. PLoS One 11: e0151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mugambi RM, Agola EL, Mwangi IN, Kinyua J, Shiraho EA, Mkoji GM, 2015. Development and evaluation of a loop mediated isothermal amplification (LAMP) technique for the detection of hookworm (Necator americanus) infection in fecal samples. Parasit Vectors 8: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiraho EA, Eric AL, Mwangi IN, Maina GM, Kinuthia JM, Mutuku MW, Mugambi RM, Mwandi JM, Mkoji GM, 2016. Development of a loop mediated isothermal amplification for diagnosis of Ascaris lumbricoides in fecal samples. J Parasitol Res 2016: 7376207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma S, Avishek K, Sharma V, Negi NS, Ramesh V, Salotra P, 2013. Application of loop-mediated isothermal amplification assay for the sensitive and rapid diagnosis of visceral leishmaniasis and post-kala-azar dermal leishmaniasis. Diagn Microbiol Infect Dis 75: 390–395. [DOI] [PubMed] [Google Scholar]
- 56.Kong Q-M, Lu S-H, Tong Q-B, Lou D, Chen R, Zheng B, Kumagai T, Wen L-Y, Ohta N, Zhou X-N, 2012. Loop-mediated isothermal amplification (LAMP): early detection of Toxoplasma gondii infection in mice. Parasit Vectors 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]