Abstract.
To improve malaria surveillance and achieve elimination, the Zambian National Malaria Elimination Program implemented a reactive test-and-treat program in Southern Province in 2013 in which individuals with rapid diagnostic test (RDT)–confirmed malaria are followed-up at their home within 1 week of diagnosis. Individuals present at the index case household and those residing within 140 m of the index case are tested with an RDT and treated with artemether–lumefantrine if positive. This study evaluated the efficiency of this reactive test-and-treat strategy by characterizing infected individuals missed by the RDT and the current screening radius. The radius was expanded to 250 m, and a quantitative polymerase chain reaction (qPCR) test was performed on dried blood spot specimens. From January 2015 through March 2016, 145 index cases were identified at health centers and health posts. A total of 3,333 individuals residing in 525 households were screened. Excluding index cases, the parasite prevalence was 1.1% by RDT (33 positives of 3,016 participants) and 2.4% by qPCR (73 positives of 3,016 participants). Of the qPCR-positive cases, 62% of 73 individuals tested negative by RDT. Approximately half of the infected individuals resided within the index case household (58% of RDT-positive individuals and 48% of qPCR-positive individuals). The low sensitivity of the RDT and the high proportion of secondary cases within the index case household decreased the efficiency of this reactive test-and-treat strategy. Reactive focal drug administration in index case households would be a more efficient approach to treating infected individuals associated with a symptomatic case.
INTRODUCTION
As malaria transmission declines, the proportion of the total infected population comprised of asymptomatic, chronically infected individuals with low parasite densities increases.1–4 Some chronically infected individuals are capable of transmitting malaria parasites in areas with competent vectors.1,3,4 Several strategies have been developed to identify and treat these individuals. Focal or mass drug administration relies on treating high-risk groups or entire populations on the assumption that malaria rapid diagnostic tests (RDTs) are insufficiently sensitive to identify infected individuals with low-level parasitemia.5,6 Active case detection, in contrast, involves screening individuals for malaria with RDTs within a defined geographic area (“hot spots”) or high-risk populations (“hot pops”) and treating those who test positive. Active case detection, and focal and mass drug administration, aim to eliminate parasites from chronically infected individuals, facilitating the interruption of local transmission.7
One method of active case detection involves reactive case detection, which leverages the underlying spatial and temporal clustering of malaria transmission.8–10 Reactive case detection includes reactive test-and-treat and reactive focal drug administration. For reactive test-and-treat, residents in the home of a symptomatic index case and those in neighboring households within a defined radius are screened with an RDT and offered treatment if positive.11–13 For reactive focal drug administration, individuals residing within an index case household, and potentially neighboring households, are treated without testing.14,15 Advantages of focal drug administration are that infected individuals who may otherwise be missed with a low-sensitivity RDT are treated, and the strategy does not increase the demand for RDTs.14,15 The disadvantage is that individuals not infected at the time of visit are exposed to antimalarial drugs. However, individuals residing in index case households are at the highest risk and administration of antimalarial drugs may provide chemoprophylaxis, particularly with the longer acting dihydroartemisinin–piperaquine combination.
The National Malaria Elimination Program of the Government of Zambia created a stepped sequence of interventions to achieve malaria elimination.16–18 Designated as Steps A through E, these interventions are to be implemented in succession depending on the parasite prevalence and case burden at health facilities.17,18 Step D consists of training volunteer community health workers to perform community case management and reactive test-and-treat within 140 m of index case households. Step D is implemented in low-transmission communities in which the parasite prevalence is approximately 1% and an average of 10 or fewer malaria cases present to a healthcare facility per week.17 In 2013, Step D activities were implemented through a phased implementation plan in selected districts in Southern Province, Zambia, with the goals of improving surveillance and interrupting transmission.16,19 As part of this study, the reactive test-and-treat radius was expanded to include all households within 250 m of the index case household and PCR-based diagnostic testing was added. This allowed us to evaluate the efficiency of Step D in identifying and treating infected individuals by including molecular detection of Plasmodium falciparum to identify infected individuals with low-level parasitemia and by extending the screening radius from 140 to 250 m.
METHODS
Study site.
The study was conducted in the rural catchment area of Macha Hospital in Choma District, Southern Province, Zambia, 70 km from the nearest town of Choma. The area has a tropical savannah climate with distinct wet and dry seasons. Malaria transmission is highest during the single rainy season from November through April, with Anopheles arabiensis as the primary vector.20,21 The hospital catchment area is populated by villagers living in small, scattered homesteads, comprised of single or multiple houses typically with extended family. The parasite prevalence as measured by active surveillance using a Pfhrp2 RDT declined over the past decade, from 9.2% in 2008 to less than 1% in 2013.22 Artemisinin combination therapy with artemether–lumefantrine was introduced as first-line antimalarial therapy in Zambia in 2002 and into the study area in 2004.23,24 Long-lasting insecticide-treated nets (LLINs) were widely distributed in the study area in 2007, and more than 11,000 LLINs were distributed from nine health centers in the catchment area of Macha Hospital in 2012, with additional LLINs distributed in 2014.20 Insecticide-treated net ownership was estimated at 83% in the study area in 2013.16
Study population.
The study population consisted of individuals residing within 250 m of an index case household. Symptomatic individuals diagnosed with malaria by RDT and eligible for reactive case detection were considered index cases. When an individual sought care at a healthcare facility and tested positive for malaria by RDT, their eligibility for follow-up with reactive test-and-treat was determined. Community health workers excluded individuals with a reported travel history as these cases were presumed to be imported. Travel was defined as staying overnight in a place outside their home district within the previous month. Rapid diagnostic test–positive individuals who had not traveled were eligible for reactive test-and-treat. Eligible index cases were to be followed up within 1 week of diagnosis.
As part of the study, health workers sent a short message service text message to the study team based at Macha Research Trust, located at Macha Hospital, within 1 week of identifying an index case to plan the reactive test-and-treat activities. A study team member accompanied the health worker to the index case household for a notification visit, at which time the head of household and other residents were informed that the health worker and study team would return the following day to perform the informed consent procedures, administer a questionnaire, and collect a fingerprick blood sample. Through the informed consent process, residents of index and neighboring households could refuse participation in the study activities.
Global positioning system (GPS) coordinates of the index houses were collected during the notification visit and were subsequently mapped using ArcGIS v.10 (ERSI, Redlands, CA). A QuickBird high-resolution satellite image of the study area obtained in 2011 was imported into ArcGIS and all households within a radius of 250 m of the index house were identified. A printed image of the index case household and eligible secondary households was provided to the study team to guide enrollment.
Study procedures.
Study enrollment began in January 2015, and continued through March 2016. Residents of households within 250 m of an index case household were eligible for the study and written informed consent was obtained from adults and parental permission from caregivers of children who agreed to participate. Questionnaires were administered to collect information on age, gender, recent history of malaria, signs and symptoms of malaria (defined as the presence of fever with either chills or headache), recent antimalarial use, bed net use, and socioeconomic status. Fingerprick blood samples were collected from participants for a P. falciparum HRP2 RDT (SD Bioline, Gyeonggi-do, Republic of Korea) and dried blood spots (Whatman 903™ Protein Saver Card; Sigma-Aldrich, Piscataway, NJ). Individuals who tested positive by RDT (other than the index case who was presumed to have been treated at the health center or health post) were offered treatment with artemether/lumefantrine in accordance with the Zambian Ministry of Health guidelines. The study was approved by the Tropical Diseases Research Center Ethics Review Committee and the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health. Local government officials were informed of the study purpose and procedures before data collection and local community acceptance was sought.
Laboratory procedures.
DNA was extracted from dried blood spots using a previously described Chelex extraction protocol.21,25 Quantitative polymerase chain reaction was performed to detect the presence of the P. falciparum cytochrome b gene (Pfcytb). Using standard genomic DNA dilution series and filter paper spotted with cultured parasites (NF54), the limit of detection was determined to be 1 parasite/μL. Primers were designed to detect the presence of Pfcytb and amplification was detected by fluorescence signal of SYBR® Green. Each reaction contained 5 μL of DNA template, 5 μL of SYBR Green PCR Master Mix (ThermoFisher, Waltham, MA), and 200 nM of forward primer (5′ CCT GAT AAT GCT ATC GTA 3′) and reverse primer (5′ TAA TAC AAT TAC TAA ACC AGC 3′). All qPCR-positive samples were evaluated on a 4% agarose gel to confirm the product size of the amplicon. Only samples confirmed by both qPCR and gel electrophoresis were considered qPCR positive.
Statistical analysis.
The primary outcomes of interest were the prevalence of individuals who were RDT positive (detected through reactive test-and-treat) and those positive by qPCR but negative by RDT (missed by reactive test-and-treat) within the index case household, within 140 m of the index case household, and between 140 and 250 m of the index case household (missed with the current screening radius). The individuals who tested positive during the rainy season (November through March) were compared with those positive during the dry season (April through October). Index cases were excluded from the analyses. Differences in proportions were tested using Fisher’s exact test. Statistical analyses were performed using STATA version 14 (StataCorp LP, College Station, TX).
RESULTS
Study households and participants.
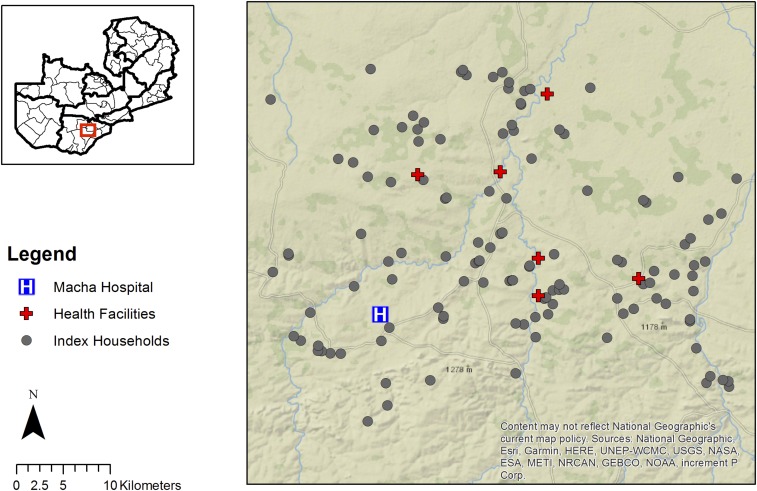
From January 2015 through March 2016, 145 index cases residing in 125 index case households were identified as eligible for reactive case detection, and the study team was notified by a health worker (Figure 1). Through reactive case detection, 146 households were identified within 140 m of the index case households, and, as part of this study, 251 households were identified between 140 and 250 m from the index case households. Thus, 24% of study households were index case households, 28% were within 140 m of the index case household, and 48% were located between 140 and 250 m of the index case household.
Figure 1.
Map of index case households included in the reactive test-and-treat program. This figure appears in color at www.ajtmh.org.
During the reactive case detection, 841 residents of the 125 index case households and 671 individuals residing in the 146 households within 140 m of the index case household were enrolled. An additional 1,480 individuals residing in the 251 households between 140 and 250 m of the index case household were enrolled as part of the study. Of the study participants, 28% (excluding index cases) lived within index case households, 22% resided in households within 140 m of the index case household, and 50% resided in households within 140–250 m from the index case household. The median age of study participants was 12 years (interquartile range: 6, 27) and 54% were female (Table 1). Household GPS coordinates were available for 2,992 of the 3,016 individuals with complete RDT and PCR data.
Table 1.
Baseline characteristics of study participants
| All participants | RDT+ | RDT− | PCR+ | PCR− | PCR+ RDT− | PCR− RDT− | |
|---|---|---|---|---|---|---|---|
| Total (%) | 3,016 | 33 (1.1) | 2,983 (98.9) | 73 (2.4) | 2,943 (97.6) | 45 (1.5) | 2,938 (98.5) |
| Median age in years (IQR) | 12 (6–27) | 12 (7–15) | 12 (6–27) | 11.5 (6–21.5) | 12 (6–27) | 11.5 (5–23.5) | 12 (6–27) |
| Female (%) | 1,624 (53.8) | 17 (51.5) | 1,593 (53.7) | 35 (48.6) | 1,575 (53.8) | 20 (44.5) | 1,573 (53.9) |
| Malaria symptoms* | |||||||
| Yes (%) | 392 (13.4) | 11 (33.3) | 379 (13.2) | 21 (30.4) | 369 (13.0) | 11 (26.8) | 368 (13.0) |
| No (%) | 2,531 (86.5) | 22 (66.7) | 2,492 (86.7) | 48 (69.6) | 2,466 (87.0) | 30 (73.2) | 2,462 (87.0) |
| Sleeps under a bed net† | |||||||
| Yes (%) | 1,527 (53.5) | 14 (45.2) | 1,503 (53.6) | 36 (51.4) | 1,481 (53.6) | 27 (61.4) | 1,476 (53.5) |
| No (%) | 1,325 (46.5) | 17 (54.8) | 1,299 (46.4) | 34 (48.6) | 1,282 (46.4) | 17 (38.6) | 1,282 (46.5) |
PCR = polymerase chain reaction; RDT = rapid diagnostic test.
Data on malaria symptoms, defined as fever with the presence of chills or headache, available for 2,923 individuals.
Bed net data available for 2,852 individuals.
Parasite prevalence.
Excluding index cases, a total of 3,188 individuals were tested for malaria by RDT and qPCR, and data on both RDT and qPCR status were available for 3,016 (95%) of these participants (Table 1). Of those 3,016 participants, the prevalence of malaria was 1.1% by RDT (33 positives of 3,016 participants) and 2.4% by qPCR (73 positives of 3,016 participants). Of the 525 households visited, 29 (6%) had at least one non-index case that tested positive by RDT and 56 (11%) households had at least one non-index case positive by qPCR. Thirty-five households (7%) had at least one individual who was qPCR positive but RDT negative. Parasite prevalence by RDT and qPCR did not vary by season, despite the seasonal transmission pattern in southern Zambia.
Efficiency of the reactive test-and-treat radius.
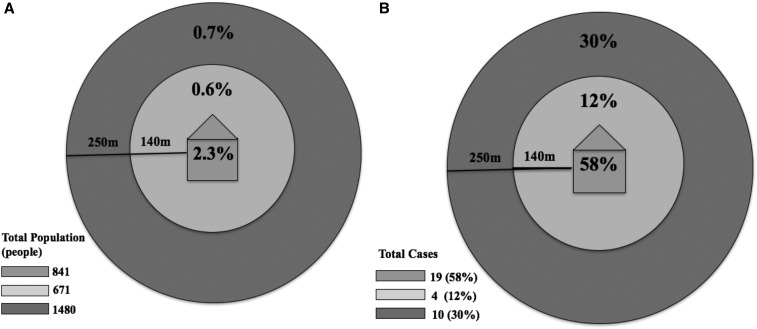
Of the RDT-positive individuals, 58% lived in the index case household, 12% lived within 140 m of the index case household, and 30% lived between 140 and 250 m of the index case household (Table 2, Figure 2). Thus, most RDT-positive individuals resided within the index case households and only a small proportion resided within the 140-m screening radius. No differences were observed in the proportion of individuals who were RDT positive in houses within 140 m (0.6%) compared with those residing 140–250 m (0.7%) of the index case household (P = 0.99) (Table 2, Figure 2A). At the household level, 59% of households with an RDT-positive resident were index case households.
Table 2.
Parasite prevalence by RDT and PCR for study participants and households, by distance from the index case household
| Index case household | Within 140 m of index case household | 140–250 m from index case household | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | RDT+ | RDT− | N | RDT+ | RDT− | N | RDT+ | RDT− | |
| Individuals | 841 | 2.3% | 97.7% | 671 | 0.6% | 99.4% | 1,480 | 0.7% | 99.3% |
| Households | 125 | 13.6% | 86.4% | 146 | 2.7% | 97.3% | 251 | 3.2% | 96.8% |
| PCR+ | PCR− | PCR+ | PCR− | PCR+ | PCR− | ||||
| Individuals | 841 | 4.2% | 95.8% | 671 | 1.5% | 98.5% | 1,480 | 1.9% | 98.1% |
| Households | 125 | 21.6% | 78.4% | 146 | 6.2% | 92.8% | 251 | 8.0% | 92.0% |
| PCR+/RDT− | PCR− | PCR+/RDT− | PCR− | PCR+/RDT− | PCR− | ||||
| Individuals | 822 | 2.2% | 97.8% | 667 | 0.9% | 99.0% | 1,470 | 1.4% | 98.6% |
| Households | 125 | 11.2% | 88.8% | 146 | 3.4% | 96.6% | 251 | 6.0% | 94.0% |
| PCR−/RDT+ | PCR−/RDT− | PCR−/RDT+ | PCR−/RDT− | PCR−/RDT+ | PCR−/RDT− | ||||
| Individuals | 806 | 0.3% | 99.7% | 661 | 0% | 100% | 1,452 | 0.2% | 99.8% |
| Households | 125 | 2.4% | 98.4% | 146 | 0% | 100% | 251 | 0.4% | 99.6% |
PCR = polymerase chain reaction; RDT = rapid diagnostic test.
Figure 2.
Proportion of study participants who tested positive by rapid diagnostic test (RDT) within each household distance category (A) and proportion all RDT-positive individuals (N = 33) living at each household distance (B).
Efficiency of reactive test-and-treat using an RDT.
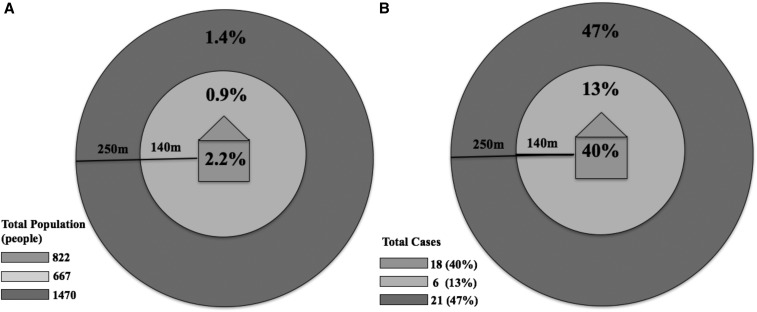
More than half of infected individuals (55% of those who were qPCR positive) were negative by RDT, indicating that most infected individuals would not be detected by a reactive test-and-treat strategy using a standard sensitivity RDT in this low-transmission setting (Table 1). At the household level, 40% of households with a resident who was RDT negative but qPCR positive were index case households, 14% were households located within 140 m of the index case household, and 46% were households located between 140 and 250 m of the index case household (Table 2). At the individual level, 40% of all individuals who were RDT negative but qPCR positive lived within the index case household, 13% lived within 140 m of the index case household, and 47% lived in households between 140 and 250 m from the index case household, outside the current screening radius (Table 2, Figure 3). No statistically significant differences were observed in the proportion of individuals who were RDT negative but qPCR positive in houses within 140 m (0.9%) compared with those residing 140–250 m (1.4%) of the index case household (P = 0.45) (Table 2, Figure 3A).
Figure 3.
Proportion of study participants who were rapid diagnostic test (RDT) negative but quantitative polymerase chain reaction (qPCR) positive within each household distance category (A) and proportion of RDT-negative but qPCR-positive participants (N = 44) living at each household distance (B).
DISCUSSION
The efficiency of a reactive test-and-treat strategy was evaluated in a low-transmission setting in southern Zambia by extending the existing screening radius from 140 to 250 m and using qPCR to detect RDT-negative, infected individuals. Overall, the sensitivity of the RDT was 45% compared with qPCR. Thus, more than half of infected individuals were missed using a standard-sensitivity RDT for screening in this low-transmission setting. The highest proportion of RDT-positive individuals resided within index case households. The proportion of infected individuals detected by either RDT or qPCR did not differ between individuals residing outside the index case household, that is, within 140 m or from 140 to 250 m of the index case household. These infected individuals may largely represent a background prevalence of asymptomatic, chronically infected individuals rather than individuals infected through local transmission associated with the index case. These findings suggest that reactive focal drug administration in the index case household may be a more efficient strategy to treat infected individuals than reactive test-and-treat in this low-transmission setting. Among the 73 infected individuals detected using qPCR, 29% resided within 140 m of the index case household and were RDT positive, and thus would have been detected and treated by the current test-and-treat strategy. By contrast, 48% of the 73 infected individuals detected using qPCR resided within an index case household and would have been treated through focal drug administration in the index case household alone. However, neither reactive focal drug administration in the index case household nor reactive case detection in a 140-m radius will identify and treat all infected individuals.
These findings are consistent with other assessments of reactive test-and-treat programs in which this strategy was shown to be an inefficient method of identifying infected individuals in low-transmission settings.26 Studies conducted in Zambia, Senegal, and Swaziland found that the number of tested individuals to identify one infected individual ranged from 37 to 250.11,12,27 In our study area, a mean of 90 RDT-negative residents were tested for each RDT-positive individual identified and a mean of 66 qPCR-negative residents were tested for each qPCR-positive, RDT-negative individual identified. A mean of 24 qPCR-negative residents of index case households were tested for each qPCR-positive, RDT-negative individual identified within an index case household. The high number of RDT-negative individuals tested for each RDT-positive individual identified provides further evidence that in low-transmission settings reactive test-and-treat using a standard sensitivity RDT is not efficient. Interestingly, the sensitivity of the PfHRP2 RDT was higher in comparison with qPCR when used for reactive case detection (45%) than active case detection (17%) in the same community, perhaps reflecting higher levels of parasitemia associated with recent focal transmission around symptomatic index cases.25
The low sensitivity of the PfHRP2 RDT for screening asymptomatic or minimally symptomatic residents during household surveys has spurned interest in the development of higher sensitivity RDTs or field-deployable molecular assays.28,29 An ultrasensitive PfHRP2 RDT was recently shown to have a 10-fold lower limit of detection for HRP2 compared with a standard RDT, with a sensitivity of 84% compared with quantitative real-time-PCR in Uganda but only 44% in Myanmar where transmission intensity is lower.30 In a low-transmission setting such as southern Zambia, where levels of parasitemia can be low in asymptomatic individuals, a similar ultrasensitive RDT is likely to have a low sensitivity as it did in Myanmar.25 An additional challenge to the use of PfHRP2 RDTs is the spread of the Pfhrp2 gene deletions in sub-Saharan Africa.31 Although Pfhrp2 deletions have not yet been reported in Zambia, they have been identified in the neighboring Democratic Republic of Congo.32
There are several limitations to this study. First, the cross-sectional study design did not allow for observations of the natural history of malaria in study participants such that, for example, RDT-negative, qPCR-positive individuals may have been recently infected and thus did not yet develop sufficient parasitemia to test positive by RDT or become symptomatic. Second, we did not attempt to identify infection with non-falciparum malaria. Plasmodium malariae has been identified in the study area but the prevalence is low and infection frequently coexists with P. falciparum.25 Last, not all household members were present during the study visits. Nonparticipation of households and individual residents is an inherent challenge to reactive test-and-treat strategies.33
The low sensitivity of current RDTs and the high proportion of secondary cases within index case households lowered the efficiency of a reactive test-and-treat strategy in a low-transmission setting in southern Zambia. Reactive focal drug administration in index case households, in which all individuals within index case households are treated without testing, appears to be a more efficient approach to treating infected individuals associated with a symptomatic case and would greatly reduce the number of RDTs needed in such low-transmission settings.
Acknowledgments:
We thank the members of the community for their volunteer participation in the surveys and the Macha Research Trust field team for conducting the surveys, without whom this research would not have been possible.
REFERENCES
- 1.Gerardin J, Ouédraogo AL, Mccarthy KA, Eckhoff PA, Wenger EA, 2015. Characterization of the infectious reservoir of malaria with an agent-based model calibrated to age-stratified parasite densities and infectiousness. Malar J 14: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood B, 1987. Asymptomatic malaria infections—do they matter? Parasite Pathol 3: 206–214. [DOI] [PubMed] [Google Scholar]
- 3.Karl S, Gurarie D, Zimmerman P, King C, Pierre T, Davis T, 2011. A sub-microscopic gametocyte reservoir can sustain malaria transmission. PLoS One 6: e20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousema JT, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 5.McMorrow ML, Aidoo M, Kachur SP, 2011. Malaria rapid diagnostic tests in elimination settings–can they find the last parasite? Clin Microbiol Infect 17: 1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisele TP, et al. 2015. Assessing the effectiveness of household-level focal mass drug administration and community-wide mass drug administration for reducing malaria parasite infection prevalence and incidence in Southern Province, Zambia: study protocol for a community randomized controlled trial. Trials 16: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickremasinghe R, Fernando S, Thillekaratne J, Wijeyaratne P, Wickremasinghe A, 2014. Importance of active case detection in a malaria elimination programme. Malar J 13: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bejon P, et al. 2010. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med 7: e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousema T, Griffin J, Sauerwein R, Smith D, Churcher T, Takken W, Ghani A, Drakeley C, Gosling R, 2012. Hitting hotspots: spatial targeting of malaria for control and eliminationtle. PLoS Med 9: e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousema T, et al. 2012. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis 201: 1764–1774. [DOI] [PubMed] [Google Scholar]
- 11.Littrell M, Sow G, Ngom A, Ba M, Mboup B, Dieye Y, Mutombo B, Earle D, Steketee R, 2013. Case investigation and reactive case detection for malaria elimination in northern Senegal. Malar J 12: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sturrock H, Novotny J, Kuene S, Dlamini S, Zulu Z, Cohen J, Hsiang M, Greenhouse B, Gosling R, 2013. Reactive case detection for malaria elimination: real-life experience from an ongoing program in Swaziland. PLoS One 8: e63830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Searle K, Shields T, Hamapumbu H, Kobayashi T, Mharakurwa S, Thuma PE, Smith DJ, Glass G, Moss WJ, 2013. Efficiency of household reactive case detection for malaria in rural southern Zambia: simulations based on cross-sectional surveys from two epidemiological settings. PLoS One 8: e70972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerardin J, Bever C, Hamainza B, Miller J, Eckhoff PA, Wenger EA, 2016. Optimal population-level infection detection strategies for malaria control and elimination in a spatial model of malaria transmission. PLoS Comput Biol 12: e1004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosha JF, et al. 2013. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malar J 12: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The President’s Malaria Initiative , 2014. Zambia: Malaria Operational Plan, FY 2015 Available at: https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy-15/fy-2015-zambia-malaria-operational-plan.pdf?sfvrsn=7. Accessed March 4, 2016.
- 17.Akros , 2013. Community Surveillance Available at: http://akros.com/malaria-prevention/community-surveillance/. Accessed March 4, 2016.
- 18.PATH , 2014. Zambia Trip Report: Project DIAMETER Available at: http://sites.path.org/dx/files/2012/11/Zambia-trip-report_forweb.pdf. Accessed October 10, 2015.
- 19.Larsen D, et al. 2015. Malaria surveillance in low-transmission areas of Zambia using reactive case detection. Malar J 14: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss WJ, Hamapumbu H, Kobayashi T, Shields T, Kamanga A, Clennon J, Mharakurwa S, Thuma P, Glass G, 2011. Use of remote sensing to identify spatial risk factors for malaria in a region of declining transmission: a cross-sectional and longitudinal community survey. Malar J 10: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi T, Chishimba S, Shields T, Hamapumbu H, Mharakurwa S, Thuma P, Glass G, Moss WJ, 2012. Temporal and spatial patterns of serologic responses to Plasmodium falciparum antigens in a region of declining malaria transmission in southern Zambia. Malar J 11: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinchoff J, Hamapumbu H, Kobayashi T, Simubali L, Stevenson J, Norris D, Calountoni E, Thuma P, Moss WJ, 2015. Factors associated with sustained use of long-lasting insecticide-treated nets following a reduction in malaria transmission in southern Zambia. Am J Trop Med Hyg 93: 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steketee R, Sipilanyambe N, Chimumbwa J, Banda J, Mohamed A, Miller J, Basu S, Miti SK, Campbell C, 2008. National malaria control and scaling up for impact: the Zambian experience through 2006. Am J Trop Med Hyg 79: 45–52. [PubMed] [Google Scholar]
- 24.Sipilantambe N, Simon J, Chanda P, Olumese P, Snow R, Hamer D, 2008. From chloroquine to artemether-lumefantrine: the process of drug policy change in Zambia. Malar J 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laban NM, Kobayashi T, Hamapumbu H, Sullivan D, Mharakurwa S, Thuma P, Schiff C, Moss WJ, 2015. Comparison of a PfHRP2-based rapid diagnostic test and PCR for malaria in a low prevalence setting in rural southern Zambia: implications for elimination. Malar J 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Eijk AM, et al. 2016. What is the value of reactive case detection in malaria control? A case-study in India and a systematic review. Malar J 15: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stresman G, Kamanga A, Moono P, Hamapumbu H, Mharakurwa S, Kobayashi T, Moss WJ, Schiff C, 2010. A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in southern province, Zambia. Malar J 9: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slater H, et al. 2015. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature 528: S94–S101. [DOI] [PubMed] [Google Scholar]
- 29.Hemingway J, Shretta R, Wells T, Bell D, Djimde A, Achee N, Qi G, 2016. Tools and strategies for malaria control and elimination: what do we need to achieve a grand convergence in malaria? PLoS Biol 14: e1002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, et al. 2017. Performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naive human challenge infections. Am J Trop Med Hyg 97: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson O, Slater H, Verity R, Parr J, Mwandagalirwa M, Tshefu A, Meshnick S, Ghani A, 2017. Modelling the drivers of the spread of Plasmodium falciparum hrp2 gene deletions in sub-Saharan Africa. Elife 6: pii: e25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parr JB, et al. 2016. Pfhrp2-deleted Plasmodium falciparum parasites in the democratic Republic of the Congo: a national cross-sectional survey. J Infect Dis 216: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Searle KM, et al. 2016. Evaluation of the operational challenges in implementing reactive screen-and-treat and implications of reactive case detection strategies for malaria elimination in a region of low transmission in southern Zambia. Malar J 15: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]