Abstract.
Eastern equine encephalitis virus (EEEV) is a mosquito-borne alphavirus found in the eastern United States. Eastern equine encephalitis virus disease in humans is rare but can result in severe, often fatal, illness. This report summarizes the national EEEV surveillance data for 2003 through 2016, including human disease cases and nonhuman infections. Over the 14-year period, 633 counties from 33 states reported EEEV activity; 88% of those counties reported EEEV activity only in nonhuman species. A total of 121 human cases of EEEV disease were reported, with a median of eight cases reported annually. The national average annual incidence of EEEV neuroinvasive disease was 0.03 cases per million population. States with the highest average annual incidence included New Hampshire, Massachusetts, Vermont, Maine, and Alabama. Eastern equine encephalitis virus neuroinvasive disease incidence was highest among males and among persons aged < 5 and > 60 years. Overall, 118 (98%) case patients were hospitalized and 50 (41%) died. The case fatality ratio was highest among case patients aged ≥ 70 years. Nonhuman surveillance data indicate that the geographic range of EEEV is much greater than human cases alone might suggest. In areas where the virus circulates, health-care providers should consider EEEV infection in the differential diagnosis for meningitis and encephalitis. Providers are encouraged to report suspected cases to their public health department to facilitate diagnosis and consider interventions to mitigate the risk of further transmission. Because human vaccines against EEEV are not available, prevention depends on community efforts to reduce mosquito populations and personal protective measures to decrease exposure to mosquitoes.
INTRODUCTION
Eastern equine encephalitis virus (EEEV), a mosquito-borne alphavirus, is one of the most severe arboviral encephalitides in North America.1 Eastern equine encephalitis virus is primarily maintained in an enzootic cycle between birds and Culiseta melanura mosquitoes, which breed in freshwater hardwood swamp environments.2 Spread of EEEV to mammals typically requires bridging mosquitoes (e.g., Aedes or Coquillettidia species) that feed on both birds and mammals.1,2 There is some evidence that alternative enzootic and epizootic cycles exist.3–6 Humans are considered dead-end hosts because they generally do not develop high enough viremia levels to allow virus transmission to feeding mosquitoes.
Most persons infected with EEEV have no apparent illness.7 An estimated < 5% of persons infected with EEEV develop meningitis or encephalitis.2,7 Systemic infection is characterized by acute onset of fever, chills, malaise, myalgia, and arthralgia.1 Signs and symptoms in patients with neuroinvasive disease include fever, headache, altered mental status, and seizures.8–10 Eastern equine encephalitis virus neuroinvasive disease is estimated to have a 30% case fatality rate and results in neurologic sequelae in more than 50% of survivors.8,11–13 Although veterinary EEEV vaccines are available for use in horses, there are no licensed vaccines or effective treatment of humans.
The first human EEEV disease cases were recognized during a 1938 outbreak in southeastern Massachusetts.8 Human EEEV disease cases have occurred sporadically and in small clusters, primarily along the Atlantic and Gulf coasts of the United States.9,11,14 The largest recorded EEEV outbreak occurred in New Jersey in 1959, with 32 laboratory-confirmed human cases during an 8-week period.11 From 1997 through 2007, a median of eight neuroinvasive disease cases were reported to the Centers for Disease Control and Prevention annually.15 This report summarizes the national EEEV surveillance data for 2003 through 2016, including human disease cases and nonhuman infections.
MATERIALS AND METHODS
Data source and case definitions.
EEEV disease is a nationally notifiable condition. Since 2003, state health departments have reported arboviral disease cases, including EEEV disease cases, to ArboNET, the national arboviral disease surveillance system. Human disease cases are reported using standard surveillance case definitions that include clinical and laboratory criteria.16 All cases classified as confirmed and probable by the reporting jurisdiction were included in this analysis.
Data routinely collected in ArboNET for human disease cases include patient age, sex, county and state of residence, date of illness onset, case status (e.g., confirmed or probable), clinical syndrome (e.g., febrile illness, meningitis, encephalitis, and acute flaccid paralysis), and outcomes (i.e., hospitalization and fatality). Cases reported as meningitis, encephalitis, acute flaccid paralysis, or other neurologic presentation are classified as neuroinvasive disease; others are considered non-neuroinvasive disease. In addition to data on human disease cases, ArboNET collects data on veterinary disease cases, and infections in mosquitoes, dead birds, and sentinel animals (e.g., chicken flocks). Nonhuman arboviral surveillance is voluntary and is performed variably across different jurisdictions. Data typically reported to ArboNET for nonhuman arboviral infections include species, state and county of collection, and date of symptom onset or specimen collection.
Data analysis.
Categorical variables were summarized using counts and proportions; continuous variables were summarized by using median and interquartile range (IQR). Descriptions of disease incidence include only neuroinvasive disease cases because non-neuroinvasive disease cases are likely underreported and reporting may be variable depending on location and other factors. Average annual incidence rates were calculated using the 2010 U.S. Census Bureau data.17 Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Maps were generated using ArcGIS version 10.3 (ESRI, Redlands, CA).
RESULTS
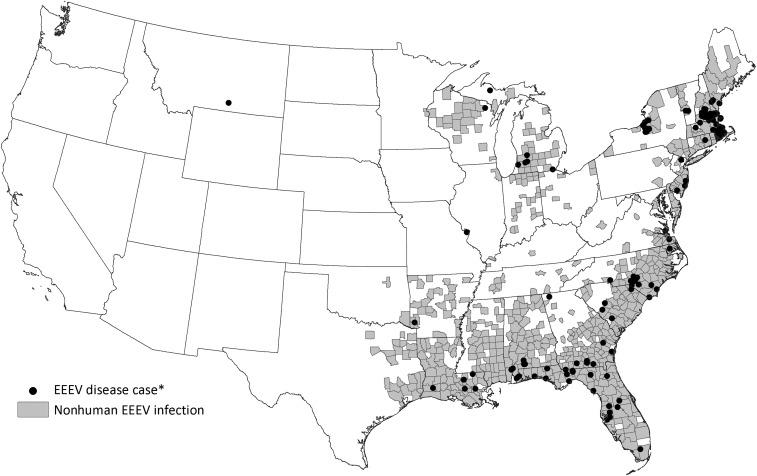
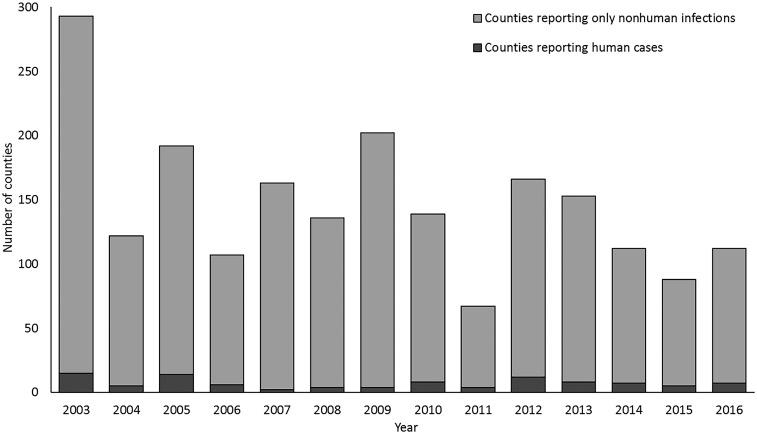
From 2003 through 2016, 633 (18%) counties from 33 (66%) states reported EEEV activity in at least one species in one or more years (Figure 1). Over the 14-year period, 559 (88%) of the 633 counties reported EEEV activity in only nonhuman species (Figure 2). Veterinary disease cases were reported from 582 counties in 31 states. Of the 3,112 veterinary cases reported, 3,016 (97%) were equids, 12 (< 1%) were camelids, 9 (< 1%) were canines, 6 (< 1%) were cervids, and 69 (2%) were reported as other species. Mosquito infections were reported from 122 counties in 23 states, dead bird infections from 84 counties in 14 states, sentinel chicken infections from 68 counties in eight states. Counties reporting activity were located primarily along the Atlantic and Gulf coasts; several counties bordering the Great Lakes also reported activity. There was no evidence of EEEV transmission in the western United States in the nonhuman data.
Figure 1.
Counties reporting Eastern equine encephalitis virus activity to ArboNET, United States, 2003–2016. *Human disease cases reported by state and county of residence.
Figure 2.
Counties reporting human Eastern equine encephalitis virus disease cases and nonhuman infections, by year, United States, 2003–2016.
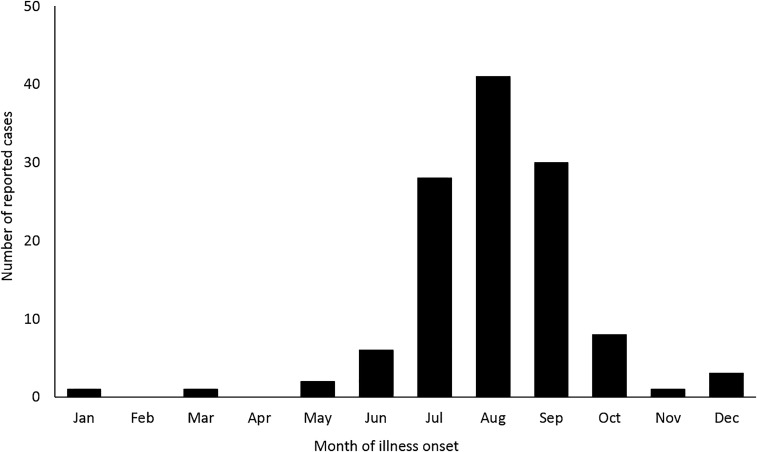
A total of 121 human cases of EEEV disease were reported from 74 counties in 20 states (Figure 1); of those, 119 (98%) were classified as neuroinvasive disease and 2 (2%) as non-neuroinvasive disease. Although there was one EEEV case reported in a Montana resident and one reported in a Missouri resident, these persons acquired their infection during travel to endemic areas. Of the 119 neuroinvasive disease cases, 110 (92%) were classified as encephalitis or meningoencephalitis (Table). A median of eight cases were reported annually (range 4–21 cases per year). Although cases occurred throughout the year, 99 (82%) case patients had illness onset during July through September (Figure 3). Eighty-five (70%) case patients were male. The median age was 52 years (IQR 13–68 years), but the majority of case patients were aged < 20 years (N = 36, 30%) or ≥ 60 years (N = 46, 38%). Overall, 118 (98%) case patients were hospitalized and 50 (41%) were fatal. Fatal case patients were significantly older than nonfatal case patients, with a median age of 63 (IQR 40–78 years), compared with a median of 45 (IQR 11–60 years) (P = 0.01). The case fatality ratio was highest among case patients aged ≥ 70 years (21 of 28; 75%); the case fatality ratio for case patients aged < 70 years was 31% (29 of 93).
Table 1.
Characteristics of reported Eastern equine encephalitis virus disease cases, United States, 2003–2016
| N = 121 | ||
|---|---|---|
| No. | (%) | |
| Male | 85 | (70) |
| Age group | ||
| < 1 years | 7 | (6) |
| 1–19 years | 29 | (24) |
| 20–39 years | 9 | (7) |
| 40–59 years | 30 | (25) |
| 60–79 years | 36 | (30) |
| ≥ 80 years | 10 | (8) |
| Clinical syndrome | ||
| Encephalitis or meningoencephalitis | 110 | (91) |
| Meningitis | 6 | (5) |
| Other neuroinvasive presentation | 3 | (2) |
| Non-neuroinvasive | 2 | (2) |
| Hospitalization | 118 | (98) |
| Fatality | 50 | (41) |
Figure 3.
Eastern equine encephalitis virus disease cases reported to Centers for Disease Control and Prevention by month of illness onset, United States, 2003–2016.
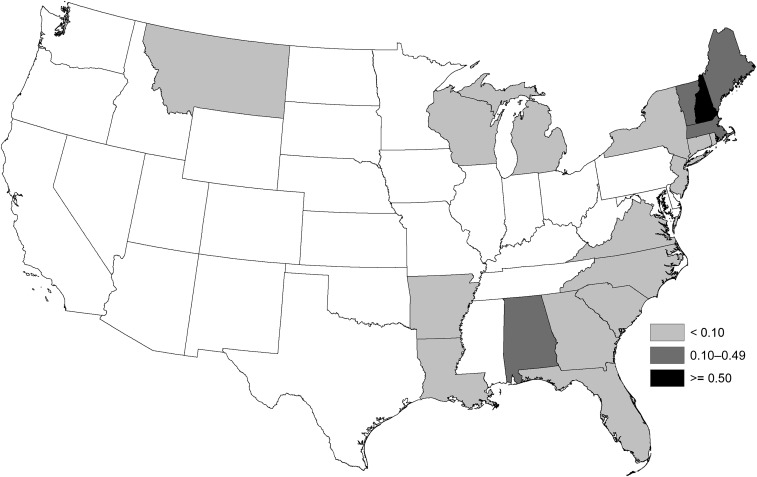
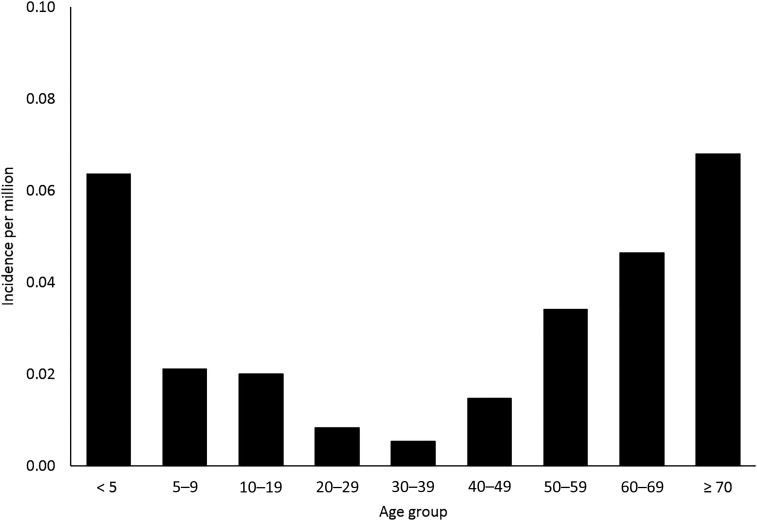
Eastern equine encephalitis virus neuroinvasive disease cases were reported from 20 states. Three states reported approximately half (52%) of the cases: Massachusetts (24 cases), Florida (18), and New Hampshire (13). The national average annual incidence of EEEV neuroinvasive disease was 0.03 cases per million population (annual range 0.01–0.07 per million). States with the highest average annual incidence included New Hampshire (0.82 per million population), Massachusetts (0.31), Vermont (0.27), Maine (0.13), and Alabama (0.12) (Figure 4). The average annual incidence of EEEV neuroinvasive disease was two times higher among males (0.04 per million) than among females (0.02 per million). Incidence was highest among persons aged < 5 and > 60 years of age and lowest among those aged 20–39 years (Figure 5).
Figure 4.
Average annual incidence of Eastern equine encephalitis virus neuroinvasive disease reported to Centers for Disease Control and Prevention per million population by state (human disease cases reported by state and county of residence), United States, 2003–2016.
Figure 5.
Average annual incidence of Eastern equine encephalitis virus neuroinvasive disease reported to Centers for Disease Control and Prevention per million population by age group, United States, 2003–2016.
DISCUSSION
Although EEEV disease continues to be a rare human disease in the United States, it can have considerable health and economic consequences. In this surveillance data set, the case fatality rate was 41%. Previous studies have estimated that long-term neurologic sequelae occur in an estimated 50% of patients and, in one study, were projected to result in lifetime expenses totaling nearly 3 million dollars per patient.18
We report very similar findings to those presented in a summary of EEEV neurologic disease in the United States from 1999 to 2007, which includes 55 cases reported in this summary.15 The common findings include an average annual incidence of 0.03 per million population, a 2-fold higher incidence among males, and a bi-modal age pattern. The relatively high percentage of EEEV disease cases among infants is a finding that has been previously reported and is notably different than trends seen in other arboviral diseases.8,19,20 The primary difference between the two reports is the increased geographic range of human disease cases; since 2007, human cases have been reported in four additional states (Arkansas, Connecticut, Maine, and Vermont).21–24
The nonhuman surveillance data reported to ArboNET indicate that the geographic range of the virus is much greater than human data alone might suggest. It is unclear why human disease has not been identified in these other areas, but it is likely the ecologic conditions necessary for EEEV to escape the enzootic cycle and enter the epizootic cycle are not present in all locations. The lack of human disease cases in some areas with reported nonhuman activity could be related to diagnostic testing and surveillance biases. If providers are not aware of virus circulation in their area, they may not consider EEEV infection in their differential diagnosis.
This report is subject to a few limitations. ArboNET is a passive surveillance system. To be reported as a disease case, a patient must seek care, a clinician must request appropriate diagnostic tests, and health-care providers and laboratories must then report cases to public health authorities. More than 90% of cases reported to national surveillance had neurologic disease presentations. Patients with less severe disease might not seek care or health-care providers may not consider the diagnosis. Therefore, cases reported to ArboNET likely underestimate the true incidence of disease, overestimate the proportion of cases that are neuroinvasive and fatal, and might not be representative of all human disease. ArboNET does not require information about clinical symptoms or laboratory findings; therefore, it is not possible to confirm that reported cases meet the national case definition. Finally, nonhuman surveillance data are reported voluntarily and, therefore, may be more subject to variability in reporting between jurisdictions.
In areas at risk for EEEV transmission, health-care providers should consider EEEV infection in the differential diagnosis of cases of aseptic meningitis and encephalitis and obtain appropriate specimens for laboratory testing. Providers are encouraged to report suspected infections to their state or local health department to facilitate diagnosis and to mitigate the risk of further transmission. Because human vaccines against EEEV are not available, prevention depends on community and household efforts to reduce vector populations (e.g., applying insecticides and reducing breeding sites) and personal protective measures to decrease exposure to mosquitoes (e.g., use of repellents and wearing protective clothing).
Acknowledgment:
We thank the staff at state and local health departments that report data to the ArboNET system.
REFERENCES
- 1.Calisher CH, 1994. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev 7: 89–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris CD, 1988. Eastern equine encephalitis. Monath TP, ed. The Arboviruses: Epidemiology and Ecology, Vol. 3. Boca Raton, FL: CRC Press, 1–20. [Google Scholar]
- 3.Mukherjee S, Moody EE, Lewokzco K, Huddleston DB, Huang J, Rowland ME, Wilson R, Dunn JR, Jones TF, Moncayo AC, 2012. Eastern equine encephalitis in Tennessee: 2002–2008. J Med Entomol 49: 731–738. [DOI] [PubMed] [Google Scholar]
- 4.Cupp EW, Klingler K, Hassan HK, Viguers LM, Unnasch TR, 2003. Transmission of eastern equine encephalomyelitis virus in central Alabama. Am J Trop Med Hyg 68: 495–500. [PMC free article] [PubMed] [Google Scholar]
- 5.Molaei G, Armstrong PM, Abadam CF, Akaratovic KI, Kiser JP, Andreadis TG, 2015. Vector-host interactions of Culiseta melanura in a focus of eastern equine encephalitis virus activity in southeastern Virginia. PLoS One 10: e0136743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingham AM, Burkett-Cadena ND, Hassan HK, Unnasch TR, 2016. Vector competence and capacity of Culex erraticus (Diptera: Culicidae) for eastern equine encephalitis virus in the southeastern United States. J Med Entomol 53: 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldfield M, Welsh JN, Taylor BF, 1968. The 1959 outbreak of eastern encephalitis in New Jersey. The inapparent infection:disease ratio. Am J Epidemiol 87: 32–38. [DOI] [PubMed] [Google Scholar]
- 8.Feemster RF, 1938. Outbreak of encephalitis in man due to the eastern virus of equine encephalomyelitis. Am J Public Health Nations Health 28: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Przelomski MM, O’Rourke E, Grady GF, Berardi VP, Markley HG, 1988. Eastern equine encephalitis in Massachusetts: a report of 16 cases, 1970–1984. Neurology 38: 736–739. [DOI] [PubMed] [Google Scholar]
- 10.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA, 1997. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 336: 1867–1874. [DOI] [PubMed] [Google Scholar]
- 11.Goldfield M, Sussman O, 1968. The 1959 outbreak of eastern encephalitis in New Jersey. I. Introduction and description of outbreak. Am J Epidemiol 87: 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Ayres JC, Feemster RF, 1949. The sequelae of eastern equine encephalomyelitis. N Engl J Med 240: 960–962. [DOI] [PubMed] [Google Scholar]
- 13.Gaensbauer JT, Lindsey NP, Messacar K, Staples JE, Fischer M, 2014. Neuroinvasive arboviral disease in the United States: 2003 to 2012. Pediatrics 134: e642–e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC , 2006. Eastern equine encephalitis–New Hampshire and Massachusetts, August–September 2005. MMWR 55: 697–700. [PubMed] [Google Scholar]
- 15.Reimann CA, Hayes EB, DiGuiseppi C, Hoffman R, Lehman JA, Lindsey NP, Campbell GL, Fischer M, 2008. Epidemiology of neuroinvasive arboviral disease–United States, 1999–2007. Am J Trop Med Hyg 79: 974–979. [PubMed] [Google Scholar]
- 16.CDC , 2015. Arboviral Diseases, Neuroinvasive and Non-Neuroinvasive: 2015 Case Definition Available at: http://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/. Accessed October 23, 2015.
- 17.US Census Bureau , 2010. Population Estimates: Intercensal Estimates 2000–2010 Available at: https://www.census.gov/programs-surveys/popest/data/tables.html. Accessed October 23, 2015.
- 18.Villari P, Spielman A, Komar N, McDowell M, Timperi RJ, 1995. The economic burden imposed by a residual case of eastern encephalitis. Am J Trop Med Hyg 52: 8–13. [DOI] [PubMed] [Google Scholar]
- 19.Farber S, Hill A, Connerly ML, Dingle JH, 1940. Encephalitis in infants and children caused by the virus of the eastern variety of equine encephalitis. JAMA 114: 1725–1731. [Google Scholar]
- 20.Letson GW, Bailey RE, Pearson J, Tsai TF, 1993. Eastern equine encephalitis (EEE): a description of the 1989 outbreak, recent epidemiologic trends, and the association of rainfall with EEE occurrence. Am J Trop Med Hyg 49: 677–685. [DOI] [PubMed] [Google Scholar]
- 21.Gibney KB, Robinson S, Mutebi JP, Hoenig DE, Bernier BJ, Webber L, Lubelczyk C, Nett RJ, Fischer M, 2011. Eastern equine encephalitis: an emerging arboviral disease threat, Maine, 2009. Vector Borne Zoonotic Dis 11: 637–639. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey NP, Lehman JA, Staples JE, Fischer M, 2014. West Nile virus and other arboviral diseases–United States, 2013. MMWR Morb Mortal Wkly Rep 63: 521–526. [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsey NP, Lehman JA, Staples JE, Fischer M, 2015. West Nile virus and other nationally notifiable arboviral diseases–United States, 2014. MMWR Morb Mortal Wkly Rep 64: 929–934. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong PM, Andreadis TG, 2013. Eastern equine encephalitis virus–old enemy, new threat. N Engl J Med 368: 1670–1673. [DOI] [PubMed] [Google Scholar]