Abstract.
A rapid, on-bead enzyme-linked immunosorbent assay for Plasmodium lactate dehydrogenase (pLDH) and Plasmodium falciparum histidine-rich protein 2 (HRP2) was adapted for use with dried blood spot (DBS) samples. This assay detected both biomarkers from a single DBS sample with only 45 minutes of total incubation time and detection limits of 600 ± 500 pM (pLDH) and 69 ± 30 pM (HRP2), corresponding to 150 and 24 parasites/μL, respectively. This sensitive and reproducible on-bead detection method was used to quantify pLDH and HRP2 in patient DBS samples from rural Zambia collected at multiple time points after treatment. Biomarker clearance patterns relative to parasite clearance were determined; pLDH clearance followed closely with parasite clearance, whereas most patients maintained detectable levels of HRP2 for 35–52 days after treatment. Furthermore, weak-to-moderate correlations between biomarker concentration and parasite densities were found for both biomarkers. This work demonstrates the utility of the developed assay for epidemiological study and surveillance of malaria.
INTRODUCTION
Quantitative laboratory measurement of malarial protein biomarkers helps define disease prevalence, distribution, and infection intensities. In the context of malaria elimination, sensitive detection methods are useful for determining response to interventions on a population level, ensuring that low-density infections are identified, and informing the development of improved point-of-care diagnostics. However, the logistics and biohazard risk of venous whole blood sample collection, preservation, and transportation from the field to the laboratory often pose challenges to large studies.
Many of these challenges are mitigated by the use of dried blood spot (DBS) cards for sample collection and preservation. In this sampling technique, which does not require specialized skills or equipment, microliter volumes of whole blood collected from a finger prick are spotted onto filter paper cards and allowed to dry at room temperature. These DBS samples are then easily stored or shipped, pose little biohazard risk, and result in improved biomarker stability compared with liquid samples.1,2 The DBS cards are often cost-effective compared with venous whole blood sample tubes and require no instrumentation to carry out the minimally invasive collection procedure.3
Because of these advantages, DBS sample cards have been used extensively in surveillance and epidemiological studies of malaria. For example, extraction and detection/sequencing of nucleic acid material from DBS have allowed for not only malaria detection in symptomatic and asymptomatic patients4–6 but also speciation,7 determination of parasite diversity,8 identification of drug-resistant strains,9,10 and evaluation of rapid diagnostic tests on a population level.11–13 Dried blood spots have also been used for the detection of antimalarial antibodies for serology-based epidemiological studies.14
Quantitation of malarial protein biomarkers from DBS samples, although less common than nucleic acid detection, is also relevant in the context of malaria elimination. Recently, two studies have measured Plasmodium falciparum histidine-rich protein 2 (HRP2), the primary protein biomarker used to diagnose malaria, in DBS patient samples. Rogier et al.15 used their DBS detection method to evaluate the accuracy of HRP2-based rapid diagnostic tests, and Gibson et al.16 demonstrated the persistence of HRP2 in patient DBS samples after treatment compared with microscopy. Although both of these studies demonstrated sensitive HRP2 quantitation from DBS, there are several disadvantages of using HRP2 alone as a diagnostic marker for malaria. First, HRP2 persists in host circulation for several weeks after parasite clearance, potentially resulting in false-positive results and unnecessary prescription of antimalarials.17 Second, HRP2 is only expressed by one of the five species of malaria known to infect humans. Third, infections with P. falciparum histidine-rich protein 2 (pfhrp2) gene deletions are increasing in prevalence and result in false-negative results on HRP2-only tests, posing a major challenge for case management.18
To address these disadvantages, we previously developed a highly sensitive, magnetic bead–based assay that simultaneously captured and sequentially detected Plasmodium lactate dehydrogenase (pLDH) and HRP2 from a single whole blood sample.19 Because pLDH is a metabolic enzyme required for parasite survival, it is present during infections from any of the five species of malaria known to infect humans.20,21 In addition, pLDH has been shown to clear within days after treatment.22 As such, this simultaneous capture and sequential detection (SCSD) assay not only distinguishes between falciparum and non-falciparum infections but also differentiates between active and resolved falciparum infections. In addition, the lower limit of detection of the SCSD assay, 2.0 parasites/μL for both pLDH and HRP2, was an order of magnitude improved over commercially available enzyme-linked immunosorbent assay (ELISA) kits and would allow for detection of individuals with asymptomatic or submicroscopic malaria infections.19
In this work, we adapt the previously developed SCSD assay for pLDH and HRP2 to detect these biomarkers from DBS and apply it to patient samples from rural Zambia. In particular, the clearance patterns of both biomarkers relative to parasite clearance are investigated. The high sensitivity of this assay is ideal for DBS sample analysis because these samples consist of just a few microliters of whole blood diluted into extraction buffer. In addition, the total protocol requires only 45 minutes of total incubation time for quantitation of both biomarkers, increasing the throughput and information yield per sample.
MATERIALS AND METHODS
Materials.
Human whole blood (K3 EDTA) was purchased from BioreclamationIVT (Hicksville, NY) (catalog no. HMWBEDTA3). Recombinant HRP2 protein was generously provided by PATH (Seattle, WA). Recombinant P. falciparum lactate dehydrogenase (rcPfLDH) was purchased from CTK Biotech (San Diego, CA) (Catalog no. A3005). Plasmodium falciparum D6 strain was cultured in the laboratory (stock concentration 18,450 parasites/μL or 43,600 parasites/μL). Anti-HRP2 antibodies were purchased from Abcam (Cambridge, United Kingdom) (ab9203, ab9206, and ab30384). Anti-pLDH antibodies were purchased from Vista Diagnostics (Kirkland, WA) (19g7 and 1201). Vista 1201 was conjugated to alkaline phosphatase (1201:AP) using Abcam ab102850 and to horseradish peroxidase (1201:HRPx) using Thermo no. 31489. BluePhos® microwell phosphatase substrate was purchased from KPL (Gaithersburg, MD) (No. 50-88-02), and TMB One was purchased from Promega (Madison, WI) (G7431). Dynabeads® MyOne™ streptavidin T1 beads were purchased from Life Technologies (Carlsbad, CA) (no. 65601). Immulon 2HB ELISA plates (14-245-61) were purchased from Fisher Scientific (Pittsburgh, PA). 903 Protein saver cards were purchased from GE Healthcare Life Sciences (Marlborough, MA) (10534612). Six-millimeter biopunches were acquired from Ted Pella Inc. (Redding, CA) (catalog no. 15111-60). All other reagents were purchased from either Fisher Scientific or Sigma Aldrich (St. Louis, MO). Dried blood spot extraction was performed with a Fisher Scientific analog vortex mixer (02-215-365). Absorbance measurements were collected on a Biotek Synergy H4 microplate reader (Vanderbilt University) or Biotek ELx808 microplate reader (Macha Research Trust).
Standardization of D6 P. falciparum culture.
Two stocks of in-house D6 P. falciparum culture (18,450 parasites/μL and 43,600 parasites/μL) were used in this study. The pLDH and HRP2 concentrations in the 18,450 parasite/μL stock were previously reported as 1.3 and 1.7 pM per parasite/μL, respectively.16,19 In addition, HRP2 in the 43,600 parasites/μL stock was previously determined to be 2.2 pM per parasite/μL.23 The pLDH concentration of in-house D6 P. falciparum culture (stock 43,600 parasites/μL) was determined to be 4.4 pM per parasite/μL using a standard well-plate ELISA, N = 6 (Supplemental Text 1).
Dried blood spot preparation and extraction.
Dried blood spot extraction was adapted from a previously reported method.16 Dried blood spot was prepared by depositing 10 μL of parasitized whole blood onto Whatman 903 protein saver cards. The spots were allowed to air-dry for a minimum of 4 hours, removed using a 6-mm biopsy punch, and placed in 2-mL microcentrifuge tubes (one spot per tube). Next, 200 μL of phosphate-buffered saline (PBS) with 0.1% Tween-20 (PBST) was added to each tube. The tubes were vortexed at 3,200 rpm for 10 minutes and then placed in a mini-centrifuge for 30–60 seconds. The supernatant was removed and saved for analysis.
Bead preparation.
Anti-pLDH and anti-HRP2 beads were prepared as reported previously.19,24 Briefly, anti-pLDH (Vista 19g7) or anti-HRP2 (Abcam ab9203) was biotinylated with EZ-Link NHS–PEG4–Biotin, No-Weigh Format (Thermo no. 21329). Unreacted NHS–PEG4–Biotin was removed using 7K MWCO Zebra spin desalting columns (Thermo no. 89882). Next, 5 mg of Dynabeads MyOne streptavidin T1 was washed three times with PBS and incubated with 500 μL of 0.4 mg/mL biotinylated antibody in PBS for 30 minutes. The beads were then washed before incubating in excess D-biotin in PBS for 30 minutes. Finally, the beads were washed and resuspended in 500 μL of PBS with 0.01% Tween-20. Stock solutions of antibody-functionalized beads were transported to Zambia in ambient conditions and stored at 4°C on arrival.
Simultaneous capture and sequential detection ELISA with DBS extracts.
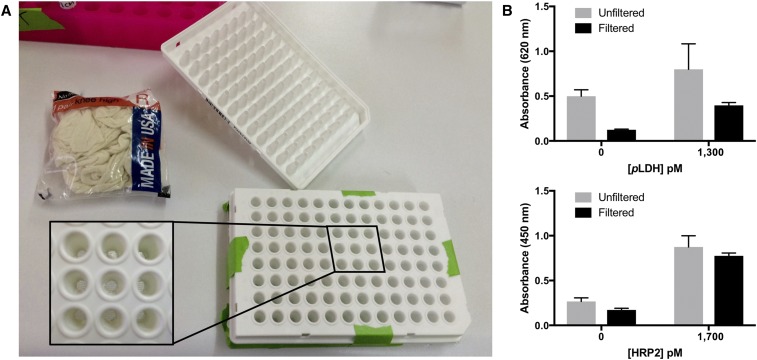
The SCSD ELISA on DBS extracts was adapted from the previously reported method.19 To avoid bead aggregation, a low-resource–filtering method was devised for removing small fibers and paper pieces from DBS extracts (Figure 1). Nylon fabric (Walmart, Bentonville, AK, No Nonsense Knee Highs) was cut to the appropriate size and taped onto a Fisherbrand flat-bottom PS 96-well plate (no. 12565501). A polymerase chain reaction (PCR) plate with the bottoms of the wells removed was then taped on top of the flat-bottom plate such that the nylon fabric was taut across the bottom of each well, forming a nylon filter between the wells of the flat-bottom and PCR plates. Next, 100 μL of DBS extract was pipetted through the nylon fabric filter into the flat-bottom plate. The nylon fabric and PCR plate were removed, and 100 μL of 10% nonfat dried milk in PBST was added to each well, followed by 4 μL of HAMA blocking reagent (Fitzgerald 85R-1001), 10 μL of 19g7-conjugated magnetic beads, 5 μL of ab9203-conjugated magnetic beads, 1.57 μL of 1201:AP (1.27 mg/mL), and 2 μL of ab30384 (0.1 mg/mL). The plate was protected from light and incubated on an orbital shaker for 15 minutes. A MagWell™ magnetic separator was used to remove the supernatant and wash the beads twice, first with 200 μL of PBST followed by 100 μL of PBST. On the second wash, the beads were moved to new wells, and 100 μL of BluePhos microwell phosphatase substrate was added to each well. The plate was protected from light and incubated for 15 minutes. The absorbance of the supernatant was measured at 620 or 630 nm (pLDH detection). Next, the beads were washed three times (100 μL PBST) and moved to new wells on the last wash before being resuspended in 100 μL TMB One solution and incubated for 5 minutes. The supernatant was then removed and the reaction was quenched with 100 μL of 2 M H2SO4. Absorbance was measured at 450 nm for detection of HRP2.
Figure 1.
(A) Affordable 96-well plate filter for use in low-resource settings. (B) Filtering improved the performance of both the Plasmodium lactate dehydrogenase (pLDH) and Plasmodium falciparum histidine-rich protein 2 (HRP2) portions of the dried blood spot simultaneous capture and sequential detection ELISA. This figure appears in color at www.ajtmh.org.
Stability study.
Dried blood spots were prepared and stored in Ziploc bags containing the desiccant at room temperature (up to 8 days) and −20°C (up to 188 days). At varying time points, DBSs were removed from storage and analyzed using the SCSD ELISA for pLDH and HRP2.
Study setting.
Patient DBS samples were collected from the Nchelenge district of Zambia as part of a separate study on parasite clearance rates in children less than 5 years of age presenting with uncomplicated malaria at a local clinic. These de-identified samples were made available to the authors for assessment of pLDH and HRP2 clearance patterns relative to parasite clearance rates using the DBS SCSD ELISA.
Patient recruitment and ethics.
Children at the clinic who tested positive for malaria (SD Bioline Pf) were recruited for this study only if a parent or guardian provided written informed consent. The samples were collected under institutional review board approval TDRC/C4/09/2014 and after approval was granted by the Zambian National Health Research Authority (MH/101/17/6).
Patient samples.
Finger-prick blood samples were collected on protein saver 903 cards. At the time of collection, parasitemia was determined by thick smear microscopy; parasites were counted per 200 white blood cells (WBC) and parasite levels were determined using an estimate of 8,000 WBC/μL. Samples were collected between December 2014 and August 2015, stored at −20°C, and analyzed by SCSD ELISA in July 2016. Patients were enrolled in the study and received treatment with artemether–lumefantrine (Coartem®) after malaria diagnosis by SD Bioline Pf RDT and confirmation of infection by thick smear. Samples (DBS and thick smears) were then collected at 15 time points after treatment: 0, 6, 12, 18, 24, 30, 36, 42, and 48 hours as well as 3, 7, 14, 21, 28, and 35 days. Samples for all time points for 15 patients were analyzed in this study.
Patient DBS sample SCSD ELISA.
All patient samples were coded, and the assays were carried out blinded to microscopy results. Patient DBS samples were extracted and analyzed via the SCSD ELISA as described previously with the following exceptions: 1) the standard curve (0–400 parasites/μL from 18,450 parasites/μL stock: 0–520 pM pLDH, 0–680 pM HRP2) consisted of 1:19 (v:v) parasitized whole blood diluted in PBST, mimicking the matrix of DBS extract, and 2) if the signal for either pLDH or HRP2 was above the linear range of the assay, the DBS extract was reanalyzed at the appropriate dilution.
Data analysis.
Biomarker concentrations in DBS extracts were interpolated from best fits of linear standard curves. All error bars shown are the standard error of measurement. Limits of detection were calculated as the biomarker concentration at sblank + 3SDblank. Intra-assay variation (%CV) was determined as the average relative standard deviation of triplicate measurements on a single plate. Inter-assay variation (%CV) was determined by finding the standard deviation of all measurements at a given concentration on different days and dividing by the average absorbance measurement at that concentration. For analysis of clearance rates across all patients, biomarker concentrations were normalized to their highest value across all time points for each patient.
RESULTS AND DISCUSSION
Dried blood spot SCSD ELISA optimization.
The protocol for the DBS sample SCSD ELISA was optimized systematically. Optimum conditions for HRP2 recovery from DBS were previously reported.16 To determine whether this method achieved sufficient elution of pLDH, the recoveries of both biomarkers were compared across multiple extraction times in PBST. The pLDH extraction efficiencies were not significantly different from those of HRP2 across all DBS extraction incubation times. In addition, increasing time did not result in significant differences in recoveries for either biomarkers (Supplemental Figure 1).
Once the DBS samples were extracted, the eluents were filtered to reduce nonspecific signal due to bead aggregation around small fibers and pieces of paper. To accomplish this, we developed an affordable, homemade filtering device that could be used in low-resource settings (Figure 1A). The filter consisted of a 96-well PCR plate with the tips of the tubes cut off. Cheap, commercially available nylon fabric covered the open bottoms of the PCR plate, which nested directly into a flat-bottomed 96-well plate. The nylon fabric was discarded after all samples and standards were filtered into the flat-bottomed plate, and the PCR plate was washed in 10% bleach, followed by three washes with deionized water, and reused with fresh nylon fabric for filtering. The total cost of the filtering device was $0.10/sample, but recycling the PCR plate decreased filtering costs to as low as $0.012/sample. As shown in Figure 1B, filtering DBS extracts through this device reduced nonspecific background signal by 4-fold for pLDH and 1.5 times for HRP2, increasing the signal-to-noise ratio from 1.6 to 3.2 and 3.3 to 4.5, respectively. In addition, filtering the samples had the benefit of decreasing the variation between repeated measurements for the pLDH portion of the assay (F test, P = 0.02).
After filtration, the SCSD ELISA was performed on DBS extracts. The previously reported protocol was performed directly in lysed whole blood.19 Because DBS extracts are more dilute than lysed whole blood, blocking conditions for the assay had to be re-optimized. It was found that adding an equal volume of 10% nonfat dried milk to DBS extracts resulted in the highest signal-to-noise ratio (Supplemental Figure 2A). Magnetic bead volumes and detection antibody concentrations used in the SCSD ELISA for pLDH and HRP2 were screened in this new matrix, and it was found that the optimized conditions for these parameters were identical to those in the original protocol for both biomarkers (Supplemental Figure 2B and C).
Dried blood spot SCSD ELISA performance.
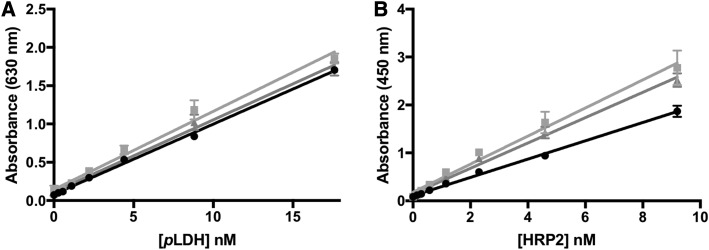
The performance of the DBS SCSD ELISA protocol was evaluated using DBS made from parasitized whole blood. The assay was performed in triplicate once per day for 3 days (Figure 2). The linear range of the assay was found to be 0.6–18 nM for pLDH and 0.15–9.5 nM for HRP2. The intra-assay variation was 10.5% for pLDH and 4.7% for HRP2. The inter-assay variation was 12.5% for pLDH and 16.9% for HRP2. All four %CV values demonstrate acceptable reproducibility. The limits of detection were 600 ± 500 pM pLDH and 69 ± 30 pM HRP2, corresponding to 150 and 24 parasites/μL in our in-house culture, respectively. It is important to note that these lower limits are reported as the biomarker concentrations in the original whole blood sample that was spotted onto the DBS card. Thus, the inherent dilution associated with DBS extraction and DBS extraction efficiency are taken into account. Although intended for laboratory use, the performance of the DBS SCSD ELISA was equal to or better than that of currently available malaria rapid diagnostic tests.
Figure 2.
Standard curves for dried blood spot on-bead simultaneous capture and sequential detection ELISA for (A) Plasmodium lactate dehydrogenase (pLDH) and (B) histidine-rich protein 2 (HRP2).
Biomarker detectability over time.
Dried blood spot cards are designed for long-term storage and preservation of biological samples. However, it has been shown that biomarker detectability from DBS changes over time.16,25 Thus, we measured pLDH and HRP2 signals from negative and positive (0 and 1,000 parasites/μL) DBS stored at both −20°C and room temperature over time. As shown in Supplemental Figure 3, neither pLDH nor HRP2 signal significantly changed after 6 months of storage at –20°C. However, for both biomarkers, recovery dramatically dropped over time when stored at room temperature. The pLDH signal at day 8 was reduced to 35% of the signal on day 0 and HRP2 signal was reduced to 31% in the same time period. This signal loss could be due to protein breakdown and loss of structure over time or to reduced extraction efficiency off the DBS card.
Patient DBS samples from rural Zambia.
Dried blood spot samples were collected over 15 time points after treatment of 15 patients; in total, 225 DBSs were analyzed for this study. Parasitemias at each time point were determined by microscopy at the time of collection. Because DBS patient samples were 1–2 years old when analyzed, it was not assumed that the extraction efficiency of these patient samples would be the same as that of freshly prepared DBS standards. Thus, rather than comparing patient DBS to a standard curve of freshly prepared DBS to determine biomarker concentrations in the original whole blood sample that was spotted onto the card, biomarker concentrations in extracts were determined. This was performed at Macha Research Trust using standard curves in parasitized whole blood diluted 1:19, approximating the DBS extract matrix. Several assays were performed each day over the course of 2 weeks (N = 14). The intra-assay variation was 9.2% for pLDH and 6.1% for HRP2 and the inter-assay variation was 19.2% and 24.5% for pLDH and HRP2, respectively. Linear ranges for the assay were 10–520 pM pLDH and 10–680 pM HRP2. The limit of detection for the pLDH portion of the assay was 9 ± 6 pM, corresponding to six parasites/μL in our in-house culture. The detection limit for HRP2 was 7 ± 6 pM, which corresponds to four parasites/μL. These detection limits were used as cutoff values for determination of positive patient samples.
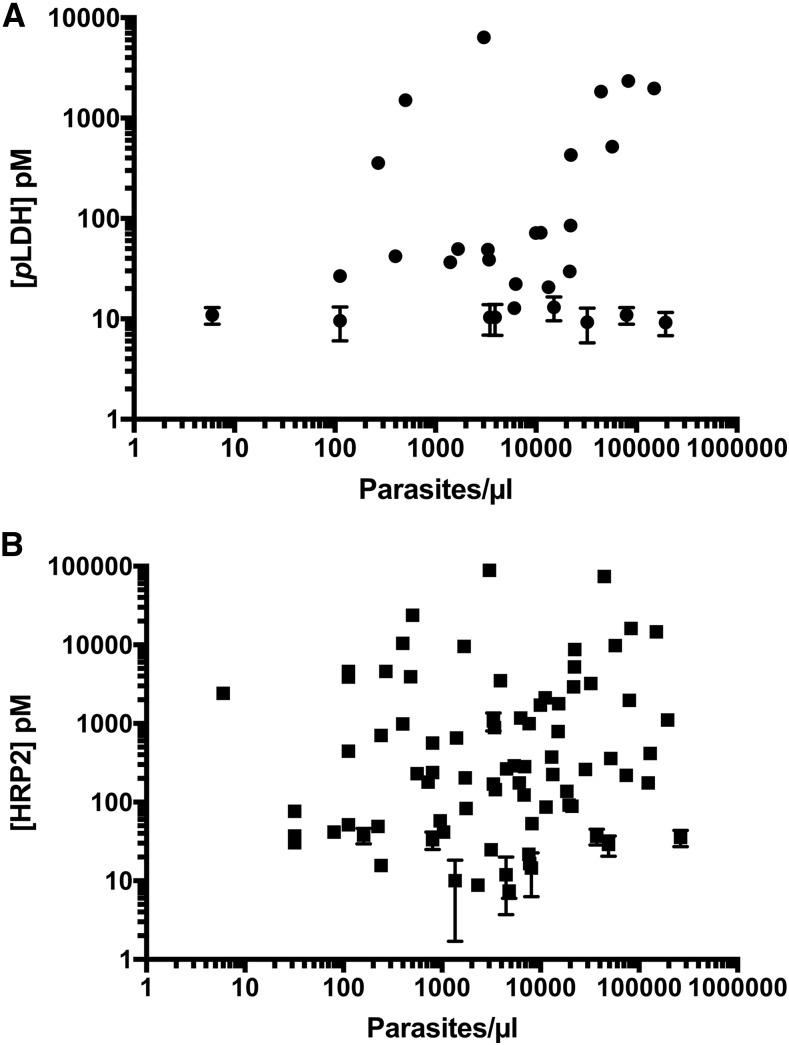
The relationships between biomarker concentrations and parasite levels for both pLDH and HRP2 based on all DBS patient samples analyzed in this study are shown in Figure 3. Similar to previous reports, HRP2 concentrations were several orders of magnitude higher than pLDH concentrations.26 Weak-to-moderate, but significant (P < 0.001), positive correlations with parasitemia were observed for both biomarkers. The Spearman correlation coefficient was 0.36 (0.23–0.47) for pLDH and parasitemia and 0.46 (0.36–0.57) for HRP2 and parasitemia, demonstrating the utility of these biomarkers for malaria diagnosis. The nonparametric Spearman correlation coefficient was chosen because the parasite densities and concentrations measured do not follow normal distributions (D’Agostino and Pearson normality tests, P < 0.001). For pLDH, direct correlations with parasitemia have been demonstrated in the literature for both P. falciparum and Plasmodium vivax malaria.26–28 The strength of the correlation found in this study is lower than that in some reports, possibly because of the lack of controlled DBS storage conditions after sample collection. Previous reports have shown that uncontrolled DBS storage can lead to reduced pLDH recovery in mock patient samples.25 Similarly, we found that DBS storage at room temperature resulted in a drastic reduction of pLDH detectability, potentially explaining why DBS from seven patients with high initial parasitemia had initial pLDH levels near or below the detection limit of the DBS SCSD ELISA. However, biological factors, such as parasite life cycle stage, also affect pLDH expression, potentially influencing the strength of the observed correlation.29
Figure 3.
Correlations between (A) Plasmodium lactate dehydrogenase (pLDH) and parasitemia and (B) histidine-rich protein 2 (HRP2) and parasitemia in patient samples from rural Zambia. Weak correlations between biomarker concentrations and parasite burdens were observed. Note: for many data points, error bars are smaller than the size of the symbol representing the mean value.
Many studies have shown correlation between HRP2 and parasitemia, although some have found no correlation.16,26,30 While uncontrolled storage conditions have a similar detrimental impact on HRP2 detectability as for pLDH, initial HRP2 concentrations were detectable for all patients in this study. However, HRP2 expression has been shown to vary with parasite stage and strain.31,32 In addition, the duration of infection and persistence of HRP2 in circulation, addressed in detail in the next section, likely influenced the strength of the correlation between biomarker concentration and parasite density.
Biomarker clearance.
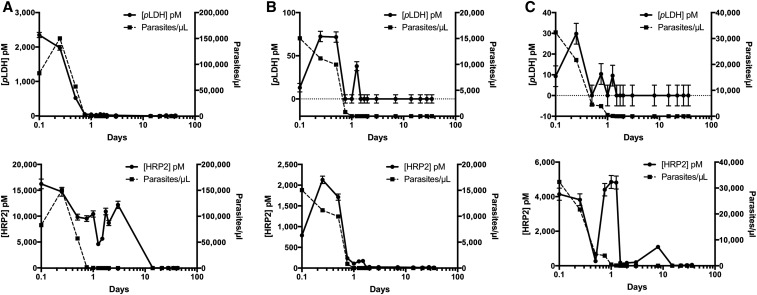
Unique parasite and biomarker clearance patterns were observed for each patient. Figure 4 shows clearance rates for three representative patients and Supplemental Figure 4 shows clearance rates for the remaining 12 patients. Overall, for the 15 patients in this study, the median parasite clearance time by microscopy after treatment with artemether–lumefantrine was 30 hours (interquartile range: 24–36 hours). Clearance times for the biomarkers were determined as the first time point in which the biomarker was undetectable for that time point and all subsequent time point measurements. The median pLDH clearance time was 36 hours (interquartile range: 6–72 hours) after treatment, following closely with parasite clearance time. In contrast, 13 of the 15 patients (87%) had measurable HRP2 levels at the final time point of this study (35–52 days after treatment). It should be noted that five of the 13 patients (38%) who were HRP2 positive at the last time point had undetectable HRP2 levels at least once at a previous time point. It is possible that uncontrolled storage conditions may have contributed to the undetectable HRP2 levels in the earlier time points. However, for one patient, pLDH levels also increased at the final time point, indicating possible reinfection or recrudescence (Supplemental Table 1, Patient 30).
Figure 4.
Biomarker clearance trends for three representative patients collected over 35 days. Plasmodium lactate dehydrogenase (pLDH) (top, solid) and histidine-rich protein 2 (HRP2) (bottom, solid) in dried blood spot extract and parasitemia (dashed) are plotted against time for (A) Patient 29, (B) Patient 55, and (C) Patient 58.
The relationship between intensity of infection and biomarker persistence was also investigated. Patient infection levels were classified based on initial parasitemias: low (0–14,999 parasites/μL, N = 7), medium (15,000–74,999 parasites/μL, N = 5), and high (≥ 75,000 parasites/μL, N = 3). Using a one-way ANOVA (df = 12), there were no significant differences in parasite clearance time (P = 0.4221), pLDH clearance time (P = 0.5543), or HRP2 clearance time (P = 0.3206) across all three groups.
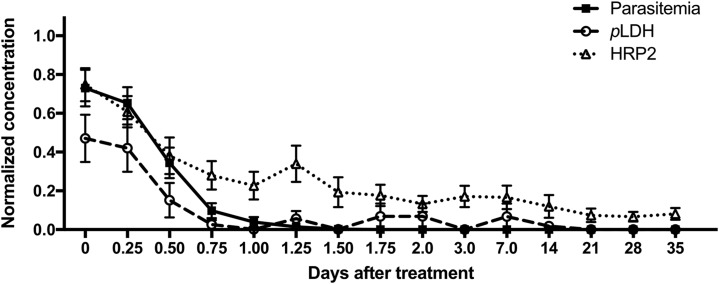
The overall clearance patterns for all patients are represented in Figure 5. For each patient, parasite and biomarker levels were normalized to the highest concentration measured. The average across all patients at each time point was calculated and plotted, allowing for a clear visualization of the overall parasite, pLDH, and HRP2 clearance patterns in this study. In general, pLDH became undetectable before infections became submicroscopic. In contrast, HRP2 remained in circulation for the duration of the study, decreasing in concentration slowly over time. The persistence and accumulation of HRP2 in circulation over the duration of an infection likely explain why HRP2 concentrations were significantly higher than pLDH concentrations for all patients in this study.
Figure 5.
Normalized parasite, Plasmodium lactate dehydrogenase (pLDH), and histidine-rich protein 2 (HRP2) clearance patterns plotted over time.
In the context of malaria elimination, an ideal evaluation tool would be positive when a patient has an active infection and negative in the absence of parasites. Distinguishing between falciparum and non-falciparum infections would also be clinically useful and inform treatment and provide useful epidemiological data. A sensitive dual pLDH and HRP2 detection method could fulfill these ideals; however, this work highlights the challenges of developing a dual assay. Although pLDH detection could overcome the lack of specificity of HRP2 that results from persistence in host circulation after parasite clearance, the relatively low levels of circulating pLDH mean that active infections could be missed in the dual format. Such a result would undermine malaria elimination efforts, allowing active infections to persist and contribute to transmission. In contrast, an HRP2-only diagnostic could result in overdiagnosis and treatment, potentially resulting in unnecessary costs and a failure to treat other serious illnesses. Thus, there is a pressing need to develop more sensitive molecular recognition elements and detection methods for pLDH.
The previously developed SCSD ELISA fills this need and is an order of magnitude more sensitive than commercially available ELISA kits for both pLDH and HRP2.19 Here, the assay was adapted for detection of both biomarkers from a single DBS. Uncontrolled DBS storage conditions and the inherent dilution when DBSs are extracted into buffer contributed to reduced analytical sensitivity of the assay, although detection limits of the DBS SCSD ELISA remain comparable to that of commercially available ELISA kits for pLDH and HRP2 applied to whole blood samples. In contrast to commercially available ELISA kits, which are singleplex and take 3–6 hours to complete, the SCSD ELISA is capable of detecting two biomarkers from a single DBS in less than 1 hour. In addition, the ease with which the antibody-functionalized beads were transported to Zambia and the possibility of lyophilizing the beads for stable ambient storage could allow the developed assay to be applied in laboratories in low-resource settings. Thus, the DBS SCSD ELISA has the potential to increase the throughput and information yield of large epidemiological or surveillance studies based on DBS samples. To this end, we have demonstrated the utility of this assay for the evaluation of biomarker clearance in a patient population from rural Zambia. In the future, the DBS SCSD ELISA will be useful for the characterization of clearance patterns in other populations and could also serve as a rapid, preliminary screening tool for parasites with pfhrp2 deletions.
CONCLUSION
In this work, the on-bead SCSD ELISA for pLDH and HRP2 was adapted for use with DBS samples. For mock DBS samples, the assay was highly reproducible and could detect pLDH as low as 600 ± 500 pM and HRP2 as low as 69 ± 30 pM, corresponding to 150 and 24 parasites/μL in our in-house culture, respectively. Using the DBS SCSD ELISA, we demonstrated the need for controlled DBS storage; the detectability of both pLDH and HRP2 from DBS decreased nearly 70% after 8 days of storage at room temperature. Next, we applied the DBS SCSD ELISA to patient DBS samples from rural Zambia to measure pLDH and HRP2. In these samples, weak-to-moderate correlations between biomarker concentration and parasite density were found for both biomarkers, and the overall concentrations of HRP2 were several orders of magnitude higher than those of pLDH. Finally, biomarker clearance patterns relative to parasite clearance were studied. It was found that pLDH clearance followed closely with parasite clearance, whereas 87% of patients had detectable levels of HRP2 for 35–52 days after treatment. This work demonstrated the utility of the SCSD ELISA for quantifying pLDH and HRP2 from DBS samples and its potential for future application in epidemiological studies.
Supplementary Material
Supplemental text, figures and table
Acknowledgments:
First and foremost, we would like to thank the children and guardians who participated in this study. We also thank Macha Research Trust for collecting and providing the dried blood spot patient samples and for the use of their on-site laboratories. We thank Kim League for culturing the D6 Plasmodium falciparum stocks used in this work and M. F. Richards for his critical comments in the preparation of the manuscript.
Note: Supplemental text, figures and table appear at www.ajtmh.org.
REFERENCES
- 1.Demirev PA, 2013. Dried blood spots: analysis and applications. Anal Chem 85: 779–789. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Jaiswal S, Shukla M, Lal J, 2014. Dried blood spots: concepts, present status, and future perspectives in bioanalysis. Drug Test Anal 6: 399–414. [DOI] [PubMed] [Google Scholar]
- 3.Parker SP, Cubitt WD, 1999. The use of the dried blood spot sample in epidemiological studies. J Clin Pathol 52: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, et al. 2014. Comparison of microscopy, nested-PCR, and real-time-PCR assays using high-throughput screening of pooled samples for diagnosis of malaria in asymptomatic carriers from areas of endemicity in Myanmar. J Clin Microbiol 52: 1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh B, Cox-Singh J, Miller AO, Abdullah MS, Snounou G, Abdul Rahman H, 1996. Detection of malaria in Malaysia by nested polymerase chain reaction amplification of dried blood spots on filter papers. Trans R Soc Trop Med Hyg 90: 519–521. [DOI] [PubMed] [Google Scholar]
- 6.Hsiang MS, Lin M, Dokomajilar C, Kemere J, Pilcher CD, Dorsey G, Greenhouse B, 2010. PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children. J Clin Microbiol 48: 3539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor SM, Juliano JJ, Trottman PA, Griffin JB, Landis SH, Kitsa P, Tshefu AK, Meshnick SR, 2010. High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol 48: 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Searle KM, et al. 2017. Distinct parasite populations infect individuals identified through passive and active case detection in a region of declining malaria transmission in southern Zambia. Malar J 16: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil JP, Nogueira F, Strömberg-Nörklit J, Lindberg J, Carrolo M, Casimiro C, Lopes D, Arez AP, Cravo PV, Rosário VE, 2003. Detection of atovaquone and Malarone resistance conferring mutations in Plasmodium falciparum cytochrome b gene (cytb). Mol Cell Probes 17: 85–89. [DOI] [PubMed] [Google Scholar]
- 10.Pimentel S, Nogueira F, Benchimol C, Quinhentos V, Bom J, Varandas L, do Rosário V, Bernardino L, 2006. Detection of atovaquone-proguanil resistance conferring mutations in Plasmodium falciparum cytochrome b gene in Luanda, Angola. Malar J 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laban NM, Kobayashi T, Hamapumbu H, Sullivan D, Mharakurwa S, Thuma PE, Shiff CJ, Moss WJ, Southern Africa International Centers of Excellence for Malaria Research , 2015. Comparison of a PfHRP2-based rapid diagnostic test and PCR for malaria in a low prevalence setting in rural southern Zambia: implications for elimination. Malar J 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndao M, Bandyayera E, Kokoskin E, Gyorkos TW, MacLean JD, Ward BJ, 2004. Comparison of blood smear, antigen detection, and nested-PCR methods for screening refugees from regions where malaria is endemic after a malaria outbreak in Quebec, Canada. J Clin Microbiol 42: 2694–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozycki CT, Umulisa N, Rulisa S, Mwikarago EI, Musabyimana JP, Habimana JP, Karema C, Krogstad DJ, 2017. False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission. Malar J 16: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corran PH, et al. 2008. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J 7: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogier E, et al. 2017. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS One 12: e0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson LE, Markwalter CF, Kimmel DW, Mudenda L, Mbambara S, Thuma PE, Wright DW, 2017. Plasmodium falciparum HRP2 ELISA for analysis of dried blood spot samples in rural Zambia. Malar J 16: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyunt MH, Kyaw MP, Win KK, Myint KM, Nyunt KM, 2013. Field evaluation of HRP2 and pan pLDH-based immunochromatographic assay in therapeutic monitoring of uncomplicated falciparum malaria in Myanmar. Malar J 12: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Q, Gatton ML, Barnwell J, Chiodini P, McCarthy J, Bell D, Cunningham J, 2014. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J 13: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markwalter CF, Ricks KM, Bitting AL, Mudenda L, Wright DW, 2016. Simultaneous capture and sequential detection of two malarial biomarkers on magnetic microparticles. Talanta 161: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown WM, et al. 2004. Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of human malarial parasites. Biochemistry 43: 6219–6229. [DOI] [PubMed] [Google Scholar]
- 21.McCutchan TF, Piper RC, Makler MT, 2008. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis 14: 1750–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iqbal J, Siddique A, Jameel M, Hira PR, 2004. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J Clin Microbiol 42: 4237–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer WS, et al. 2017. Rapid concentration and elution of malarial antigen histidine-rich protein II using solid phase Zn(II) resin in a simple flow-through pipette tip format. Biomicrofluidics 11: 034115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markwalter CF, Davis KM, Wright DW, 2016. Immunomagnetic capture and colorimetric detection of malarial biomarker Plasmodium falciparum lactate dehydrogenase. Anal Biochem 493: 30–34. [DOI] [PubMed] [Google Scholar]
- 25.Versteeg I, Mens PF, 2009. Development of a stable positive control to be used for quality assurance of rapid diagnostic tests for malaria. Diagn Microbiol Infect Dis 64: 256–260. [DOI] [PubMed] [Google Scholar]
- 26.Martin SK, Rajasekariah G-H, Awinda G, Waitumbi J, Kifude C, 2009. Unified parasite lactate dehydrogenase and histidine-rich protein ELISA for quantification of Plasmodium falciparum. Am J Trop Med Hyg 80: 516–522. [PubMed] [Google Scholar]
- 27.Jang JW, Cho CH, Han ET, An SS, Lim CS, 2013. pLDH level of clinically isolated Plasmodium vivax and detection limit of pLDH based malaria rapid diagnostic test. Malar J 12: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piper R, Lebras J, Wentworth L, Hunt-Cooke A, Houzé S, Chiodini P, Makler M, 1999. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am J Trop Med Hyg 60: 109–118. [DOI] [PubMed] [Google Scholar]
- 29.Vivas L, Easton A, Kendrick H, Cameron A, Lavandera JL, Barros D, de las Heras FG, Brady RL, Croft SL, 2005. Plasmodium falciparum: stage specific effects of a selective inhibitor of lactate dehydrogenase. Exp Parasitol 111: 105–114. [DOI] [PubMed] [Google Scholar]
- 30.Rubach MP, et al. 2012. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian children. PLoS One 7: e35985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desakorn V, Dondorp AM, Silamut K, Pongtavornpinyo W, Sahassananda D, Chotivanich K, Pitisuttithum P, Smithyman AM, Day NP, White NJ, 2005. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans R Soc Trop Med Hyg 99: 517–524. [DOI] [PubMed] [Google Scholar]
- 32.Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, Luchavez J, Bell D, Cheng Q, 2005. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis 192: 870–877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental text, figures and table