Abstract
Background
The increasing incidence of oropharynx squamous cell cancer (OPSCC) is well established. However, up-to date incidence estimates and trends for head and neck squamous cell cancers (HNSCC) overall including major anatomic sites and non-oropharyngeal (non-OP) HNSCCs by sex, race and age in the United States (US) are not well described.
Methods
Retrospective analysis of incident HNSCCs during 1992-2014 using Surveillance, Epidemiology, and End Results (SEER) database was used to evaluate incidence of HNSCCs overall, OPSCC and non-OP HNSCC (larynx, oral cavity, hypopharynx, nasopharynx, nasal cavity). Incidence rates were calculated overall and by subgroups of interest, and incidence rate ratios (IRR) were used to compare rates between groups. The incidence rates presented are per 100,000 people and age-adjusted to the 2000 US Standard Population (19 age groups – Census P25-1130). Annual percent change (APC) was modeled with and without joinpoints
Results
Incidence of HNSCC overall declined (average APC [aAPC] -0.8, p<0.001) in spite of significant increases in incidence of OPSCCs, most notably between 2000-2014 (APC=2.1, p<0.001). Indeed, significant declines in incidence were observed for all non-OP HNSCC sites, for both women and men (each p<0.001). Among women, risk of OPSCC also significantly decreased (aAPC -0.8, p=0.002), while among men OPSCC risk was stable during 1992-2001 (APC 0.4, p=0.42), then significantly increased during 2001-2014 (APC 2.7, p<0.001). Decreases in non-OP HNSCC risk were especially large for Black women (aAPC -2.6, p<0.001) and men (aAPC -3.0, p<0.001). While incidence of HNSCC used to be highest among Blacks, since 2009 HNSCC incidence is higher among Whites than Blacks.
Conclusions
Incidence of HNSCC is declining, especially for non-OP HNSCC and Blacks.
Introduction
A decade ago it became apparent that the epidemiology of head and neck squamous cell cancers (HNSCC) had changed.1 Although incidence of HNSCC was highest among Blacks, notable increases in incidence of oropharyngeal squamous cell cancer (OPSCC) were observed,1, 2 especially among younger age cohorts, men and Whites.1, 3,4 At the time an “epidemic” of HNSCC was described. Despite these observations, it is unknown whether incidence of HNSCC is commensurate with the projected epidemic and remains higher among Blacks than Whites.
Recent data support the need for renewed epidemiologic analysis of HNSCC. Incidence of OPSCC is increasing among older, not just younger individuals,5 and HPV is responsible for an increasing proportion of OPSCCs among women and non-Whites.6 The incidence of oral cavity SCC in younger women and more recent birth cohorts of White men and women also appears to be increasing,7–9 however the incidence of non-oropharyngeal HNSCC overall decreased between 1995-2005.10 There is no recent examination of incidence of the major non-oropharyngeal anatomic sites overall, by sex, or race. We therefore, performed a comprehensive investigation of HNSCC incidence trends using the most recently available United States registry data to understand the evolving epidemiology of HNSCC.
Methods
Data from Surveillance, Epidemiology, End Results (SEER) on incident diagnosis of HNSCC between 1992-2014 from all 13 U.S. registries were analyzed.11 Data was restricted to squamous cell cancer histology (ICD0-3 codes: 8050 to 8076, 8078, 8083, 8084, and 8094). Oropharyngeal tumor site included: ICD0-3 C01.9 [BOT], C02.4 [lingual tonsil], C09.0-C09.9 [tonsil], C10.0 [vallecula], C10.1 [anterior surface of epiglottis], C10.2-C10.9 [oropharynx], C14.2 [waldeyers ring], C14.0 [pharynx NOS], C05.1[soft palate], C05.2 [uvula]. Oral cavity tumor site included ICD0-3 C0.3-00.6, C0.9, C2.0-2.3, C2.8-3.1, C3.9-4.1, C4.8-5.0, C5.8-6.2, C6.8-6.9. Nasopharyngeal tumor site included ICDO-3 C11.0-C11.3, C11.8-C11.9. Nasal cavity ICDO-3 was C30.0. Hypopharyngeal tumor site included ICDO-3 C12.9-C13.2, C13.8-C13.9. Laryngeal tumor site included ICDO-3 C32.0-C32.3, C32.8-C32.9. Oral cavity, larynx, hypopharynx, nasal cavity and nasopharynx were grouped together as non-oropharyngeal (non-OP) for some analyses.
The distribution of OP and non-OP cases was compared across risk factors by Pearson chi square (SAS software, NC).12 Incidence rates were calculated overall and by subgroup, and incidence rate ratios (IRR) were used to compare the rates between groups. The incidence rates presented are per 100,000 people and age-adjusted to the 2000 US Standard Population (19 age groups – Census P25-1130).13 Annual percent change (APC) in incidence was modelled with and without joinpoints. Models which best represent the data were used. Average APC (aAPC) represents change in incidence between 1992-2014, while APC for specified shorter time periods is derived from joinpoint models. Race/ethnicity was categorized as White Non-Hispanic (White), Black Non-Hispanic (Black), Asian Non-Hispanic (Asian), Hispanic, Asian Pacific Islander (API) and American Indians/Alaskan Natives and Asians (AIAN).
Results
There were 98,856 incident HNSCC cases diagnosed between 1992-2014, including 30,792 OPSCC (31%) and 68,064 (69%) non-OP HNSCC. HNSCC patient characteristics are summarized in Supplementary Table 1. The majority were 50 years of age or older (87.0%), men (73.1%), White (73.4%) and ever married (77.4%). The most common anatomic site of HNSCC was the oropharynx (30,792 cases; 31.1%) followed by the oral cavity (28,797; 29.1%) and larynx (28,234 cases; 28.6%). Cancers of the nasal cavity, nasopharynx and hypopharynx were uncommon (range 1.3-5.2%). OPSCC patients were more likely than non-OP HNSCC to be young (<50 years of age: 15.0% vs 12.1%), men (78.7% vs. 70.7%), White (76.6% vs. 71.9%), and present with regional disease (66.6% vs. 41.9%), (p<0.001 for each; Supplementary Table 1).
The number of OPSCC cases tripled between 1992 and 2014. In the past 10 years alone, the number of OPSCCs increased 56%, from 5,964 cases in 2000-2004 to 9,291 cases in 2010-2014. The number of non-OP HNSCC cases also increased, but more modestly (by 11%), from 14,058 in 2000-2004 to 15,671 cases in 2010-2014. This was driven primarily by increases in nasal cavity (43% increase from 240 in 2000-2004 to 344 in 2010-2014), nasopharynx (32% increase from 628 in 2000-2004 to 829 in 2010-2014), and oral cavity (21% increase from 5,954 in 2000-2004 to 7,178 in 2010-2014) but also included decreases in larynx (0.5% decrease from 5,945 in 200-2004 to 5,915 in 2010-2014) and hypopharynx (0.4% decrease from 1,232 in 200-2004 to 1,227 in 2010-2014).
Incidence
The incidence rate of HNSCC overall during the study period was 11.2 per 100,000. Incidence of oropharynx, oral cavity, and larynx SCC were 3.4, 3.3, and 3.2 per 100,000, respectively. The incidence of other non-oropharyngeal HNSCC sites was less than 1.0 per 100,000 each. In Table 1, incidence rates are summarized by sex, age-groups and race/ethnicity. The rate of HNSCC overall was significantly higher among men than women (17.9 vs. 5.5 per 100,000, p<0.001), and at every anatomic site. Risk of OPSCC was 4-fold higher among men than women, while risk for non-oropharyngeal HNSCC was 3-fold higher for men than women. For non-oropharyngeal HNSCC the magnitude of the sex difference in risk was most notable for larynx and hypopharynx cancers (IRRs 4.5-5.1, p<0.001 for each) and attenuated for oral cavity, nasopharynx and nasal cavity (IRRs 1.8-2.7).
Table One.
Incidence rate (IR)£ and incidence rate ratio (IRR)Ω of oropharyngeal squamous cell cancer (OPSCC) and non-oropharyngeal head and neck squamous cell cancer per 100,000, by sex, age, and race from 1992–2014. Average APC (aAPC)¥ also shown.
| All HNSCC | OPSCC | Non-oropharyngeal HNSCC | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Incidence rate per 100,000 (95% CI) |
IRR | aAPC p-value |
Incidence rate per 100,000 (95% CI) |
IRR | aAPC, p-value |
Incidence rate per 100,000 (95% CI) |
IRR | aAPC, p-value |
|
|
| |||||||||
| Overall | 11.2 (11.1–11.3) | −0.8, p<.001 | 3.4 (3.4–3.5) | 1.2, p<.001 | 7.8 (7.7–7.8) | −1.6, p<.001 | |||
|
| |||||||||
| Sex | |||||||||
| Women | 5.5 (5.5–5.6) | Reference | −1.2, p<.001 | 1.4 (1.3–1.4) | Reference | −0.8, p=.002 | 4.1 (4.1–4.2) | Reference | −1.0, p=.003 |
| Men | 17.9 (17.8–18.0) | 3.2 | −0.8, p<.001 | 5.8 (5.7–5.9) | 4.2 | 1.7, p<.001 | 12.1 (12.0–12.2) | 2.9 | −2.0, p<.001 |
|
| |||||||||
| Age | |||||||||
| < 30 | 0.1(0.1–0.1) | 0.0 | 0.04, p=.95 | 0.0 (0.0–0.0) | 0.0 | - | 0.1 (0.1–0.1) | 0.0 | −0.1, p=.89 |
| 30–39 | 1.5 (1.4–1.5) | 0.1 | −0.7, p=.02 | 0.3 (0.3–0.4) | 0.0 | −0.3, p=.70 | 1.1 (1.1–1.2) | 0.1 | −0.8, p=.02 |
| 40–49 | 7.7 (7.6–7.9) | 0.3 | −1.0, p<.001 | 3.1 (3.0–3.1) | 0.3 | 0.5, p=.17 | 4.7 (4.6–4.8) | 0.3 | −2.0. p<.001 |
| 50–59 | 23.9 (23.6–24.2) | Reference | −0.6, p<.001 | 9.3 (9.1–9.5) | Reference | 2.0, p<.001 | 14.6 (14.3–14.8) | Reference | −2.0, p<.001 |
| 60–69 | 41.5 (41.1–42.0) | 1.7 | −1.1, p<.001 | 13.4 (13.1–13.6) | 1.4 | 1.5, p<.001 | 28.2 (27.8–28.6) | 1.9 | −2.4, p<.001 |
| 70–79 | 47.0 (46.3–47.6) | 2.0 | −1.0, p<.001 | 11.4 (11.1–11.7) | 1.2 | 1.4, p=.29 | 35.6 (35.0–36.1) | 2.4 | −1.9, p=.02 |
| ≥80 | 38.5 (37.8–39.2) | 1.6 | −0.02, p=.90 | 7.0 (6.7–7.3) | 0.7 | 0.7, p=.10 | 31.5 (30.9–32.2) | 2.2 | −0.2, p=.12 |
|
| |||||||||
| Race/ethnicity | |||||||||
| White NH* | 12.2 (12.1–12.3) | Reference | −0.2, p=.02 | 4.0 (3.9–4.0) | Reference | 2.0, p<.001 | 8.2 (8.2–8.3) | Reference | −1.4, p<.001 |
| Black NH | 14.3 (14.0–14.5) | 1.2 | −2.8, p<.001 | 4.4(4.3–4.6) | 1.1 | −1.8, p<.001 | 9.8 (9.6–10.1) | 1.2 | −3.3, p<.001 |
| Hispanic | 7.2 (7.0–7.4) | 0.6 | −0.9, p=.04 | 1.9 (1.8–2.0) | 0.5 | 1.0, p=.13 | 5.3 (5.1–5.4) | 0.6 | −1.5, p<.001 |
| Asian NH | 6.8 (6.6–6.9) | 0.6 | −1.3, p=.01 | 1.2 (1.2–1.3) | 0.3 | −0.3, p=.63 | 5.5 (5.4–5.7) | 0.7 | −1.1, p=.11 |
| AIAN NH | 8.6 (7.9–9.3) | 0.7 | 0.5, p=.24 | 2.4 (2.1–2.8) | 0.6 | - | 6.2 (5.6–6.8) | 0.7 | - |
|
| |||||||||
| Calendar Period | |||||||||
| 1992–1999 | 12.2 (12.1–12.3) | Reference | −1.6, p<.001 | 3.1 (3.0–3.2) | Reference | −0.3, p=.55 | 9.1 (9.0–9.2) | Reference | −2.0, p<.001 |
| 2000–2009 | 10.7 (10.6–10.8) | 0.9, p<.001 | −0.49, p=.24 | 3.4 (3.3–3.4) | 1.1 p<.001 | 2.3, p<.001 | 7.4 (7.3–7.4) | 0.8 p<.001 | −1.7, p<.001 |
| 2010–2014 | 10.8 (10.6–10.9) | 0.9, p<.001 | −0.1, p=.73 | 3.9 (3.8–4.0) | 1.3 p<.001 | 2.6, p=.02 | 6.9 (6.8–7.0) | 0.8 p<.001 | −1.6, p=.009 |
Non-Hispanic
An incidence rate (IR) is the risk of a specified diagnosis, calculated as the number of new diagnoses per 100,000 people in the specific sex, age or race group who do not previously have this diagnosis) during the time period of interest. For example, an IR of 10 signifies for each 100,000 people without HNSCC, 10 were diagnosed with HNSCC during the time period of 1992–2014. The IR in this table were age-adjusted to the 2000 US Standard Population (19 age groups – Census P25-1130), providing a population-based estimate for the rate of diagnosis.
Incidence rate ratios (IRR) compare risk between variables. For example, IRR of 3.2 for men indicates that the incidence rate was 3.2 times higher among men than women during the time period.
Average annual percent change (aAPC) provides an estimate of the change in the rate of diagnosis during that time period. A significant aAPC indicates an increase (>0) or decrease (<0) in incidence (risk) during the time period, while a non-significant aAPC indicates that incidence rates were stable during that time period.
Incidence of HNSCC overall increased with age, with ~2-fold higher risk among older age groups (60-69, 70-79 and 80+ compared to 50-59 year olds). This increase in risk among those aged 60-79 was more modest for OPSCC (IRRs 1.2-1.4; Table 1) than for non-oropharyngeal HNSCC (IRRs 1.9-2.4). As expected, risk of both OPSCC and non-oropharyngeal HNSCC was substantially lower for those under 50 years of age (Table 1).
When considering race, HNSCC risk was significantly higher among Blacks compared to Whites during 1992-2014 overall (IRR 1.2, p<0.001), but became higher among Whites than Blacks beginning in 2009. The risk of HNSCC was significantly lower among Hispanics and AIANs, compared to Whites (RRs 0.6-0.7, p<0.001 for each). Similar patterns by race were observed for OPSCC and non-oropharyngeal HNSCCs, and when examining incidence during the last decade.
Incidence trends
When considering changes in incidence during the study period, the rate of HNSCC overall declined (aAPC -0.8, p<0.001; Figure 1). This observed decrease was in spite of significant increases in the incidence of OPSCCs, most notably between 2000-2014 (APC=2.1, p<0.001). Significant declines were observed for each of the non-oropharyngeal sites (oral cavity, larynx, nasopharynx, hypopharynx, nasal cavity; Figure 1). For non-oropharyngeal HNSCCs a decrease was observed between 1992-2014 (aAPC of -1.6, (p<0.001) and faster decreases between 1996-2003 (APC= -3.1, p<0.001). Given the similarity in trends observed for each of these non-oropharyngeal sites, hereafter they will be referred to collectively as non-oropharyngeal HNSCC.
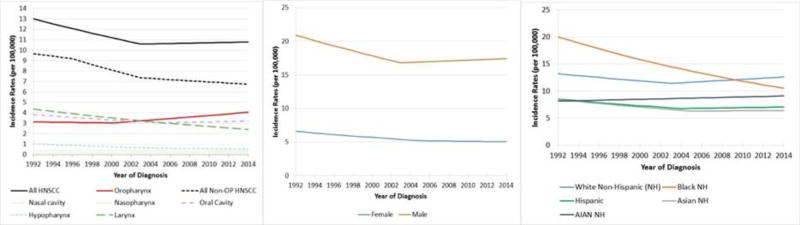
Figure 1. Age-adjusted Incidence Rates and Trends Over Time for HNSCC Overall, by Site (A), Sex (B) and Race (C), between 1992 and 2014.
Incidence rates are represented on the y-axis (per 100,000) by calendar years using selected joinpoint model 1992-2014. For all HNSCC, there was a decline from 1992 to 2003 (APC -1.8, p<0.001) and stable incidence from 2003–2014 (APC=0.2, p=0.148). For oropharynx, incidence rates were stable from 1992-2000 (APC -0.4, p=0.415), and increased from 2000-2014 (APC 2.1, p <0.001). For non-OP HNSCC, the incidence rates declined during 1992-1996 (APC -1.2, p=0.041), 1996-2003 (APC -3.1, p<.001) and 2003-2014 (APC -0.8, p<0.001). Larynx decreased from 1992-2014 (APC -2.7, p<0.001). Oral cavity decreased from 1992-2005 (APC -1.8, p<.001) and was stable from 2005-2014 (APC 0.6, p=0.104). Hypopharynx decreased from 1992-2006 (APC= -3.8, p<0.001) and was stable from 2006-2014 (APC-1.8, p=0.063). Nasal cavity was stable over the period 1992-2014 (APC 0.04, p=0.994). Nasopharynx was stable over the period 1992-2014 (APC -0.7, p=0.075). Incidence trends for HNSCC by sex and race are shown in panels B and C, respectively. Incidence rates are age-adjusted to the 2000 US Standard Population (19 age groups – Census P25-1130).
The significant declines in HNSCC incidence overall were observed for both women and men (p<0.001 for both; Figure 1). Risk of OPSCC, however, significantly decreased for women (average APC -0.8, p=0.002) while among men risk was stable from 1992 to 2001 (APC 0.4, p=0.42), then significantly increased between 2001 to 2014 (APC 2.7, p<0.001; Figure 2A). Risk of non-oropharyngeal HNSCC significantly decreased for both women and men in the study period, although at a faster rate for men (aAPC -2.0) than women (aAPC -1.0); p<0.001 for each; Figure 2B). For men, the rate of decline was more modest in more recent calendar period (2004 to 2014; APC -0.9, p<0.001) than that observed between 1992 and 2004 (APC -2.9, p<0.001).
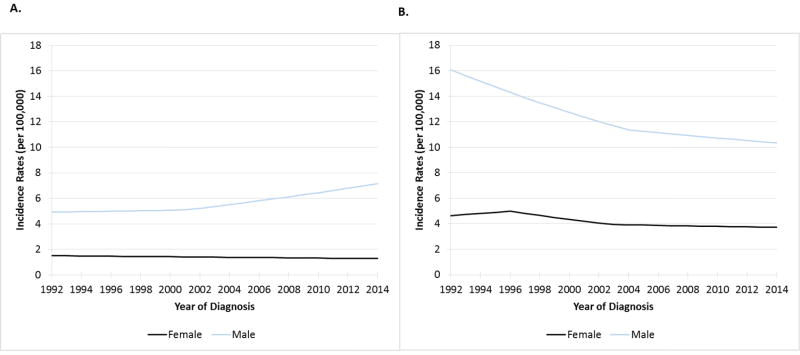
Figure 2. Age-adjusted Incidence rates and trends over time for oropharynx squamous cell cancer (Figure 2A) and non-oropharyngeal head and neck squamous cell cancer (non-OP HNSCC, Figure 2B), by sex.
Incidence rates for females and males are shown in black and blue lines, respectively. Incidence on y-axis (per 100,000) and year of diagnosis on x-axis. In Figure 2A, incidence of OPSCC during 1992-2001 was stable for men (p=0.419), but increased 2001-2014 (APC 2.7, p<0.001). Incidence of OPSCC declined significantly for women (APC -0.8, p=0.002). In Figure 2B, incidence of non-OP HNSCC declined significantly during 1992-2004 (APC -2.9, p<0.001) and 2004-2014 (APC -0.9, p<0.001) for men. Among women incidence of non-OP HNSCC was stable during 1992-1996 and 2003-2014 (p>0.10 for each), but decreased significantly 1996-2003 (APC -3.3, p<0.001). Incidence rates are age-adjusted to the 2000 US Standard Population (19 age groups – Census P25-1130).
To account for the observed differences by sex, subsequent analyses exploring incidence trends by age and race were sex-stratified. Between 1992 and 2014, OPSCC incidence in women was stable for those under 60 years, but decreased significantly among both 60-69 (average APC -1.6, p=0.02) and 70-79 year olds (average APC -1.1, p=0.005; Figure 3A). By contrast, there were significant increases in incidence of OPSCC among men aged 50-79 (average APCs 2.1-2.5; Figure 4A). Of note, for some ages the increases in incidence were more dramatic during specific time periods (e.g. 70-79 year old men had an APC of 4.8 between 1992-1998). For non-oropharyngeal HNSCC incidence decreased among all age groups of men and most age groups of women (except 30-39 year old aAPC=1.3, p=0.03) and non-significant declines were observed among 40-49 and 70+ year old women. This increase in 30-39 year old women was driven by increasing oral cavity incidence.
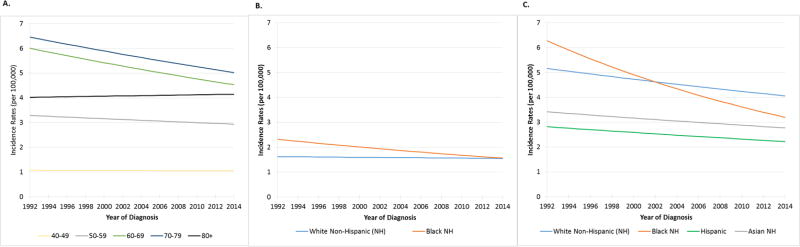
Figure 3. Age-adjusted Incidence rates among women for oropharynx squamous cell cancer by age (Figure 3A) and race/ethnicity (Figure 3B) and for non-oropharynx head and neck squamous cell cancer by race/ethnicity (Figure 3C).
Incidence on y-axis (per 100,000) and year of diagnosis on x-axis. In Figure 3A, in zero joinpoint model non-significant changes were seen for ages 40-49, 50-59, and 80+ (p>0.147 for each). Significant decreases in incidence were observed for OPSCC among 60-69 (APC -1.3, p=0.003) and 70-79 (APC -1.1, p=0.005). In Figure 3B. Selected joinpoint model incidence rates during 1992-2014 are shown for Whites in blue (p=0.4) and Blacks (APC-1.8, p=0.006) in orange. In Figure 3C, incidence for non-OP HNSCC is depicted using zero joinpoint model during 1992-2014. Significant declines in incidence are shown for Whites in blue (APC -1.0, p<0.001), Blacks in red (APC -3.0, p<0.001), Hispanics in green (APC -1.1, p=0.016) and Asians in grey (APC -0.9, p=0.025). Incidence rates are age-adjusted to the 2000 US Standard Population (19 age groups – Census P25-1130).
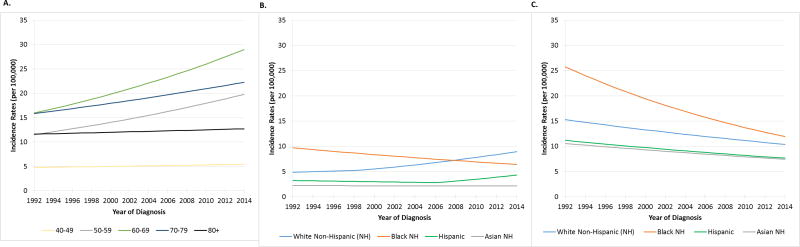
Figure 4. Age-adjusted Incidence rates among men for oropharynx squamous cell cancer by age (Figure 4A) and race/ethnicity (Figure 4B) and for non-oropharynx head and neck squamous cell cancer by race/ethnicity (Figure 4C).
Incidence on y-axis (per 100,000) and year of diagnosis on x-axis. Blue, green, red, and grey indicate respective races: Whites, Blacks, Hispanic and Asian. In Figure 4A, in zero joinpoint model non-significant changes in OPSCC incidence were seen for ages 40-49, and 80+ (p>0.114 for each). Incidence of OPSCC increased significantly in the study period for 50-59 (APC 2.5, p<0.001), 60-69 (APC 2.7, p<0.001), and 70-79 (APC 1.6, p<0.001) year old men. In joinpoint analysis, significant increases for 60-69 year old men were observed 1999-2014 (APC 3.8, p<0.001) and for 70-79 year old men during 1992-1998 (APC 4.8, p=0.02) and 2001-2014 (APC 4.2, p<0.001). In Figure 4B, in selected joinpoint models, OPSCC incidence increased for Whites during 1999-2014 (APC 3.5, p<0.001) and Hispanics during 2006-2014 (APC 5.6, p=0.003), while decreasing for Blacks during 1992-2014 (APC-1.9, p<0.001). In Figure 4C, incidence rates for non-OP HNSCC are shown in zero joinpoint model. There were significant decreases for Whites (APC -1.7, p<0.001), Blacks (APC -3.4, p<0.001), Hispanics (APC -1.7, p<0.001), and Asians (APC -1.6, p<0.001). In joinpoint modeling, significant declines in incidence for Whites were noted during 1992-2003 (APC -2.8, p<0.001) and 2003-2014 (APC -0.7, p=0.002) and for Asians during 1996-2006 (APC -3.6, p<0.001). Incidence rates are age-adjusted to the 2000 US Standard Population (19 age groups – Census P25-1130).
We next explored incidence trends by race and ethnicity. Incidence of HNSCC overall decreased among women across all race categories (Supplementary Figure 1). The rate of decrease was fastest among Black women (aAPC -2.6, p<0.001). Similarly, among men incidence of HNSCC overall decreased for all race categories (p<0.001 for all except API and AIAN) with the fastest decrease among Black men (aAPC -3.0, p<0.001; Supplemental Figure 2). OPSCC incidence decreased significantly among Black women (average APC -1.8, p=0.006), but was stable among White women (p=0.42; Figure 3b). Given the small numbers in other race categories, trends in OPSCC incidence could not be evaluated. Among men, the incidence of OPSCC significantly decreased among Black men (aAPC -1.9, p<0.001), however a significant increase was observed among White men (aAPC 2.8, p<0.001) and a more modest increase was observed among Hispanic men (aAPC=1.4, p=0.096; Figure 4B). Examining only the past 10 years (2005-2014), similar trends were observed with increasing OPSCC among White (aAPC=3.5, p<0.001) and Hispanic (aAPC=4.8, p<0.001) men but not for Black men (aAPC= -1.9, p<0.001), White women (aAPC= -0.2, p=0.42) or Black women (aAPC= -1.8, p=0.006). For non-oropharyngeal HNSCCs, incidence decreased significantly among women and men of every race (except for stable incidence among Asian men). These decreases were largest for Black men (aAPC= -3.4). In 2014, incidence of non-oropharyngeal HNSCC remained higher among Black men than men of other race and ethnicities, although the disparities in rates were substantially less than in prior years (Figure 4C). Incidence of non-oropharyngeal HNSCC also decreased substantially among Black women (aAPC= -3.0), first dropping below the rate in White women in 2003, with rates by 2014 of 3.2 vs 4.2 per 100,000 (p=0.02) in Black vs White women (Figure 3C).
Site-specific trends in non-oropharyngeal HNSCC including decreased incidence of oral cavity cancers (men: aAPC= -1.1, p=<.001; women: aAPC= -0.4, p=0.02), larynx (men: aAPC= -2.7, p=<0.001; women: aAPC= -3.1, p<0.001), hypopharynx (men: aAPC= -3.1, p<0.001; women: aAPC= -3.9, p<0.001), nasopharynx (men: aAPC= -0.6, p=0.15; women: aAPC= -1.0, p=0.06), and nasal cavity (men: aAPC=0.4, p=0.57; women: aAPC cannot be calculated; Supplemental Figure 3). When exploring sex- and race-stratified incidence for these non-oropharyngeal anatomic sites, incidence was decreasing in all groups, except for oral cavity, where an increasing incidence was observed between 2006-2014 among White men (APC=1.2, p=0.04), but not men of other races, or among women overall. Among women 50-59 years old oral cavity SCC incidence also appeared to increase between 2006-2014 (APC=3.3. p=0.035), but this increase was not observed among women of other age groups.
Discussion
This analysis is a comprehensive evaluation of population-based incidence trends for HNSCC using the most current data available. Despite the consistent increases in incidence of OPSCC, which now represents 40% of HNSCCs in the US, incidence of head and neck cancers are declining overall, most notably among Black men and women. Indeed, each of the major anatomic sites of HNSCCs can be considered rare cancers, as incidence is lower than 6 cases per 100,000 individuals per year. The clinical importance of incidence trends is the risk of being diagnosed with the condition of interest. Therefore, herein we show that the risk of HNSCC has decreased overall, but that the risk of diagnosis with OPSCC has actually increased.
A rapid decline in incidence of HNSCC occurred from 1992 to 2003. Beginning around 2003, however, the incidence of HNSCC overall began to increase modestly, driven by larger increases in OPSCC among men. No recent analysis has included cancers of the hypopharynx, nasal cavity, and nasopharynx to describe current incidence trends of HNSCC overall or of non-oropharyngeal HNSCCs. Prior work has shown a decline in HPV-unrelated cancers of the oral cavity and oropharynx,1, 14, 15 and a decline in larynx cancer using data up 1997.16 This analysis is a more accurate representation of the comprehensive changes in incidence for all HPV-unrelated cancers: incidence of non-oropharyngeal HNSCCs consistently declined between 2003-2014 in all subgroups (i.e. by sex, race, and tumor site, with few exceptions).
The incidence of HNSCC has historically been higher among Blacks than Whites in the US. However, the incidence of HNSCC is now for the first time lower among Blacks than Whites, beginning around 2008 (Figure 1). Further, over the past 10 years the incidence of non-oropharyngeal HNSCCs has continued to dramatically decline among men (Figure 2B). These changing trends in case burden mark a change in HNSCC patients, with more OPSCC and fewer non-oropharyngeal HNSCC cases. Evaluation of these changes are important to inform the need of specialty-specific head and neck oncologic providers (e.g. staffing, training), as the continuing decline in non-oropharyngeal HNSCCs heralds a shift in practice for clinicians. Whereas prior literature has forecasted an epidemic, this analysis highlights that non-oropharyngeal HNSCCs are becoming increasingly rare in the US and OPSCC remain rare. This in combination with the extensive data regarding survival benefits for high-volume centers17–19 support the need for centralized care of HNSCC patients.
Several novel findings by race were observed including an increase in OPSCC among Hispanic men. While OPSCC incidence remains lower among Hispanic than White men, it is steadily increasing among Hispanic men in parallel fashion to White men with dramatic increase in recent years (2006-2014: APC 5.4, p=0.003). Prior work has shown that prevalence of HPV-positive tumors has increased over time across races6, indicating that HPV is also driving incidence trends among these other racial groups. Among Blacks, the proportion of OPSCC caused by HPV is increasing6, suggesting the dramatic reduction in incidence of OPSCC among Blacks driven by reduction in tobacco and alcohol related cancers would be even larger if not for the increasing contribution of HPV-positive cancers in Blacks. Among women, the previous racial disparity in OPSCC incidence between Whites and Blacks no longer exists. These differences by race are important to consider both by clinicians and public health practitioners as there are well-established differences in access to care by race20, 21 and prognostic differences.22
When considering changes in the landscape of non-oropharyngeal HNSCC, incidence has declined in both women and men, although these declines are more dramatic among men. The incidence of non-oropharyngeal HNSCC among White women surpasses that of Black women beginning in 2008, with continued widening reverse disparity of cancer between the two races since that time. In men the decrease in incidence has been steady and significant for Black men, but less prominent for Whites, and the burden of non-oropharyngeal HNSCC remains higher for Blacks. These more rapid declines among Blacks may be explained by the larger decrease in daily smoking in the U.S. among Blacks vs. Whites in recent decades.23
In this analysis, incidence trends of cancers of the nasal cavity, nasopharynx, oral cavity, larynx and hypopharynx had similar trends and were thus considered collectively as non-oropharyngeal HNSCCs. While these are rare cancers, determining changes in incidence is important to understand the contribution of etiologic agents. There were non-significant declines in incidence of nasal cavity and nasopharynx, while cancers of the oral cavity, larynx and hypopharynx are declining significantly. A significant increase in oral cavity cancers was confirmed in younger women, however these cancers remain rare with an unknown etiology driving this trend24.
In younger age cohorts, increases in OPSCC incidence were observed (consistent with prior reports)3, 8 but decreases of similar magnitude in non-oropharyngeal HNSCC. This declining non-oropharyngeal HNSSC incidence among younger age-cohorts is the results of decades of success in reducing tobacco use in the US. However, recent US population-based data observed higher smoking prevalence among men and women 18-44 compared to 45-64 year olds (19% vs 8%)25, suggesting non-oropharyngeal HNSCC incidence may increase in the future once this younger cohort ages, unless tobacco cessation efforts can decrease their use.
This study had several limitations including the use of registry data from 13 states and lack of HPV data. Bias due to geographic variations is possible. Intrinsic to SEER data is the possibility of site misclassification. Site categories used were largely consistent with the literature, with few modifications to reduce potential misclassification and those with potential overlap were excluded, however misclassification as a source of confounding remains. Although this is a comprehensive analysis of HNSCC, other head and neck cancers (ie salivary gland, cutaneous, thyroid, etc) are not included.
This paper offers a comprehensive analysis, by sex, race, and anatomic site of the epidemiology of HNSCC over 22 years in the US. We document a striking prolonged decrease in the incidence of HNSCC among both men and women and increases in OPSCC not only among Whites but among some other groups as well.
Supplementary Material
Acknowledgments
Oral Cancer Foundation
Footnotes
Conflicts of Interest: None
Author contributions: Drs. Fakhry and D’Souza are responsible for the overall content. Mr. Krapcho contributed with regard to planning, analysis and editing. Dr. Eisele contributed to planning, interpretation and writing.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Souza G, Cullen K, Bowie J, et al. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PloS One. 2014;9:e86023. doi: 10.1371/journal.pone.0086023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of Oropharyngeal Cancer Among Elderly Patients in the United States. JAMA Oncol. 2016;2:1617–1623. doi: 10.1001/jamaoncol.2016.1804. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza G, Westra WH, Wang SJ, et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 8.Tota JE, Anderson WF, Coffey C, et al. Rising incidence of oral tongue cancer among white men and women in the United States, 1973–2012. Oral Oncol. 2017;67:146–152. doi: 10.1016/j.oraloncology.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Joseph LJ, Goodman M, Higgins K, et al. Racial disparities in squamous cell carcinoma of the oral tongue among women: a SEER data analysis. Oral Oncol. 2015;51:586–592. doi: 10.1016/j.oraloncology.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Cole L, Polfus L, Peters ES. Examining the incidence of human papillomavirus-associated head and neck cancers by race and ethnicity in the U.S., 1995–2005. PloS One. 2012;7:e32657. doi: 10.1371/journal.pone.0032657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [cited 2017 Jul 13];Registry Groupings for Analyses - SEER Registries [Internet] Available from: https://seer.cancer.gov/registries/terms.html.
- 12. [cited 2017 Jul 13];SAS® Help Center: Chi-Square Tests and Statistics [Internet] Available from: http://documentation.sas.com/?cdcId=statcdc&cdcVersion=14.2&docsetId=statug&docsetTarget=statug_freq_details08.htm&locale=en.
- 13. [cited 2018 Jan 26];19 Age Groups - Standard Populations - SEER Datasets [Internet] Available from: https://seer.cancer.gov/stdpopulations/stdpop.19ages.html.
- 14.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel MA, Blackford AL, Rettig EM, et al. Rising population of survivors of oral squamous cell cancer in the United States. Cancer. 2016;122:1380–1387. doi: 10.1002/cncr.29921. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho AL, Nishimoto IN, Califano JA, et al. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114:806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 17.David JM, Ho AS, Luu M, et al. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer. 2017 doi: 10.1002/cncr.30843. [DOI] [PubMed] [Google Scholar]
- 18.Wuthrick EJ, Zhang Q, Machtay M, et al. Institutional Clinical Trial Accrual Volume and Survival of Patients With Head and Neck Cancer. J Clin Oncol. 2015;33:156–164. doi: 10.1200/JCO.2014.56.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourin CG, Forastiere AA, Sanguineti G, et al. Impact of Surgeon and Hospital Volume on Short-Term Outcomes and Cost of Oropharyngeal Cancer Surgical Care. The Laryngoscope. 2011;121:746–752. doi: 10.1002/lary.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AY, Schrag NM, Halpern MT, et al. The impact of health insurance status on stage at diagnosis of oropharyngeal cancer. Cancer. 2007;110:395–402. doi: 10.1002/cncr.22788. [DOI] [PubMed] [Google Scholar]
- 21.Chen AY, Halpern M. Factors predictive of survival in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1270–1276. doi: 10.1001/archotol.133.12.1270. [DOI] [PubMed] [Google Scholar]
- 22.Fakhry C, Westra WH, Wang SJ, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123:1566–1575. doi: 10.1002/cncr.30353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014 SurgeonGeneral.gov [Internet]Available from: https://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html.
- 24.Li R, Faden DL, Fakhry C, et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke. Head Neck. 2015;37:1642–1649. doi: 10.1002/hed.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. [cited 2017 Jul 17];Early Release of Selected Estimates on Data From the January–June 2013 National Health Interview Survey [Internet] Available from: https://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201312_08.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.