Abstract
Objective
Integration of robotic surgical technology into skull base surgery is limited due to minimum angle requirements between robotic tools (narrow funnel effect), steep angle of approach, and instrumentation size. The objectives of this study were to systematically analyze surgical approach portals using a computer model, determine optimal approaches, and assess feasibility of the derived approaches on robotic surgical systems.
Study Design
Computer analysis on 10 computed tomography scans was performed to determine approach trajectories, angles between robotic tools, and distances to specified skull base target locations for transorbital and transnasal surgical approach portals.
Setting
Dry laboratory and cadaver laboratory.
Subjects and Methods
The optimal combinations were tested on the da Vinci and Raven robotic systems.
Results
Multiportal analyses showed the angles between 2 robotic tools were 14.7, 28.3, and 52.0 degrees in the cases of 2 transnasal portals, combined transnasal and medial orbit portals, and bilateral superior orbit portals, respectively, approaching a prechiasmatic target. The addition of medial and superior transorbital portals improved the skull base trajectory angles 21 and 27 degrees, respectively. Two robotic tools required an angle of at least 20 degrees between them to function effectively at skull base targets.
Conclusion
Technical feasibility of robotic transorbital and transnasal approaches to access sella and parasellar target locations was demonstrated. This technique addresses the 2 major drawbacks of (1) the narrow funnel effect generated from portals in close proximity and (2) the steep angle of approach to the skull base, as observed in previous studies analyzing transoral, transcervical, transmaxillary, and transhyoid portals.
Keywords: robotic surgery, skull base, transorbital, endoscopic, pituitary
Robotic technology holds tremendous potential for allowing skull base surgeons the ability to dexterously manipulate slender instruments through narrow portals, maintaining 7 degrees of freedom while performing finely controlled movements at the target location. To accomplish this also requires additional refinements, including enhanced visual magnification, 3-dimensional visualization, motion scaling, and tremor reduction.
The most common use of this technology thus far in head and neck surgery has been in posterior oropharyngeal exposures; the generous size of the oral cavity offers a surgical portal large enough to permit access with the endoscope as well as 2 separate tools for instrumentation. However, inherent anatomical constraints in other surgical sites impede current robotic systems’ effectiveness, both in terms of the distance of separation between surgical portals and in the angle between instruments. Numerous investigators using currently available robotic systems have encountered significant limitations in the anterior skull base.1–5 In these studies, anterior skull base robotic access was obtained through transoral, transcervical, transhyoid, and maxillary antrostomy surgical portals. Technical feasibility was demonstrated in specific skull base locations, but 2 major limitations were noted: (1) when portals were in close proximity to each other, the robotic arms did not perform well due to collisions between each other, which has been referred to as the “narrow funnel effect,” and (2) there was limited access to the anterior portion of the skull base due to the steep approach vectors defined by the surgical portals.
In an effort to improve methods of surgical access and target manipulation, we have investigated new techniques for analyzing and developing surgical approaches.6,7 We have found that, particularly for the parasellar region and the central anterior cranial fossa, multiple transorbital surgical approaches can potentially be exploited for use in robotic surgery.8,9 These approaches can be combined with other surgical portals in multiportal approaches to optimize parameters such as approach angle and visualization of pathology, as well as minimize collateral tissue damage.
In using the current Food and Drug Administration (FDA)–approved da Vinci (Intuitive Surgical, Sunnyvale, California) robotic platform, the narrow funnel effect must be overcome by choosing surgical portals that are adequately spaced apart. In addition and of equal importance, the surgical portal must provide excellent visualization, allow adequate access, and have a favorable approach vector to skull base targets.
The purposes of this study were several-fold: (1) to determine the optimal approaches for robotic surgery using computer modeling, (2) to perform an anatomic and engineering feasibility study to determine how this technology could be clinically applicable to skull base surgery, and (3) to identify which features of the technology and instrumentation require further modification before use in vivo.
Materials and Methods
A computer analysis of multiple surgical approaches was performed on 10 cadaver computed tomography (CT) scans. Since previous studies have not evaluated combined transnasal and transorbital portals, these were selected as the focus of the analysis. (x,y,z) coordinate data that characterized the surgical approaches and critical neurovascular structures were collected to compute distance to target, angle between robotic tools, and angle with respect to anatomical planes as previously described (Figure 1).6 In addition, qualitative information from the modeling was incorporated analyzing virtual endoscopy routes through the various surgical portals. Based on the analysis, the favorable approaches were tested on a dry skull using the da Vinci Si robotic system. The skull was prepared for bilateral medial (precaruncular) and superior (superior lid crease) transorbital approaches by removing a 10 × 15-mm window of the lamina papyracea, the nasal septum, and the anterior and posterior sphenoid walls. Two da Vinci instruments (8- and 5-mm Needle Drivers) with standard grasping and wrist capability and the 8.5-mm 3-dimensional endoscope were used, testing all combinations of the following portals: (1) bilateral medial orbit, (2) bilateral superior orbit, and (3) bilateral transnasal. White clay lined the posterior sphenoid and sella, and the pituitary fossa was filled in with red clay. Instruments were manipulated in the pituitary fossa to remove the red-colored clay. Robotic arm tool range of motion was recorded, and video data were collected.
Figure 1.
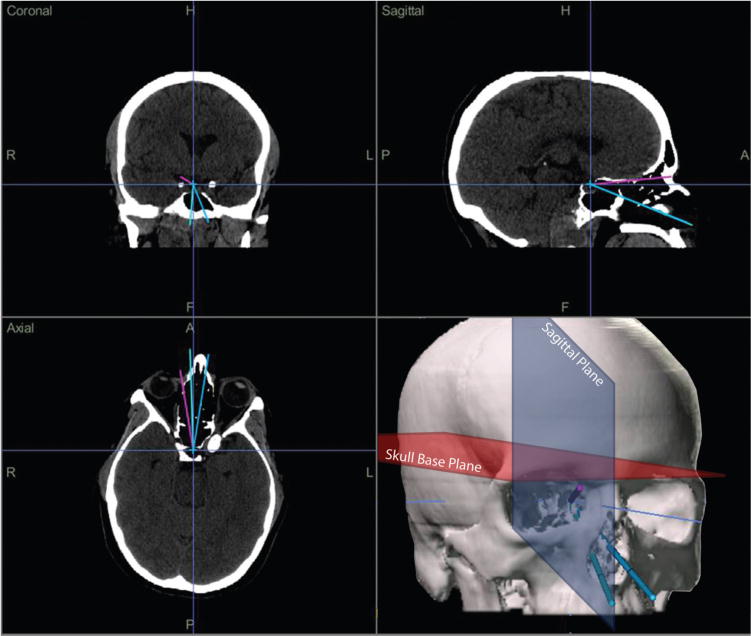
Screenshot of the graphical user interface of the iNtellect Cranial Navigation (Stryker Corporation, Kalamazoo, Michigan) used to visualize approach trajectories and collect (x,y,z) coordinate data. In the lower right quadrant, skull base and sagittal planes are depicted.
A cadaver study was performed using a research surgical robotic platform, the Raven (BioRobotics Laboratory, Seattle, Washington).10 It is a compact, open-source surgical robot controlled with Phantom Omni (Geomagic, Wilmington, Massachusetts) devices that can manipulate da Vinci tools. Currently, there are more than 10 Raven surgical robotic systems at major research institutions throughout the United States.
The angles between robotic arms were determined to access the following target locations in the sella and parasellar region: (1) prechiasmatic, (2) postchiasmatic, and (3) bilateral cavernous sinus. The minimum angle was determined between robotic arms prior to collision. Using the same robotic instruments and a 5-mm 0-degree endoscope, the system was tested on 3 cadaver specimens. The combinations through the above 6 surgical portals were tested to access the target locations (Table 1).
Table 1.
Entry portals and target pathology locations used in the analysis for robotic tools to access the skull base.
| Surgical Portals | Target Locations |
|---|---|
| Right transnasal | Prechiasmatic |
| Left transnasal | Postchiasmatic |
| Right medial orbit | Right cavernous sinus |
| Left medial orbit | Left cavernous sinus |
| Right superior orbit | |
| Left superior orbit |
Standard sinus surgery instruments were used to perform transnasal sphenoidotomy and gain exposure to the pituitary fossa, pre- and postchiasmatic cisterns, and cavernous sinuses. Transorbital (medial and superior) approaches were performed as previously described.11 The robotic instruments were manipulated easily into all of the portals. They were manually placed through the incision on the skin or conjunctiva and then advanced with remote robotic control. Combinations of all 6 portals for 2 robotic tools and an endoscope were tested at the target locations.
Institutional review board approval was not required because no live human subjects were studied.
Results
Computer Analysis Results
The computer analysis showed significant gains in working angles between instruments and decreases in distances to target locations when a standard transnasal approach was supplemented with transorbital portals. Two robotic tools placed in the right and left nares working at a prechiasmatic target location had an angle between themselves of 14.7 degrees; addition of a medial orbit portal increased this to 28.3 degrees. Using bilateral superior orbit approaches to the prechiasmatic target location, this angle further increased to 52.0 degrees (Table 2). Distances to target locations were reduced with the addition of medial and superior orbit portals, from 93.2 mm to 73.4 mm in the case of the prechiasmatic target location and medial orbit portal. With respect to a midsagittal plane, transnasal instruments approached the prechiasmatic target location at 8.0 degrees, compared with 14.5 and 26.5 degrees for medial and superior orbit portals, respectively. With a skull base plane defined by the posterior clinoid processes and tuberculum sellae, approach vectors through the portals had a range of 33.9 degrees for the prechiasmatic location. The nasal portals approached from an angle of 22 degrees below the skull base plane (denoted as negative in Table 2), the medial orbit portal 5.5 degrees above, and the superior orbit portal 11.7 degrees above the skull base plane.
Table 2.
Averaged (n = 10 computed tomography scans) angle and distance results for the multiportal approach to skull base target locations.
| Prechiasmatic | Postchiasmatic | Cavernous Sinus | |
|---|---|---|---|
| Angles between instruments, mean (SD), deg | |||
| Bilateral transnasal | 14.69 (1.89) | 13.20 (1.72) | 13.86 (1.88) |
| Nasal and opposite medial orbit | 28.31 (3.59) | 22.66 (3.56) | 25.16 (3.89) |
| Nasal and opposite superior orbit | 41.91 (4.62) | 41.37 (3.63) | 43.77 (4.88) |
| Bilateral medial orbit | 28.63 (3.17) | 24.32 (2.5) | 24.88 (2.67) |
| Bilateral superior orbit | 52.02 (3.52) | 46.03 (3.62) | 43.45 (3.18) |
| Distances to target, mean (SD), mm | |||
| Nasal | 93.22 (5.61) | 103.74 (7.04) | 97.88 (6.05) |
| Medial orbit | 59.95 (2.36) | 70.28 (3.57) | 67.32 (3.47) |
| Superior orbit | 73.41 (2.38) | 82.41 (3.67) | 86.80 (4.22) |
| Approach angle with respect to midsagittal plane, mean (SD), deg | |||
| Nasal | 8.01 (1.39) | 7.14 (1.28)a | 15.91 (2.72) |
| Medial orbit | 14.69 (1.73) | 12.50 (1.4)a | 24.40 (2.85) |
| Superior orbit | 26.48 (1.53) | 23.29 (1.73)a | 32.93 (2.25) |
| Approach angle with respect to skull base plane, mean (SD), deg | |||
| Nasal | −22.20 (6.02) | −21.25 (5.78) | −17.17 (6.21) |
| Medial orbit | 5.47 (4.17) | 5.90 (4.62) | 4.57 (3.18) |
| Superior orbit | 11.66 (5.93) | 8.39 (5.22) | 10.61 (6.22) |
Contralateral approach angles shown.
Robotic Feasibility Results
The results from the da Vinci robotic system applied to dry skull showed a robotic tool range of motion of 28 to 46 degrees for bilateral medial orbit portals (Figure 2). Right and left nasal portals were not feasible due to robotic tool collision. The robotic arms could function in portals separated by a distance as small as 3.5 cm.
Figure 2.
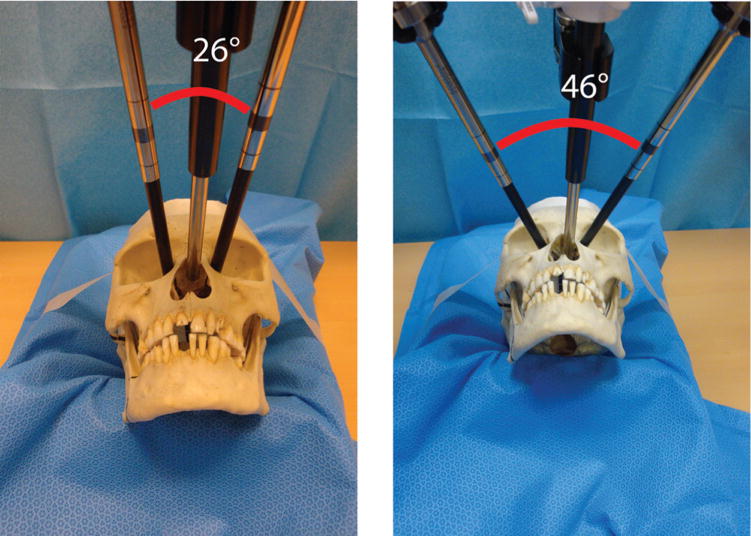
da Vinci robotic system applied to a dry skull to assess gross geometric feasibility. Range in angulation between robotic arms was 26 to 46 degrees.
Clay surgical targets were set up in the anterior cranial fossa (Figure 3). Removal of all targets was performed without difficulty through the combined transnasal, medial, and superior orbit portals (see video, available at otojournal.org). There were no major robotic tool collisions that prevented access to all of the target locations. It was observed that only the tool tips were visible endoscopically and that the operator had little awareness of the position of the proximal tool shaft, which during certain maneuvers translated medially or laterally. As a result, we have also developed methods to prevent inappropriate target contact (in preparation).
Figure 3.
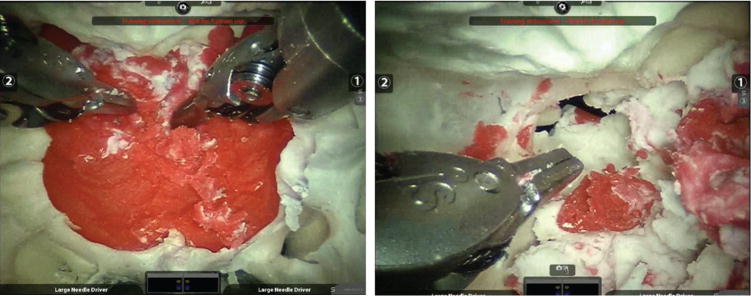
Modeled (red clay) pituitary targets accessed by transorbital da Vinci robotic instruments. (A) Transnasal endoscope, instruments transorbital, and (B) left medial orbit endoscope, instruments through contralateral medial orbital and transnasal portals. Video available online at otojournal.org.
The cadaver operations performed with the Raven surgical robotic platform confirmed the findings on a dry skull. Figure 4 is a photograph of the multiportal arrangement. The surgical robotic tools adequately accessed the target locations in the sella and parasellar regions (Figure 5). The robotic tool range of motion in the case of bilateral medial orbit portals was 18 to 40 degrees (Figure 6).
Figure 4.
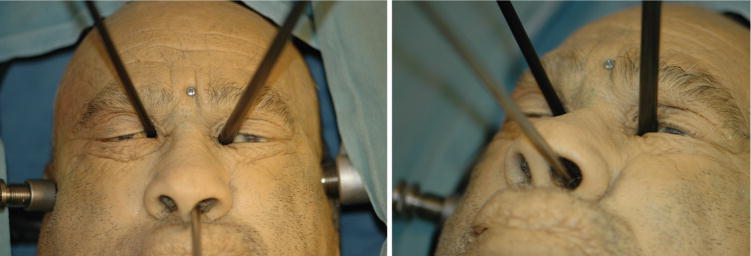
Multiportal technique with the Raven robot. The endoscope was placed transnasally and robotic tools were placed through bilateral medial orbit portals to access the anterior cranial fossa.
Figure 5.
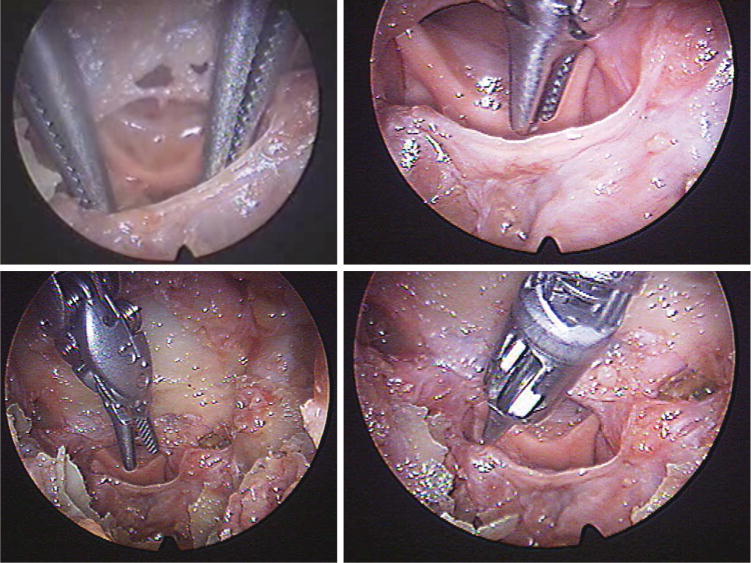
Robotic instruments accessing the prechiasmatic target location.
Figure 6.
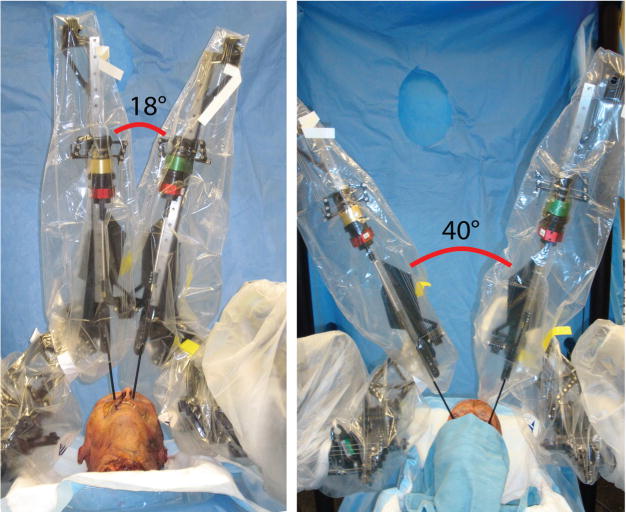
Raven surgical robotic system tool range of motion (18–40 degrees) in bilateral medial orbit portals to access sella and parasellar target locations.
Discussion
This preliminary study demonstrated technical feasibility of robotic transorbital and transnasal approaches to access sella and parasellar target locations. This multiportal technique addresses the (1) narrow funnel effect generated from portals in close proximity and the (2) steep angle of approach to the skull base, as observed in the previous studies analyzing the transoral, transcervical, transmaxillary, and transhyoid portals.
The narrow funnel effect appears to be solved by the addition of transorbital portals. The computer analysis showed a wide range of angulation possible between robotic tools using medial and superior orbit portals—2- to 3-fold more than through transnasal portals alone (28 and 52 degrees vs 14 degrees). This range was confirmed both on the dry skull and cadaver surgical procedures. The use of bilateral nasal portals was not a feasible option for 2 robotic tools due to instrument collision. The computer analysis showed that the angulation between robotic tools would be 14 degrees in this case, if there were no collisions proximally. Based on these data and observations of the success at bilateral medial orbit portals (28 degrees), it is estimated that the angle required between portals is approximately 20 degrees for adequate function of 2 robotic tools. The second issue of steep angle of approach is also ameliorated in that, depending on the target location, a transnasal and transorbital portal approach trajectory can be selected from a range of more than 30 degrees. This includes options above the skull base plane (superior orbit), at the skull base plane (medial orbit), and below the skull base plane (transnasal).
The da Vinci surgical robotic system has multiple drawbacks for use in the skull base. Its original design was for cardiac surgery and thus not optimized for use in spaces with 2 portals separated by only several centimeters. The instrumentation is too large in its current state to be used safely; however, with future design improvements and miniaturization of the instrumentation and 3-dimensional endoscope, feasibility of use in skull base surgery may improve. The process for development of a new surgical system is significant and takes many years. While new systems can be developed in parallel, it is also important to fully explore the potential advantages of the available system. The transorbital portals in this study were only evaluated in combination with transnasal portals. Although this offered some solutions to previous barriers, a more thorough analysis would have also included transorbital portals in combination with the other portals previously studied (transoral, transcervical, transmaxillary, and transhyoid). Future work will further define the 3-dimensional working space and complete range of motion within each surgical portal.
The use of the open-source Raven surgical robot in addition to the da Vinci offers the ability to simultaneously maximize the clinical use of the currently available system and develop improvements in the system that can be rapidly integrated and made available to other researchers. For example, one opportunity for improvement in a robotic system for this application is to implement boundaries on the motion of the surgical tools (including the entire length of the tool shaft). It was observed that the operator has no knowledge of the location of the proximal robotic tool shaft in the current system, as the endoscope only visualizes the instrument tips. Certain maneuvers may cause the proximal shaft location to translate at the location along the shaft that is adjacent to the orbit. Placing a constraint in the range of motion at this level will serve to protect the orbit, as is done with transorbital endoscopic surgery without robotic assistance. We have subsequently developed methods to prevent proximal tool shaft translation so to protect soft tissue structures such as the orbital contents. This technology has been integrated into the Raven surgical system and reported in the literature.11
Conclusion
This preclinical surgical and engineering study demonstrates that transorbital multiportal robotic skull base surgery is feasible and overcomes the 2 major barriers described previously of the narrow funnel effect and the steep approach angle to the skull base. We found that an angle of approximately 20 degrees between 2 portals is necessary for adequate function of 2 robotic arms. The computer analysis accurately predicted the angles measured on dry skulls and cadavers. Thus, the computer analysis technique is useful in analyzing new surgical portals. The use of combined transorbital and transnasal portals permits a wide range of angles between instruments that were not possible with previous approaches. In the case of combined transnasal and transorbital portals approaching a prechiasmatic target, the angle between instruments was 28 degrees; in the case of bilateral superior orbital portals, it was 52 degrees. Similarly, using combined transnasal and transorbital portals, there is a wider range of approach vectors compared with previously reported portals. Transorbital portals permit a wider approach angle of 26 degrees for a superior orbit portal relative to a midsagittal plane, compared with 8 degrees for a transnasal approach. Relative to the skull base plane, a superior transorbital portal approaches a prechiasmatic target from 12 degrees above the plane, compared with a transnasal portal that approaches that same target from 22 degrees below the plane. This provides a large range of approach vectors from which to choose, based on the location and characteristics of the lesion. This study was limited to procedures on dry skull and cadaver specimens but did demonstrate gross feasibility and opportunities for improvement, including the implementation of boundary conditions to minimize tissue injury along the surgical corridor.
Supplementary Material
Acknowledgments
Sponsorships: None.
Funding source: This study was supported by grant NIH T32 DC00018 from the National Institutes of Health (Dr Bly), the American Academy of Otolaryngology—Head and Neck Surgery Foundation (Dr Bly), and the University of Washington Housestaff Association (Dr Bly).
Footnotes
This article was presented at the 2013 AAO-HNSF Annual Meeting & OTO EXPO; September 29 to October 3, 2013; Vancouver, BC, Canada.
Author Contributions
Randall A. Bly, study design, data collection and analysis, manuscript writing and preparation; David Su, study design, data collection, manuscript revisions; Thomas S. Lendvay, study design, data collection, manuscript revisions; Diana Friedman, study design, data collection, manuscript revisions; Blake Hannaford, study design, data collection, manuscript revisions, Manuel Ferreira, study design, data collection, manuscript revisions; Kris S. Moe, study design, data collection and analysis, manuscript revisions.
Competing interests: Kris S. Moe is a founder and shareholder for SPI Surgical, Inc.
Supplemental Material
Additional supporting information may be found at http://oto.sagepub.com/content/by/supplemental-data
References
- 1.Hanna EY, Holsinger C, DeMonte F, Kupferman M. Robotic endoscopic surgery of the skull base: a novel surgical approach. Arch Otolaryngol Head Neck Surg. 2007;133:1209–1214. doi: 10.1001/archotol.133.12.1209. [DOI] [PubMed] [Google Scholar]
- 2.McCool RR, Warren FM, Wiggins RH, III, Hunt JP. Robotic surgery of the infratemporal fossa utilizing novel suprahyoid port. Laryngoscope. 2010;120:1738–1743. doi: 10.1002/lary.21020. [DOI] [PubMed] [Google Scholar]
- 3.O’Malley BW, Weinstein GS. Robotic anterior and midline skull base surgery: preclinical investigations. Int J Radiat Oncol Biol Phys. 2007;69(suppl 2):S125–S128. doi: 10.1016/j.ijrobp.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, O’Malley BW, Jr, Newman JG, et al. Transoral robotic surgery of the skull base: a cadaver and feasibility study. ORL J Otorhinolaryngol Relat Spec. 2010;72:181–187. doi: 10.1159/000276937. [DOI] [PubMed] [Google Scholar]
- 5.Kupferman M, Demonte F, Holsinger FC, et al. Transantral robotic access to the pituitary gland. Otolaryngol Head Neck Surg. 2009;141:413–415. doi: 10.1016/j.otohns.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Bly RA, Su D, Hannaford B, Ferreira M, Moe KS. Computer modeled multiportal approaches to the skull base. J Neurol Surg B. 2012;73(6):415–423. doi: 10.1055/s-0032-1329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bly RA, Chang S, Cudejkova M, Liu JJ, Moe KS. Computer-guided orbital reconstruction to improve outcomes. JAMA Facial Plast Surg. 2013;15:113–120. doi: 10.1001/jamafacial.2013.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moe KS, Bergeron CM, Ellenbogen RG. Transorbital neuroendoscopic surgery. Neurosurgery. 2010;67(3 suppl):ons16–28. doi: 10.1227/01.NEU.0000373431.08464.43. [DOI] [PubMed] [Google Scholar]
- 9.Balakrishnan K, Moe KS. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol Head Neck Surg. 2011;144:815–820. doi: 10.1177/0194599810397285. [DOI] [PubMed] [Google Scholar]
- 10.Hannaford B, Rosen J, Friedman D, et al. Raven-II: an open platform for surgical robotics research. IEEE Trans Biomed Eng. 2013;60:954–959. doi: 10.1109/TBME.2012.2228858. [DOI] [PubMed] [Google Scholar]
- 11.Ryden F, Kosari SN, Chizeck HJ. A computer vision approach to virtual fixtures in surgical robotics. Paper presented at: RSS 2012 Workshop on Algorithmic Frontiers in Medical Robotics: Manipulation in Uncertain, Deformable, Heterogenous Environments; July 9–10, 2012; Sydney, Australia. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


