Abstract
Spontaneous coronary artery dissection is a nonatherosclerotic etiology of acute coronary syndrome, including sudden cardiac death, which frequently affects younger women. This review highlights contemporary knowledge regarding spontaneous coronary artery dissection demographics, prevalence, diagnosis, presentation, and associated conditions and risks, inpatient treatment, major adverse clinical events, and outpatient management decisions.
Keywords: Acute Coronary Syndrome, Acute Myocardial Infarction, Pregnancy, Spontaneous Coronary Artery Dissection, Women
1. INTRODUCTION
Heart disease is the leading cause of morbidity and mortality among women.1 As compared with men, women have differing clinical presentation, risk factors, optimal treatment strategies, and outcomes.2 Spontaneous coronary artery dissection (SCAD) is increasingly distinguished as a pertinent etiology of myocardial infarction (MI) among women. SCAD MI is caused by lack of myocardial blood flow due to partial or complete occlusion of the coronary artery from a vessel‐wall tear and/or hematoma. Diagnosis, treatment, and future care of SCAD patients can be challenging, hence the need for continued attention and research. This review aims to discuss current knowledge regarding SCAD and what future work is needed.
2. DEMOGRAPHICS AND PREVALENCE
SCAD predominantly affects women, who account for 81% to 92% of the patients in recent series (Table 1).3, 4, 5, 6, 7, 8 Reported prevalence of SCAD is 0.07% to 1.1%,9 but these data predate contemporary heightened diagnostic awareness and the availability of intracoronary imaging, which can demonstrate features of SCAD in otherwise ambiguous cases on coronary angiogram.9 Among women, SCAD is the etiology of acute coronary syndrome (ACS) in as many as 35% of cases8, 10 and the most common etiology of MI associated with pregnancy.11, 12
Table 1.
Comparison of clinical characteristics, presentation, coronary involvement, management, and outcomes in recent SCAD cohorts
| Cohort | Tweet, 201712, a | Saw, 20175 | Lettieri, 20156, b | Rogowski, 20177 | Nakashima, 20168 |
|---|---|---|---|---|---|
| No. of patients | 323 | 327 | 134 | 64 | 63 |
| Mean age, y | 45 ± 9.6 | 52.5 ± 9.6 | 52 ± 11 | 53 ± 11.2 | 46 ± 10 |
| Female sex | 100 | 91 | 81 | 94 | 94 |
| White race | 95 | 82 | — | — | — |
| BMI, kg/m2 | 24.9 ± 5 | 24.4 (21.5–28.3) | 24.3 ± 3.5 | 24.8 ± 4.8 | 22.4 ± 4.2 |
| DM | 0.9 | 4.6 | 2 | 0 | 0 |
| Dyslipidemia | 35 | 26 | 33 | 52 | 23 |
| HTN | 27 | 36 | 51 | 45 | 33 |
| Current smoker | 0.6 | 10 | 34 | 28 | 32 |
| Pregnancy associated | 17 | 2 | — | 5 | 8 |
| History of infertility therapy | 18 | — | — | — | — |
| History of hormonal birth control | 86 | — | — | — | 0 |
| History of postmenopausal HT | 15 | 12 | — | — | 2 |
| Migraines | 30 | 36 | — | — | — |
| History of depression | 31 | 23 | — | — | — |
| History of anxiety | 31 | 13.5 | — | 2c | — |
| Extreme emotion/stress | 13 | 48 | — | — | 29 |
| Extreme exertion | 12 | 28 | — | 19 | 10 |
| FMD | 30d | 63 | — | 8e | 8f |
| EVA | 36d | — | — | — | — |
| Connective‐tissue disease | 2 | 5 | — | — | 2 |
| STEMI | 40 | 26 | 49 | 30 | 87 |
| NSTEMI | 58 | 74 | 40 | 69 | 13 |
| CV arrest | 10 | VT/VF 9 | 2.8 | —g | Shock/arrest 16 |
| Artery involvementf | |||||
| LM | 8 | 0.6 | 2.8 | 3 | 0 |
| LAD | 62 | 54 | 36 | 48 | 59 |
| LCx | 15 | 38 | 15 | 16 | 6 |
| RCA | 14 | 27 | 27 | 9 | 24 |
| Ramus/OM/PDA/PLA | 22 | — | 19 | 31 | — |
| Multivessel | 18 | 14 | 13 | 9 | 11 |
| PCI | 45 | 17 | 38 | 11 | 54 |
| PCI lack of success | 23 | 31 | 28 | 14 | 9 |
| CABG | 10 | 2.1 | 6 | 1.6 | 1.6 |
| In‐hospital death | 0 | 0 | 2.2 | 1.6 | — |
| In‐hospital urgent revascularization | 2 | 4.3 | 1.5 | 0 | — |
| Follow‐up | |||||
| Median F/U time | 2.34 y (1.04–5.06 y) | 3.1 y (1.49–5.49 y) | 22 months (1–166 months) | 4.5 y (1.8–8.4 y) | 34 months (3–160 months) |
| F/U MI | 18%; 21% 5‐y KM | 16.8%; 4.8%/y | 2 (1.6%) | 4 (6.3%)g | 18 (28.6%) |
| F/U SCAD | 16%; 20% 5‐y KM | 10.4%; 2.8%/y | 4.7% | 3 (4.7%) | 22%; 27% 5‐y KM |
| F/U HF | 2%; 3% 5‐yr KM | — | 5 (3.9%) | 0 | — |
| F/U death | 0 | 0.3%/y | 4 (3.1%) | 0b | 1 (1.6%) |
Abbreviations: CABG, coronary artery bypass grafting; CV, cardiovascular; EVA, extracoronary vascular abnormalities; FMD, fibromuscular dysplasia; F/U, follow‐up; HF, heart failure; HT, hormone therapy; HTN, hypertension; IQR, interquartile range; KM, Kaplan–Meier; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LM, left main coronary artery; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; OM, obtuse marginal artery; PCI, percutaneous coronary intervention; PDA, posterior descending artery; PLA, posterolateral artery; SCAD, spontaneous coronary artery dissection; SD, standard deviation; STEMI, ST‐segment elevation myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia.
Data are presented as %, mean ± SD, or median (IQR), unless otherwise noted.
Due to this study's focus on pregnancy‐associated SCAD, all patients included in this cohort were women.
11.9% of the patients in this cohort had atherosclerosis.
Documented as panic disorder.
If calculating percentages based on those imaged systematically for arteriopathies (n = 161), the percentage of FMD is 61% and EVA is 72%. EVA includes FMD but also noncoronary dilations, tortuosity, and dissection, as described elsewhere.21
Five patients of the 40 imaged had FMD (13%).
Five patients of the 24 imaged had FMD (21%).
One patient had in‐hospital cardiac arrest.
Denominators of the coronary artery percentages are total number of patients.
One patient with persistent SCAD had out‐of‐hospital cardiac arrest 16 days after initial diagnosis but was successfully resuscitated.
SCAD can affect persons who are in their late teens to octogenarians, but overall the mean age among women has been reported as 42 to 53 years.3, 5, 6, 7, 8, 12, 13 Classic modifiable risk factors for atherosclerosis are not common among SCAD patients. In 2 recent series with >300 SCAD patients each, diabetes mellitus was present in only 0.9% to 4.6%, smoking in 0.6% to 10%, hypertension in 27% to 36%, and mean body mass index was 24 kg/m2.5, 12 Although SCAD has been reported in virtually every race and ethnicity and in most world geographical regions, most reported SCAD patients in contemporary series and registries are white women. This may be related to selection bias, access to healthcare and research, pathophysiology of SCAD, or other unknown factors.5, 12
3. PRESENTATION
Women with SCAD present with signs and symptoms of ACS such as ST‐segment elevation myocardial infarction, non–ST‐segment elevation myocardial infarction, and sudden cardiac death.3, 5, 6, 7, 8, 12 Although SCAD patients may present with chest pain and other ischemic symptoms, the initial electrocardiogram and troponin levels may be normal; thus, serial evaluations are advised in those with concerning symptoms.14 Some patients with history of SCAD report stuttering chest pain or a possible inciting trigger in the days prior to their ACS, suggesting that the SCAD may evolve before causing clinically urgent myocardial injury.
4. DIAGNOSIS
SCAD is usually diagnosed on invasive coronary angiography; intracoronary imaging can be used for confirmation in indeterminate cases, often by visualizing an intramural hematoma.15 In some, intravascular imaging demonstrates the presence of hematoma without a discrete tear in the coronary wall, suggesting that SCAD may also arise from spontaneous bleeding within the vasa vasorum. The most commonly affected coronary vessel is the left anterior descending coronary artery, although any of the coronary arteries and branches can be affected.9 In 9% to 18% of cases, SCAD is present in >1 coronary artery at presentation, which is referred to as multivessel SCAD.3, 5, 6, 7, 8, 12
Unfortunately, SCAD can be diagnosed on autopsy although it can be missed on macroscopic inspection.16 Cardiac computed tomography angiography is a noninvasive study that can also visualize SCAD.17 However, much remains to be understood about the appearance of acute SCAD on cardiac computed tomography angiography, as it can be inadvertently overlooked or not well visualized.17, 18, 39
5. ASSOCIATED CONDITIONS AND RISKS
Several SCAD‐associated factors and conditions have been identified. For instance, as many as 12% to 48% of patients report extreme exertion or intense stress/emotion prior to their SCAD ACS.5, 12 Examples include swimming in cold lakes, downhill skiing, recent unemployment, or death of a close relative/friend. Although the association of SCAD with extreme exertion has been reported more commonly among men,3, 19 this association is also observed among women.
Compared with matched controls, those with SCAD are 4× more likely to have coronary artery tortuosity.20 Extracoronary vascular arteriopathies such as dilatation, tortuosity, aneurysm, dissection, and fibromuscular dysplasia (FMD) are common (>60% when assessed systematically; Figure 1).5, 12, 21 FMD, a noninflammatory, nonatherosclerotic arteriopathy, is the most common vascular abnormality.5, 12, 21 More recently, in a Mayo Clinic series of 586 SCAD patients consisting primarily of women, there was a 40% lifetime and 27% 1‐year prevalence of migraine.22 Those with migraines reported more anxiety, depression, chest pain, and concern for future SCAD events.
Figure 1.
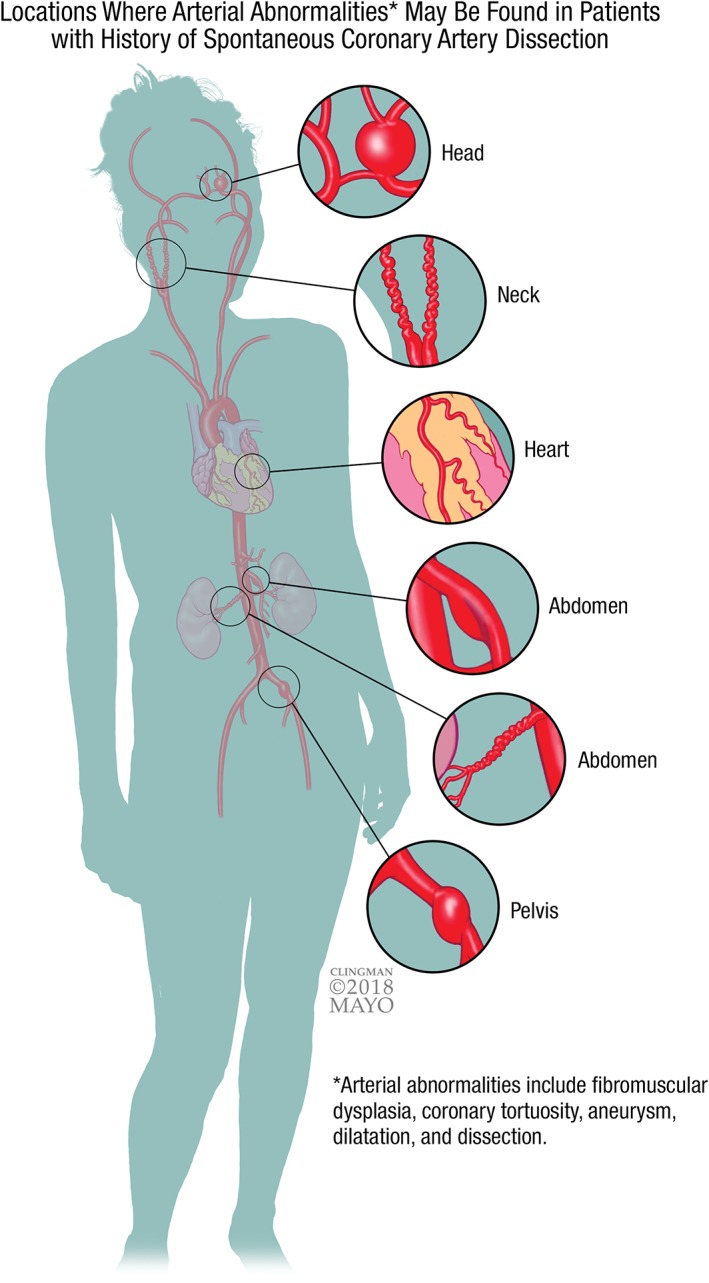
Although any arterial bed can be affected, this figure demonstrates common locations of where arterial abnormalities such as FMD, coronary artery tortuosity, aneurysm, dilatation, and dissection may be found in patients with history of SCAD. Abbreviations: FMD, fibromuscular dysplasia; SCAD, spontaneous coronary artery dissection
Additionally, approximately 5% of patients have heritable connective‐tissue disorders such as Marfan, Ehlers‐Danlos, and Loeys‐Dietz syndromes.5, 12, 23 In the Mayo Clinic “Virtual” Multicenter SCAD Registry study, 1% of 412 patient enrollees with SCAD had a family member with angiographically or pathologically confirmed SCAD. Variable inheritance patterns (eg, mother‐daughter, sisters, aunt‐niece) suggest complex genetic correlations.24
SCAD is one of the most common etiologies of MI among pregnant and postpartum women,11 accounting for ≤18% of all SCAD cases among women.3 Importantly, pregnancy‐associated SCAD (P‐SCAD) presents more severely than SCAD occurring in patients without pregnancy, including a higher prevalence of ST‐segment elevation myocardial infarction, SCAD involving the left main coronary artery or multiple vessels simultaneously, and reduced left ventricular systolic function.12 P‐SCAD may occur any time during or following pregnancy, but it is most commonly reported within the first month, often during the first week, postpartum. Some women noted onset of SCAD symptoms when breastfeeding. Compared with the general US maternal population, women with P‐SCAD more often had history of multiple childbirths, infertility treatment, and preeclampsia.12
6. INPATIENT TREATMENT
Upon diagnosis of SCAD, the therapeutic approach should take into account differences in outcomes with percutaneous coronary intervention (PCI) in SCAD compared with atherosclerotic MI. Recent studies importantly identified that PCI for SCAD is more frequently unsuccessful than PCI for atherosclerotic‐related MI.3, 4, 5, 6, 7, 8 Challenges include wire insertion into the false lumen of the dissection, expansion and extension of the dissection or hematoma, and the necessity of inserting more stents than would otherwise be anticipated.4
In those patients who are stable or have preserved coronary blood flow, observation is currently preferred instead of attempts at revascularization. Unlike coronary atherosclerosis, SCAD can heal sometimes within days. Of note, however, an important minority (≤10%) of patients do experience SCAD progression within the first 1 to 6 days with interval reduction in coronary blood flow. For this reason, even conservatively managed patients may benefit from inpatient monitoring for several days immediately following SCAD.4, 5
Revascularization with PCI should be considered for women who are unstable or have poor (Thrombolysis In Myocardial Infarction flow 0–1) coronary blood flow. Coronary artery bypass grafting (CABG) is another possible revascularization approach, although surgeons anecdotally comment on the challenges related to friability of the dissected coronary. In a cohort of 189 patients, 20 patients (18 women) had CABG during the initial hospitalization, and 32 of 34 of target vessels were successfully bypassed. The 2 that were not successful were small vessels (distal left anterior descending and diagonal coronary arteries). Of the patients with CABG who had coronary angiography during follow‐up, 11 of 16 grafts were occluded, presumably because of competitive coronary blood flow due to native‐vessel healing.4 Despite graft “failure” on follow‐up, CABG can be a beneficial revascularization technique for limiting extent of myocardial injury at time of MI.
What exactly constitutes ideal medical management for SCAD is not clear. Medical therapies that are generally beneficial after MI, such as β‐blockers and angiotensin‐converting enzyme inhibitors, are encouraged especially when there is left ventricular systolic dysfunction. Although acute anticoagulation and aggressive antiplatelet therapies may prevent development of an intracoronary thrombus, such benefit must be balanced by the theoretical risk of hematoma expansion. Dual antiplatelet therapy is necessary for those who undergo acute PCI. However, many women managed conservatively are de‐escalated to low‐dose aspirin only, although this practice is largely based on expert opinion. SCAD itself is not considered an indication for chronic anticoagulation,9 but chronic anticoagulation may be appropriately used for indications such as left ventricular apical thrombus in a patient with an akinetic apex secondary to SCAD.
The role of statin therapy is not well understood. Unlike atherosclerotic MI, statins have not been shown to prevent recurrent SCAD.5 Therefore, statin use in the setting of SCAD is generally reserved for individuals who merit primary prevention of atherosclerosis based on risk algorithms and guidelines.5 Statin therapy may also be considered in those with a concurrent diagnosis of endothelial dysfunction.2
7. MAJOR ADVERSE CARDIAC EVENTS
Although in‐hospital death is uncommon in SCAD, there is a considerable burden of major adverse cardiac events in follow‐up (Table 1). Methodology and time to follow‐up varies according to cohort, with recurrent MI occurring in 1.6% to 18% of patients (21% 5‐year Kaplan–Meier estimates, 4.8%/y); recurrent SCAD occurring in 4.7% to 22% of patients (20% 5‐year Kaplan–Meier estimates, 2.8%/y); heart failure in 2% to 3.9% (3% 5‐year Kaplan–Meier estimates), and death ranging from 0% to 3.1% (0.3%/y).5, 6, 7, 8, 12 More than half of women have recurrent chest pains, even without demonstrable ischemia or worsening angiographic epicardial coronary appearance.5, 17, 25
8. OUTPATIENT CARE
Because of the unique aspects of SCAD and challenges patients can encounter, it is imperative that the outpatient clinician is well informed and discusses pertinent concerns in follow‐up (Figure 2). Referral to a center with cardiologists and vascular medicine physicians with experience caring for women with SCAD and/or FMD can be beneficial for individualizing a care plan for patients.
Figure 2.
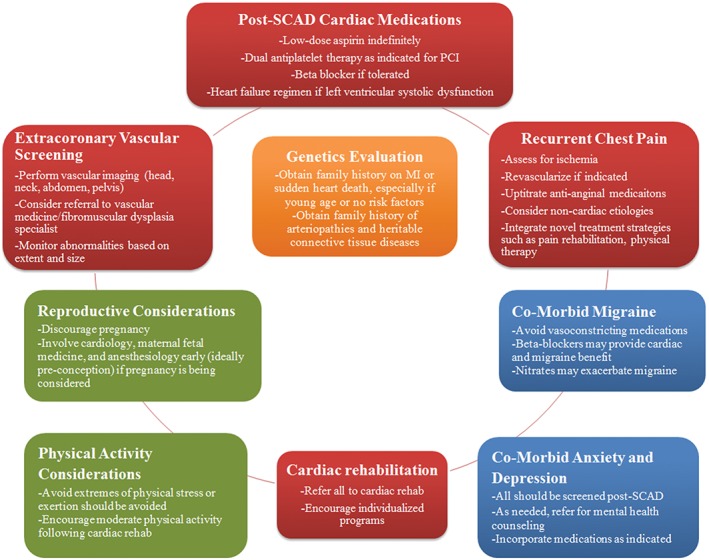
Pertinent considerations when assessing a woman with history of SCAD. Abbreviations: MI, myocardial infarction; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection
8.1. Extracoronary vascular arteriopathy
Because of the notable prevalence of extracoronary vascular arteriopathies, including subclinical intracerebral aneurysms (14%–23%)5, 21, 26 and FMD,5, 21 at least 1‐time vascular imaging is currently appropriate for women with SCAD.9 Though a portion of this evaluation may be performed in the catheterization laboratory (eg, renal angiogram), comprehensive outpatient computed tomography angiography (CTA) or magnetic resonance angiography may be used to assess head, neck, abdomen, and pelvis vasculature after hospital dismissal. CTA protocols dedicated for SCAD patients have been developed to limit radiation exposure and scanning time.27 Although magnetic resonance angiography does not involve ionizing radiation, it is limited by image resolution and speed of acquisition.28 Ultrasound imaging can detect elevated flow velocities in regions of interest (eg, renal arteries in those with renal FMD) and may be used to describe anatomy, but it is not recommended for initial screening studies.
Management of arterial abnormalities detected during screening must be individualized. For instance, asymptomatic small aneurysms and localized dissections may be monitored; the suggested frequency and modality is usually determined by location and severity. Ultrasound following a CTA diagnosis can be particularly useful for detecting significant stenosis in regions of FMD or dissection with associated luminal narrowing. These decisions should be guided by a vascular medicine specialist, particularly one with expertise in FMD who is aware of current research and practice recommendations.28
Even if imaging does not identify arteriopathy, there remains the possibility of undetected abnormalities. For instance, in one study, a patient with a negative CTA of the head, neck, chest, abdomen, and pelvis was found to have FMD of the brachial artery identified during coronary angiography with radial access.21 As such, some patients are counseled about avoidance of specific activities. Among SCAD patients with FMD, the likelihood of future vascular events is relatively low,29 but discussion regarding symptoms of possible stroke (eg, spontaneous carotid dissection) and when to seek care may be prudent.
8.2. Familial predisposition
Genetic studies are ongoing to better understand familial predisposition to SCAD. At this point, because it is rare for multiple family members to be affected, patients are counseled to inform relatives about signs and symptoms of MI. Beyond providing counseling, education, and when possible engaging in current research initiatives, clinicians usually do not conduct specific evaluation or additional monitoring of family members.
8.3. Genetics evaluation
Although only a small proportion of SCAD patients have heritable connective‐tissue diseases overall, the diagnosis of connective‐tissue disorders such as Marfan, Loeys‐Dietz, and Ehlers‐Danlos vascular subtype syndromes can have significant implications for the patient and family. Therefore, it is appropriate to refer patients with SCAD for medical genetics evaluation with discussion and consideration of genetic panel testing for known mutations. Such evaluation presents another opportunity to screen for other family members with history suspicious for SCAD (eg, early cardiac death of uncertain etiology), FMD, aneurysms, and other vascular abnormalities.
8.4. Reproductive counseling
SCAD often affects women of childbearing age. Due to the association of SCAD with pregnancy and uncertain risk of subsequent pregnancy related to recurrent SCAD, pregnancy after SCAD is generally discouraged. For many women, this represents a difficult loss. Among 8 women with pregnancy following SCAD, 1 had a recurrent, severe SCAD involving the left main coronary artery at 9 weeks postpartum, requiring emergency CABG. Her initial SCAD was not associated with pregnancy.25, 30 If a woman decides to pursue pregnancy, preconception involvement of a multidisciplinary team including cardiology, maternal‐fetal medicine, and anesthesiology is key to facilitate proactive monitoring strategies and contingency plans regarding the pregnancy.31
8.5. Recurrent chest pain
A large proportion of women (>50% in some cohorts25) report chest pain after SCAD, including a subgroup of women who experience premenstrual chest pain.32 In patients with new symptoms concerning for angina, evaluation for ischemia with stress testing and/or coronary imaging should be conducted. Some patients, for instance, have in‐stent restenosis requiring revascularization. However, many women have unremarkable studies. The differential includes endothelial dysfunction, microvascular disease, pericarditis, and noncardiac pain such as gastrointestinal reflux or musculoskeletal strain. Often, patients respond to antianginal therapies such as long‐acting nitrates, calcium channel blockers, or β‐blockers. However, symptomatic hypotension and other side effects can limit their use. Additional treatment options include ranolazine, L‐arginine, gabapentin, antidepressants or antianxiolytics, physical therapy, pain rehabilitation or pain clinic, spinal stimulation, or chest‐wall massage.
Because of the chest pain associated with menstruation, one might hypothesize a therapeutic role for exogenous hormone treatment following SCAD; however, evidence is lacking to validate such use. Due to the poorly understood role of the hormonal milieu and the association of SCAD with pregnancy, exogenous hormone use after SCAD may be harmful. At present, until evidence emerges to elucidate this matter, alternatives to exogenous systemic hormones for contraception or treatment of menopausal symptoms are preferred.
8.6. Migraines
Migraines are common among women with SCAD (lifetime prevalence estimated at 40%). Women with SCAD and migraine history report more chest pain, depression, anxiety, and concern for recurrent SCAD than other SCAD patients.22 Avoidance of medications with vasoconstrictive properties, such as triptans, is generally advised. β‐Blockers can be considered as a preventive strategy. Sometimes management of migraines can be challenging or limit titration of cardiac medications (eg, long‐acting nitrate may precipitate migraine) and collaborating with a neurology/headache team for alternative treatment options (eg, botulinum toxin) can be considered.
8.7. Cardiac rehabilitation and physical activity
Referral for cardiac rehabilitation is recommended for all persons following MI.33 However, in a survey of >350 SCAD patients, 96% of whom were women, the most common reason given by those who did not participate in cardiac rehabilitation (67%) was lack of referral.34 Among those who did participate, most experienced physical and mental health benefits. Cardiac rehabilitation in women with SCAD is both safe and beneficial,35, 36 especially if it is a tailored program, as many of these patients are physically fit at baseline.37
In light of the association of SCAD with extreme physical exertion, most women are advised against subsequent participation in competitive or contact sports, prolonged high‐intensity activities (especially leading to exhaustion), body‐building, or heavy weightlifting involving straining or performance of the Valsalva maneuver. There is currently little evidence to guide these recommendations, however. More restrictive guidelines might be employed in women with exercise‐triggered SCAD, recurrent SCAD, or in the presence of other dissections or aneurysms. Nevertheless, excessive restrictions on physical activity may be counterproductive or even harmful and can subject the individual to risks of other health problems such as depression, obesity, diabetes, and osteoporosis. All are encouraged to engage in moderate‐intensity, consistent physical activity, ideally guided by a regimen developed during cardiac rehabilitation.
8.8. Anxiety and depression
Anxiety and depression, including posttraumatic stress disorder, can occur after SCAD. Some women have preexisting diagnoses of anxiety and depression, and as many as 48% have SCAD events precipitated by emotional stress.5 SCAD is a life‐changing event requiring healthy coping strategies. Therefore, it is imperative that patients have access to appropriate screening, counseling with incorporation of mental health specialists, and medications when indicated.
8.9. Outpatient management of cardiac medications
Similar to acute management strategies, much remains to be learned about outpatient medication management following SCAD and current practice is often based on experience and cohort studies. Most patients are continued on indefinite low‐dose aspirin, extrapolated from known benefits among others at risk for MI. However, if a PCI was not performed, dual antiplatelet therapy is typically not continued, particularly for women with menorrhagia. β‐Blockers are beneficial in those with reduced left ventricular systolic function, and a recent Canadian study found that β blockade was associated with fewer recurrent events.5 Similarly, angiotensin‐converting enzyme inhibitors (or angiotensin receptor blockers) are considered beneficial in those with reduced left ventricular systolic function. Antianginals such as nitrates, ranolazine, calcium channel blockers, and β‐blockers can be helpful for treatment of angina.
9. THE FUTURE OF SCAD
Over the past 5 years, improvement in awareness and diagnosis of SCAD has prompted renewed efforts to better understand underlying pathophysiology, natural history, and optimal acute and long‐term treatments. Ongoing research efforts include cohorts such as the Mayo Clinic “Virtual” Multicenter SCAD Registry and DNA Biorepository38 and the Canadian SCAD Registries,5 among others.40, 41 Most recently, the American Heart Association and the European Society of Cardiology published a scientific statement and position paper on SCAD, respectively. Ultimately, these efforts aim to further knowledge of SCAD, improve future care, and perhaps even prevent events among patients.
10. CONCLUSION
Understanding the nuances of SCAD, a nonatherosclerotic etiology of ACS, is critical to improving delivery of care to women. This includes recognizing associated conditions such as extracoronary vascular abnormalities, coronary tortuosity, connective‐tissue diseases, family history, migraines, and potential triggers such as extreme emotion/exertion and pregnancy/postpartum. Management and counseling of SCAD patients is different than for individuals with atherosclerosis. Depending on the needs of the individual patient, incorporation of a multidisciplinary team including specialists in cardiology, vascular medicine, genetics, psychiatry, pain management, neurology, cardiac rehabilitation, radiology, and obstetrics can facilitate personalized care.
Conflicts of interest
The authors declare no potential conflicts of interest.
Tweet MS, Kok SN, Hayes SN. Spontaneous coronary artery dissection in women: What is known and what is yet to be understood. Clin Cardiol. 2018;41:203–210. 10.1002/clc.22909
Funding information National Institutes of Health, Grant/Award number: HD65987
REFERENCES
- 1. Mehta LS, Beckie TM, DeVon HA, et al; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation. 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 2. Tweet MS, Best P, Hayes SN. Unique presentations and etiologies of myocardial infarction in women. Curr Treat Options Cardiovasc Med. 2017;19:66. [DOI] [PubMed] [Google Scholar]
- 3. Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- 4. Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–786. [DOI] [PubMed] [Google Scholar]
- 5. Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 6. Lettieri C, Zavalloni D, Rossini R, et al. Management and long‐term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116:66–73. [DOI] [PubMed] [Google Scholar]
- 7. Rogowski S, Maeder MT, Weilenmann D, et al. Spontaneous coronary artery dissection: angiographic follow‐up and long‐term clinical outcome in a predominantly medically treated population. Cathet Cardiovasc Interv. 2017;89:59–68. [DOI] [PubMed] [Google Scholar]
- 8. Nakashima T, Noguchi T, Haruta S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the Angina Pectoris–Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol. 2016;207:341–348. [DOI] [PubMed] [Google Scholar]
- 9. Tweet MS, Gulati R, Hayes SN. What clinicians should know αbout spontaneous coronary artery dissection. Mayo Clin Proc. 2015;90:1125–1130. [DOI] [PubMed] [Google Scholar]
- 10. Saw J, Aymong E, Mancini GB, et al. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–819. [DOI] [PubMed] [Google Scholar]
- 11. Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy‐associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–1702. [DOI] [PubMed] [Google Scholar]
- 12. Tweet MS, Hayes SN, Codsi E, et al. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–435. [DOI] [PubMed] [Google Scholar]
- 13. Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68:297–312. [DOI] [PubMed] [Google Scholar]
- 14. Lindor RA, Tweet MS, Goyal KA, et al. Emergency department presentation of patients with spontaneous coronary artery dissection. J Emerg Med. 2017;52:286–291. [DOI] [PubMed] [Google Scholar]
- 15. Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073–1079. [DOI] [PubMed] [Google Scholar]
- 16. Desai S, Sheppard MN. Sudden cardiac death: look closely at the coronaries for spontaneous dissection which can be missed. A study of 9 cases. Am J Forensic Med Pathol. 2012;33:26–29. [DOI] [PubMed] [Google Scholar]
- 17. Tweet MS, Gulati R, Williamson EE, et al. Multimodality imaging for spontaneous coronary artery dissection in women. JACC Cardiovasc Imaging. 2016;9:436–450. [DOI] [PubMed] [Google Scholar]
- 18. Eleid MF, Tweet MS, Young PM, et al. Spontaneous coronary artery dissection: challenges of coronary computed tomography angiography. Eur Heart J Acute Cardiovasc Care. 2017. doi: 10.1177/2048872616687098. [DOI] [PubMed] [Google Scholar]
- 19. Fahmy P, Prakash R, Starovoytov A, et al. Pre‐disposing and precipitating factors in men with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9:866–868. [DOI] [PubMed] [Google Scholar]
- 20. Eleid MF, Guddeti RR, Tweet MS, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7:656–662. [DOI] [PubMed] [Google Scholar]
- 21. Prasad M, Tweet MS, Hayes SN, et al. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672–1677. [DOI] [PubMed] [Google Scholar]
- 22. Kok SN, Hayes SN, Cutrer FM, et al. Outcomes and prevalence of migraines in patients with spontaneous coronary artery dissections: a cohort study. Circulation. 2017;136:A20967. [Google Scholar]
- 23. Henkin S, Negrotto SM, Tweet MS, et al. Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart. 2016;102:876–881. [DOI] [PubMed] [Google Scholar]
- 24. Goel K, Tweet M, Olson TM, et al. Familial spontaneous coronary artery dissection: evidence for genetic susceptibility. JAMA Intern Med. 2015;175:821–826. [DOI] [PubMed] [Google Scholar]
- 25. Tweet MS, Olin JW. Insights into spontaneous coronary artery dissection: can recurrence be prevented? J Am Coll Cardiol. 2017;70:1159–1161. [DOI] [PubMed] [Google Scholar]
- 26. Lather HD, Gornik HL, Olin JW, et al. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: a report from the US registry for fibromuscular dysplasia. JAMA Neurol. 2017;74:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liang JJ, Prasad M, Tweet MS, et al. A novel application of CT angiography to detect extracoronary vascular abnormalities in patients with spontaneous coronary artery dissection. J Cardiovasc Comput Tomogr. 2014;8:189–197. [DOI] [PubMed] [Google Scholar]
- 28. Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions. A Scientific Statement From the American Heart Association. 2014;129:1048–1078. [DOI] [PubMed] [Google Scholar]
- 29. Kadian‐Dodov DL, Goldfinger J, Hairston J, et al. Natural history of cervical artery fibromuscular dysplasia and associated neurovascular events. J Am Coll Cardiol. 2016;67:2268. [DOI] [PubMed] [Google Scholar]
- 30. Tweet MS, Hayes SN, Gulati R, et al. Pregnancy after spontaneous coronary artery dissection: a case series. Ann Intern Med. 2015;162:598–600. [DOI] [PubMed] [Google Scholar]
- 31. Codsi E, Tweet MS, Rose CH, et al. Spontaneous coronary artery dissection in pregnancy: what every obstetrician should know. Obstet Gynecol. 2016;128:731–738. [DOI] [PubMed] [Google Scholar]
- 32. Tweet MS, Codsi E, Best PJ, et al. Menstrual chest pain in women with history of spontaneous coronary artery dissection. J Am Coll Cardiol. 2017;70:2308–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013. ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 34. Krittanawong C, Tweet MS, Hayes SE, et al. Usefulness of cardiac rehabilitation after spontaneous coronary artery dissection. Am J Cardiol. 2016;117:1604–1609. [DOI] [PubMed] [Google Scholar]
- 35. Silber TC, Tweet MS, Bowman MJ, et al. Cardiac rehabilitation after spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2015;35:328–333. [DOI] [PubMed] [Google Scholar]
- 36. Chou AY, Prakash R, Rajala J, et al. The first dedicated cardiac rehabilitation program for patients with spontaneous coronary artery dissection: description and initial results. Can J Cardiol. 2016;32:554–560. [DOI] [PubMed] [Google Scholar]
- 37. Naderi S, Weinberg I, Lindsay M, et al. Spontaneous coronary artery dissection patients significantly more fit than the average patient referred for exercise stress testing. J Am Coll Cardiol. 2015;65(suppl A):A315. [Google Scholar]
- 38. Tweet MS, Gulati R, Aase LA, et al. Spontaneous coronary artery dissection: a disease‐specific, social networking community–initiated study. Mayo Clin Proc. 2011;86:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tweet MS, Akhtar NJ, Hayes SN, Best PJ, Gulati R, Araoz PA. Spontaneous coronary artery dissection: Acute findings on coronary computed tomography angiography. Eur Heart J Acute Cardiovasc Care. 2018. Jan 1:2048872617753799 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hayes SN, Kim ESH, Saw J, et al. Spontaneous coronary artery dissection: Current state of the Science: A scientific statement from the American Heart Association. Circulation, 2018. Feb 22 [Epub ahead of print] pii: CIR.0000000000000564. 10.1161/CIR.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018. Feb 22. 0, 1–21 [Epub ahead of print] 10.1093/eurheartj/ehy080 [DOI] [PMC free article] [PubMed] [Google Scholar]


