Abstract
Pyrethroid-treated clothing is commonly worn for protection against mosquitoes; pyrethroids are both insecticides and repellents. Pyrethroid resistance has become increasingly common in Aedes aegypti, the vector of dengue, Zika, and other arboviruses, but it is not clear whether resistance is associated with reductions in repellency. In order to determine whether long-lasting permethrin impregnated (LLPI) clothing is protective, we used Aedes aegypti from New Orleans, LA (pyrethroid-sensitive) and San Juan, PR (resistant) to measure both lethality and repellency. PCR and Sanger sequencing were used to confirm resistance status by detecting mutations in the kdr gene at positions 1016 and 1534. Arm-in-cage trials of 100 Aedes aegypti females from both populations were performed for 10 minutes to bare arm or an arm clothed in untreated military camouflage or military camouflage impregnated with deltamethrin, permethrin, or etofenprox. Trials were repeated 4–5 times on different days. Number of landings, number of blood meals, and immediate and 24-hour mortality were recorded. Mortality was extremely low in all trials. Compared to untreated cloth, mosquitoes demonstrated a trend towards a 2%-63% reduction in landings and a statistically significant 78–100% reduction in blood feeding on pyrethroid-treated cloth for most insecticides. Effects were observed in both pyrethroid-sensitive and pyrethroid-resistant mosquito populations. Our data show that kdr mutations are associated with pyrethroid resistance but are likely not the only contributors. Pyrethroids appear to maintain repellent effect against resistant mosquitoes. This finding suggests that even in places where pyrethroid resistance is widespread, permethrin still has a role for use as a repellent on clothing to protect against mosquito bites.
Introduction
Aedes aegypti mosquitoes are the vectors of several important human infections including dengue, Zika, chikungunya, and yellow fever, which affect hundreds of millions of people each year globally in tropical and subtropical regions, causing a burden of disease equal to more than four million disability-adjusted life years in 2013.[1,2] Vector control remains a key component in preventing these illnesses, and pyrethroid insecticides have been among the most widely used vector control tools because of their efficacy and favorable mammalian toxicity profile. Pyrethroid insecticides are used for indoor and outdoor spraying and currently are the only class used for treating bed nets;[3] additionally, they are gaining popularity for use in clothing.[4–8] The widespread use of pyrethroids, however, has led to increasing resistance to some or all pyrethroids in many Aedes populations.
Pyrethroids exert their insecticidal effect on the voltage-gated sodium channel (VGSC) located on the membrane of neurons. When pyrethroids bind an open channel, they prevent its closure, thus prolonging the action potential and resulting in the insect’s rapid paralysis, known as “knockdown” or kdr, and death. Several point mutations in the Aedes aegypi VGSC have been identified, though only a few have been definitively linked to a resistant phenotype.[9–14] Two SNPs in domain II and domain III, at positions 1016 and 1534, respectively, have been well studied. In Latin American Ae. aegypti populations, isoleucine replaces valine at position 1016 and cysteine replaces phenylalanine at position 1534. Both mutations have been associated with high-level kdr resistance, though the evolution of and interaction between these two mutations are not well-understood.[15] While there is substantial geographic variability in the distribution of particular kdr mutations, this mechanism of pyrethroid resistance has become widespread,[16] compromising the utility of these insecticides for vector control.
In addition to their insecticidal effect, pyrethroids have both spatial repellent and contact irritant effects,[17–19] though excitorepellency is probably the most important effect when pyrethroids are used on clothing. Limited evidence suggests that pyrethroid-resistant populations may actually be more strongly repelled by pyrethroids and other repellents than fully susceptible mosquitoes.[20–22] If pyrethroids have potent repellent effect against both susceptible and resistant Ae. aegypti, then they may remain useful tools for personal protection against mosquitoes despite the increasing prevalence of pyrethroid resistance worldwide.
In this study, we tested repellent activity of military-grade cloth factory-impregnated with three different pyrethroid insecticides (permethrin, deltamethrin, and etofenprox) against susceptible and resistant strains of Ae. aegypti. Genetic markers of resistance, specifically kdr mutations, were documented in these same populations. Our results demonstrate that pyrethroid-impregnated cloth can exhibit repellent activity even against pyrethroid-resistant mosquito populations.
Materials and methods
Mosquito colony origin and maintenance
Susceptible Aedes aegypti eggs were collected in New Orleans, LA in 2008,[23,24] and generation F12-13 mosquitoes (NO mosquitoes) were used in tests. Resistant Ae. aegypti eggs were collected from San Juan, Puerto Rico in 2016,[25] and generation F1-2 adult females (PR mosquitoes) were used in bioassays. Although mosquito colonies were derived from eggs collected in different years, all experiments were performed over a brief period in the fall and winter of 2016–2017 using adult mosquitoes descended from the original collected mosquito eggs. Mosquito colonies were maintained as described previously.[23,26] Colonies were maintained in separate containers at approximately 26°C and at a relative humidity of ≈75% under a photo regime of 14 light:10 dark hours. The light phase included two 30-min crepuscular periods (40-watt incandescent bulb) daily. Larvae were fed a 2:1 mixture of liver powder:baker’s yeast on a standardized schedule. Adults were housed in 30 × 30 × 30-cm Plexiglas cages fitted with cotton surgical stocking tops and fed 10% sucrose solution ad libitum.[26] Adult females were not permitted to blood-feed prior to experiments.
Mosquito DNA extraction
Ae. aegypti mosquitoes used for kdr genotyping were field collected from Puerto Rico (resistant) and New Orleans (susceptible). Mosquito DNA was extracted using published protocols.[27,28] Briefly, individual mosquitoes were manually homogenized in 100 μl PBS and 10 μl 10% saponin solution. The mosquito homogenate was incubated for 20 minutes at room temperature then centrifuged at 20,000 x g for 2 minutes. The pellet was resuspended in 200 μl PBS and centrifuged again; the supernatant was removed and the pellet was resuspended in 75 μl deionized water and 25 μl Chelex then gently vortexed and incubated in a 95–99°C water bath for 13 minutes. Finally, the solution was centrifuged for 2 minutes at 20,000 x g and the supernatant containing gDNA was transferred to a clean tube and stored at -20°C.
Gene amplification and sequencing
The voltage-gated sodium channel (kdr) gene from Ae. aegypti was amplified using a protocol adapted from Sayano, et al.[29] The IIS6 and IIIS6 regions of the kdr gene were amplified using the following primers: KasaikdraegSCF20 5’-GACAATGTGGATCGCTTCCC-3’ and KasaiddraegSCR21 5’-GCAATCTGGCTTGTTAACTTG-3’ (domain II) and KasaikdraegSCF7 5’-GAGAACTCGCCGATGAACTT-3’ and KasaikdraegSCR7 5’GACGACGAAATCGAACAGGT-3’ (domain III) and FastStart High Fidelity PCR system (Roche, Mannheim, Germany). PCR reactions contained 5 μl template DNA in a mixture of 12.75 μl water, 2.5 μl 10X FastStart High Fidelity Reaction Buffer, 0.25 μl FastStart HighFidelity Enzyme Blend, 0.5 μl 10mM dNTPs, 2 μl forward primer, and 2 μl reverse primer. Amplification was performed under the following conditions: 95°C for 2 minutes; 35 cycles of 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds; 72°C for 5 minutes and a 4°C hold.
Sanger sequencing was then performed on PCR products using the primers aegSCF3 5’-GTGGAACTTCACCGACTTCA-3’ for domain II and aegSCR8 5’-TAGCTTTCAGCGGCTTCTTC-3’ for domain III (Eton). Sequences were aligned with published sequences (Genbank IDs KJ957878-KJ957893 and KF537414-15, JF4796611-12, and JX275501) using Mega software (www.megasoftware.net).[30]
Arm-in-cage testing
Mosquito knock-down testing was performed using United States military cloth (6.8 oz/yd2 50% nylon/50% cotton rip stop printed with current ACU camouflage pattern) either untreated or chemically impregnated with 0.52% insecticide (deltamethrin, permethrin, or etofenprox) by weight as an even application, or by bare arm. The arm was cleaned with 70% ethanol and allowed to dry between trials. The forearm of a volunteer was covered with a piece of the candidate cloth. A cover constructed from white EPDM (60 mil) rubber roofing membrane was attached over the cloth with Velcro straps. The cover had an opening (33 x 150 mm) that exposed the candidate cloth. The volunteer’s hand and wrist were covered with a nitrile glove. At the start of each test, the volunteer inserted his forearm into a Plexiglas cage (30 cm on each side) through a cloth sleeve. The cage contained exactly one hundred 5–7 day old female Aedes aegypti mosquitoes (either NO or PR strain). In a few cases, 1 or 2 mosquitoes died before exposure to the clothing; in these cases, analyses were adjusted for the slight decrease in number of exposed mosquitoes by dividing by counts by the proportion alive at the beginning of each trial. Each bioassay was conducted for 10 minutes during which the number of mosquitoes landing on the cloth was counted. A mosquito was counted as landing if it touched the cloth for at least 2 seconds. The number of mosquitoes that probed the cloth and obtained a blood meal was also counted. Mosquito mortality was determined 24 hours after each bioassay was terminated. Tests were repeated for each fabric on four different days for New Orleans mosquitoes and on five different days for Puerto Rico mosquitoes, with one replicate per day. Untreated cloth was used as the control each day. Bare arm control was used in two trials to assess any repellent effect of the untreated cloth by itself. All experiments were performed between October 2016 and February 2017. Temperature for the trials ranged from 25.7 to 28.1°C and relative humidity ranged from 67.4 to 88.0%. Two male human subjects were used for the test but were not systematically rotated. Subjects provided both written and verbal informed consent (North Carolina State University IRB protocol 2925, annual renewal approved 6/21/2017).
Statistical analyses
Associations between mosquito strain (NO or PR) and the presence of kdr mutations were assessed using Chi-squared tests. Insecticidal effect was defined as the increase in 24-hour mortality between control (untreated) and treated cloth. Repellency was measured two ways: 1) reduction in number of landings and 2) reduction in number of blood meals between control and treatment tests performed on the same day. These numbers were standardized to the number of live mosquitoes present at the beginning of a given test. Results were compared using Student’s t-test, Kruskal-Wallis equality-of-populations rank test, Wilcoxon rank sum test (Mann Whitney two sample statistic), and Wilcoxon matched pairs signed rank test with untreated cloth as the referent. Repellency was expressed as reduction in landing or blood feeding behavior calculated by the following equation:
| (1) |
for landings or blood meals. The control count used for each calculation was the one performed on the same day as the treatment count.
Ethics
Protocol 2925 was approved by the North Carolina State University Institutional Review Board. The approved procedure involved arm-in-cage studies of pyrethroid insecticides against Aedes aegypti mosquitoes. This procedure has been reviewed and approved by the North Carolina State University Institutional Review Board (IRB Protocol 2925, annual renewal approved 6/21/2017). Both verbal and written informed consent were obtained from study participants.
Results
The gene for the Ae. aegypti voltage-gated sodium channel (kdr) was amplified from 19 mosquitoes from the pyrethroid-susceptible New Orleans population (NO) and 22 mosquitoes from the pyrethroid-resistance Puerto Rico population (PR). The kdr gene was successfully sequenced at domain II (1016 location) from 19 NO mosquitoes and 18 PR mosquitoes and at domain III (1534 location) from 19 NO and 15 PR mosquitoes. Results are summarized in Table 1. Heterozygosity was common in both populations at both SNPs, though both kdr mutations were more common in the PR mosquitoes, as expected. While PR mosquitoes were more likely to have either kdr mutation, this relationship was only statistically significant for the presence of at least one 1016 mutation (OR 13.71, 95% CI 2.04, 145.62). No mosquitoes were homozygous for kdr mutations at both SNPs. All PR mosquitoes had at least one kdr mutation, while only 11/19 (58%) of NO mosquitoes had at least one kdr mutation (p = 0.01). PR mosquitoes were more likely to have both mutations, though this was not statistically significant (p = 0.1). Thus, kdr alleles were present in both populations but were found in all members of the PR population.
Table 1. kdr (voltage gated sodium channel) gene sequencing from susceptible and resistant mosquito populations.
| Wild type | Heterozygous | kdr | No sequence | No kdr | 1 kdr | Both kdr | |
|---|---|---|---|---|---|---|---|
| Susceptible (NO) | 8 | 6 | 5 | ||||
| 1016 | 12 | 6 | 1 | 0 | |||
| 1534 | 10 | 9 | 0 | 0 | |||
| Resistant (PR) | 0 | 5 | 6 | ||||
| 1016 | 2 | 14 | 2 | 4 | |||
| 1534 | 3 | 9 | 3 | 7 |
Arm-in-cage tests were performed on nine different days. There was wide variability in biting behavior between days despite the temperature- and humidity-controlled environment. In a multivariate linear regression model, neither temperature nor relative humidity was associated with landing and feeding behavior, adjusted for mosquito strain and clothing treatment (untreated vs. treated).
Compared to bare arm, untreated cloth protected against landing and blood feeding (p = 0.03 for both by Wilcoxon rank-sum test). Untreated cloth was used as a control for the evaluation of pyrethroid-treated fabric in arm-in-cage tests, shown in Table 2. By Kruskal-Wallis test, there was no statistically significant difference in reduction in landings between clothing treatments for the NO mosquito strain (p = 0.2), but insecticides did have a statistically significant effect on landing for the resistant PR strain (p = 0.02). The reduction in number of blood meals taken was significantly different for PR mosquitoes (p = 0.02), with a trend towards significance in the NO group (p = 0.06). When fabrics impregnated with different insecticides were compared to untreated cloth using pair-wise Wilcoxon signed rank test, we observed a protective effect of pyrethroid-treated clothing against landing and blood-feeding, but the difference did not reach statistical significance in most cases (Table 2). While all treated clothing reduced the number of landings compared to untreated clothing (Figs 1 and 2), this effect was only significant for the reduction in landings by permethrin. However, the reduction in blood meals compared to untreated control was statistically significant in most trials, with the exception of deltamethrin in the NO group (Table 2). Mosquito mortality at 24 hours was surprisingly low in all trials (0–11%), with no significant differences between resistant and susceptible populations or between untreated and insecticide-impregnated cloth.
Table 2. Results of arm-in-cage testing for pyrethroid-susceptible (NO) and pyrethroid-resistant (PR) mosquitoes.
Results are expressed as the median (range) of the trials. Four replications were performed for trials of NO mosquitoes. Fiver replications were performed for PR mosquitoes, with the exception of 4 trials for deltamethrin. All % reduction measurements are made compared to same-day untreated cloth controls.
| Landings | % reduction in landings | Blood meals | % reduction in blood meals | % landings that blood fed | % reduction in blood meals/landing | 24-hour mortality (%) | |
|---|---|---|---|---|---|---|---|
| Susceptible (NO) | |||||||
| Untreated | 273 (48–442) | . | 5.5 (1–14) | . | 2.1 (1.0–4.0) | . | 0.5 (0–2) |
| Permethrin | 129 (19–227)# | 44 (34–60)† | 0 (0–1)# | 100 (89–100)* | 0 (0–0.4)# | 100 (78–100)* | 0 (0–3) |
| Deltamethrin | 135 (54–225) | 31 (-159-88) | 0.5 (0–1) | 94 (-1-100) | 0.4 (0–1.9)# | 80 (9–100)* | 1.5 (0–4) |
| Etofenprox | 256 (44–368) | 4 (-88-63) | 1 (0–3)# | 93 (67–100)* | 0.3 (0–1.9)# | 93 (9–100)* | 0 (0–4) |
| Resistant (PR) | |||||||
| Untreated | 200 (127–357) | . | 4 (0–15) | . | 1.7 (0–7.9) | . | 0 (0–1) |
| Permethrin | 78 (38–125)† | 63 (46–78)# | 0 (0–0)# | 100 (100–100)† | 0 (0–0)# | 100 (100–100)† | 0 (0–7) |
| Deltamethrin | 107 (62–213)# | 44 (32–71)# | 0 (0–1)# | 100 (66–100)* | 0 (0–1.0) | 100 (-17, 100) | 0 (0–3.1) |
| Etofenprox | 182 (22–308) | 2 (-4-83) | 1 (0–3)# | 78 (0–100)* | 0.3 (0–2.7) | 78 (-221-100) | 0 (0–11) |
†p≤0.01
*p≤0.05
#p≤0.10
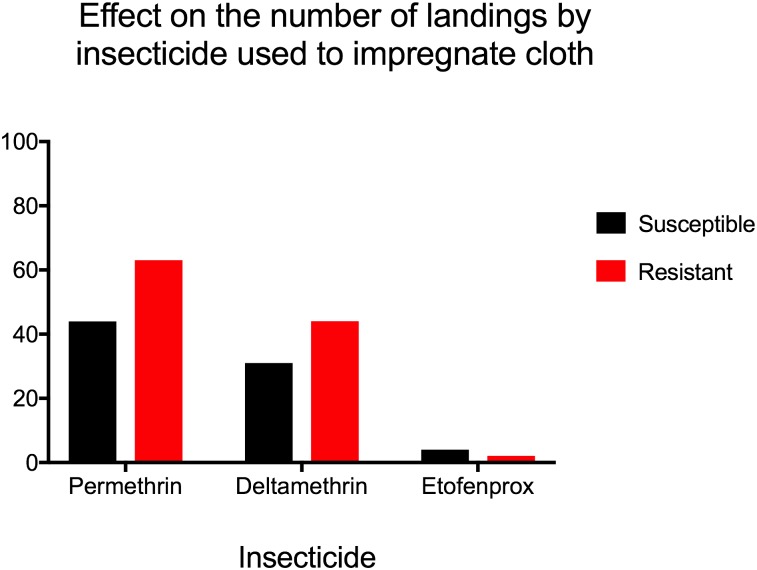
Fig 1. Effect on the number of landings by insecticide used to impregnate cloth.
Referent group is untreated cloth. All three insecticides reduced number of landings.
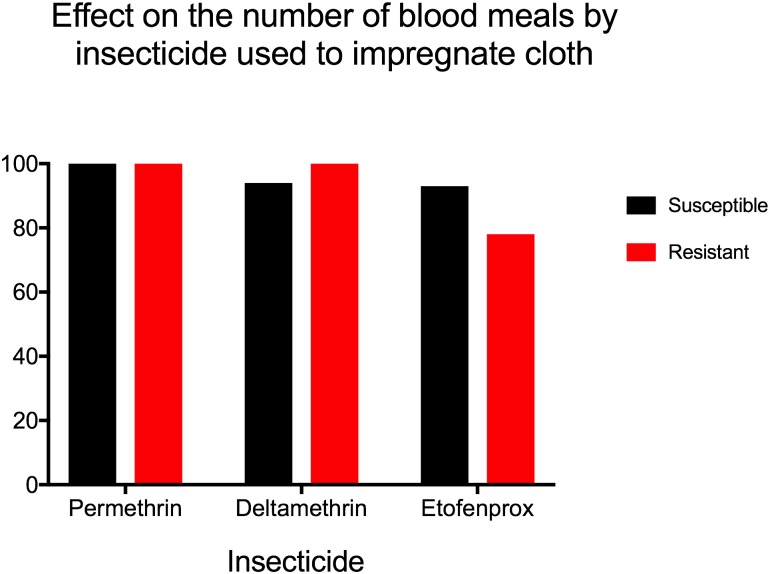
Fig 2. Effect on the number of blood meals by insecticide used to impregnate cloth.
Referent group is untreated cloth. All three insecticides reduced the number of mosquitoes taking a blood meal by 78–100%, with similar results for sensitive (NO) and resistant (PR) mosquito populations.
There was not a significant difference in reduction of mosquito landings or blood meals by deltamethrin or etofenprox between strains; however; permethrin caused a significantly stronger reduction in landing for the PR mosquitoes (p = 0.05). Additionally, the effect of pyrethroid-treated cloth was even stronger in reducing blood feeding than in preventing landings, almost completely abolishing blood feeding activity in the case of permethrin and deltamethrin (Table 2, Figs 1 and 2).
Discussion
Here we demonstrate that even in the absence of significant insecticidal activity, clothing impregnated with the pyrethroids permethrin, deltamethrin, and etofenprox exhibited the ability to repel Aedes aegypti mosquitoes. This effect was observed for both pyrethroid-sensitive and pyrethroid-resistant strains of mosquito.
Mosquito resistance to pyrethroids has become a global problem. Of the major mosquito-borne diseases, only yellow fever has a highly effective vaccine available. Thus, vector control remains the cornerstone of interventions to prevent arboviruses and malaria. As illustrated by the incomplete protection afforded by currently available vaccines for dengue and malaria [31–37] and by the vaccine shortages complicating control of the recent yellow fever outbreaks in Brazil, Angola, and the Democratic Republic of Congo,[38–43] vector control remains a crucial public health intervention for prevention and control of mosquito-borne infections. Because of pyrethroids’ safety and potency, they are widely used as adulticides, leading over time to the development of high levels of pyrethroid resistance in many areas most affected by arboviruses and malaria.
Pyrethroids are also widely used for community and household protection via indoor and outdoor area spraying and for personal protection through the use of insecticide impregnated for bed nets and clothing. While area spraying’s effect is mainly realized by reduction in the population of mosquitoes in the area, pyrethroid-treated bed nets and clothing can provide complete personal protection from vector-borne disease simply by preventing bites. Even if mosquitoes are pyrethroid-resistant, bed nets and clothing may remain effective if pyrethroids maintain their repellent effect. In this study, we demonstrated that several pyrethroids maintain repellent effect against resistant mosquitoes, implying that pyrethroid-treated clothing could remain an important tool for personal protection against mosquito-borne infection.
Unexpectedly, we were not able to detect any statistical difference in mortality between NO and PR mosquitoes exposed to any of the pyrethroids, but this is likely because of extremely low mortality even in the susceptible population. Although the NO mosquitoes used in this study are documented to be pyrethroid-susceptible,[24] it is possible that they are not completely sensitive to the insecticides used here, given that we found kdr mutations in a large proportion of the tested mosquitoes and observed surprisingly low mortality after arm-in-cage testing.
In our arm-in-cage tests, untreated cloth itself did not reduce landings by mosquitoes, but based on experiments with pyrethroid-sensitive mosquitoes, it did appear to reduce mosquito biting and feeding, likely through its barrier function. Despite low mortality in all tests, cloth treated with all three pyrethroids exhibited strong effect to reduce number of landings and number of blood meals compared to control, though this difference only reached statistical significance for blood meals (most tests) and for landings by NO mosquitoes exposed to permethrin. Permethrin and deltamethrin appeared to be stronger repellents than etofenprox, though though we lacked power to test this relationship; however, all three insecticides had repellent effect against blood meals compared to untreated cloth or bare arm. The difference suggested by our data corroborates a recent study finding that etofenprox is less protective against blood feeding even in pyrethroid-resistant mosquito populations.[44]
Our results are in agreement with other studies that have shown that resistant mosquitoes are repelled by pyrethroids. One in vitro study demonstrated a lack of association between Aedes aegypti mortality and contact irritant effect (measured by proportion of mosquitoes fleeing from a treated surface after contact) for a variety of insecticides including several pyrethroids.[21,45] Agossa et al. showed that wild Anopheles mosquitoes in areas with known pyrethroid resistance were spatially repelled by indoor residual spraying of huts in semi-field conditions, with 22–28% fewer mosquitoes entering pyrethroid-treated huts compared to control huts. Blood-feeding was not significantly different inside the treated huts, but human baits did not sleep under nets or use repellents.[46] Our observation that deltamethrin and etofenprox-treated clothing were not superior to permethrin-treated clothing supports the possibility that permethrin-resistance does not affect repellency.
The repellent effect of permethrin against even pyrethroid-resistant mosquitoes is likely to be most important for personal protective measures such as insecticide-impregnated clothing. The efficacy of permethrin-treated clothing against mosquitoes has been demonstrated in many laboratory studies and short-duration field trials.[4,6,7,44,45,47–60] Another laboratory study showed a similar reduction in landing and blood feeding behavior with permethrin-treated clothing compared to untreated clothing using slightly different methods.[45,51] As in our work, they found little difference in landing and blood feeding between resistant and susceptible mosquitoes. Interestingly, in an arm-in-cage experiment in which the arm was only partially covered by cloth, providing mosquitoes with a choice of bare or clothed skin to land and feed on, they observed that resistant mosquitoes rested longer on permethrin-treated cloth and were less likely to move to more bite-susceptible bare arm than susceptible mosquitoes. They interpreted this as a reduction in repellent effect on resistant mosquitoes; but when the arm was fully covered, they did not see a difference in landing or blood feeding, suggesting that repellent effect is robust for both strains.[45]
Field studies of permethrin-impregnated clothing also support a repellent effect for treated clothing, though by design they are unable to examine differences in effectiveness between pyrethroid-resistant and susceptible mosquitoes. A US-based study in park rangers documented a significant difference in antibody titer to Aedes spp. salivary antigen between treated and control groups, suggesting that this clothing did exert a sizable protective effect against mosquito biting.[50] The largest field-based trial of factory-treated clothing found no effect on 5-month dengue incidence of permethrin-impregnated school uniforms in a cohort of 1811 Thai children, but poor quality cloth that permitted rapid washout of insecticide with laundering was a major limitation to this study. However, they did detect a reduction in the number of Ae. aegypti mosquitoes captured in classrooms randomized to treated clothing during the first month of the study, prior to washout of the permethrin, suggesting that the clothing did have a repellent effect on mosquitoes.[8]
Our study has several limitations. First, we are comparing two mosquito populations which have been bred in the laboratory for different periods of time since egg collection. This difference likely affects the populations’ heterozygosities and possibly their biology and behavior. Second, based on genotyping results, kdr mutations are found frequently in “susceptible” NO mosquitoes, raising questions about the effect of pyrethroids on this population. Although kdr mutations associated with pyrethroid resistance were detected even in phenotypically susceptible populations, sequencing confirmed that phenotypically resistant PR mosquitoes were significantly more likely than susceptible NO mosquitoes to have one or both of two kdr mutations, 1016I or 1534C. While we did not perform genetic testing on the exact same mosquitoes that were used for arm-in-cage tests, they came from the same population and would be expected to have a similar distribution; however, this limited our ability to correlate specific kdr mutations or combinations of mutations with mortality or with landing and feeding behavior. Second, we did not look for other mutations in the VGSC gene, which might have affected resistance to or repellency by the pyrethroids used in this study. Third, we were unable to examine another common mode of resistance, upregulation of detoxifying enzymes such as CYP 450 enzymes,[61] and thus cannot determine how any insecticide resistance due to metabolic changes affects the repellent activity of pyrethroids. In at least one study, however, kdr mutations were the more important mechanism of resistance to pyrethroids in adult Ae. aegypti.[13] Finally, limited sample size restricts our ability to make robust statistical inferences.
Dengue incidence has increased since 2005 despite economic development and vector control efforts.[62] Recent Zika and chikungunya virus epidemics have demonstrated that new arboviral threats are likely to emerge. Because vaccines are not yet widely available for most Aedes-transmitted infections and their treatment is supportive, public health institutions will continue to rely on vector control activities to limit arboviral disease transmission. Increasing prevalence of pyrethroid resistance in mosquitoes threatens the effectiveness of vector control activities worldwide. We examined the repellent effect of pyrethroid-impregnated clothing on resistant and susceptible strains of Ae. aegypti. Although there was not a statistically significant, the magnitude of the repellent effect against landing, blood meals, and blood meals per landing was greater for the resistant mosquitoes. These findings provide evidence that even in the absence of an insecticidal or knockdown effect, pyrethroid-treated cloth can protect against mosquito bites and disease transmission. Both susceptible NO and resistant PR mosquitoes were repelled by treated cloth, implying that for person protection, pyrethroid-impregnated cloth for use in bed nets and clothing will likely remain efficacious even in areas where pyrethroid-resistant mosquitoes are abundant. All in all, our data suggest that LLPI clothing may play an important role in personal protection against mosquito-borne diseases.
Supporting information
The first tab (Puerto Rico) provides results from the pyrethroid-resistant Puerto Rico strain. The second tab (New Orleans) provides results from the pyrethroid-sensitive New Orleans strain.
(XLSX)
Provides data from arm-in-cage trials.
(XLSX)
Acknowledgments
Insect Shield (Greensboro, NC) provided the cloth used for arm-in-cage testing. Insect Shield had no input on study design, data analysis, or manuscript preparation. We thank Andreea Waltmann for assistance with the figures.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond Engl. 2015. August 22;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015. January 10;385(9963):117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO/Department of control of neglected tropical diseases. Pesticides and their application, for the control of vectors and pests of public health importance. 6th ed World Health Organization; 2006. 114 p. [Google Scholar]
- 4.Lane RS. Treatment of clothing with a permethrin spray for personal protection against the western black-legged tick, Ixodes pacificus (Acari: Ixodidae). Exp Appl Acarol. 1989. May;6(4):343–52. [DOI] [PubMed] [Google Scholar]
- 5.Faulde M, Uedelhoven W. A new clothing impregnation method for personal protection against ticks and biting insects. Int J Med Microbiol IJMM. 2006. May;296 Suppl 40:225–9. [DOI] [PubMed] [Google Scholar]
- 6.Schreck CE, Mount GA, Carlson DA. Pressurized sprays of permethrin on clothing for personal protection against the lone star tick (Acari: Ixodidae). J Econ Entomol. 1982. December;75(6):1059–61. [DOI] [PubMed] [Google Scholar]
- 7.Vaughn MF, Meshnick SR. Pilot study assessing the effectiveness of long-lasting permethrin-impregnated clothing for the prevention of tick bites. Vector Borne Zoonotic Dis Larchmt N. 2011. July;11(7):869–75. [DOI] [PubMed] [Google Scholar]
- 8.Kittayapong P, Olanratmanee P, Maskhao P, Byass P, Logan J, Tozan Y, et al. Mitigating Diseases Transmitted by Aedes Mosquitoes: A Cluster-Randomised Trial of Permethrin-Impregnated School Uniforms. PLoS Negl Trop Dis. 2017. January;11(1):e0005197 doi: 10.1371/journal.pntd.0005197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Fernandez-Salas I, et al. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol. 2007. December 1;16(6):785–98. doi: 10.1111/j.1365-2583.2007.00774.x [DOI] [PubMed] [Google Scholar]
- 10.Wuliandari JR, Lee SF, White VL, Tantowijoyo W, Hoffmann AA, Endersby-Harshman NM. Association between Three Mutations, F1565C, V1023G and S996P, in the Voltage-Sensitive Sodium Channel Gene and Knockdown Resistance in Aedes aegypti from Yogyakarta, Indonesia. Insects. 2015. July 23;6(3):658–85. doi: 10.3390/insects6030658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris AF, Rajatileka S, Ranson H. Pyrethroid Resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg. 2010. August 5;83(2):277–84. doi: 10.4269/ajtmh.2010.09-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanola J, Somboon P, Walton C, Nachaiwieng W, Prapanthadara L. A novel F1552/C1552 point mutation in the Aedes aegypti voltage-gated sodium channel gene associated with permethrin resistance. Pestic Biochem Physiol. 2010. March 1;96(3):127–31. [Google Scholar]
- 13.Haddi K, Tomé HVV, Du Y, Valbon WR, Nomura Y, Martins GF, et al. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: a potential challenge for mosquito control. Sci Rep. 2017. April 19;7:srep46549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Nomura Y, Zhorov BS, Dong K. Sodium Channel Mutations and Pyrethroid Resistance in Aedes aegypti. Insects. 2016. October 31;7(4):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black WC Iv. Coevolution of the Ile1,016 and Cys1,534 Mutations in the Voltage Gated Sodium Channel Gene of Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2015. December;9(12):e0004263 doi: 10.1371/journal.pntd.0004263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith LB, Kasai S, Scott JG. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic Biochem Physiol. 2016. October;133:1–12. doi: 10.1016/j.pestbp.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyay P, Dhiman S, Devi KA, Banerjee S, Rabha B, Chaurasia A, et al. Ultra low concentration deltamethrin loaded patch development and evaluation of its repellency against dengue vector Aedes (S) albopictus. Parasit Vectors. 2013. September 28;6:284 doi: 10.1186/1756-3305-6-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manda H, Shah P, Polsomboon S, Chareonviriyaphap T, Castro-Llanos F, Morrison A, et al. Contact Irritant Responses of Aedes aegypti Using Sublethal Concentration and Focal Application of Pyrethroid Chemicals. PLoS Negl Trop Dis. 2013. February 28;7(2):e2074 doi: 10.1371/journal.pntd.0002074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miot HA, Ferreira DP, Mendes FG, Carrenho FRH, de Oliveira Amui I, Carneiro CAS, et al. Efficacy of topical permethrin as repellent against Aedes aegypti’s bites. Dermatol Online J. 2008. July 15;14(7):1 [PubMed] [Google Scholar]
- 20.Thanispong K, Achee NL, Bangs MJ, Grieco JP, Suwonkerd W, Prabaripai A, et al. Irritancy and repellency behavioral responses of three strains of Aedes aegypti exposed to DDT and alpha-cypermethrin. J Med Entomol. 2009. November;46(6):1407–14. [DOI] [PubMed] [Google Scholar]
- 21.Achee NL, Sardelis MR, Dusfour I, Chauhan KR, Grieco JP. Characterization of Spatial Repellent, Contact Irritant, and Toxicant Chemical Actions of Standard Vector Control Compounds. J Am Mosq Control Assoc. 2009. June 1;25(2):156–67. doi: 10.2987/08-5831.1 [DOI] [PubMed] [Google Scholar]
- 22.Sathantriphop S, Thanispong K, Sanguanpong U, Achee NL, Bangs MJ, Chareonviriyaphap T. Comparative Behavioral Responses of Pyrethroid-Susceptible and -Resistant Aedes aegypti (Diptera: Culicidae) Populations to Citronella and Eucalyptus Oils. J Med Entomol. 2014. November 1;51(6):1182–91. doi: 10.1603/ME13191 [DOI] [PubMed] [Google Scholar]
- 23.Witting-Bissinger BE, Stumpf CF, Donohue KV, Apperson CS, Roe RM. Novel arthropod repellent, BioUD, is an efficacious alternative to deet. J Med Entomol. 2008. September;45(5):891–8. [DOI] [PubMed] [Google Scholar]
- 24.Stell FM, Roe RM, Arellano C, Kennedy L, Thornton H, Saavedra-Rodriguez K, et al. Proof of concept for a novel insecticide bioassay based on sugar feeding by adult Aedes aegypti (Stegomyia aegypti). Med Vet Entomol. 2013. September;27(3):284–97. doi: 10.1111/j.1365-2915.2012.01048.x [DOI] [PubMed] [Google Scholar]
- 25.Ponce-García G, Del Río-Galvan S, Barrera R, Saavedra-Rodriguez K, Villanueva-Segura K, Felix G, et al. Knockdown Resistance Mutations in Aedes aegypti (Diptera: Culicidae) From Puerto Rico. J Med Entomol. 2016. November;53(6):1410–4. doi: 10.1093/jme/tjw115 [DOI] [PubMed] [Google Scholar]
- 26.Trexler JD, Apperson CS, Zurek L, Gemeno C, Schal C, Kaufman M, et al. Role of Bacteria in Mediating the Oviposition Responses of Aedes albopictus (Diptera: Culicidae). J Med Entomol. 2003. November 1;40(6):841–8. [DOI] [PubMed] [Google Scholar]
- 27.Mharakurwa S, Kumwenda T, Mkulama MAP, Musapa M, Chishimba S, Shiff CJ, et al. Malaria antifolate resistance with contrasting Plasmodium falciparum dihydrofolate reductase (DHFR) polymorphisms in humans and Anopheles mosquitoes. Proc Natl Acad Sci U S A. 2011. November 15;108(46):18796–801. doi: 10.1073/pnas.1116162108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lardeux F, Tejerina R, Aliaga C, Ursic-Bedoya R, Lowenberger C, Chavez T. Optimization of a semi-nested multiplex PCR to identify Plasmodium parasites in wild-caught Anopheles in Bolivia, and its application to field epidemiological studies. Trans R Soc Trop Med Hyg. 2008. May;102(5):485–92. doi: 10.1016/j.trstmh.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 29.Sayono S, Hidayati APN, Fahri S, Sumanto D, Dharmana E, Hadisaputro S, et al. Distribution of Voltage-Gated Sodium Channel (Nav) Alleles among the Aedes aegypti Populations In Central Java Province and Its Association with Resistance to Pyrethroid Insecticides. PLOS ONE. 2016. March 3;11(3):e0150577 doi: 10.1371/journal.pone.0150577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016. July;33(7):1870–4. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHJM, Chotpitayasunondh T, Chua MN, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet Lond Engl. 2014. October 11;384(9951):1358–65. [DOI] [PubMed] [Google Scholar]
- 32.Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med. 2015. September 24;373(13):1195–206. doi: 10.1056/NEJMoa1506223 [DOI] [PubMed] [Google Scholar]
- 33.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N Engl J Med. 2015. January 8;372(2):113–23. doi: 10.1056/NEJMoa1411037 [DOI] [PubMed] [Google Scholar]
- 34.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet Lond Engl. 2015. July 4;386(9988):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014. July;11(7):e1001685 doi: 10.1371/journal.pmed.1001685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BGNO, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012. December 13;367(24):2284–95. doi: 10.1056/NEJMoa1208394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011. 17;365(20):1863–75. doi: 10.1056/NEJMoa1102287 [DOI] [PubMed] [Google Scholar]
- 38.Goldani LZ. Yellow fever outbreak in Brazil, 2017. Braz J Infect Dis. 2017. March;21(2):123–4. doi: 10.1016/j.bjid.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraemer MUG, Faria NR, Reiner RC, Golding N, Nikolay B, Stasse S, et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: a modelling study. Lancet Infect Dis. 2017. March;17(3):330–8. doi: 10.1016/S1473-3099(16)30513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yellow fever urban outbreak in Angola and the risk of extension. Releve Epidemiol Hebd. 2016. April 8;91(14):186–92. [PubMed] [Google Scholar]
- 41.Otshudiema JO, Ndakala NG, Mawanda E-TK, Tshapenda GP, Kimfuta JM, Nsibu L-RN, et al. Yellow Fever Outbreak—Kongo Central Province, Democratic Republic of the Congo, August 2016. MMWR Morb Mortal Wkly Rep. 2017. March 31;66(12):335–8. doi: 10.15585/mmwr.mm6612a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu JT, Peak CM, Leung GM, Lipsitch M. Fractional dosing of yellow fever vaccine to extend supply: a modelling study. Lancet Lond Engl. 2016. 10;388(10062):2904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yellow fever vaccine: WHO position on the use of fractional doses–June 2017. Releve Epidemiol Hebd. 2017. 23;92(25):345–50. [PubMed] [Google Scholar]
- 44.Agramonte NM, Bloomquist JR, Bernier UR. Pyrethroid resistance alters the blood-feeding behavior in Puerto Rican Aedes aegypti mosquitoes exposed to treated fabric. PLoS Negl Trop Dis. 2017. September;11(9):e0005954 doi: 10.1371/journal.pntd.0005954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orsborne J, Banks SD, Hendy A, Gezan SA, Kaur H, Wilder-Smith A, et al. Personal Protection of Permethrin-Treated Clothing against Aedes aegypti, the Vector of Dengue and Zika Virus, in the Laboratory. PLOS ONE. 2016. May 17;11(5):e0152805 doi: 10.1371/journal.pone.0152805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agossa FR, Gnanguenon V, Anagonou R, Azondekon R, Aïzoun N, Sovi A, et al. Impact of Insecticide Resistance on the Effectiveness of Pyrethroid-Based Malaria Vectors Control Tools in Benin: Decreased Toxicity and Repellent Effect. PLOS ONE. 2015. December 16;10(12):e0145207 doi: 10.1371/journal.pone.0145207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faulde MK, Uedelhoven WM, Malerius M, Robbins RG. Factory-based permethrin impregnation of uniforms: residual activity against Aedes aegypti and Ixodes ricinus in battle dress uniforms worn under field conditions, and cross-contamination during the laundering and storage process. Mil Med. 2006. June;171(6):472–7. [DOI] [PubMed] [Google Scholar]
- 48.Schreck CE, Mount GA, Carlson DA. Wear and wash persistence of permethrin used as a clothing treatment for personal protection against the lone star tick (Acari: Ixodidae). J Med Entomol. 1982. March 24;19(2):143–6. [DOI] [PubMed] [Google Scholar]
- 49.Wallace JW, Nicholson WL, Perniciaro JL, Vaughn MF, Funkhouser S, Juliano JJ, et al. Incident Tick-Borne Infections in a Cohort of North Carolina Outdoor Workers. Vector Borne Zoonotic Dis Larchmt N. 2016. May;16(5):302–8. [DOI] [PubMed] [Google Scholar]
- 50.Londono-Renteria B, Patel JC, Vaughn M, Funkhauser S, Ponnusamy L, Grippin C, et al. Long-Lasting Permethrin-Impregnated Clothing Protects Against Mosquito Bites in Outdoor Workers. Am J Trop Med Hyg. 2015. October;93(4):869–74. doi: 10.4269/ajtmh.15-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaughn MF, Funkhouser SW, Lin F-C, Fine J, Juliano JJ, Apperson CS, et al. Long-lasting permethrin impregnated uniforms: A randomized-controlled trial for tick bite prevention. Am J Prev Med. 2014. May;46(5):473–80. doi: 10.1016/j.amepre.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 52.Most B, de Santi VP, Pagès F, Mura M, Uedelhoven WM, Faulde MK. Long-lasting permethrin-impregnated clothing: protective efficacy against malaria in hyperendemic foci, and laundering, wearing, and weathering effects on residual bioactivity after worst-case use in the rain forests of French Guiana. Parasitol Res. 2017. February;116(2):677–84. doi: 10.1007/s00436-016-5333-6 [DOI] [PubMed] [Google Scholar]
- 53.Schreck CE, Snoddy EL, Spielman A. Pressurized sprays of permethrin or deet on military clothing for personal protection against Ixodes dammini (Acari: Ixodidae). J Med Entomol. 1986. July 28;23(4):396–9. [DOI] [PubMed] [Google Scholar]
- 54.Faulde MK, Uedelhoven WM, Robbins RG. Contact toxicity and residual activity of different permethrin-based fabric impregnation methods for Aedes aegypti (Diptera: Culicidae), Ixodes ricinus (Acari: Ixodidae), and Lepisma saccharina (Thysanura: Lepismatidae). J Med Entomol. 2003. November;40(6):935–41. [DOI] [PubMed] [Google Scholar]
- 55.Mount GA, Snoddy EL. Pressurized sprays of permethrin and deet on clothing for personal protection against the lone star tick and the American dog tick (Acari: Ixodidae). J Econ Entomol. 1983. June;76(3):529–31. [DOI] [PubMed] [Google Scholar]
- 56.Jordan RA, Schulze TL, Dolan MC. Efficacy of plant-derived and synthetic compounds on clothing as repellents against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2012. January;49(1):101–6. [DOI] [PubMed] [Google Scholar]
- 57.Miller NJ, Rainone EE, Dyer MC, González ML, Mather TN. Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol. 2011. March;48(2):327–33. [DOI] [PubMed] [Google Scholar]
- 58.Banks SD, Orsborne J, Gezan SA, Kaur H, Wilder-Smith A, Lindsey SW, et al. Permethrin-Treated Clothing as Protection against the Dengue Vector, Aedes aegypti: Extent and Duration of Protection. PLoS Negl Trop Dis. 2015. October 6;9(10):e0004109 doi: 10.1371/journal.pntd.0004109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans SR, Korch GW, Lawson MA. Comparative field evaluation of permethrin and deet-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990. September;27(5):829–34. [DOI] [PubMed] [Google Scholar]
- 60.Faulde M, Scharninghausen J, Tisch M. Preventive effect of permethrin-impregnated clothing against ticks and biting insects. Int J Med Microbiol. 2008;298(S1):321–4. [Google Scholar]
- 61.Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans [Internet]. [cited 2017 Jul 21]. http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed]
- 62.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016. October 8;388(10053):1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The first tab (Puerto Rico) provides results from the pyrethroid-resistant Puerto Rico strain. The second tab (New Orleans) provides results from the pyrethroid-sensitive New Orleans strain.
(XLSX)
Provides data from arm-in-cage trials.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.