Abstract
Background
Mineralocorticoid receptor antagonists (MRAs) are the recommended medical therapy for primary aldosteronism (PA). Whether this recommendation effectively reduces cardiovascular risk is not well understood.
Methods
We conducted a cohort study including 602 PA patients treated with MRAs and 41853 age-matched patients with essential hypertension (EH) to investigate the risk for incident cardiovascular events (composite of myocardial infarction, heart failure hospitalization, stroke), atrial fibrillation, diabetes, and death in patients with PA compared with EH.
Findings
Both groups had comparable cardiovascular risk profiles and blood pressures throughout the study. The incidence rate of cardiovascular events was higher among PA patients on MRAs compared with EH: 56·3 (48·8, 64·7) versus 26·6 (26·1, 27·2) events per 1,000 person-years (adjusted HR=1·91 [1·63, 2·25]; adjusted 10-year cumulative incidence difference: 14·1 [10·1, 18·0] excess events per 100 persons). PA patients also had higher adjusted risks for incident mortality (HR=1·34 [1·06, 1·71]), diabetes (HR=1·26 [1·01, 1·57]), and atrial fibrillation (HR=1·93 [1·54, 2·42]). Compared with EH, the excess risk for cardiovascular events and mortality was limited to PA patients whose renin activity remained suppressed (<1 µg/L/h) on MRAs (adjusted HR=2·83 [2·11, 3·80] and 1·79 [1·14, 2·80] respectively) whereas patients who were treated with higher MRA doses and had unsuppressed renin (≥1 µg/L/h) had no significant excess risk.
Interpretation
Current practice of MRA therapy in PA is associated with significantly higher risk for incident cardiometabolic events and death independent of blood pressure control. Targeting MRA therapy to raise renin may mitigate this excess risk.
Funding
National Institutes of Health
Keywords: Primary aldosteronism, cardiovascular events, renin, hypertension, mineralocorticoid receptor antagonists
Introduction
Primary aldosteronism (PA), characterized by autonomous aldosterone secretion that is independent of renin,(1, 2) increases the risk for cardiovascular and metabolic disease via activation of the mineralocorticoid receptor (MR).(2, 3) It is estimated that 4–19% of patients with hypertension,(3–5) and 3–14% of patients with normotension,(6–8) may have PA. Although a notable subset of patients with PA have disease that can be cured by surgical adrenalectomy, the majority of patients with PA are treated medically with MR antagonists.(2, 9)
Although MR antagonists are the expert consensus recommended medical therapy for PA,(2, 9) there are no large longitudinal or intervention studies demonstrating the efficacy of MR antagonists for reducing the risk for cardiovascular or metabolic diseases in PA, nor any specific guidelines regarding optimal treatment targets when prescribing MR antagonists for PA.(2, 9) Prior studies have suggested that MR antagonist therapy for PA may not effectively reduce cardiometabolic risk; however, these have been small case-control studies(10–12) or longitudinal cohort studies with low event rates.(13–15)
Herein, we conducted a large cohort study to investigate the risk for incident cardiometabolic outcomes and death in patients with PA treated with MR antagonists compared with patients with essential hypertension.
Methods
Data Source and Study Cohort
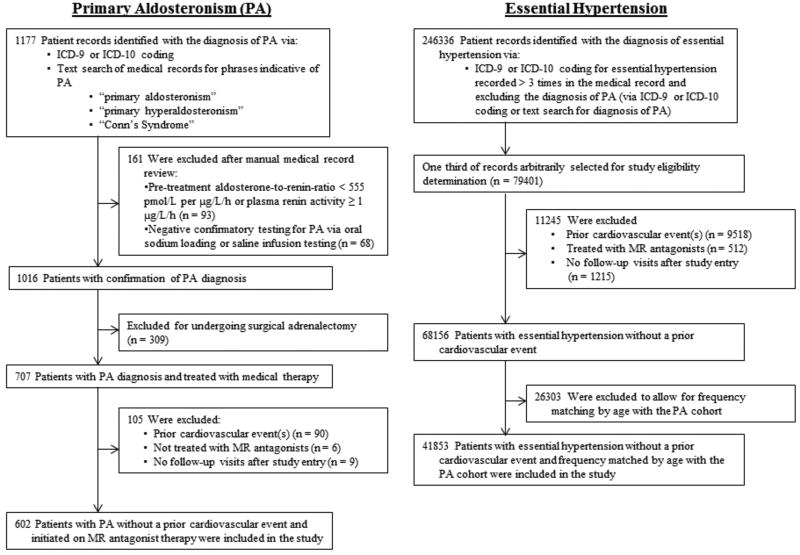
The study cohort was derived from a research registry of patients at the Brigham and Women’s Hospital, the Massachusetts General Hospital, and their affiliated partner hospitals (Figure 1). Eligibility for the current study required that patients had to be seen between the years 1991–2016 and be ≥ 18 years of age. Entry into the study was defined as the date immediately after the first follow-up visit: 1–6 months after initiating an MR antagonist (for PA patients) or after the diagnosis of essential hypertension was first entered into the medical record (for essential hypertension patients).
Figure 1. Derivation of Study Cohort.
The registry was searched for all potential patients with PA using International Classification of Disease, 9th and 10th Revisions (ICD-9 and ICD-10) codes as well as using the following text search terms: “primary aldosteronism”, “primary hyperaldosteronism”, and “Conn’s Syndrome”. Manual medical record review was performed to exclude patients whose baseline laboratory data were inconsistent with the diagnosis of PA (see Definition of Exposures below). Potential patients with PA were also excluded if they underwent surgical adrenalectomy, had a prior cardiovascular event (see Definition of Outcomes below), were not treated with MR antagonists, or had no follow-up visits after study entry (Figure 1). 602 patients with PA treated longitudinally with MR antagonists were included in the analyses.
The registry was then searched for patients with essential hypertension using ICD-9 and ICD-10 codes with the search exclusion of the diagnosis of PA. Essential hypertension patients were excluded if they had a prior cardiovascular event, were treated with MR antagonists on or before the date of study entry, or had no follow-up visits after study entry. The essential hypertension population was frequency matched by decade of age at study entry to the PA study population. There were 41853 patients with essential hypertension included in the analyses (Figure 1). Patient cohort data were extracted from our institutional registry and collated into a de-identified database for this study. This approach and research design was approved by the authors’ institutional human research and ethics committees.
Definition of Exposure
The main exposure was the diagnosis of PA treated with an MR antagonist. The primary comparison group was patients diagnosed with essential hypertension who were frequency matched by age (see Supplementary Appendix for detailed information on exposure definitions).
Exposures for secondary analyses included plasma renin activity (PRA) while on MR antagonist therapy and pre-treatment serum aldosterone levels in the PA cohort. We categorized PA patients who had at least one PRA measured more than one month after starting MR antagonists by whether the first PRA measurement on MR antagonist therapy was unsuppressed (≥ 1·0 µg/L/h) or remained suppressed (< 1·0 µg/L/h). For this secondary analysis, entry into the study for PA patients treated with MR antagonists was defined as the date of the first PRA measurement. We also categorized PA patients by a pre-treatment serum aldosterone concentration (defined as the most recent value prior to starting MR antagonist therapy) that was high (≥ 555 pmol/L) or low (< 555 pmol/L).
Definition of Covariates
The following covariates at the time of study entry were considered as potential confounders: age, sex, race, body mass index (BMI), smoking status, history of diabetes mellitus, hemoglobin A1C, history of atrial fibrillation, LDL cholesterol, statin use, estimated glomerular filtration rate, daily aspirin use, systolic blood pressure, diastolic blood pressure, and number of antihypertensive medications prescribed (excluding MR antagonists).
Definition of Outcomes
The primary outcome was an incident cardiovascular event defined as a composite of incident myocardial infarction (MI) or coronary revascularization, congestive heart failure (CHF) hospitalization, or stroke (see detailed information on outcome assessments in the Supplementary Appendix). Secondary outcomes were the individual components of the composite cardiovascular outcome, as well as incident atrial fibrillation, incident diabetes mellitus, and death.
Statistical Analysis
The primary analysis investigated whether patients with PA treated with an MR antagonist had a higher risk of incident composite cardiovascular events compared with patients with essential hypertension. Secondary analyses investigated the risk of the individual components of the composite cardiovascular outcome, atrial fibrillation, diabetes, and death between these two populations, and whether PRA or aldosterone measurements in the PA group influenced this risk. We used multivariate Cox regression models (proc PHREG in SAS v9·4, Cary, NC) to estimate adjusted hazard ratios and 95% confidence intervals. All models were adjusted for the aforementioned covariates at the time of study entry. The analyses for incident atrial fibrillation and diabetes mellitus excluded any patients with a pre-existing diagnosis of the condition at study entry. The proportional hazard assumption was evaluated by testing significance of the exposure-time interactions in the Cox models. The standardized cumulative incidence of composite cardiovascular events was one minus a standardized survival function (proc PHREG baseline statement in SAS v9·4), which was estimated by averaging the estimated individual-specific survival curves based on the multivariate Cox models mentioned above over the distributions of the aforementioned covariates in our cohort. We used this model to calculate differences in standardized cumulative incidence of cardiovascular events at 3, 5, and 10 years of follow-up between patients with PA and patients with essential hypertension.
Patients were censored on the date of the specified outcome occurrence or, if they did not develop the outcome during follow-up at our institutions, at the date of their final recorded follow-up visit. The cardiovascular outcomes of incident MI/coronary revascularization, CHF hospitalization, and stroke were treated as competing events and analyzed based on the cause-specific Cox regression models.(16) All p-values are two-sided.
Exploratory Analysis
In an exploratory analysis which included the initially excluded 205 patients with PA who underwent surgical adrenalectomy and did not have a prior cardiovascular event (Figure S1), we investigated the risk for incident composite cardiovascular events comparing PA treated with surgical adrenalectomy, PA treated with MR antagonist therapy, and essential hypertension.
Role of the Funding Source
The funding sources for this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient Characteristics
PA patients treated with MR antagonists were more likely to be male, black, and have lower serum potassium, compared with patients with essential hypertension (Table 1). Age and other cardiovascular risk factors were similar between the two groups. Prior to study entry, PA patients had higher blood pressures compared with patients with essential hypertension; however, at the time of study entry, and throughout the duration of the study, mean blood pressures were comparable (Table S1). Similarly, the mean number of prescribed antihypertensive medications, not including MR antagonists, was also comparable. The majority of PA patients were treated with spironolactone and had bilateral disease (Table 1).
Table 1.
Baseline Characteristics of the Study Cohort.
| Primary Aldosteronism |
Essential Hypertension |
|
|---|---|---|
| (N = 602) | (N = 41853) | |
| Age – yr | 58 (12) | 57 (12) |
| Sex – Female (n/%) | 271/45 | 21484/51 |
| Race or Ethnic Group (n/%) | ||
| White | 336/56 | 27544/66 |
| Black | 153/25 | 6775/16 |
| Hispanic | 54/9 | 2693/6 |
| Othera | 59/10 | 4841/12 |
| Body Mass Index – kg/m2 | 31·1 (6·0) | 29·8 (6·4) |
| Follow-up Time – yr | 7·0 (4·7) | 8·8 (5·6) |
| Primary Aldosteronism Characteristicsb | ||
| Serum Aldosterone – pmol/L | 638 (472–943) | -- |
| Plasma Renin Activityc (n/%) | ||
| ≤ 0·60 µg/L/h | 548/91 | -- |
| 0·61 – 0·99 µg/L/h | 54/9 | -- |
| ≥ 1·00 µg/L/h | 0/0 | -- |
| Aldosterone-to-Renin Ratio – pmol/L per µg/L/h | 1689 (987–4239) | -- |
| Serum Potassium – mmol/L | 3·6 (0·5) | 4·1 (0·5) |
| Potassium supplementation (n/%) | 275/46 | 442/1 |
| CT or MRI Imaging (n/%) | 581/97 | -- |
| Unilateral Adrenal Abnormality (n/%) | 220/38 | -- |
| Bilateral Adrenal Abnormalities (n/%) | 65/11 | -- |
| Normal Appearing Adrenal Glands (n/%) | 296/51 | -- |
| Adrenal Vein Sampling (n/%) | 344/57 | -- |
| Lateralization (n/%) | 54/16 | -- |
| No Lateralization (n/%) | 239/69 | -- |
| Unsatisfactory/Indeterminate (n/%) | 51/15 | -- |
| Blood Pressure Prior to Study Entryd | ||
| Systolic Blood Pressure – mm Hg | 148 (18) | 138 (19) |
| Diastolic Blood Pressure – mm Hg | 86 (13) | 81 (11) |
| Blood Pressure at Time of Study Entrye | ||
| Systolic Blood Pressure – mm Hg | 137 (19) | 135 (18) |
| Diastolic Blood Pressure – mm Hg | 81 (13) | 80 (11) |
| Antihypertensive Medication Use | ||
| MR Antagonist Use (n/%) | 602/100 | 0/0 |
| Spironolactone | 500/83 | -- |
| Eplerenone | 102/17 | -- |
| Mean Spironolactone Total Daily Dose (mg) | 45 (30) | -- |
| Mean Eplerenone Total Daily Dose (mg) | 54 (36) | -- |
| Mean Number of Non-MR Antagonist Antihypertensives | 2·9 (1·4) | 2·7 (1·4) |
| ACE Inhibitor/Angiotensin II Receptor Blocker (n/%) | 407/68 | 30396/73 |
| Calcium Channel Blocker (n/%) | 405/67 | 17247/41 |
| Beta Blocker (n/%) | 395/66 | 23743/57 |
| Diuretics | ||
| Thiazide (n/%) | 243/40 | 21864/52 |
| Loop (n/%) | 42/7 | 6767/16 |
| Potassium-sparing (non-MR Antagonist) (n/%) | 112/19 | 3857/9 |
| Otherf (n/%) | 137/23 | 7227/17 |
| Other Cardiovascular Risk Factors | ||
| Daily Aspirin (n/%) | 213/35 | 12689/30 |
| LDL – mmol/L | 2·80 (0·85) | 2·87 (0·96) |
| Statin use (n/%) | 204/34 | 17492/42 |
| Diabetes Mellitus (n/%) | 118/20 | 8364/20 |
| Hemoglobin A1C – proportion of total hemoglobin | 0·060 (0·011) | 0·061 (0·010) |
| Serum Creatinine – µmol/L | 80·8 (44·2) | 78·5 (57·2) |
| Estimated Glomerular Filtration Rate – mL/s/1·73m2 | 1·32 (0·38) | 1·35 (0·37) |
| Atrial Fibrillation (n/%) | 24/4 | 1761/ 4 |
| Smoking Status (n/%) | ||
| Never | 330/55 | 19478/47 |
| Former | 176/29 | 9683/23 |
| Current | 34/6 | 3935/9 |
| Not known | 62/10 | 8757/21 |
Unless otherwise specified, normally distributed continuous variables are reported as mean (SD); non-normally distributed continuous variables are reported as median (25th – 75th percentile IQR); categorical variables are reported as percentages. MR = mineralocorticoid receptor; ACE = angiotensin-converting enzyme; LDL = low-density lipoprotein.
Other race includes Asian, Native American, other, and unknown.
Laboratory values most recent prior to study entry.
For much of the study period, the hospital-affiliated laboratories reported a minimum plasma renin activity of < 0·60 µg/L/h. For study purposes, these minimum values were recorded as 0·59 µg/L/h.
Refers to the last blood pressure recorded prior to study entry (i.e. just prior to starting MR antagonist [PA] or just prior to ICD-9/10 coding for essential hypertension).
Refers to the first blood pressure recorded 1–6 months after starting MR antagonist therapy (PA) or initial ICD-9/10 coding for essential hypertension.
Other antihypertensive medication includes hydralazine, clonidine, alpha blockers, nitrates, minoxidil, methyldopa, and direct renin inhibitors.
Risk for Incident Cardiometabolic Outcomes and Mortality
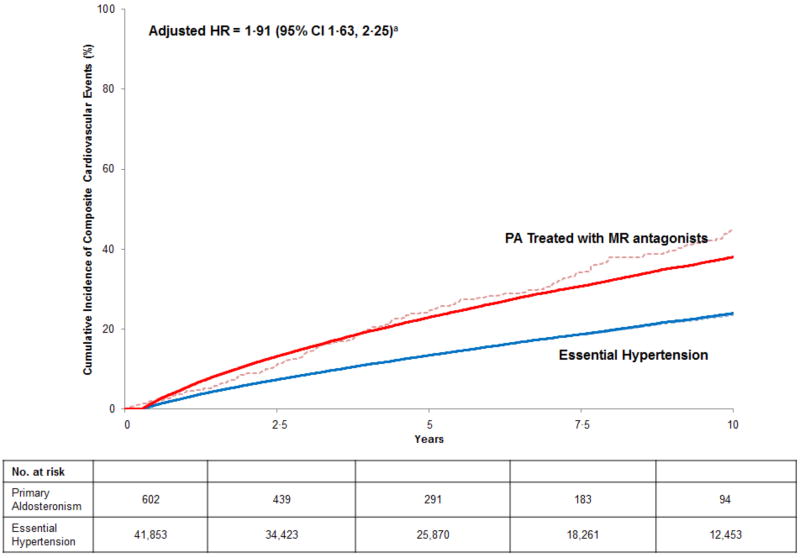
PA patients treated with MR antagonists had a nearly two-fold higher unadjusted incidence rate of composite cardiovascular events (56·3 [95% CI 48·8, 64·7] vs. 26·6 [95% CI 26·1, 27·2] per 1,000 person years) (Table 2). The adjusted hazard ratio for incident composite cardiovascular events was 1·91 (95% CI 1·63, 2·25) (Figure 2), with each individual component of the composite being associated with higher risk (Table 2). Patients with PA also had a significantly higher risk of death and for developing incident atrial fibrillation and diabetes compared with patients with essential hypertension (Table 2). The adjusted 10-year cumulative incidence difference for developing cardiovascular events indicated that PA patients treated with MR antagonists had 14·1 (95% CI 10·1, 18·0) excess events per 100 individuals compared with essential hypertension, and these findings did not materially change when analyses included death as a competing risk (Table 3).
Table 2.
Incidence Rates of Cardiometabolic Outcomes and Mortality in Medically Treated Primary Aldosteronism and Essential Hypertension.
| Primary Aldosteronism | Essential Hypertension | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes |
No. at Risk |
Events |
Person- years |
Incidence per 1,000 Person-yr (95% CI) |
No. at Risk |
Events |
Person- years |
Incidence per 1,000 Person-yr (95% CI) |
Multivariable Adjusted Hazard Ratio (95% CI)a |
P value | |
| Composite Cardiovascular Events | 602 | 192 | 3410 | 56·3 (48·8, 64·7) | 41853 | 8600 | 322835 | 26·6 (26·1, 27·2) | 1·91 (1·63, 2·25) | <0·0001 | |
| MI or Coronary Revascularizationb | 602 | 71 | 3410 | 20·8 (16·4, 26·1) | 41853 | 3170 | 322835 | 9·8 (9·5, 10·2) | 1·81 (1·39, 2·37) | <0·0001 | |
| CHF Hospitalizationb | 602 | 46 | 3410 | 13·5 (10·0, 17·8) | 41853 | 2443 | 322835 | 7·6 (7·3, 7·9) | 1·61 (1·17, 2·22) | 0·0038 | |
| CVA/TIAb | 602 | 75 | 3410 | 22·0 (17·4, 27·4) | 41853 | 2987 | 322835 | 9·3 (8·9, 9·6) | 2·38 (1·83, 3·08) | <0·0001 | |
| Atrial Fibrillationc | 578 | 98 | 3748 | 26·2 (21·3, 31·7) | 40092 | 4276 | 335189 | 12·8 (12·4, 13·1) | 1·93 (1·54, 2·42) | <0·0001 | |
| Diabetes Mellitusd | 484 | 97 | 3015 | 32·2 (26·2, 39·1) | 33489 | 6721 | 257906 | 26·1 (25·4, 26·7) | 1·26 (1·01, 1·57) | 0·041 | |
| Mortality | 602 | 81 | 5270 | 15·4 (12·3, 19·0) | 41853 | 6443 | 442232 | 14·6 (14·2, 14·9) | 1·34 (1·06, 1·71) | 0·016 | |
MI = myocardial infarction; CHF = congestive heart failure; CVA = cerebrovascular accident; TIA = transient ischemic attack.
Hazard ratios adjusted for the following variables at the time of study entry: age, sex, race/ethnicity, BMI, smoking status, diabetes mellitus, hemoglobin A1C, atrial fibrillation, LDL cholesterol, statin use, daily aspirin use, estimated glomerular filtration rate, systolic blood pressure, diastolic blood pressure, and number of antihypertensive medications.
The cardiovascular outcomes of incident MI/coronary revascularization, CHF hospitalization, and stroke were treated as competing events in that once a patient had one of these outcomes, they were censored and no longer “at risk” for the other outcomes.
Patients with known atrial fibrillation at the time of study entry were excluded. The multivariate adjusted hazard ratio did not include atrial fibrillation as a covariate.
Patients with known diabetes mellitus at the time of study entry were excluded. The multivariate adjusted hazard ratio did not include diabetes mellitus as a covariate.
Figure 2. Standardized Cumulative Incidence Curve of Composite Cardiovascular Events in Medically Treated Primary Aldosteronism and Essential Hypertension.
Solid lines = adjusted cumulative incidence; dashed lines = unadjusted cumulative incidence.
Incident composite cardiovascular events defined as a composite of incident myocardial infarction/coronary revascularization, congestive heart failure hospitalization, and transient ischemic attack/cerebrovascular accident. PA = primary aldosteronism; MR = mineralocorticoid receptor.
a Hazard ratios adjusted for, and cumulative incidence curve standardized to the distribution of, the following variables at the time of study entry in our cohort: age, sex, race/ethnicity, BMI, smoking status, diabetes mellitus, hemoglobin A1C, atrial fibrillation, LDL cholesterol, statin use, daily aspirin use, estimated glomerular filtration rate, systolic blood pressure, diastolic blood pressure, and number of antihypertensive medications.
Table 3.
Standardized Cumulative Incidence of Cardiovascular Events in Medically Treated Primary Aldosteronism and Essential Hypertension.
| Primary Aldosteronism |
Essential Hypertension |
Difference in Cumulative Incidence |
|
|---|---|---|---|
| 3-Year Cumulative Incidence (per 100 persons) | |||
| Unadjusted (95% CI) | 16·9 (14·7, 19·1) | 8·6 (8·3, 8·9) | 8·3 (6·1, 10·5) |
| Adjusted (95% CI)a | 15·3 (13·2, 17·4) | 8·7 (8·4, 9·0) | 6·6 (4·5, 8·6) |
| 5-Year Cumulative Incidence (per 100 persons) | |||
| Unadjusted (95% CI) | 25·6 (22·5, 28·7) | 13·4 (13·1, 13·7) | 12·2 (9·1, 15·3) |
| Adjusted (95% CI)a | 23·0 (20·2, 25·8) | 13·5 (13·2, 13·8) | 9·5 (6·6, 12·3) |
| 10-Year Cumulative Incidence (per 100 persons) | |||
| Unadjusted (95% CI) | 42·4 (37·9, 46·9) | 23·6 (23·1, 24·1) | 18·8 (14·3, 23·3) |
| Adjusted (95% CI)a | 38·1 (34·2, 42·0) | 24·0 (23·5, 24·5) | 14·1 (10·1, 18·0) |
Standardized to the distributions at the time of study entry in our cohort for the following variables: age, sex, race/ethnicity, BMI, smoking status, diabetes mellitus, hemoglobin A1C, atrial fibrillation, LDL cholesterol, statin use, daily aspirin use, estimated glomerular filtration rate, systolic blood pressure, diastolic blood pressure, and number of antihypertensive medications.
Renin Activity as a Biomarker of MR Antagonist Treatment Efficacy
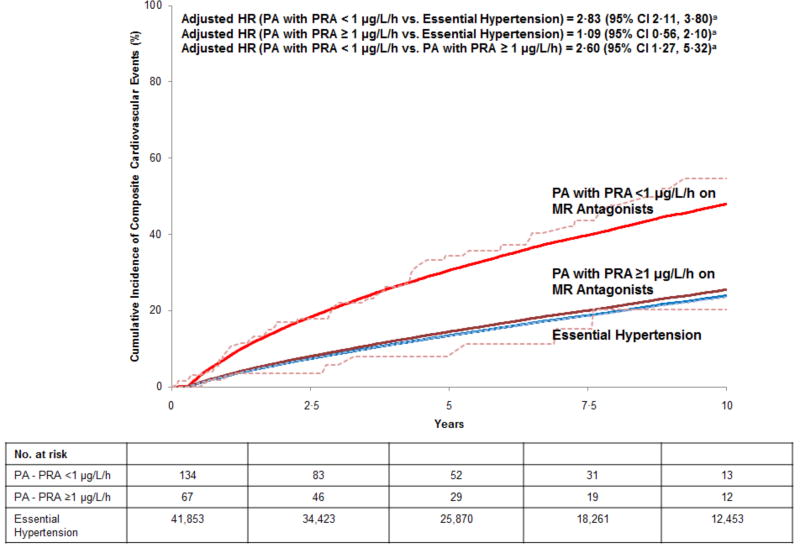
Of the 602 PA patients in the study, 201 had their first PRA measured at least one month after initiation of MR antagonist therapy and did not have cardiovascular events. Among these patients, 67 achieved an unsuppressed PRA (≥ 1 µg/L/h); the PRA of the remaining 134 patients remained suppressed (< 1 µg/L/h) (Figure 3). The median time to the first PRA measurement was similar in those with suppressed (0·56 years [IQR 0·21, 2·08 years]) and unsuppressed PRA measurements (0·62 years [IQR 0·21, 2·58 years]). Compared with patients with essential hypertension, PA patients whose PRA remained suppressed (<1 µg/L/h) while on MR antagonists had a nearly three-fold higher risk for incident composite cardiovascular events (adjusted HR=2·83 [95% CI 2·11, 3·80]) (Figure 3) and a significantly higher risk for death (adjusted HR=1·79 [95% CI 1·14, 2·80]) (Figure S2). In contrast, achieving an unsuppressed PRA (≥1 µg/L/h) resulted in no statistically significant difference in incident cardiovascular events (Figure 3) or death (Figure S2) compared with patients with essential hypertension. Repeating these analyses based on the second and third PRA measurements did not materially change the results (Table S2). Importantly, the mean longitudinal blood pressures of patients with suppressed and unsuppressed renin were comparable throughout the study period (Table S3); however, patients who had an unsuppressed renin activity were started on higher doses of MR antagonists (initial dose: spironolactone 50 [27] vs 43 [28] mg, eplerenone 65 [27] vs. 53 [48] mg) (Table S4).
Figure 3. Standardized Cumulative Incidence Curve of Composite Cardiovascular Events in Medically Treated Primary Aldosteronism Stratified by Plasma Renin Activity Achieved with Mineralocorticoid Receptor Antagonists and Essential Hypertension.
Solid lines = adjusted cumulative incidence; dashed lines = unadjusted cumulative incidence.
Incident composite cardiovascular events defined as a composite of incident myocardial infarction/coronary revascularization, congestive heart failure hospitalization, and transient ischemic attack/cerebrovascular accident. PA = primary aldosteronism; PRA = plasma renin activity; MR = mineralocorticoid receptor.
a Hazard ratios adjusted for, and cumulative incidence curve standardized to the distribution of, the following variables at the time of study entry in our cohort: age, sex, race/ethnicity, BMI, smoking status, diabetes mellitus, hemoglobin A1C, atrial fibrillation, LDL cholesterol, statin use, daily aspirin use, estimated glomerular filtration rate, systolic blood pressure, diastolic blood pressure, and number of antihypertensive medications.
In contrast, stratified analyses of PA patients by serum aldosterone level at the time of PA diagnosis (prior to initiation of MR antagonist therapy) showed no significant difference in the risk for incident composite cardiovascular events between PA patients with high vs. low pre-treatment serum aldosterone levels (Figure S3).
Exploratory Analysis: Medical versus Surgical Therapy for PA and Incident Outcomes
Surgically treated patients were younger and had biochemically more severe PA; however, the blood pressure upon entry into the analysis was comparable across sub-groups (Table S5). Patients with surgically treated PA had a significantly lower risk for incident composite cardiovascular events compared with those with essential hypertension (adjusted HR = 0·58 [95% CI 0·35, 0·97]) (Figure S4).
Discussion
The current study demonstrates that even when patients with PA are treated with MR antagonists to achieve similar blood pressures as patients with essential hypertension with comparable cardiovascular risk profiles, they had an approximately two-fold higher risk for incident cardiovascular outcomes, as well as higher risks for incident diabetes, atrial fibrillation, and death. Importantly, this higher risk appeared to be limited to PA patients whose renin remained suppressed on MR antagonist therapy; in contrast, the risk in PA patients who were treated with higher doses of MR antagonists and had a substantial rise in renin was not different from patients with essential hypertension. Since the suppression of renin in PA is a result of excessive MR activation and volume expansion,(1, 2, 8) the rise in renin with MR antagonist therapy reflects sufficient antagonism of the renal-MR (and potentially extra-renal MR).(17–19) Together, the current study suggests an alarming clinical practice of insufficient MR blockade and excessive cardiometabolic risk in medically treated PA patients, and raises the distinct possibility that a novel practice recommendation may improve outcomes in patients with PA: in addition to blood pressure lowering, titration of MR antagonists to target a rise in renin may be the most effective indicator of adequate MR antagonism that corresponds to cardiovascular risk reduction and survival that is comparable to patients with essential hypertension and approaching that of surgically treated patients with PA.
Since PA was first described by Jerome Conn, it has been observed that when possible, surgical therapy to eliminate the source of aldosterone excess remarkably improves blood pressure and risk for adverse cardiovascular outcomes.(13, 20, 21) In contrast, since most studies assessing the efficacy of MR antagonist therapy have been either cross-sectional or longitudinal (10, 11, 14, 15) and small in size, the general recommendation to use MR antagonists by professional society guidelines(2, 9) has been based on the assumption that this approach may be as effective as medical therapy for essential hypertension. For example, perhaps the two most influential studies in this regard are those by Catena et al.(14) and Reincke et al..(15) Catena et al. conducted a longitudinal analysis investigating the risk for incident cardiovascular events (similar composite outcome to our current study) in 54 patients with PA treated with a mixture of surgical or MR antagonist therapy compared with 323 patients with essential hypertension.(14) Although they observed a higher prevalence of cardiovascular disease in PA patients at baseline, there were only 10 incident events in the PA population and 19 incident events in the essential hypertension population, resulting in no significant difference in incident outcomes, and no observable differences in outcomes between medically and surgically treated PA patients. Reincke et al. investigated long-term mortality in a longitudinal cohort study comparing PA patients treated with a mixture of surgery or MR antagonists with essential hypertension and found that patients with PA had no difference in all-cause mortality (22 deaths) compared with essential hypertension (74 deaths); however, in secondary analyses cardiovascular mortality was higher in PA patients (50% vs. 34% of deaths).(15)
Our current findings substantially extend those of others before us. The lack of large and robust longitudinal data assessing the effectiveness of MR antagonists in PA has resulted in the absence of specific recommendations outlining the optimal approach to medical therapy in PA: should MR antagonists be titrated to normalize blood pressure, normalize potassium, raise renin, or target a different factor? As our findings show, although physicians at well-regarded and large academic centers routinely prescribed MR antagonists for PA, and achieved blood pressure control to a similar degree as comparable patients with essential hypertension, their approach was associated with a substantially higher risk for cardiometabolic outcomes and death compared with essential hypertension and surgically cured PA. In this regard, our study suggests that it may not be appropriate to assume that surgical and medical treatments for PA, or that blood pressure control for PA and essential hypertension, are similarly effective and the need to discuss these options more candidly with patients. Further, the size and scope of our study design permitted analyses that indicated that focusing only on controlling blood pressure with MR antagonists may be insufficient to reduce long-term cardiometabolic risk in PA. Activation of the renal and extra-renal MR by aldosterone is associated with not only hypertension, but also myocardial fibrosis, left ventricular hypertrophy, nephropathy, and death;(12, 15, 17, 22) therefore, a strategy that targets more aggressive dosing of MR antagonists and uses a rise in renin as a crude physiologic biomarker to assess sufficient blockade of the renal-MR may be the most effective way to reduce cardiovascular risk in medically treated PA.
It should be noted that the risk for incident cardiovascular events we observed among patients with moderate-to-severe essential hypertension (on average using 3 antihypertensive medications) was remarkably similar to that previously reported in comparable cohorts of essential hypertension.(23, 24) Thus, it is alarming that despite having similar blood pressures at baseline and throughout longitudinal follow-up while on MR antagonists, our cohort of PA patients had 14·1 more cardiovascular events per 100 persons at 10 years of follow-up when compared with essential hypertension. In addition, PA patients treated with MR antagonists also had a significantly higher risk for incident atrial fibrillation and diabetes, conditions that have previously been suspected to be due to aldosterone excess.(25–28)
Our results must be interpreted within the context of the study design. First, this study was observational. Although blood pressure and other cardiovascular risk factors were similar at the time of study entry and our models adjusted for important confounders, there may still be residual confounding and misclassification. Since our study used an open cohort design, we could not capture nonfatal cardiovascular events that occurred outside of our healthcare system which may have resulted in an underestimation of cumulative incidence rates. Second, only a subset of study participants had renin measured in follow-up, the timing and frequency of renin measurements were variable, and it was unclear if and when any of the renin values were used to guide therapy. Further, although our data suggest that higher MR antagonist dosing may be required to achieve a rise in renin, the heterogeneity in the type and dosing of MR antagonists limit generalizeability. Thus, we cannot firmly conclude that the intent-to-treat to a rise in renin is beneficial, nor can we affirm what level of renin activity best reflects sufficient MR antagonism to reduce cardiovascular risk. Third, our observational study design did not prospectively control blood pressure in each exposure group. Although patients with PA had higher blood pressures prior to study entry, upon initiation of therapy at study entry, blood pressure control and use of antihypertensive medications were comparable to those with essential hypertension, the longitudinal control of blood pressure was comparable, and our models adjusted for the treated blood pressure at study entry. Fourth, although our models adjusted for number of antihypertensive medications, we did not have sufficient information to adjust for all longitudinal changes in daily doses and frequencies of antihypertensive medications; however, repeating our analyses with adjustments for individual antihypertensive medication subclasses did not appreciably change the results. Fifth, we could not adequately account for the duration and degree of antecedent hypertension. However, our findings suggest that even if antecedent hypertension was a risk factor, treatment to modulate renin activity may mitigate its effect. Sixth, although our sample sizes precluded us from conducting further subset analyses, future studies should investigate whether our findings apply similarly to unilateral and bilateral forms of PA. Finally, PA patients in our study did not undergo systematic assessments for subclinical hypercortisolism since this is not a routinely recommend practice in PA.(2, 9) Although cortisol co-secretion in PA and by adrenal tumors may contribute to cardiometabolic disease,(29, 30) cortisol is a mineralocorticoid and a glucocorticoid, thus MR antagonists should abrogate most of the cardiovascular consequences related to cortisol.
In conclusion, PA is characterized by renin-independent aldosterone secretion that results in excessive MR activation and cardiovascular disease. When possible, surgical adrenalectomy to cure the aldosterone excess in PA mitigates this excess risk; however, the majority of patients with PA are treated medically with MR antagonists that are presumed to be an effective alternative to surgery. The current study demonstrates that the current clinical practice of using MR antagonists for PA may fail to abrogate the excess risk for cardiometabolic outcomes (primary outcome) as well as death, atrial fibrillation, and diabetes (secondary outcomes) compared with similar patients with essential hypertension and, therefore, a more aggressive and multi-faceted approach to medical therapy may be needed. Targeting a rise in renin as a biomarker for adequate MR blockade may serve as an important clinical benchmark for providers who treat PA patients with MR antagonists.
Supplementary Material
Research in Context.
Evidence before this study
Primary aldosteronism (PA) is the most common modifiable form of hypertension and substantially increases the risk for cardiometabolic disease and death. The vast majority of patients with PA are treated medically with mineralocorticoid receptor antagonists (MRAs) rather than adrenalectomy. Although international societies and expert committees strongly recommend this use of MRAs as the main medical therapy for PA, there are no large longitudinal or intervention studies demonstrating the efficacy of MR antagonists for reducing the risk for cardiovascular or metabolic diseases in PA, nor any specific guidelines regarding optimal treatment targets when prescribing MR antagonists for PA. We considered evidence assessing the efficacy of MRAs in PA using The Endocrine Society Clinical Practice Guidelines on the management of PA and citations within it, and we searched for articles using a PubMed query for: primary aldosteronism, mineralocorticoid receptor antagonist, cardiovascular events, and renin.
Added value of this study
The current study is the largest longitudinal study to assess whether the current use of MRAs in PA is effective at lowering the risk for incident cardiometabolic disease and death. The findings of this study show that when compared with patients with essential hypertension who had comparable demographic profiles and longitudinal blood pressure trends, patients with PA treated with MRAs had significantly higher risks for incident cardiovascular events, death, diabetes, and atrial fibrillation. The large sample size of this study permitted robust statistical significance and the ability to assess that the risks for these events were independent of blood pressure, thereby implicating the independent and deleterious effects of aldosterone excess and mineralocorticoid receptor activation. Further, this study demonstrated that patients with PA who were treated with higher doses of MRAs, such that their renin activity substantially increased, had decreased risk for cardiovascular events and death such that the risk for these outcomes was no different than that of patients with essential hypertension.
Implications of all the available evidence
The current use of MRAs for the medical treatment of PA is associated with significantly higher risk for incident cardiometabolic events and death, and these risks are independent of blood pressure control. Titrating MRA doses to raise renin activity may represent the most effective approach to mitigate this excess risk. Clinical practice guidelines for PA should consider recommending that blood pressure control with MRA therapy alone may be insufficient at mitigating excess cardiovascular risk; rather, targeting a rise in renin activity as a clinical benchmark for adequate mineralocorticoid receptor blockade may be the most efficient therapeutic approach.
Acknowledgments
The authors acknowledge their funding sources. AV was supported by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01 DK107407, by Grant 2015085 from the Doris Duke Charitable Foundation, and by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL111771. The National Institute of Digestive and Diabetes and Kidney Diseases of the National Institutes of Health supported GCC under Award Number K24DK091417 and GLH under Award Number F32 DK114953.
Footnotes
Contributors:
GLH, GCC, and AV developed the project concept and designed the study. GLH and NY collected data and performed medical record review to determine both exposure and outcome status. GLH, MW, and AV performed the statistical analyses. GLH and AV wrote the first draft of the report. All authors made critical revisions of the report.
Declaration of Interests:
All authors declare no competing interests.
References
- 1.Conn JW. Plasma Renin Activity in Primary Aldosteronism. Importance in Differential Diagnosis and in Research of Essential Hypertension. JAMA. 1964;190:222–5. [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF., Jr The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889–916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 3.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69(14):1811–20. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary Sodium Restriction Increases the Risk of Misinterpreting Mild Cases of Primary Aldosteronism. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2016-1963. jc20161963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piaditis G, Markou A, Papanastasiou L, Androulakis II, Kaltsas G. Progress in aldosteronism: a review of the prevalence of primary aldosteronism in pre-hypertension and hypertension. Eur J Endocrinol. 2015;172(5):R191–203. doi: 10.1530/EJE-14-0537. [DOI] [PubMed] [Google Scholar]
- 6.Baudrand R, Guarda FJ, Fardella CE, Hundemer G, Brown J, Williams GH, Vaidya A. Continuum of Renin-Independent Aldosteronism in Normotension. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markou A, Pappa T, Kaltsas G, Gouli A, Mitsakis K, Tsounas P, Prevoli A, Tsiavos V, Papanastasiou L, Zografos G, Chrousos GP, Piaditis GP. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: A very high odds ratio for progression into arterial hypertension. Journal of Clinical Endocrinology and Metabolism. 2013;98:1409–16. doi: 10.1210/jc.2012-3353. [DOI] [PubMed] [Google Scholar]
- 8.Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, De Boer IH, Vaidya A. The Spectrum of Subclinical Primary Aldosteronism and Incident Hypertension: A Cohort Study. Annals of Internal Medicine. 2017 doi: 10.7326/M17-0882. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaidya A, Malchoff CD, Auchus RJ, Committee AAS. An Individualized Approach to the Evaluation and Management of Primary Aldosteronism. Endocr Pract. 2017;23(6):680–9. doi: 10.4158/EP161717.RA. [DOI] [PubMed] [Google Scholar]
- 10.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–33. doi: 10.1210/jc.2013-2805. [DOI] [PubMed] [Google Scholar]
- 11.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62–9. doi: 10.1161/HYPERTENSIONAHA.113.01316. [DOI] [PubMed] [Google Scholar]
- 13.Catena C, Colussi G, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50(5):911–8. doi: 10.1161/HYPERTENSIONAHA.107.095448. [DOI] [PubMed] [Google Scholar]
- 14.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Archives of internal medicine. 2008;168:80–5. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 15.Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, Endres S. Observational study mortality in treated primary aldosteronism: The German conn’s registry. Hypertension. 2012;60:618–24. doi: 10.1161/HYPERTENSIONAHA.112.197111. [DOI] [PubMed] [Google Scholar]
- 16.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–54. [PubMed] [Google Scholar]
- 17.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345(23):1689–97. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 18.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18(9):1429–33. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res. 2016;118(6):935–43. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strauch B, Petrak O, Zelinka T, Wichterle D, Holaj R, Kasalicky M, Safarik L, Rosa J, Widimsky J., Jr Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21(10):1086–92. doi: 10.1038/ajh.2008.243. [DOI] [PubMed] [Google Scholar]
- 21.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF, Jr, Gomez-Sanchez CE, Funder JW, Reincke M Primary Aldosteronism Surgery Outcome i. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. The lancet Diabetes & endocrinology. 2017 doi: 10.1016/S2213-8587(17)30135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, Catena C. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295(22):2638–45. doi: 10.1001/jama.295.22.2638. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O’Connor PJ, Selby JV, Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–42. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez CJ, Swett K, Agarwal SK, Folsom AR, Fox ER, Loehr LR, Ni H, Rosamond WD, Chang PP. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: the atherosclerosis risk in communities study. JAMA internal medicine. 2014;174(8):1252–61. doi: 10.1001/jamainternmed.2014.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seccia TM, Caroccia B, Adler GK, Maiolino G, Cesari M, Rossi GP. Arterial Hypertension, Atrial Fibrillation, and Hyperaldosteronism: The Triple Trouble. Hypertension. 2017;69(4):545–50. doi: 10.1161/HYPERTENSIONAHA.116.08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS, Group TS. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35(8):1698–708. doi: 10.1097/HJH.0000000000001361. [DOI] [PubMed] [Google Scholar]
- 27.Rossi GP, Seccia TM, Gallina V, Muiesan ML, Leoni L, Pengo M, Ragazzo F, Caielli P, Belfiore A, Bernini G, Cipollone F, Cottone S, Ferri C, Giacchetti G, Grassi G, Letizia C, Maccario M, Olivieri O, Palumbo G, Rizzoni D, Rossi E, Sechi L, Volpe M, Mantero F, Morganti A, Pessina AC. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): rationale and study design. J Hum Hypertens. 2013;27(3):158–63. doi: 10.1038/jhh.2012.21. [DOI] [PubMed] [Google Scholar]
- 28.Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–9. doi: 10.1210/jc.2005-1733. [DOI] [PubMed] [Google Scholar]
- 29.Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” Adrenal Tumors and the Risk for Incident Diabetes and Cardiovascular Outcomes: A Cohort Study. Ann Intern Med. 2016;165(8):533–42. doi: 10.7326/M16-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, Riester A, Asbach E, Hughes BA, O’Neil DM, Bidlingmaier M, Tomlinson JW, Hassan-Smith ZK, Rees DA, Adolf C, Hahner S, Quinkler M, Dekkers T, Deinum J, Biehl M, Keevil BG, Shackleton CHL, Deeks JJ, Walch AK, Beuschlein F, Reincke M. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2(8) doi: 10.1172/jci.insight.93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.