Abstract
Background
Current Human papillomavirus (HPV) L1 VLP vaccines protect against HPV-16 and HPV-18-associated cancers, in females and males. Although correlates of protection have not been identified, HPV specific antibodies at sites of infection are thought to be the main mechanism of protection afforded by vaccination. Oral sampling has gained increased attention as a potential alternative to serum in monitoring immunity to vaccination and understanding local immunity in oral cancers.
Methods
Serum was collected via venipuncture, and saliva was collected via oral rinses and Merocel® sponges from healthy volunteers: 16 unvaccinated females, 6 females (ages 24–41) and 6 midadult aged male (ages 27–45) recipients of three doses of the HPV-16/18/6/11 vaccine (Gardasil®). Mid-adult male vaccine trial participants were compared to female participants. Samples were tested for anti-HPV-16 and anti-HPV-18 immunoglobulin G levels by an L1 virus-like particle-based enzyme-linked immunosorbent assay (ELISA).
Results
All vaccinated participants had detectable serum anti-HPV-16 and anti-HPV-18 antibodies. Optimal standard concentration range and sample serial dilutions for oral rinses were determined. The standard curve was not affected by the type of solution examined. Reproducibility of HPV-16 and HPV-18 antibody titers in mouthwash (overall CV<10%) or in Merocel® extraction buffer was robust (CV<13%). Excellent assay linearity (R2>0.9) was observed for sera spiked controls in both solutions. HPV-16 and HPV-18 specific antibodies were detectable in saliva from vaccine recipients, both in mouthwash and in Merocel® sponges but levels were several logs lower than those in serum.
Conclusions
This study confirms the application of HPV-16 and HPV-18 ELISAs currently used in sero-epidemiological studies of immunogenicity of HPV vaccines for use with oral samples. Oral samples may be a useful resource for the detection of HPV-16 and HPV-18-specific antibodies in saliva following vaccination.
Introduction
In 2017, it is estimated that 49,670 people will be diagnosed with oral and oropharyngeal cancer in the United States of America (USA), and about 9,700 people will die from this disease [1]. The incidence rates of oral and oropharyngeal cancer are increasing and are more than twice as high in men as women [2]. Persistent infection with oncogenic human papillomavirus (HPV) types, especially HPV-16, is strongly associated with oropharyngeal cancer [3]. Currently in the USA more than 70% of oropharyngeal cancers are attributed to HPV infection, particularly HPV-16 and HPV-18 [4].
Three virus-like particle (VLP) late 1 (L1) based prophylactic vaccines targeting up to nine HPV oncogenic types have been approved by USA Food and Drug Administration (FDA) [5–7]. Gardasil®, first approved in 2006, is comprised of L1 major capsid protein-based VLPs of HPV-6, 11, 16, and 18. The vaccine is highly efficacious at preventing HPV-16 and HPV-18 infections in males and females as well as associated cervical, vaginal, vulvar, and anal lesions [8–11]. Cervarix®, a HPV vaccine comprised of L1 based VLP for HPV-16 and HPV-18 demonstrates robust efficacy against genital HPV-16 and -18 infections in females and in a post-hoc analysis, efficacy at the oral cavity in females [12]. Although the underlying mechanisms of protection have not been fully elucidated, HPV specific neutralizing antibodies at sites of infection are thought to be the main mechanism of protection against infection [13–15].
Traditionally, serum samples have been the specimen of choice for biomarker detection and immune monitoring of vaccines. However, the site of infection where the cancer originates is of critical importance. Saliva has gained increased attention as an attractive alternative collection site to serum, since oral sampling collection is simple, painless, non-invasive, and presents no risks [16]. Many biomarkers have been measured in saliva, such as hormones, cytokines, and vaccine-induced antibodies, making it a promising matrix for monitoring immune responses both systemically and locally in the oral cavity of vaccinated individuals [17–19]. Previous studies have found oral HPV-specific IgG levels in natural infection; however, the levels are low and only modestly correlate with serum HPV-specific IgG levels [20–24]. Detection of anti-HPV-16 antibodies in saliva from vaccine recipients has been previously reported. However, in that study a luminex bead-based assay was used, and data were reported without the use of a standard curve [25]. Given the interest in measuring HPV-specific antibodies in saliva in Clinical/epidemiological studies, we adapted the serum enzyme linked immunoabsorbent assay (ELISA) protocol to assess HPV-specific antibody levels in saliva.
Previously, our lab monitored the immunogenicity of HPV vaccines by measuring serum anti-HPV-16 and anti-HPV-18 IgG antibody levels in sero-epidemiological studies using a standardized VLP-based direct ELISA [26, 27]. Here, we evaluated whether the standardized L1 VLP-based direct ELISA could be used to detect HPV type specific antibodies in oral samples collected using two types of collection methods, mouthwash and Merocel® sponges following vaccination. Our hypothesis was that the HPV vaccine would induce antibodies against HPV at mucosal sites, in this case saliva, that could be detected by an L1 VLP ELISA. However, because levels at mucosal sites were expected to be much lower than in serum, the assay had to be optimized and qualified for the new matrix: saliva. This study serves as a methods validation paper, accompanying our previous findings of HPV antibodies detected in the oral cavity of vaccinated males [28]. A mouthwash sample is comprised of both oral cells and saliva, and is the standard method for collecting oral specimens for HPV analysis [29]. Mouthwash specimens are commonly archived in many HPV studies; therefore, we chose to assess if this specimen was adequate for oral antibody testing. Merocel® sponges served as a device that collects saliva. The data described here indicate that the standardized L1 VLP-based ELISA can reliably detect HPV-16 and HPV-18 antibodies in both mouthwash and Merocel® sponges. Having an assay that allows for accurate detection of HPV specific antibodies in saliva provides an alternative means of determining efficacy of HPV vaccines for the prevention of oral cancer.
Materials and methods
Samples
Saliva from healthy research donor volunteers (Occupational Health Services, FNLCR, Frederick, MD), 16 unvaccinated (ages 32–63) and 6 female (ages 24–41) recipients of three doses of the HPV-16/18/6/11 vaccine (Gardasil®) were collected in mouthwash (Target) and in Merocel® (Beaver-Visitec International, Inc.) sponges. HPV vaccinated donors were chosen based solely on vaccination status, and unvaccinated donors were chosen based on prescreening and testing as HPV seronegative. Six serum samples from male recipients of Gardasil® were used from the Mid-Adult Male Vaccine Study - The MAM Study (www.clinicaltrials.gov, NCT01432574). This is a single-arm intervention trial that enrolled and vaccinated men ages 27–45, and assessed the antibody responses to Gardasil [30]. Subjects were vaccinated intramuscularly with Gardasil at day 1 of the study and at months 2 and 6. The six male subjects were selected out of 150 men from Tampa, Florida, and Cuernavaca, Mexico, who met eligibility criteria (male sex, age 27–45 years, and completion of 4 years of follow-up in the HPV Infection in Men study) and received at least one dose of vaccine. The six samples chosen were from month 30 of the study (24 months after vaccination).
Mouthwash Samples
50 mL tubes (Corning Cat# 352098) were filled with 15 mL of mouthwash solution and donors were asked to swish the mouthwash vigorously for 30-45 seconds and to expel the mouthwash into an empty collection tube [31]. Samples were centrifuged, aliquoted and stored at −80°C.
Merocel® Sponges
Subjects placed a Merocel® sponge against the central part of the inner cheek for a total of 30 seconds, 15 seconds for each side. The sponge was then placed into a sterile 15 mL cryovial. Vials were stored at −80°C until extraction. The sponges were extracted using a buffer containing PBS (Gibco Cat# 14190-136), 256 mM NaCl (Fisher Scientific Cat# S271-10) and 100 μg/mL aprotinin (Sigma Cat# A-4529-25MG). Extracts were aliquoted and stored at −80°C. A dilution factor, based on the weight of the collected material, was calculated for each sample, as previously reported [32].
Serum
10 mL of blood was collected in a red top tube (BD Cat# 366430). Following centrifugation, sera was aliquoted into cryovials and stored at −80°C until testing.
ELISA
HPV-16 and HPV-18-specific IgG antibody titers were determined by the L1 VLP ELISA. Microtiter plates (Maxisorp, NUNC, Cat# 439454) were coated with HPV VLPs produced in our laboratory, as previously described [33, 34]. Starting at 1:2, Saliva was serially diluted 2-fold to 1:256, in the blocking buffer (PBS (Gibco, Cat# 14190-136), 4% Milk (BD, Cat# 232100), 0.2% Tween 20 (VWR, Cat# EM-PX1296-1) was plated and assayed. Positive controls for HPV ELISAs were generated by spiking HPV-antibody positive serum obtained from an HPV vaccine recipient, and diluted into mouthwash or sponge extraction buffer, at ratios of 1:16,666, 1:50,000, and 1:150,000. Negative controls were obtained from Occupational Health Services donors whose serum tested below detection cut offs for HPV-16 and -18 antibodies (negative for HPV-16 and -18 antibodies). Serial dilutions of samples, standards and quality controls were included in each plate and absorbance was measured. The mean optical density (OD) of saliva samples, from HPV-16 and HPV-18 seronegative, plus 3 standard deviations were used to define the cutoff. Antibody levels, expressed as ELISA units (EU)/mL, were calculated by interpolation of OD values from the standard curve by averaging the calculated concentrations from all dilutions that fall within the working range of the standard curve. Assay reproducibility and linearity were determined using mouthwash or sponge extraction buffer spiked with three different known levels of HPV-16 and HPV-18 antibodies (Low: 0.027 EU/mL; Medium: 0.08 EU/mL; High: 0.267 EU/mL). Limit of quantitation (LOQ) was determined by testing 15 seronegative samples, diluted 1:2, and run on three separate days. The mean of the 15 samples plus three times the standard deviation represents LOQ for the assay. For limit of detection (LOD), absolute mouthwash was diluted 1:2, and ran on 6 separate plates, in three different days. The LOD represents the mean plus two times the standard deviation for the assay (data not shown). The LOQ values were in turn used to interpolate cut off values for seropositivity. Lower cut points for serum were set at 19EU/mL for anti-HPV-16, while 18EU/mL was set for anti-HPV-18 ELISA. Cut points for anti-HPV16 mouthwash ELISA were set to 0.042EU/mL, and 0.032 EU/mL for anti-HPV-18. Merocel® extraction buffer cut points were set to 0.030 EU/mL for anti-HPV-16, and 0.036 EU/mL for anti-HPV-18 [28].
Statistical Methods
Proc varcomp (SAS Institute, Inc.) was used to calculate the coefficient of variation (CV) between duplicate plates and among the different days. The linearity of the assay was evaluated using the least squares method.
Results
Standards Concentration range of Saliva HPV L1 VLP ELISA: The HPV standard concentration range was determined by serial dilution of HPV L1 VLP standards for both saliva and serum samples (Table 1). Diluted standards, prepared in mouthwash or Merocel® extraction buffer were tested within two different plates over three consecutive days (Figure 1A, C). The results demonstrate that HPV-16 and HPV-18 ELISA assays are very consistent within different plates and among different days. To determine if standards values were influenced by dilution buffer, standards were diluted in mouthwash or Merocel® extraction buffer. There was no significant difference in the standard curve generated using mouthwash or Merocel® extraction buffer (Figure 1B, 1D) as diluents.
Table 1.
Optimal Standards Concentration Range
| Standards | Saliva ELISA (EU/mL) | Serum ELISA (EU/mL) | ||
|---|---|---|---|---|
| HPV-16 | HPV-18 | HPV-16 | HPV-18 | |
| STD 1 | 0.640 | 0.393 | 2.56 | 1.57 |
| STD 2 | 0.320 | 0.196 | 1.28 | 0.785 |
| STD 3 | 0.160 | 0.098 | 0.64 | 0.393 |
| STD 4 | 0.080 | 0.049 | 0.32 | 0.196 |
| STD 5 | 0.040 | 0.024 | 0.16 | 0.098 |
| STD 6 | 0.020 | 0.012 | 0.08 | 0.049 |
| STD 7 | 0.010 | 0.006 | 0.04 | 0.025 |
| STD 8 | 0.005 | 0.003 | 0.02 | 0.012 |
Standard concentration range for HPV-16 and HPV-18 ELISAs were determined by serial dilution of HPV VLP standards for both saliva and serum samples.
Figure 1.
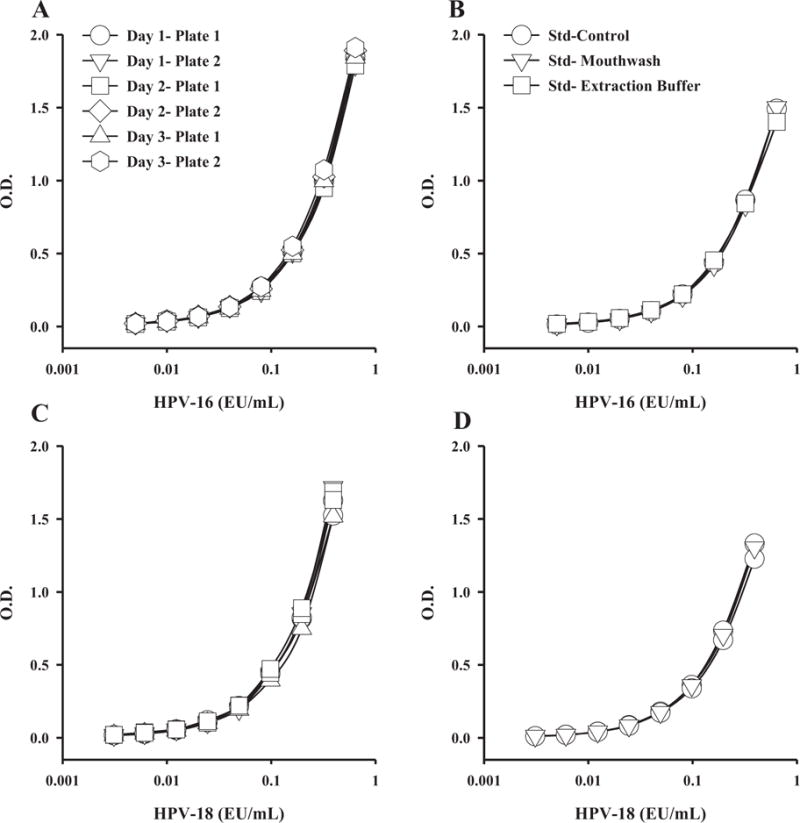
Standard performance for HPV-16 or HPV-18 in different days (A, C), or in different buffer type (B, D). Mouthwash and extraction buffer were tested for anti-HPV-16 and anti-HPV-18 antibodies by ELISA. Testing over different days and matrices did not impact HPV-16 or HPV-18 assay standard performance.
Determination of Cutoff Values of Saliva HPV L1 VLP ELISA: Saliva samples collected in mouthwash and Merocel® sponges from 15 HPV-16 and HPV-18 sera antibody-negative females were tested by ELISA. The antibody levels, expressed as ELISA Units (EU/mL), were calculated by interpolation of Optical Density (OD) values from the standard curve by averaging the calculated concentrations from all dilutions that fall within the working range of the standard curve. The mean OD or EU/mL of these samples plus 3 standard deviations were set as the cutoff values for these assays (Table 2).
Table 2.
Optical Density and Concentration Cutoffs
| O.D. | Concentration (EU/mL) | |||
|---|---|---|---|---|
| HPV-16 | HPV-18 | HPV-16 | HPV-18 | |
|
Mouthwash (n=15, 3 days) |
0.066 | 0.073 | 0.042 | 0.032 |
|
Extraction Buffer (n=15, 2 days) |
0.049 | 0.083 | 0.03 | 0.036 |
Cutoff values were set by taking the mean OD or EU/ml of 15 mouthwash and sponges HPV-16 and HPV-18 seronegative female samples, plus 3 standard deviations.
Reproducibility and Linearity of Saliva HPV L1 VLP ELISA: To determine reproducibility over the range of the assay, positive controls of three different levels of anti-HPV-16 and HPV-18 antibodies were spiked into mouthwash or Merocel® extraction buffer and were tested on 2 different plates over 3 different days. In addition to overall CV, the CVs between different plates and among the different days of these positive controls for both HPV-16 and HPV-18 were calculated. The HPV-16 and HPV-18 antibody titers in mouthwash or in Merocel® extraction buffer were reproducible with overall CVs of less than 12.1% (Table 3).
Table 3.
Reproducibility of Mouthwash and Extraction Buffer Spiked with Different Levels of HPV-16 and HPV-18 antibodies.
| Within Day CV (%) | Between Day CV (%) | Overall CV (%) | ||||
|---|---|---|---|---|---|---|
| HPV-16 | HPV-18 | HPV-16 | HPV-18 | HPV-16 | HPV-18 | |
| Mouthwash Low | 9.3 | 6.5 | 4.4 | 12.2 | 9.6 | |
| Mouthwash Medium | 8.0 | 6.9 | 2.6 | 12.3 | 8.1 | 7.1 |
| Mouthwash High | 3.9 | 4.1 | 4.1 | 4.1 | 4.0 | 4.1 |
| Extraction Buffer Low | 12.1 | 7.5 | 14.0 | 5.5 | 12.1 | 7.7 |
| Extraction Buffer Medium | 7.8 | 5.7 | 16.5 | 6.5 | 8.4 | 6.0 |
| Extraction Buffer High | 5.2 | 4.0 | 6.3 | 6.7 | 5.6 | 4.1 |
Reproducibility was determined by spiking mouthwash or sponge extraction buffer with three different known levels of HPV-16 and HPV-18 antibodies (Low: 0.027 EU/mL; Medium: 0.08 EU/mL; High: 0.267 EU/mL).
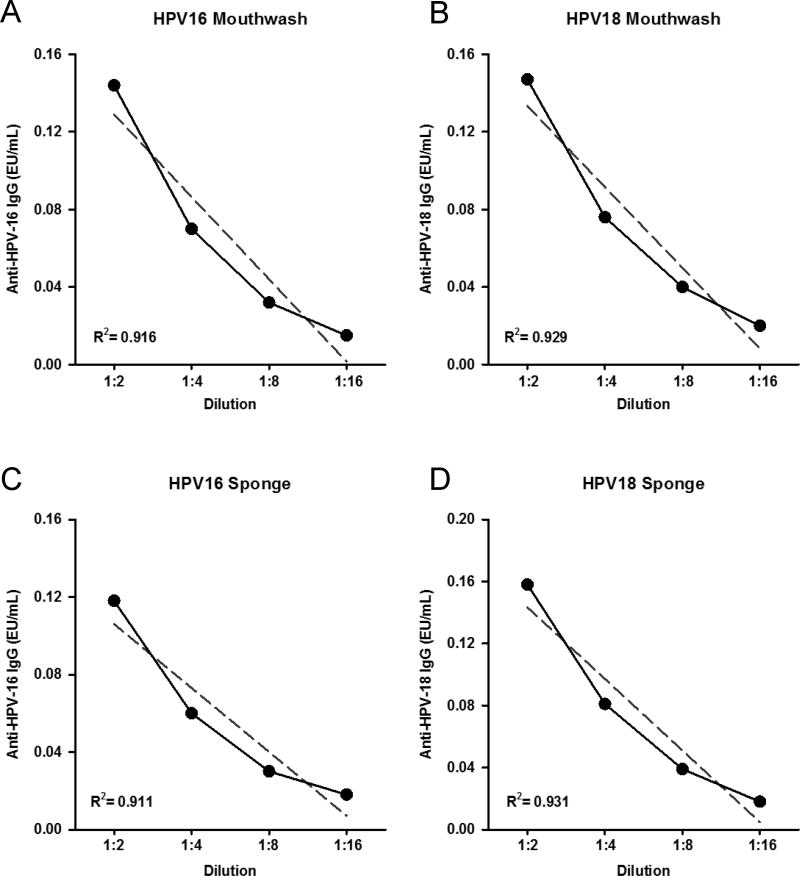
Sera with high levels of HPV-16 and HPV-18 antibodies were spiked into mouthwash or Merocel® extraction buffer and tested by ELISA for assay linearity. Linear regression analysis found an excellent assay linearity (R2>0.9) for spiked controls in both matrices (Figure 2). Assessment of linear regression showed strong linearity when averaged between 12 samples (Table 4).
Figure 2.
Assay linearity in mouthwash (A, B) and in sponge extraction buffer (C, D) for HPV-16 and HPV-18. Mouthwash and Merocel® extraction buffers spiked with HPV-16 and HPV-18 antibody positive sera display robust linearity. R2 is representative of 1 sample serially diluted.
Table 4.
Assay Linearity in Mouthwash and in Extraction Buffer
| R2 | CV | |
|---|---|---|
| HPV-16 Mouthwash | 0.919 | 1.0% |
| HPV-16 Extraction Buffer | 0.923 | 0.9% |
| HPV-18 Mouthwash | 0.925 | 1.4% |
| HPV-18 Extraction Buffer | 0.921 | 0.7% |
Assay linearity was assessed by spiking in HPV-16 and HPV-18 seropositive samples into mouthwash and extraction buffer. R2 obtained from 12 samples.
HPV ELISA with Saliva from Vaccine Recipients: This study is focused on the optimization of the HPV ELISA system and evaluation of its performance for testing a challenging mucosal sample: saliva samples. A small set of samples selected were used as a proof of principle for the application of serological methods for use in saliva samples collected in clinical trials. HPV-16 and HPV-18 ELISAs were performed in mouthwash and in Merocel® sponge saliva samples, collected from six females, and six male Gardasil® vaccinated volunteers. HPV-16 and HPV-18 specific antibodies were detectable in saliva from vaccinated recipients, both in mouthwash and in Merocel® sponges (Figure 3). All volunteers had detectable antibodies against HPV-16 and HPV-18 in serum (HPV-16 median was 3005.8 EU/mL for males and 886.0 EU/mL for females; HPV-18 median was 817.6 EU/mL for males and 201.7 EU/mL for females).
Figure 3.
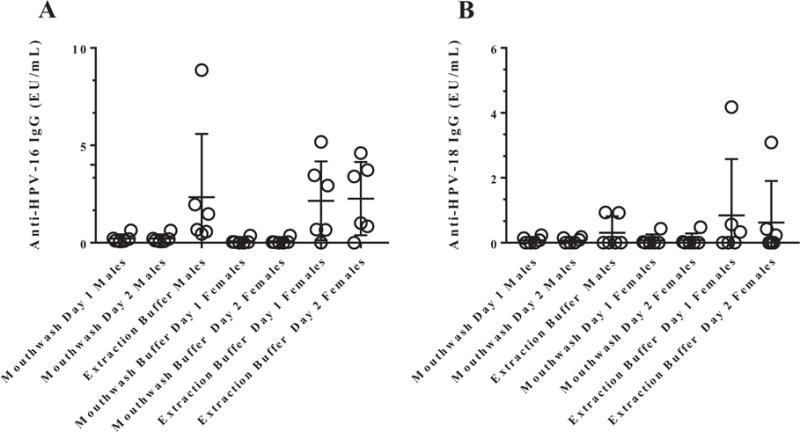
HPV-16 (A) and HPV-18 (B) antibodies can be detected by ELISA in saliva from vaccine recipients. ELISA cutoffs were determined by using mouthwash and sponge samples from 15 HPV-16 and HPV-18 seronegative females, as tested by ELISA. The antibody levels, expressed as ELISA Units (EU/ml), were calculated by interpolation of OD values from the standard curve by averaging the calculated concentrations from all dilutions that fall within the working range of the standard curve. The mean OD or EU/ml of these samples plus 3 standard deviations were set as the cutoff values for these assays (Table 2). Large variations exist in antibody levels as the collection of blood from male samples were part of a clinical trial, collected at 30 months post vaccination, while females were healthy volunteers from the Occupational Health Services that self-reported vaccination and were not part of a clinical trial.
Discussion
HPV prophylactic vaccine correlates of protection have not been formally identified, although the vaccine has shown excellent efficacy over a range of serum antibody titers [35–38]. Preclinical and clinical studies indicate that neutralizing antibodies are the major effector of protection against infection [13, 39–45]. Thus, there has been an increased interest in measuring antibody levels, particularly at sites of infection, including cervical secretions and oral fluids [28, 33, 46, 47]. Antibody levels observed at mucosal secretions are known to be logs lower than serum levels [28, 48]. This represents a challenge for quantitative assessments of anti-HPV antibodies, as assays used for sera may lack sensitivity for detection in mucosal secretions.
Despite the relevance and interest of mucosal measurements of vaccine induced immune markers, there are no standardized procedures for saliva collection for antibody measurements. Commonly used methods for saliva collection have been passive drool or spit [49]. Saliva can be obtained, in the absence of stimulation (unstimulated) or by stimulation using various agents or techniques [50]. Unstimulated saliva is preferred in most biomarker studies as the composition of stimulated saliva can be influenced by the materials or techniques used during collection. In this study, saliva was collected using mouthwash, a solution typically used in epidemiological studies for collection of samples for HPV DNA or other biomarkers [31]. Merocel® sponges, a device previously used with success in biomarker studies for cervical secretion collections were also assessed in this study [51]. Our results demonstrate that anti-HPV-16 and HPV-18 antibodies can be reliably detected by ELISA in saliva collected using these two common collection methods in epidemiological studies.
Overall, CVs were comparable to assay CVs described previously for sera ELISA [28]. Using this assay, we recently reported that the HPV quadrivalent vaccine induces significant antibody levels in saliva from men that received three vaccine doses [28]. In addition, a strong correlation was observed between antibody levels in serum and oral fluid of men. These findings are consistent with findings from other vaccines and suggest that antibodies found in the oral cavity transudate from the peripheral blood, suggesting that serum levels are a good proxy for mucosal secretions [52, 53]. In previous studies, HPV-specific antibodies were measured in saliva and oral mucosal transudate (OMT), using an Ora Sure device [25]. However, the assay used in that study did not include a standard, and results were reported in MFI (median fluorescent intensity). We have adapted our HPV L1 VLP-based ELISA to measure antibodies in oral fluids. The results indicate that our ELISA can be used reliably to measure antibody levels in saliva collected in mouthwash or in Merocel® sponges, and that these matrices do not affect the standard curve or assay performance.
Currently, there are no established HPV-16 or HPV-18 standards nor controls for saliva HPV-antibody positive assays. For this study, standards and positive controls were generated by spiking HPV positive serum into the solutions (mouthwash and extraction buffer) used for saliva collection. The results suggest that the different extraction buffers used did not affect detection of the standards. The negative control cutoff points for both HPV-16 and HPV-18 were determined from the saliva of seronegative females. The cutoff values found were different between mouthwash and sponge. This variation is most likely due to the difference in recoveries of the antibodies from varying samples using the two different devices. Assay reproducibility and linearity in saliva were robust, supporting the application of this assay in future large epidemiologic studies.
Finally, we tested our ELISA in the saliva samples from vaccine recipients. Both HPV-16 and HPV-18 specific antibodies were detected in the saliva samples from both mouthwash and sponge extraction buffer, of males and females. Overall, the antibody levels in oral samples were 500-fold lower compared to serum samples [28]. In conclusion, our ELISA can be reliably used for measurement of HPV-16 and HPV-18 antibody levels in saliva from vaccinated individuals using two common collection methods.
Acknowledgments
We thank Merck Research Laboratories, for supplying the quadrivalent HPV vaccine (MAM Study); individuals who made significant contributions to this study, including Kimberly Isaacs-Soriano, Christina Gage, Andrea Bobanic, and Kayoko Kennedy; and all study participants, without whom this study would not have been possible.
Financial Support: This work was supported by the Investigator-Initiated Studies Program of Merck Sharp and Dohme (research grant to A. R. G.); the Miles for Moffitt program, and the Moffitt Cancer Center. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health under contract No.HHSN261200800001E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. The opinions expressed in this paper are those of the authors and do not necessarily represent those of the funder. The funders of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Potential Conflicts of interest: ARG reports receiving grants from Merck during the conduct of the study and other grants from Merck outside the submitter work. All other authors reported no potential conflicts.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza G, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardasil®, [package insert] United States Food and Drug Administration. Initial approval 2006. Revised 03/2014. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111263.pdf. Accessed June 2014.
- 6.Gardasil9®, [package insert] United States Food and Drug Administration. Initial approval 2014. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111263.pdf. Accessed August 2017.
- 7.Cervarix®, [package insert] United States Food and Drug Administration. Initial approval 2009. http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM186981.pdf. Accessed June 2014.
- 8.Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199(7):936–44. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 9.Munoz N, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–39. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 10.Brown DR, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199(7):926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AR, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrero R, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitburd F, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzich JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92(25):11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118(1 Suppl):S12–7. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson S, et al. Comparison of biomarkers in blood and saliva in healthy adults. Nurs Res Pract. 2012;2012:246178. doi: 10.1155/2012/246178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wade SE, Haegele AD. Time-integrated measurement of corticosteroids in saliva by oral diffusion sink technology. Clin Chem. 1991;37(7):1166–72. [PubMed] [Google Scholar]
- 18.Streckfus C, Bigler L, O’Bryan T. Aging and salivary cytokine concentrations as predictors of whole saliva flow rates among women: a preliminary study. Gerontology. 2002;48(5):282–8. doi: 10.1159/000065250. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowski PA, et al. Mucosal vaccination strategies for women. J Infect Dis. 1999;179(Suppl 3):S493–8. doi: 10.1086/314810. [DOI] [PubMed] [Google Scholar]
- 20.Buchinsky FJ, et al. Comparison of oral fluid and serum ELISAs in the determination of IgG response to natural human papillomavirus infection in university women. J Clin Virol. 2006;35(4):450–3. doi: 10.1016/j.jcv.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Cameron JE, et al. Human papillomavirus-specific antibody status in oral fluids modestly reflects serum status in human immunodeficiency virus-positive individuals. Clin Diagn Lab Immunol. 2003;10(3):431–8. doi: 10.1128/CDLI.10.3.431-438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marais DJ, et al. Oral antibodies to human papillomavirus type 16 in women with cervical neoplasia. J Med Virol. 2001;65(1):149–54. [PubMed] [Google Scholar]
- 23.Passmore JA, et al. Cervicovaginal, oral, and serum IgG and IgA responses to human papillomavirus type 16 in women with cervical intraepithelial neoplasia. J Med Virol. 2007;79(9):1375–80. doi: 10.1002/jmv.20901. [DOI] [PubMed] [Google Scholar]
- 24.Marais DJ, et al. More men than women make mucosal IgA antibodies to Human papillomavirus type 16 (HPV-16) and HPV-18: a study of oral HPV and oral HPV antibodies in a normal healthy population. BMC Infect Dis. 2006;6:95. doi: 10.1186/1471-2334-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowhani-Rahbar A, et al. Antibody responses in oral fluid after administration of prophylactic human papillomavirus vaccines. J Infect Dis. 2009;200(9):1452–5. doi: 10.1086/606026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrin DM, et al. Comparison of adaptive and innate immune responses induced by licensed vaccines for Human Papillomavirus. Hum Vaccin Immunother. 2014;10(12):3446–54. doi: 10.4161/hv.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safaeian M, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6(11):1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto LA, et al. Quadrivalent Human Papillomavirus (HPV) Vaccine Induces HPV-Specific Antibodies in the Oral Cavity: Results From the Mid-Adult Male Vaccine Trial. J Infect Dis. 2016;214(8):1276–83. doi: 10.1093/infdis/jiw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawton G, et al. Human papillomaviruses in normal oral mucosa: a comparison of methods for sample collection. J Oral Pathol Med. 1992;21(6):265–9. doi: 10.1111/j.1600-0714.1992.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 30.Giuliano AR, et al. Immunogenicity and safety of Gardasil among mid-adult aged men (27–45 years)-The MAM Study. Vaccine. 2015;33(42):5640–6. doi: 10.1016/j.vaccine.2015.08.072. [DOI] [PubMed] [Google Scholar]
- 31.Pierce Campbell CM, et al. Quantification of secretory leukocyte protease inhibitor (SLPI) in oral gargle specimens collected using mouthwash. J Immunol Methods. 2013;400–401:117–21. doi: 10.1016/j.jim.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemp TJ, et al. Oral Immunoglobulin Levels are Not a Good Surrogate for Cervical Immunoglobulin Levels. Front Oncol. 2012;2:61. doi: 10.3389/fonc.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemp TJ, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26(29–30):3608–16. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dessy FJ, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4(6):425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 35.Group, F.I.S. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 36.Paavonen J, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 37.Garland SM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 38.Joura EA, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 39.Munoz N, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373(9679):1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 40.Nygard M, et al. Evaluation of the Long-Term Anti-Human Papillomavirus 6 (HPV6), 11, 16, and 18 Immune Responses Generated by the Quadrivalent HPV Vaccine. Clin Vaccine Immunol. 2015;22(8):943–8. doi: 10.1128/CVI.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsson SE, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10):696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 42.Romanowski B, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: Five-year clinical data and modeling predictions from a randomized study. Hum Vaccin Immunother. 2016;12(1):20–9. doi: 10.1080/21645515.2015.1065363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz TF, et al. Four-year follow-up of the immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine when administered to adolescent girls aged 10–14 years. J Adolesc Health. 2012;50(2):187–94. doi: 10.1016/j.jadohealth.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Villa LL, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24(27–28):5571–83. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 45.Day PM, et al. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J Virol. 2007;81(16):8784–92. doi: 10.1128/JVI.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nardelli-Haefliger D, et al. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95(15):1128–37. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 47.Petaja T, et al. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer. 2011;129(9):2147–57. doi: 10.1002/ijc.25887. [DOI] [PubMed] [Google Scholar]
- 48.Scherpenisse M, et al. Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Hum Vaccin Immunother. 2013;9(2):314–21. doi: 10.4161/hv.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 50.Topkas E, et al. Evaluation of saliva collection devices for the analysis of proteins. Clin Chim Acta. 2012;413(13–14):1066–70. doi: 10.1016/j.cca.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 51.Kemp TJ, et al. Evaluation of two types of sponges used to collect cervical secretions and assessment of antibody extraction protocols for recovery of neutralizing anti-human papillomavirus type 16 antibodies. Clin Vaccine Immunol. 2008;15(1):60–4. doi: 10.1128/CVI.00118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brokstad KA, et al. IgA, IgA subclasses, and secretory component levels in oral fluid collected from subjects after parental influenza vaccination. J Infect Dis. 1995;171(4):1072–4. doi: 10.1093/infdis/171.4.1072-a. [DOI] [PubMed] [Google Scholar]
- 53.Brokstad KA, et al. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis. 2002;185(7):878–84. doi: 10.1086/339710. [DOI] [PubMed] [Google Scholar]