Abstract
BACKGROUND
The Diabetes-Depression Care-Management Adoption Technologies Trial is a translational study of safety-net primary care predominantly Hispanic patients with type 2 diabetes in collaboration with the Los Angeles County Department of Health Services (LACDHS).
OBJECTIVES
To evaluate cost effectiveness of an information and communication technologies (ICT) facilitated depression care management program.
STUDY DESIGN
Cost-effectiveness of the ICT-facilitated care (TC) delivery model was evaluated relative to usual care (UC) and a supported care (SC) model. TC added automated low-intensity periodic depression assessment calls to patients. Patient reported outcomes included the 12-item Short Form Survey (SF-12) converted into quality adjusted life years (QALYs) and 9-item Patient Health Questionnaire (PHQ-9)-calculated depression-free days (DFDs). Costs and outcomes data were collected over a 24-month period (−6 to 0 months baseline, 0 to 18 months study intervention).
RESULTS
A sample of 1,406 patients (484 in UC, 480 in SC, 442 in TC) was enrolled in the non-randomized trial. TC had a significant improvement in DFDs (17.3; P = .011) and significantly greater SF-12 utility improvement (2.1%, P = 0.031) compared to UC. Medical costs were statistically significantly lower for TC (−$2,328; P=.001) relative to UC but not significantly lower than SC. TC had a greater than 50% probability of being cost effective relative to SC at willingness to pay thresholds over $50,000 per QALY.
CONCLUSIONS
A technology-facilitated depression care delivery model improved quality-adjusted life years, depression free days and medical costs. It was cost-effective compared to supported care and dominant compared to usual care.
Keywords: primary care, disease management, depression, direct health care costs, telemedicine, health technology assessment, automated assessment, cost–utility analysis, cost effectiveness analysis
Introduction
Depression, an often-ignored comorbidity for those with chronic illness [1], creates significant challenges for primary care systems because it worsens health status and outcomes, increases health care utilization and costs, and elevates suicide risk [2–6]. This is particularly true for Hispanic patients served by safety-net primary care providers, who often find it challenging to engage patients with major depression, particularly when accompanied by concurrent chronic illnesses, because it requires active and ongoing depression symptoms assessment and management on top of managing other medical conditions such as diabetes [7–10]. Concurrently, Hispanics are less likely to receive guideline-congruent depression care even after controlling for clinical and economic factors [11], more likely to be served by physicians who fail to detect existing mental health problems [12,13], and at higher risk of discontinuing antidepressant use during the first 30 days of treatment [14,15].
An increasingly popular supported care delivery model involves team-based support for chronic care functions and uses patient disease registry information systems to support guideline- and protocol-based clinical decisions [8]. Despite its effectiveness [16–19], integrating depression comorbidity care remains a substantial challenge, especially in terms of proactive screening, treatment follow-up, and long-term monitoring and management, because of the intensive labor and time needed to collect, summarize, and review individual or aggregate patient data to facilitate care [8].
Harnessing advanced information and communication technologies (ICT) to automate key aspects of depression care is a promising approach to facilitating adoption of the team-based collaborative depression care model [8]. For example, automated speech recognition telephonic assessment technology [20,21] combined with an electronic decision rules and priorities management system [22] can automate depression assessments, patient self-management behavior prompting, optimization of treatment follow-up, and ongoing monitoring and management. This approach can fill gaps in current implementation of depression care to facilitate optimal adaptive depression management in primary care that could simultaneously improve health care outcomes at reduced costs, reduce the physical or economic burden on patients, and be responsive to patient choice.
The Diabetes-Depression Care-Management Adoption Trial (DCAT) [8,23–29] is a translational study conducted in collaboration with the Los Angeles County Department of Health Services (LACDHS), the second-largest safety-net care system in the United States. Using a comparative effectiveness research design, this quasi-experimental nonrandomized study compared three delivery models in three groups: usual care (UC), team-supported care (SC), and ICT-facilitated care (TC). This paper evaluates the cost effectiveness of the ICT-facilitated care delivery model, implemented in the TC group of the DCAT, in a predominantly Hispanic safety-net primary care population with type 2 diabetes from a government program or payer perspective.
Methods
As described by Wu and colleagues [8], the DCAT was conducted from 2011 to 2013 in collaboration with LACDHS. Institutional review board approval was obtained from the University of Southern California, Olive View–UCLA Medical Center, and Los Angeles Biomedical Research Institute.
Eight public ambulatory care clinics were selected to participate in the DCAT based on criteria that reflect geographic and diabetes care model diversity. The UC group included two community clinics and represents the status quo of clinical practice, in which primary care physicians and their staffs perform the translation and adoption of depression care evidence. The SC group used disease management team staff members, including physicians, nurse practitioners, nurses, and social workers, acquainted with guidelines and protocols to support diabetes care management for high-risk or high-use patients. The SC model included a homegrown, web-based chronic disease management registry (DMR) system to support clinical assessment and decisions. The TC group involved a fully automated telephone assessment (ATA) system provided in Spanish or English and linked with the DMR to trigger depression care management calls based on patient medical records, call history, and patient preferences [23]. This model was designed to assist time-pressured clinical social workers and medical and nursing providers by routinely screening and monitoring patient depression symptoms, treatment adherence, and communication with providers.
Trained bilingual study recruiters identified type 2 diabetes patients from database and clinical records. Study-eligible patients were 18 years old or older, had been diagnosed with type 2 diabetes, had a working phone number, spoke English or Spanish, and could read and understand the consent form. Patients with possible suicidal ideation, cognitive impairment, alcohol abuse, or recent lithium or antipsychotic medication use at baseline were ineligible for the trial.
As previously described, the DCAT compared three delivery models in three groups: UC, SC, and TC. During the recruitment of TC participants, the recruiters demonstrated ATA calls to participants and assessed their preferences (e.g., language, call time, password-protected access).[8] The DCAT project assistant configured patient enrollment and baseline information in the DMR. An algorithm-driven electronic rule and priorities management system then processed DMR clinical and patient preferences data to determine automated call characteristics for each patient (including frequency of call, applicable modules, questions to be asked, language, and call time). Patients then received low-intensity periodic calls assessing depression symptoms and treatment adherence. For patients without prior history nor current diagnosis of depression, the ATA calls were made once per quarter; otherwise once per month. Patient responses to the ATA calls were automatically documented in the DMR. If patients exhibited depressive symptoms, self-harm intentions, or concerns about medication, automated task or alerts would engage providers (e.g., nurse care managers, social workers, emergency responders) to provide appropriate care management. Prior publications have detailed the technology design [23] and evaluated patient acceptance [24] and engagement [28] in the TC approach.
Data Collection
The complete set of data collection instruments are described in detail elsewhere [8]. Patients were surveyed at baseline (−6 to 0 months) and outcomes were reported at 6-month intervals thereafter (0 to 18 months). We evaluated cost and cost-effectiveness outcomes during the 18-month follow-up evaluation period relative to the baseline period.
The DCAT study aimed to accelerate the adoption of the collaborative care depression model. Two prior studies of this care model, the Improving Mood-Promoting Access to Collaborative (IMPACT) randomized controlled trial and the Multifaceted Diabetes and Depression Program (MDDP) study, have conducted cost-effectiveness analyses to establish the economic values of the collaborative care model.[30, 31] These studies used SF-12 and DFDs as the predetermined outcome measures. To be consistent and comparable to the prior studies, the DCAT also chose SF-12 and DFDs as outcome measures.
Depression-free days (DFDs) were calculated using the 9-item Patient Health Questionnaire (PHQ-9). A PHQ-9 score less than 5 indicated that the patient had 1 DFD, whereas a PHQ-9 score greater than 14 indicated 0 DFDs. Scores between 5 and 14 reflected linearly interpolated (0–1) depression scores between remission and major depression [32]. The PHQ-9 was used because it provides both a dichotomous diagnosis of major depression and a continuous severity score and has been found to have high sensitivity and specificity for a diagnosis of major depressive disorder based on a structured psychiatric interview [33,34]. Health-related quality of life was assessed using the Medical Outcomes Study Short-Form Health Survey (SF-12) physical and mental component summaries fitted to the Brazier and Roberts SF-6D utility scale [35]. As with the prior collaborative depression care studies, these utility scores and DFDs were used to estimate quality-adjusted life years (QALYs) gained during the evaluation period relative to baseline.
Medical care costs and utilization were obtained from LACDHS electronic medical services records for all study participants, based on the International Classification of Diseases (9th edition), Diagnosis-Related Group, National Drug Code, and Current Procedures Terminology (4th edition) coding. Because county payments are confidential and to make the cost analysis generalizable beyond Southern California, we used 2013 Medicare prices to measure medical service costs per unit. Medicare prices (payment amounts allowed by Medicare) were attached to these medical services based on the RBRVS EZ-Fees software program, which creates and analyzes physician payments using Medicare’s Resource-Based Relative Value Scale for all services except pharmaceuticals [31]. Because the same prices were assigned to all medical services regardless of time period, medical cost inflation was not relevant to the cost estimates. Given the 18 month follow up period, no discounting was done to costs or outcomes, since the effects would be negligible. Medication dispensing date, drug name, National Drug Code, and drug cost were obtained from LACDHS electronic pharmacy records.
Intervention costs were measured as actual budget-based costs (not charges) for the ATA intervention. The automated calls cost 10 cents per minute. Calls averaged 5.28 minutes for the 1,738 completed ATA calls made to TC intervention patients, which was approximately 50% response rate. ATA calls triggered an emergency responder to contact patients to assess suicide or self-harm risks 35 times, at a cost of $100 per response. The data sources for the cost evaluation also included the ATA call records and a survey of 12 providers taking part in the DCAT who estimated the time (in minutes) needed to screen for depression using the PHQ-9 and assess medication adherence and other issues during a phone call. As detailed in the online appendix the average cost to complete a human depression assessment call using the SC approach was $18.72, compared to $0.57 for the ATA system. The average additional study implementation patient cost per quarter was $28.60 for SC patients and $18.49 for TC patients compared to UC patients (see online appendix for details).
The ATA call and provider notification system cost about $120,000 to develop. These system development costs would not be applicable to an ATA system already in place or ready for implementation, and were thus excluded from the cost comparison analysis. If such development costs were amortized over the TC intervention group, they would add about $5.50 per call, assuming that the ATA system has a useful life of 10 years with a 10% straight line depreciation rate. That cost per call would still make the ATA contact system substantially less expensive than the $18.72 per call for conducting a human depression assessment in the SC group. Moreover, this is a negligible amount given that the annual predicted medical costs per study patient exceed $6,000 (see Table 1).
Table 1.
Comparison of baseline characteristics of 1,406 patients enrolled in the DCAT, Los Angeles, 2011–2013.
| UC | SC | TC | SC vs. UC | TC vs. UC | TC vs. SC | |
|---|---|---|---|---|---|---|
| (n = 484) | (n = 480) | (n = 442) | ||||
| M (SD) or n (%) | P | |||||
| Age | 55.05 (9.27) | 52.09 (9.25) | 52.59 (8.90) | < .001 | < .001 | .40 |
| Female | 334 (69.0) | 285 (59.3) | 273 (61.8) | .002 | .02 | .50 |
| Hispanic or Latino | 454 (94.2) | 400 (83.3) | 400 (90.7) | < .001 | .06 | .001 |
| Spanish as preferred language | 429 (88.6) | 373 (77.7) | 361 (81.7) | < .001 | .004 | .16 |
| Body mass index | 32.40 (7.00) | 32.66 (7.63) | 33.16 (7.18) | .58 | .11 | .31 |
| Less than high school education | 364 (75.4) | 298 (62.1) | 315 (71.3) | < .001 | .18 | .004 |
| Unemployed | 327 (67.6) | 324 (67.5) | 293 (66.3) | .99 | .73 | .75 |
| Economic status | 3.96 (2.44) | 3.77 (1.96) | 4.33 (2.09) | .18 | .01 | < .001 |
| Stressors | 2.14 (2.19) | 2.55 (2.32) | 2.50 (2.10) | .005 | .01 | .70 |
| Sum of stress level | 14.48 (16.24) | 19.18 (19.66) | 16.88 (16.81) | < .001 | .03 | .06 |
| Predicted annual health cost | 6469.04 (3449.31) | 6884.32 (3831.27) | 6319.77 (3954.15) | .08 | .54 | .03 |
| Age at onset of diabetes | 45.03 (10.45) | 41.93 (10.26) | 42.23 (9.93) | < .001 | < .001 | .65 |
| Insulin use | 132 (72.7) | 322 (67.1) | 288 (65.2) | < .001 | < .001 | .58 |
| SF-12 physical | 43.04 (11.17) | 45.81 (11.02) | 43.95 (10.85) | < .001 | .21 | .01 |
| SF-12 mental | 50.05 (12.17) | 49.03 (14.39) | 50.49 (12.39) | .23 | .58 | .10 |
| Diabetes complications | 0.71 (0.45) | 0.74 (0.44) | 0.65 (0.48) | .32 | .05 | .004 |
| Whitty-9 diabetes symptoms scale | 1.67 (0.62) | 1.72 (0.65) | 1.56 (0.53) | .19 | .005 | < .001 |
| Diabetes emotional burden | 2.76 (1.96) | 3.70 (2.07) | 2.52 (1.88) | < .001 | .05 | < .001 |
| Diabetes regimen stress | 2.61 (1.91) | 3.61 (2.10) | 2.40 (1.85) | < .001 | .10 | < .001 |
| Diabetes self-care | 4.00 (1.34) | 4.75 (1.24) | 4.23 (1.24) | < .001 | .006 | < .001 |
| PHQ-9 | 6.67 (5.50) | 6.93 (6.49) | 6.37 (5.95) | .50 | .43 | .17 |
| Brief Symptom Inventory | 1.35 (2.97) | 1.30 (3.25) | 0.97 (2.69) | .81 | .04 | .09 |
| Sheehan Disability Scale | 2.24 (2.83) | 2.13 (3.02) | 2.04 (2.86) | .28 | .55 | .64 |
| Dysthymia | 68 (14.1) | 123 (25.6) | 65 (14.7) | < .001 | .86 | < .001 |
| Previous diagnosis of major depressive disorder | 26 (5.4) | 77 (16.0) | 17 (3.8) | < .001 | .34 | < .001 |
| Chronic pain | 146 (30.2) | 135 (28.1) | 73 (16.5) | .53 | < .001 | < .001 |
| Overall patient satisfaction | 4.62 (0.73) | 4.82 (0.49) | 4.67 (0.53) | < .001 | .18 | < .001 |
| A1c value | 8.39 (1.94) | 9.57 (2.21) | 9.72 (1.94) | < .001 | < .001 | .30 |
A1c, glycated hemoglobin; DCAT, Diabetes-Depression Care-Management Adoption Trial; PHQ-9, 9-item Patient Health Questionnaire; SC, supported care; SF-12, Short Form-12 Health Survey; TC, technology-facilitated care; UC, usual care.
Statistical Methods
The key outcomes of interest for the cost-effectiveness analysis were medical and intervention costs, DFDs, and SF-12 utility. We conducted the primary cost-effectiveness analysis in terms of cost per QALY from a payer perspective, with additional consideration of the overall intervention impacts on medical costs, quality of life, and DFDs.
Intent-to-treat analysis was conducted to evaluate all intervention effects. Difference-in-differences regression models were estimated to evaluate systematic cost and utilization differences among UC, SC, and TC at 6-, 12- and 18-month follow-ups [38,39]. This regression analysis method is a powerful strategy for adjusting for any individual-specific unobservable factors that are time invariant and account for variation in the outcomes.
To implement this difference-in-differences regression analysis, each patient’s baseline six month costs and outcomes measures was subtracted from that patient’s corresponding cost and outcomes values during each subsequent six month period during the study intervention period (6, 12 and 18 months). This created a panel data set with three observations per patient representing the three differences from baseline in six-month costs and outcomes for each patient. To flexibly adjust for any time trends, time indicators for the 12-month and 18-month periods were included as additional regressors. The regressions generate predicted six-month period contributions to costs and outcomes for 3 periods per patient (6, 12, 18 months). These are then averaged into annualized costs, DFDs and QALY gains for each patient which are reported in the Results.
This econometric approach is demonstrated in the following equations. This study examined the regression specification for an outcome or cost Oit, where i is the subscript for individual i and t is the subscript for time period t (Oi0 represents outcomes or costs measured at the six month preintervention baseline for individual i). Assume a (1 x J) vector of J observable exogenous characteristics Xit, with the j characteristic Xijt for individual i at time t. Assume an additional (1 x K) vector of K time-invariant unobservable individual-level exogenous characteristics Ii (e.g., underlying health, personal attitudes and behaviors, personality traits, aptitude, background, etc.) with the k unobservable characteristic Iik. Let eit represent the residual random error for each individual i at each time point t. The panel data regression specification for Oit can be written as:
-
1
Oi0 = β0Xi0 + δIi + ei0 (t = 0)
-
2
Oit = β0Xi0 + δIi + βXit + γTreatment + ξ1Time + eit
(t = 6, 12, 18 months)
In Equation 2, γ is the treatment effect parameter and ξ1 captures a time trend.
We can combine Equations 1 and 2 into a differencing estimation equation:
-
3
O*it = (Oit − Oi0) = βXit + γTreatment + ξ1Time + e*it
(t = 6, 12, 18 months)
In this equation, e*it = (eit − ei0). Using this differencing specification in Equation 3, the β0 and δ parameters for all baseline exogenous characteristics (both observed and unobserved), which are unnecessary for estimating treatment effects, are netted out of the final estimation equation.
Because patients were not randomly assigned to treatment, treatment assignment was unbalanced among the UC, SC, and TC treatment groups, with many baseline characteristics being statistically significantly different across treatment assignment. To adjust for time-varying variable confounding between treatment assignment and cost or other outcome variables, we generated both ordinary least squares and propensity score-adjusted regression estimates [40]. The propensity score was the predicted probability of treatment assignment from a multinomial logistic regression of actual treatment assignment on all available observed patient baseline characteristics. The model used study group as the dependent variable and 27 baseline characteristics as the observed independent variables: age; sex; preferred language; body mass index; education level; employment status; economic status; total stressors; sum stress level; predicted future health costs; age at onset of diabetes; insulin use; SF-12 physical and mental health scores (scored 0–100, wherein higher scores indicate a higher level of health); number of diabetes complications; Whitty-9 diabetes symptoms scale score (scored 1–5, wherein higher scores indicate more severe diabetes); diabetes emotional burden; diabetes regimen distress; mean Toobert diabetes self-care score (scored 0–7, wherein higher scores indicate better diabetes self-care); PHQ-9 score (scored 0–27, wherein higher scores indicate worse depression); Brief Symptom Inventory total score (scored 0–24, wherein higher scores indicate worse anxiety); mean Sheehan Disability Scale score (scored 0–10, wherein higher scores indicate more significant functional impairment); dysthymia; previous diagnosis of major depressive disorder; chronic pain; overall patient satisfaction; and hemoglobin A1c value. Because treatment assignment in the trial was not balanced on observable baseline factors, it is appropriate to use a regression method that captures and adjusts for variation in baseline observable factors.
In addition to the propensity score correction for time-invariant unobservable confounders, all regression models adjusted for patient characteristics including patient age, gender, Hispanic ethnicity, marital status, education, comorbidities, disease severity as measured by the Charlson Index and Chronic Disease Score, smoking status, prior hemoglobin A1C, pain, and utility measures. The regression models were performed both with and without the study group propensity scores, which had little impact on findings.
Results
A sample of 1,406 patients (484 in UC, 480 in SC, 442 in TC) was enrolled in the DCAT. Comparison of baseline characteristics among study groups is shown in Table 1. A majority of the patients were Hispanic or Latino and female. Unbalanced samples were expected because of the quasi-experimental design.
Table 2 provides the difference-in-differences regression-adjusted medical cost comparisons for TC and SC intervention patients compared to UC patients. Separate regressions were run for total medical costs and each cost subcategory. Medical cost subcategories included medications, emergency department, outpatient, inpatient, and laboratory or other (included home care, durable medical equipment, and additional medical costs not otherwise specified) in 6-month intervals for the 18-month study duration. The TC delivery model had a substantial 6-month savings compared to the UC group in total medical costs of −$776 (P = .001; 95% CI = −$1,242 to −$310), with outpatient costs substantially and significantly lower than for the UC group. All other cost categories were not significantly different, with the exception of laboratory or other miscellaneous costs, which were significantly higher than the UC group by $20 for each 6-month period. The SC care delivery model also demonstrated significantly lower total, inpatient, and outpatient medical costs than the UC group, although the point estimate for total medical cost savings was $105 less than for the TC group (not statistically significant).
Table 2.
Regression-adjusted 6 month medical care cost differences from baseline relative to usual care.
| Technology-facilitated care | Supported care | |||||
|---|---|---|---|---|---|---|
| DID | P | 95% CI | DID | P | 95% CI | |
| Medications | −$174 | .177 | −$427, $79 | $174 | .193 | −$88, $435 |
| Emergency department | −$8 | .426 | −$29, $12 | $7 | .504 | −$14, $28 |
| Outpatient | −$763 | .000 | −$985, −$541 | −$288 | .014 | −$518, −$59 |
| Inpatient | $149 | .334 | −$154, $452 | −$584 | < .001 | −$897, −$271 |
| Laboratory and other | $20 | < .001 | $18, $22 | $20 | < .001 | $18, $22 |
| Total medical costs | −$776 | .001 | −$1,242, −$310 | −$671 | .006 | −$1,153, −$189 |
CI, confidence interval; DID, difference-in-differences from baseline period (−6 to 0 months) for each patient relative to UC patients across all intervention time periods (0 to 18 months).
Note. Regression models adjusted for propensity scores, age, gender, ethnicity, marital status, education, and baseline assessed comorbidities, disease severity, smoking, prior hemoglobin A1C, pain, and utility measures. Laboratory or other costs include home care, durable medical equipment, and additional medical costs not otherwise specified. Total and category medical costs are estimated in separate regressions so cost category point estimates don’t add up to the total medical cost point estimates.
As shown in Table 3, the difference-in-difference results revealed that DFDs were significantly higher in each 6-month period for the TC group relative to UC (17.3; P = .011; 95% CI = 3.97–30.68). For the SC group, the 6-month difference in DFDs was not statistically different from the UC group (−6.9; P = .33; 95% CI = −20.7 to 6.9). Regarding SF-12 utility scores, the 6-month differences were significant for both the TC (2.1%; P = .031; 95% CI = 0.002–0.041) and SC (2.9%; P = .005; 95% CI = 0.009–0.050) groups.
Table 3.
Regression-adjusted 6 month outcome differences from baseline relative to usual care.
| Technology-facilitated care | Supported care | |||||
|---|---|---|---|---|---|---|
| DID | P | 95% CI | DID | P | 95% CI | |
| Depression-free days | 17.325 | .011 | 3.971, 30.679 | −6.893 | .328 | −20.694, 6.908 |
| SF-12 utility | 0.021 | .031 | 0.002, 0.041 | 0.029 | .005 | 0.009, 0.050 |
CI, confidence interval; DID, difference in differences; SF-12, Short Form-12 Health Survey.
Note. Regression models adjusted for propensity scores, age, gender, ethnicity, marital status, education, and baseline assessed comorbidities, disease severity, smoking, prior hemoglobin A1C, pain, and utility measures.
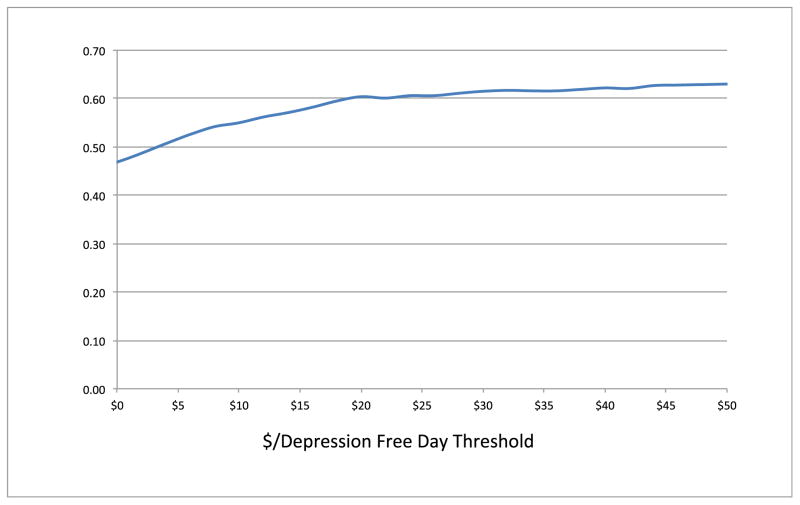
Although costs, QALYs, and DFDs were all significantly better in the TC group relative to the UC group, they were not statistically significantly better than those for the SC group. To assess whether TC was cost effective relative to SC, we used the bootstrap method to evaluate the relative net monetary benefits of TC compared to SC using various willingness-to-pay thresholds for both QALYs and DFDs using the patient-level costs and outcomes trial results. Figure 1 shows that TC had greater than a 50% probability of being cost effective compared to SC as long as the willingness to pay for QALYs is greater than $40,000. Because the current U.S. societal willingness to pay for QALYs is generally thought to be greater than $50,000 and probably between $100,000 and $200,000 per QALY [41–43], this suggests that the TC model is likely to be cost effective relative to the SC model. Figure 2 shows that the TC model had greater than a 50% probability of being cost effective as long as a DFD is worth more than $4. Katon et al. [46] stated that DFDs are valued at approximately $10 per day with a lower bound of $5 a day, implying that the TC model is cost effective in producing DFDs. Because the statistical confidence intervals overlap for the SC and TC outcomes, as shown in Figures 1 and 2 the probability that TC will produce cost effective QALYs or DFDs relative to SC will not approach 100% at any willingness to pay threshold.
Figure 1.
Technology-facilitated care versus supported care cost-effectiveness acceptability curve using cost by quality-adjusted life year threshold.*
*Y-axis: Frequency of PSA Model Replication Results
Figure 2.
Technology-facilitated care versus supported care cost-effectiveness acceptability curve using cost by depression-free day threshold. *
* Y-axis: Frequency of PSA Model Replication Results
Discussion
Principal Finding
To our knowledge, this is the first economic evaluation of a trial of an ATA system delivered in both English and Spanish for depression symptoms assessment and treatment monitoring that serves urban safety-net primary care populations with diabetes. The findings suggest that this ICT-facilitated care delivery model resulted in significant improvements in quality of life and DFDs compared to UC or SC in a large public health care system. The ICT-facilitated care model dominated UC, showing both statistically significantly lower costs and better patient outcomes. The results were quite robust and were not meaningfully different whether estimated with or without propensity score adjustment for non-random treatment assignment. They were also not much different when patients with major depressive disorders were excluded from the regression estimates (results available upon request).
The ICT-facilitated care model showed nonsignificantly better medical costs and quality of life than SC but significantly better DFDs. Given that the TC intervention was less expensive for contacting patients undergoing episodes of depression than SC, the ICT-facilitated care delivery model is likely to be cost effective relative to the SC model, despite the overlap in cost and outcome statistical confidence intervals for the TC and SC interventions. Moreover, since we have repeated patient-level observations on costs and outcomes, the cost effectiveness results are estimated directly using robust distribution-free bootstrapping methods, rather than hypothetical parameter distributions. For this reason, the Acceptability Curves in Figures 1 and 2 reflect the actual distributions of the relative costs and outcomes between the TC and SC groups, rather than model cost effectiveness frequencies based on assumed parameter distributions.
Comparison to Prior Work
The main contribution of this paper is the demonstration that a TC delivery model harnessing advanced ICTs to automate key aspects of depression care is cost effective relative to UC and an SC model involving team-based collaborative depression care for patients with diabetes. Prior studies have evaluated the cost effectiveness of team-supported collaborative care compared to UC for depression and diabetes [38–42]. Katon et al. [38] showed the collaborative care intervention is associated with high clinical benefits at no greater cost than UC for U.S. older adults with depression and diabetes. Cost effectiveness of the collaborative care for depression and diabetes outside the United States was established by Johnson et al. [39] and Camacho et al. [40]. Katon et al. [41] showed that collaborative care is associated with a high probability of achieving savings in long-term (i.e., 5-year) total ambulatory medical costs compared to UC. In that study, higher costs associated with providing team-supported mental health care were offset by greater savings in outpatient and total medical costs [41]. Finally, Hay et al. [31] demonstrated the cost effectiveness of team-supported collaborative care compared to UC in U.S. safety-net populations with diabetes. Nevertheless, substantial challenges remain in safety-net health care systems to integrate collaborative depression care, including the intensive labor and time needed to provide proactive depression screening, treatment follow-up, and long-term monitoring and management [8,23]. The DCAT ICT-facilitated care model is likely to be a cost-effective approach relative to the team-supported collaborative care model. An ATA system tethered to an electronic clinical decision support system offers a promising solution to achieve cost effectiveness and thus scale evidence-based collaborative depression care to large populations, especially in resource-constrained environment like safety-net health care systems.
Approaches to facilitating chronic disease management such as automated remote monitoring ICTs, electronic clinical decision support, and artificial intelligence are receiving increased research attention. However, evidence from economic evaluations of such ICT-facilitated interventions is scarce. Choi-Yoo et al. [47] compared the cost effectiveness of UC and an ATA system with care management for pain and depression in patients with cancer. This prior research and the current study of ATA interventions found the system to be associated with increased DFDs, but only the DCAT saved costs. The DCAT is the first trial of an ATA system for depression and diabetes designed for dissemination in safety-net settings and that compared the ATA system not only with UC but also SC. In the DCAT, the ATA intervention achieved dominance in cost effectiveness compared to UC and was likely to be cost effective compared to SC.
Limitations
The main study limitation relevant to our cost-effectiveness results is the nonrandom assignment of patients to the intervention groups. This imbalance necessitated exploration of alternative propensity score-adjusted regression estimation methods to control for potential treatment assignment bias. The propensity score used for treatment assignment deals with the appropriate source of observable confounding. Alternative approaches to control for treatment assignment bias (e.g., Heckman selection methods or instrumental variables) can create additional model misspecification and are unlikely to improve parameter estimates in this case, since there were no observable variables explaining treatment selection that are unlikely to be correlated with treatment costs and outcomes.[48,49] Although findings were robust regarding alternative regression specifications based on available study variables, and even when patients with major depressive disorders were excluded, we cannot claim the same degree of robustness for these results as we could have if baseline randomization had been used to balance treatment groups across all potential confounders. Nevertheless, there are offsetting limitations associated with randomized study designs as well, including additional study implementation costs, external validity and Hawthorne effects.[50]
Another limitation is the relatively short follow-up period in the DCAT (i.e., 18 months). Given that our findings show significant improvements in costs and outcomes, throughout the study period, even in the final six-month period (13–18 months), it is likely that the cost effectiveness of the TC intervention would have been even more favorable, had additional study follow up been possible. Thus our results are conservative estimates of the value of the TC intervention.
Further studies are needed to ascertain whether improving outcomes of depression in patients with diabetes decreases lifetime medical costs in this population. Moreover, outpatient costs are substantially and significantly lower and laboratory and other miscellaneous costs are slightly but significantly higher in the TC and SC treatment groups than the UC group. There is no evidence that inpatient costs, emergency department costs, medication costs, treatment adherence or hypoglycemic events were significantly different across the study groups. Larger and longer-term studies are also needed to establish the specific impacts of such interventions on patient treatment adherence, adverse events, or changes in patient disability, disease complications, and overall survival.
Conclusion
ICT-facilitated depression care is both cost saving and effective in improving depression and quality of life outcomes in a predominantly Hispanic safety-net primary care population with type 2 diabetes.
Supplementary Material
Acknowledgments
Source of financial support: This study was supported by the Assistant Secretary for Planning and Evaluation for the U.S. Department of Health and Human Services (1R18AE000054-01). Trial Registration: ClinicalTrials.gov NCT01781013
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li C, Ford ES, Zhao G, Ahluwalia IB, Pearson WS, Mokdad AH. Prevalence and correlates of undiagnosed depression among U.S. adults with diabetes: the Behavioral Risk Factor Surveillance System, 2006. Diabetes Res Clin Pract. 2009;83(2):268–279. doi: 10.1016/j.diabres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 2.van Dooren FEP, Nefs G, Schram MT, Verhey FRJ, Denollet J, Pouwer F. Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PloS One. 2013;8(3):e57058. doi: 10.1371/journal.pone.0057058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- 4.Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry. 2013;35(3):217–225. doi: 10.1016/j.genhosppsych.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egede LE, Walker RJ, Bishu K, Dismuke CE. Trends in costs of depression in adults with diabetes in the United States: Medical Expenditure Panel Survey, 2004–2011. J Gen Intern Med. 2016;31(6):615–622. doi: 10.1007/s11606-016-3650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck AT, Steer RA, Beck JS, Newman CF. Hopelessness, depression, suicidal ideation, and clinical diagnosis of depression. Suicide Life Threat Behav. 1993;23(2):139–145. [PubMed] [Google Scholar]
- 7.Alegría M, Chatterji P, Wells K, et al. Disparity in depression treatment among racial and ethnic minority populations in the United States. Psychiatr Serv. 2008;59(11):1264–1272. doi: 10.1176/appi.ps.59.11.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Ell K, Gross-Schulman SG, et al. Technology-facilitated depression care management among predominantly Latino diabetes patients within a public safety net care system: comparative effectiveness trial design. Contemp Clin Trials. 2014;37(2):342–354. doi: 10.1016/j.cct.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Katon W. Collaborative depression care models: from development to dissemination. Am J Prev Med. 2012;42(5):550–552. doi: 10.1016/j.amepre.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Katon W, Unützer J, Wells K, Jones L. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456–464. doi: 10.1016/j.genhosppsych.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. Mental Health: Culture, Race, and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services; 2001. [Google Scholar]
- 12.Borowsky SJ, Rubenstein LV, Meredith LS, Camp P, Jackson-Triche M, Wells KB. Who is at risk of nondetection of mental health problems in primary care? J Gen Intern Med. 2000;15(6):381–388. doi: 10.1046/j.1525-1497.2000.12088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez HP, Chen J, Rodriguez MA. A national study of problematic care experiences among Latinos with diabetes. J Health Care Poor Underserved. 2010;21(4):1152–1168. doi: 10.1353/hpu.2010.0923. [DOI] [PubMed] [Google Scholar]
- 14.Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry. 2006;163(1):101–108. doi: 10.1176/appi.ajp.163.1.101. [DOI] [PubMed] [Google Scholar]
- 15.Pinto-Meza A, Fernandez A, Serrano-Blanco A, Haro JM. Adequacy of antidepressant treatment in Spanish primary care: a naturalistic six-month follow-up study. Psychiatr Serv. 2008;59(1):78–83. doi: 10.1176/ps.2008.59.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Ell K, Katon W, Cabassa LJ, et al. Depression and diabetes among low-income Hispanics: design elements of a socioculturally adapted collaborative care model randomized controlled trial. Int J Psychiatry Med. 2009;39(2):113–132. doi: 10.2190/PM.39.2.a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ell K, Katon W, Xie B, et al. Collaborative care management of major depression among low-income, predominantly Hispanic subjects with diabetes: a randomized controlled trial. Diabetes Care. 2010;33(4):706–713. doi: 10.2337/dc09-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ell K, Xie B, Quon B, Quinn DI, Dwight-Johnson M, Lee PJ. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26(27):4488–4496. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ell K, Xie B, Kapetanovic S, et al. One-year follow-up of collaborative depression care for low-income, predominantly Hispanic patients with cancer. Psychiatr Serv. 2011;62(2):162–170. doi: 10.1176/ps.62.2.pss6202_0162. [DOI] [PubMed] [Google Scholar]
- 20.Rabiner L, Juang BH. Fundamentals of Speech Recognition. Englewood Cliffs, NJ: Prentice-Hall; 1993. [Google Scholar]
- 21.Steeneken HJM. Assessment for automatic speech recognition: I. comparison of assessment methods. Speech Commun. 1993;12(3):241–246. [Google Scholar]
- 22.Business rules engine. [Accessed March 8, 2017];Wikipedia. Available from: https://en.wikipedia.org/wiki/Business_rules_engine.
- 23.Wu S, Vidyanti I, Liu P, et al. Patient-centered technological assessment and monitoring of depression for low-income patients. J Ambulatory Care Manage. 2014;37(2):138–147. doi: 10.1097/JAC.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez M, Wu S, Jin H, et al. Automated remote monitoring of depression: acceptance among low-income patients in diabetes disease management. JMIR Ment Health. 2016;3(1):e6. doi: 10.2196/mental.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin H, Wu S, Di Capua P. Development of a clinical forecasting model to predict comorbid depression among diabetes patients and an application in depression screening policy making. Prev Chronic Dis. 2015;12:150047. doi: 10.5888/pcd12.150047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin H, Wu S, Vidyanti I, Di Capua P, Wu B. Predicting depression among patients with diabetes using longitudinal data: a multilevel regression model. Methods Inf Med. 2015;54(6):553–559. doi: 10.3414/ME14-02-0009. [DOI] [PubMed] [Google Scholar]
- 27.Wu B, Jin H, Vidyanti I, Lee PJ, Ell K, Wu S. Collaborative Depression Care Among Latino Patients in Diabetes Disease Management, Los Angeles, 2011–2013. Preventing chronic disease. 2014:11. doi: 10.5888/pcd11.140081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidyanti I, Wu B, Wu S. Low-income minority patient engagement with automated telephonic depression assessment and impact on health outcomes. Quality of Life Research. 2015 May 1;24(5):1119–29. doi: 10.1007/s11136-014-0900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Capua P, Wu B, Sednew R, Ryan G, Wu S. Complexity in Redesigning Depression Care: Comparing Intention Versus Implementation of an Automated Depression Screening and Monitoring Program. Population health management. 2016 Oct 1;19(5):349–56. doi: 10.1089/pop.2015.0084. [DOI] [PubMed] [Google Scholar]
- 30.Katon WJ, Unützer J, Fan MY, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29(2):265–270. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- 31.Hay JW, Katon WJ, Ell K, Lee PJ, Guterman JJ. Cost-effectiveness analysis of collaborative care management of major depression among low-income, predominantly Hispanics with diabetes. Value Health. 2012;15(2):249–254. doi: 10.1016/j.jval.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vannoy SD, Arean P, Unützer J. Advantages of using estimated depression-free days for evaluating treatment efficacy. Psychiatr Serv. 2010;61(2):160–163. doi: 10.1176/appi.ps.61.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21(6):547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed June 2009];RBRVS EZ-Fees. 2011 Available from: http://www.rbrvs.net.
- 37.U.S. Department of Veterans Affairs. [Accessed June 2009];Pharmaceutical prices. Available from: http://www.pbm.va.gov/DrugPharmaceuticalPrices.aspx.
- 38.Myer B. Natural and quasi-experiments in economics. J Bus Econ Stat. 1995;13(2):151–161. [Google Scholar]
- 39.Bertrand M, Duflo E, Mullainathan S. How much should we trust differences-in-differences estimates? Q J Econ. 2004;119(1):249–275. [Google Scholar]
- 40.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 41.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year in search of a standard. Med Decis Making. 2000;20(3):332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 42.Neumann PJ, Cohen JT, Weinstein MC. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. Macroeconomics and Health: Investing in Health for Economic Development. Geneva, Switzerland: Commission on Macroeconomics and Health; 2001. [Accessed Apr 14, 2017]. Available from: http://apps.who.int/iris/bitstream/10665/42435/1/924154550X.pdf. [Google Scholar]
- 44.Johnson JA, Lier DA, Soprovich A, Al Sayah F, Qui W, Majumdar SR. Cost-effectiveness evaluation of collaborative care for diabetes and depression in primary care. Am J Prev Med. 2016;51(1):e13–e20. doi: 10.1016/j.amepre.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Camacho EM, Ntais D, Coventry P, et al. Long-term cost-effectiveness of collaborative care (vs usual care) for people with depression and comorbid diabetes or cardiovascular disease: a Markov model informed by the COINCIDE randomized controlled trial. BMJ Open. 2016;6:e012514. doi: 10.1136/bmjopen-2016-012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katon WJ, Russo JE, Von Korff M, Lin EH, Ludman E, Ciechanowski PS. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31(6):1155–119. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi Yoo SJ, Nyman JA, Cheville AL, Kroenke K. Cost effectiveness of telecare management for pain and depression in patients with cancer: results from a randomized trial. Gen Hosp Psychiatry. 2014;36(6):599–606. doi: 10.1016/j.genhosppsych.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madalla GS. Limited-Dependent and Qualitative Variables in Econometrics. Cambridge University Press; New York: 1983. [Google Scholar]
- 49.Greene WH. Econometric Analysis. 7. Prentice Hall; New York: 2012. [Google Scholar]
- 50.Avorn J. Powerful Medicines: The Benefits, Risks, and Costs of Prescription Drugs. Knopf Doubleday Publishing Group; New York: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.