Abstract
Tissue-resident memory (TRM) CD8+ T-cells are non-recirculating, long-lived cells housed in tissues that can confer protection against mucosal pathogens. HIV-1 is a mucosal pathogen and the gastrointestinal tract is an important site of viral pathogenesis and transmission. Thus, CD8+ TRM cells may be an important effector subset for controlling HIV-1 in mucosal tissues. This study sought to determine the abundance, phenotype, and functionality of CD8+ TRM cells in the context of chronic HIV-1 infection. We found that the majority of rectosigmoid CD8+ T-cells were CD69+CD103+S1PR1− and T-betLowEomesoderminNeg, indicative of a tissue-residency phenotype similar to that described in murine models. HIV-1-specific CD8+ TRM responses appeared strongest in individuals naturally controlling HIV-1 infection. Two CD8+ TRM subsets, distinguished by CD103 expression intensity, were identified. CD103Low CD8+ TRM primarily displayed a transitional memory phenotype and contained HIV-1-specific cells and cells expressing high levels of Eomesodermin, whereas CD103High CD8+ TRM primarily displayed an effector memory phenotype and were EomesoderminNeg. These findings suggest a large fraction of CD8+ T-cells housed in the human rectosigmoid mucosa are tissue-resident and that TRM contribute to the anti-HIV-1 immune response. Further exploration of CD8+ TRM will inform development of anti-HIV-1 immune-based therapies and vaccines targeted to the mucosa.
INTRODUCTION
The gastrointestinal mucosa is an important site of HIV-1 pathogenesis, as it serves both as a portal of entry and site of HIV-1 persistence throughout chronic infection1. Accordingly, immune-based strategies to prevent and/or eradicate HIV-1 infection will likely require durable and robust HIV-1-specific immune responses in the rectosigmoid mucosa and other vulnerable tissues2, 3, 4, 5. Characterized as long-lived, non-recirculating effector memory T-cells localized to tissues including the gastrointestinal tract, tissue-resident memory T-cells (TRM) represent a potential immunotherapeutic target for combating mucosal pathogens such as HIV-16, 7, 8, 9.
First described in the murine model, TRM are thought to develop from killer cell lectin like receptor G1 (KLRG1)-negative precursor effector T-cells following migration into peripheral tissues10. Here, exposure to tissue-specific cytokine mixtures involving TGF-β drives expression of early activation marker CD69 and integrin αE(CD103)β7, which promote tissue accumulation and retention and are considered hallmarks of the TRM phenotype6, 11, 12, 13, 14. Interestingly, although T-box transcription factors Eomesodermin and T-bet regulate CD8+ T-cell development and effector function, a feature of CD103+ CD8+ TRM conserved across models of infection is strong down-regulation of Eomesodermin (Eomes)12, 15. Recently, using a murine Herpes simplex virus-1 model, skin CD8+ TRM were shown to display low T-bet and negligible Eomes expression16. Unlike circulating effector memory CD8+ T-cells, TRM in the gastrointestinal tract appear to be maintained independently of cognate antigen for long periods7, 11, 17, 18. Positioned at sites of pathogen exposure, TRM initiate rapid and robust defenses upon reinfection, notably cytokine production, mobilizing both innate and adaptive arms of the immune system10. Studies of lung, skin, genital mucosa, and small intestine have all demonstrated the protective capacity of TRM against a range of pathogens after secondary infection and during reactivation of latent viral infection17, 19, 20, 21, 22, 23, 24. Together, these data suggest TRM may be beneficial in controlling HIV-1 infection in peripheral, non-lymphoid tissues like the gastrointestinal mucosa.
Although the protective attributes of TRM have been characterized extensively in murine models, a knowledge gap exists regarding their role in HIV-1 infection. Previous characterization of mucosal CD8+ T-cells in chronic HIV-1 infection revealed them to be phenotypically and functionally different from CD8+ T-cells circulating in blood. Rectosigmoid CD8+ T-cells displayed weak perforin-mediated cytotoxicity and diminished expression of T-bet and Eomesodermin compared to their blood counterparts25, 26, 27. Rather, rectosigmoid CD8+ T-cells were primarily T-betLowEomesoderminNeg and displayed robust cytokine/chemokine polyfunctionality characteristic of TRM. Strong polyfunctional HIV-1-specific CD8+ T-cell responses in rectosigmoid mucosa have been identified as a correlate of immune control as they are particularly robust in individuals who naturally control HIV-1 (i.e. “controllers”)26. Whether these observations reflect an abundance of canonical ‘tissue-resident’ CD8+ T-cells in human gastrointestinal mucosa and participation of TRM in controlling HIV-1-infection is unknown.
The goal of this study was to determine whether gastrointestinal HIV-1-specific CD8+ T-cells in chronically HIV-1-infected and healthy participants share the characteristics of tissue resident T-cells as described in murine models of infectious disease, and to better understand the implications of this understudied population for HIV-1 pathogenesis, immune control, and vaccine design.
RESULTS
Most CD8+ T-cells in human rectosigmoid mucosa display a tissue-resident phenotype
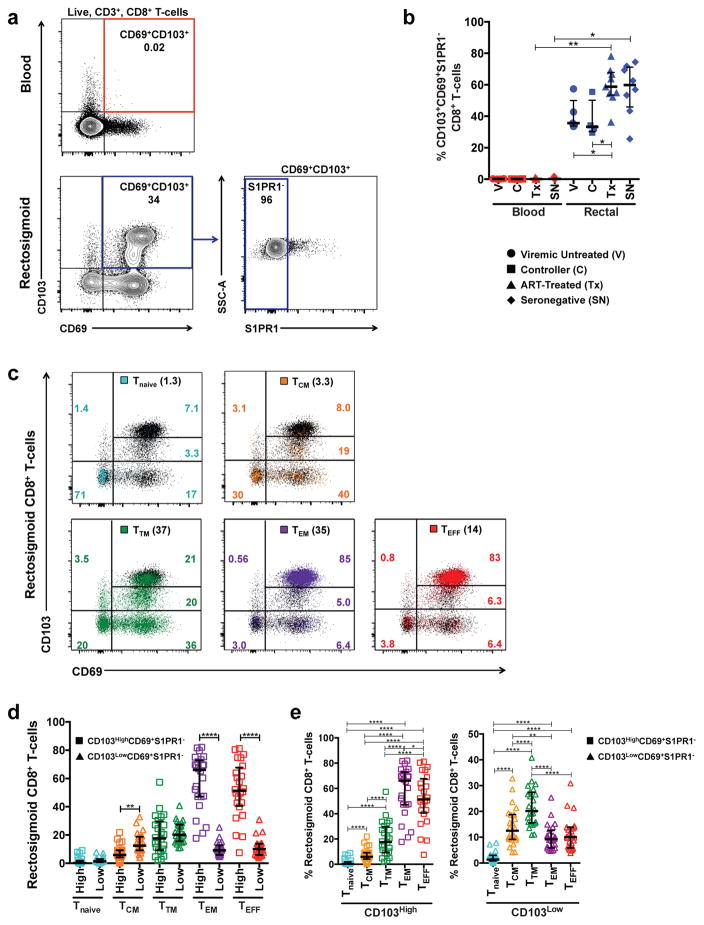
TRM can be distinguished from recirculating populations via expression of tissue-specific markers and the absence of recirculation markers8, 19. Co-expression of CD69 and αE integrin (CD103) in the absence of sphingosine-1-phosphate receptor (S1PR1) and C–C motif chemokine receptor 7 (CCR7) are commonly used to identify tissue-resident CD8+ T-cells in a range of tissues6, 13, 14, 28. To determine whether this strategy also identifies tissue-resident CD8+ T-cells in human rectosigmoid mucosa, we used flow cytometry to analyze cells for expression of CD103, CD69, and S1PR1 in chronically HIV-1-infected and seronegative participants.
Irrespective of HIV-1 disease status, large fractions of rectosigmoid CD8+ T-cells were CD103+CD69+S1PR1−; in contrast, this subset was negligible in blood (Figure 1a–b). In tissues, effector memory CD8+ T-cells often display a tissue-residency phenotype 6, 28, 29, 30, 31; however, the extent to which other memory subsets display such a phenotype is not known. We next analyzed expression of CD103, CD69, and S1PR1 within rectosigmoid CD8+ T-cell memory subsets in HIV-1+ and seronegative participants. Memory subsets were defined as follows: naïve (CD45RO−CD27+CCR7+), central memory (CD45RO+CD27+CCR7+), transitional memory (CD45RO+CD27+CCR7−), effector memory (CD45RO+CD27−CCR7−), and effector (CD45RO−CD27−CCR7−)(Supplementary Figure 1a–b)32, 33. Although the abundance of naïve (Tnaive) and central memory (TCM) CD8+ T-cells in mucosa was low compared to effector (TEFF) and effector memory (TEM) T-cells, all memory subsets contained some CD103+CD69+S1PR1− CD8+ T-cells, the proportion of which increased with memory differentiation. Only a small fraction of Tnaive and TCM CD8+ T-cells were CD103+CD69+S1PR1− (medians 2.16% and 19.5%, respectively); in contrast, large proportions of TEFF and TEM were CD103+CD69+S1PR1− (medians 64.7% and 74.9%, respectively)(Figure 1c–d). We also observed CD103 expressed at high and low median fluorescence intensities (MFI) on rectosigmoid CD8+ T-cells. While the majority of rectosigmoid tissue-resident CD8+ T-cells were CD103High, a small percentage was CD103Low; this percentage varied with memory differentiation. The proportion of CD103Low CD69+S1PR1− putative tissue-resident CD8+ T-cells was highest among TTM and TCM, and lowest among Tnaive (Figure 1c–e).
Figure 1.
Characterization and distribution of tissue-resident CD8+ T-cells in the rectosigmoid mucosa of chronically HIV-1-infected and seronegative participants. (a) Representative flow cytometry plot displaying surface staining of tissue-resident markers and gating strategy used to identify live, CD3+, tissue resident CD8+ T-cells as CD103+CD69+S1PR1− in blood (red) and rectosigmoid mucosa (blue). (b) Differences in the frequency of CD103+CD69+S1PR1− CD8+ T-cells between blood and rectosigmoid mucosa across HIV-1 disease status. (c) Representative flow cytometry plot demonstrating the difference in CD103 fluorescence intensity and CD69 expression in rectosigmoid naïve (Tnaive, aqua), central memory (TCM, orange), transitional memory (TTM, green), effector memory (TEM, purple), and effector (TEFF, red) CD8+ T-cells. Numbers in parentheses indicate the percentage of the memory subset in the total rectosigmoid CD8+ T-cell population; colored numbers indicate frequency of the memory subset in each quadrant. Memory/effector subsets were first identified by differential expression of CD45RO, CD27, and CCR7 as described in the text. (d) Comparison of the frequencies of rectosigmoid CD103High and CD103Low CD69+S1PR1− CD8+ T-cells within each memory subset, or (e) between memory subsets in a combined group of chronically HIV-1-infected and seronegative participants. Horizontal bars represent median, whiskers indicate interquartile ranges with the level of significance shown with asterisks as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
CD103 binds β7 to form the heterodimeric integrin αEβ7, which interacts with E-cadherin on epithelial cells, facilitating accumulation of tissue-resident T-cells in close proximity to the epithelium34, 35, 36. We used immunohistochemistry and quantitative image analysis to confirm the distribution of CD103Neg, CD103Low, and CD103High CD8+ T cells. Cells were quantified in three anatomical compartments within rectosigmoid mucosa: intraepithelial lymphocytes (IE), lamina propria (LP), and areas bordering (B) the IE and LP (Supplementary Figure 2a). CD103Neg CD8+ T cells were located primarily in the LP, in contrast, greater than 60% of CD103High CD8+ T cells and approximately 50% of CD103Low CD8+ T cells were intraepithelial (Supplementary Figure 2a–c). These findings corroborate previous observations concerning the localization of CD103-expressing CD8+ T-cells in human intestine34, 35 and reveal that a large proportion of rectosigmoid CD8+ T-cells resemble tissue-resident cells described previously. In human rectosigmoid mucosa, this population is composed mainly of CD103High effector and effector memory cells.
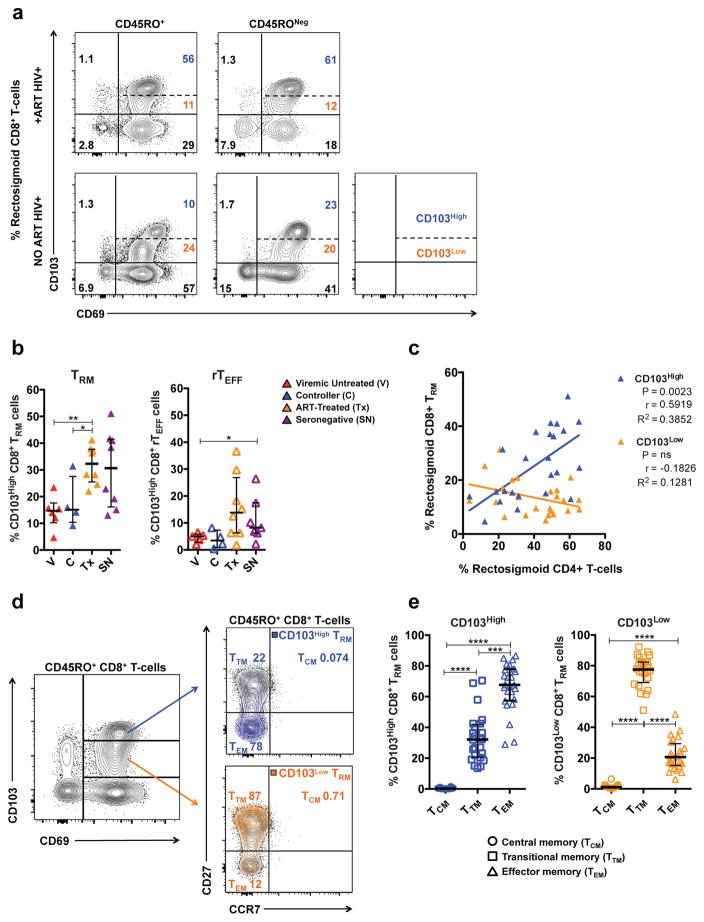
CD103High and CD103Low tissue-resident CD8+ T-cells differ in abundance across HIV-1 disease status, association with rectosigmoid CD4+ T-cells, and expression of memory markers
Disruption of the gut epithelial barrier and dramatic depletion of gut CD4+ T-cells are hallmarks of HIV-1 infection5, 37. CD4+ T-cells have been shown to aid development of lung CD103High CD8+ TRM during influenza infection10, 38. Accordingly, we hypothesized that the abundance of CD103High tissue-resident CD8+ T-cells might be lower in chronically HIV-1-infected compared to seronegative participants. To test this, we evaluated expression of tissue-resident markers on unstimulated rectosigmoid CD8+ T-cells in the following groups: HIV-1+ controllers (C); HIV-1+ viremic individuals not on antiretroviral therapy (V); HIV-1+ individuals on antiretroviral therapy (Tx); and seronegative controls (SN) (Table 1). As CD8+ T-cells displaying a tissue-resident phenotype were primarily observed in effector memory and effector subsets, we investigated both CD45RO+ and CD45RONeg populations; referring to CD45RO+ CD103+CD69+S1PR1− cells as tissue-resident memory cells (TRM) and CD45RONeg CD103+CD69+S1PR1− cells as tissue-resident effector cells, abbreviated rTEFF.
Table 1.
Participant Characteristics
| Gender | Race | Plasma viral load, RNA copies/mL* [Median] [Range] |
CD4 count, Cells/mm3 [Median] [Range] |
Time Post HIV+ Diagnosis, years [Median] [Range] |
Age, Years [Median] [Range] |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Controllers (n = 4) | M, 2 | AA, 3 | 943 | 769 | 14 | 59 |
| F, 1 | C, 1 | <40 – 1,497 | 431 – 1,128 | 7 – 31 | 50 – 63 | |
| T**, 1 | ||||||
|
| ||||||
| Viremic Untreated (n = 6) | M, 6 | AA, 4 | 57,995 | 324 | 14 | 48 |
| C, 2 | 9,436 – 308,383 | 198 – 516 | 3 – 31 | 33 – 54 | ||
|
| ||||||
| ART-Treated (n = 8) | M, 6 | AA, 4 | <40 | 770 | 9 | 48 |
| F, 2 | C, 4 | NA | 564 – 2,658 | 6 – 30 | 28 – 67 | |
|
| ||||||
| Seronegatives (n = 8) | M, 7 | AA, 5 | NA | 764 | NA | 49 |
| F, 1 | C, 3 | NA | 406 – 2,269 | NA | 26 – 60 | |
M, Male; F, Female; T, Transgender;
male-to-female.
AA, African American; C, Caucasian; NA, Not applicable.
Median determined using only detectable viral load data points.
As expected, HIV-1+ participants not on antiretroviral therapy (ART) displayed lower proportions of CD103High CD8+ TRM and rTEFF compared to HIV-1+ participants on ART and seronegatives, reaching statistical significance for ART-treated compared to controllers and viremic untreated individuals for TRM, and between viremic untreated and seronegatives for rTEFF (Figure 2a–b). No differences between subject groups were observed in the proportions of CD103Low TRM and rTEFF (data not shown). When all participant groups were combined, a strong positive correlation was observed between the percentage of rectosigmoid CD4+ T-cells and that of CD103High but not CD103Low TRM (Figure 2c). A similar correlation was seen when all HIV-1+ participants were considered together (P=0.0048, R2=0.44); however, when each subgroup (including seronegatives) was examined individually, no significant differences or trends were observed (not shown). No correlations were observed between rectosigmoid CD4+ T-cells and the percentages of CD103High or CD103Low rTEFF cells (Supplementary Figure 3a).
Figure 2.
Distribution, association with rectosigmoid CD4+ T-cells, and maturation status of CD103High and CD103Low tissue-resident CD8+ T-cells. (a) Representative flow cytometry plot showing differences in the proportion of CD103High (blue) and CD103Low (orange) CD45RO+ and CD45RONeg CD8+ T-cells between a chronically HIV-1-infected individual on ART (top panel) and a chronically HIV-1-infected individual not on ART (bottom panel). (b) Differences in the frequency of rectosigmoid CD103High CD8+ TRM and rTEFF cells across HIV-1-disease status. (c) Association between the frequency of rectosigmoid CD4+ T-cells and the frequency of rectosigmoid CD103High and CD103Low TRM CD8+ T-cells in all study participants. (d) Representative flow cytometry plot showing differences in the expression of memory markers CD27 and CCR7 between CD103High and CD103Low rectosigmoid CD8+ TRM cells. (e) Distribution of central memory (TCM), transitional memory (TTM), and effector memory (TEM) subsets within CD103High and CD103Low rectosigmoid CD8+ TRM in a combined group of all study participants. Associations calculated using non-parametric Spearman correlation and linear regression analyses. Horizontal bars represent median, whiskers indicate interquartile range with the level of significance indicated with asterisks as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
CD103High and CD103Low tissue-resident CD8+ T-cells also differed in memory phenotype. The majority of CD103High TRM displayed an effector memory phenotype (TEM median 67.7%, TTM median 32.2%, TCM median 0.225%); in contrast, CD103Low TRM cells mainly displayed a transitional memory phenotype characterized by expression of CD27 (TTM median 77.6%, TEM median 20.6%, TCM median 1.05%)(Figure 2d–e). Among rTEFF, CD103High cells primarily displayed the canonical effector phenotype (CD45RO−CD27−CCR7−, median 73.5%), whereas a substantial fraction of CD103Low rTEFF expressed CD27 (CD45RO−CD27+CCR7− median 55.4%) (Supplementary Figure 3b–c). Collectively, these data reveal lower proportions of highly differentiated TEM/TEFF CD103High tissue-resident CD8+ T-cells in HIV-1+ individuals not on ART compared to ART-treated and seronegative groups, and a strong association between CD103High CD8+ TRM cells and rectosigmoid CD4+ T-cells.
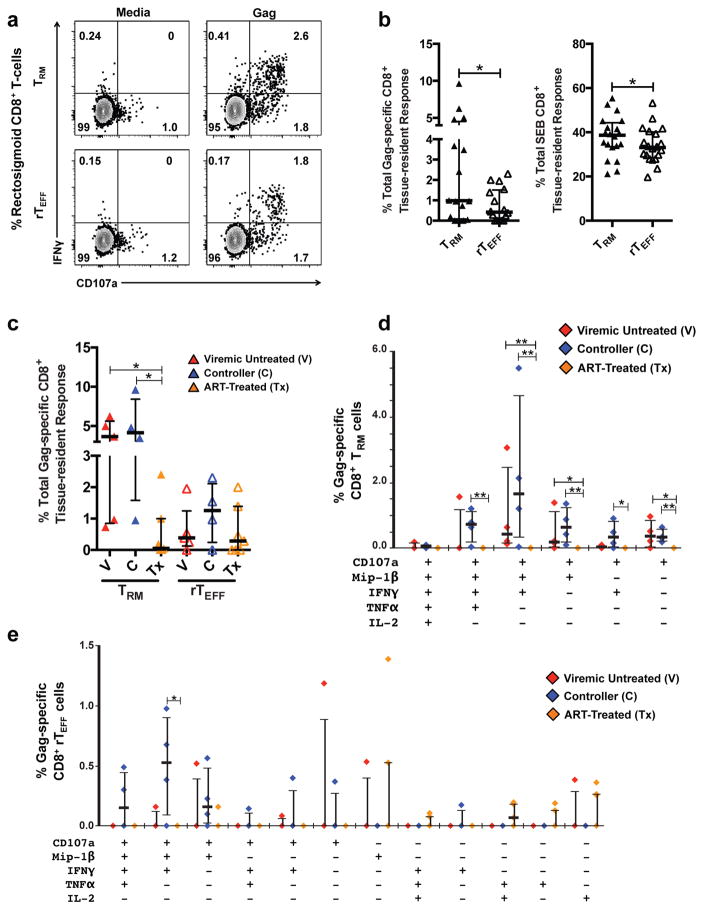
HIV-1 gag-specific tissue-resident CD8+ T-cell responses originate from CD103Low cells and appear strongest in controllers
Although tissue-resident T-cells represent a potential immunotherapeutic target for HIV-1 eradication, little is known about the contribution these cells to the HIV-1-specific immune response during chronic infection. This and previous work have revealed the presence of HIV-1 Gag-specific, MHC class I tetramer-binding CD8+ T-cells expressing αEβ7 in human rectosigmoid mucosa30 (Supplementary Figure 2d), and studies in rhesus macaques demonstrated vaccine-inducible long-lasting mucosal T-cell responses, suggesting TRM contribute to the HIV-1-specific immune response18, 39. We investigated Gag-specific tissue-resident CD8+ T-cell responses and their contribution to the total rectosigmoid Gag-specific CD8+ T-cell response across HIV-1-disease status using an ex vivo 6h stimulation assay followed by intracellular staining for effector molecules.
Degranulation (CD107a) and cytokine production in response to Gag-peptide stimulation were detected in rectosigmoid CD8+ TRM and rTEFF, with TRM displaying significantly higher percentages of Gag and SEB-responding cells compared to rTEFF (Figure 3a–b). Both controllers and viremic participants not on ART displayed significantly greater total and polyfunctional Gag-specific tissue-resident CD8+ T-cell responses compared to ART-treated participants (Figure 3c–e). This parallels earlier cross-sectional studies, in which we found low percentages of total and polyfunctional Gag-specific T-cells in mucosal tissues of participants on ART, particularly those with viral suppression25, 26, 27. Although the total HIV-1 Gag-specific response did not differ significantly in magnitude between controllers and viremic untreated participants, the highest median polyfunctional responses (3- and 4-function) were detected in controllers (Figure 3d–e). This was true for both TRM (Figure 3d) and rTEFF (Figure 3e). The strongest polyfunctional responses elicited by both TRM and rTEFF involved CD107a, MIP-1β, and IFN-γ, with all notable polyfunctional responses involving CD107a (Figure 3d–e). With the exception of IL-2-producing cells, Gag-responding CD8+ TRM were primarily CD103Low, reaching statistical significance for CD107a- and IFN-γ-producing TRM; in contrast, SEB-responding TRM were primarily CD103High (Supplementary Figure 4a–b). Similar trends were observed for Gag-responding CD8+ rTEFF (Supplementary Figure 4c).
Figure 3.
Responses of rectosigmoid CD8+ TRM cells to antigenic stimulation. (a) Representative flow cytometry plot demonstrating degranulation (CD107a) and IFN-γ-production following stimulation with an HIV-1 Gag-peptide pool or medium containing DMSO (negative control) by rectosigmoid CD8+ TRM (top) and rTEFF (bottom) from an HIV-1+ controller. (b) Difference in the frequency of CD8+ TRM and rTEFF cells responding to Gag-peptide and SEB stimulation. (c) Total percentage of rectosigmoid CD8+ TRM and rTEFF responding in any way (TNF-α, IFN-γ, IL-2, CD107a, and/or MIP-1β) to HIV-1 Gag stimulation. For each subject, the total HIV-1 Gag-specific response shown in (c) was calculated by combining all non-overlapping Boolean categories that are displayed individually in panels (d), for TRM, and (e), or rTEFF. (d) Difference in percentages of poly- and monofunctional Gag-specific rectosigmoid CD8+ TRM cells across HIV-1 disease status. (e) Difference in percentages of poly-and monofunctional Gag-specific rectosigmoid CD8+ rTEFF cells across HIV disease status. Response data were analyzed using Boolean gating of responding populations within live, CD3+, CD8+, CD45RO+ or CD45RONeg, CD103+CD69+S1PR1− gates. Co-expression analyses were generated using SPICE software. Poly- and monofunctional combinations with negligible responses were omitted. Horizontal bars represent medians, whiskers indicate interquartile ranges with the level of significance shown with asterisks as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
Previous work has demonstrated robust polyfunctional HIV-1-specific CD8+ T-cell responses in rectosigmoid mucosa of controllers26; however, it is unknown whether these responses originate from resident and/or circulating populations. To understand the extent to which tissue-resident cells contribute to the total HIV-1 Gag-specific CD8+ T-cell response in rectosigmoid mucosa, we analyzed expression of CD69, CD103, and S1PR1 on Gag-specific polyfunctional CD8+ T-cells, focusing on the CD107a+MIP-1β+IFN-γ+ (7+M+F+) response as it had the highest median frequency (Supplementary Figure 5). Rectosigmoid Gag-specific 7+M+F+ CD8+ T-cells displayed three main patterns: the canonical tissue-resident phenotype (CD103+CD69+S1PR1−), CD69 single positive (CD103−CD69+S1PR1−), and absence of all three markers (CD103−CD69−S1PR1−); the proportions differed between viremic individuals not on ART and controllers. In viremic individuals, rectosigmoid Gag-specific 7+M+F+ CD8+ T-cells were primarily tissue-resident (median 35.9%) or CD69 single positive (median 38.7%) with a smaller fraction lacking all three markers (median 11.6%). In controllers, the majority of 7+M+F+ CD8+ T-cells were CD69 single positive (median 52.2%) with much smaller proportions exhibiting tissue-residency (median 16.0%) or absence of all three markers (median 16.7%)(Supplementary Figure 5a–c). These trends were conserved across all polyfunctional responses (data not shown). The results presented here, in conjunction with previous work, demonstrate that HIV-1-specific tissue-resident CD8+ T-cells are present in rectosigmoid mucosa and capable of polyfunctional responses. In the context of chronic HIV-1 infection, Gag-specific tissue-resident CD8+ T-cells appear to be mainly CD103Low. Notably, although tissue-resident Gag-specific CD8+ T-cell responses appeared strongest in controllers, the tissue-resident population constituted a smaller proportion of the total rectosigmoid Gag-specific CD8+ T-cell response in controllers compared to viremic participants not on ART.
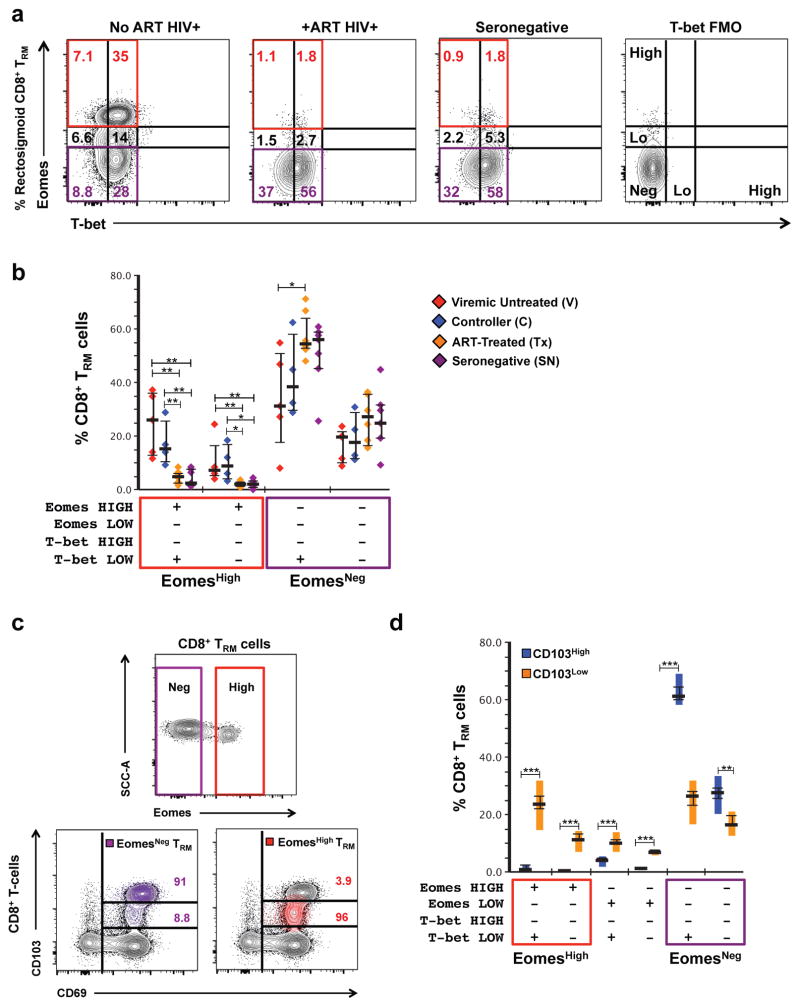
Eomesodermin expression in tissue-resident CD8+ T-cells is associated with low CD103 fluorescence intensity and is greatest in HIV-1+ participants not on ART
Previous work demonstrated that although human rectosigmoid CD8+ T-cells typically had a T-betLowEomesoderminNeg phenotype characteristic of TRM, HIV-1+ individuals not on ART displayed greater Eomes expression compared to ART-treated and healthy participants27. Whether this reflects up-regulation of Eomes by tissue-resident CD8+ T-cells or an influx of EomesHigh CD8+ T-cells from circulation is unknown. To elucidate the origin of EomesHigh CD8+ T-cells in rectosigmoid mucosa and understand modulations in T-bet and Eomes expression during chronic HIV-1 infection, we used flow cytometry to detect intracellular T-bet and Eomes, in conjunction with tissue-resident markers, in unstimulated rectosigmoid CD8+ T-cells from HIV-1+ individuals and healthy controls (Figure 4; Supplementary Figure 6).
Figure 4.
Expression of T-bet and Eomesodermin in tissue-resident CD8+ T-cells varies with HIV-1-disease status and CD103 fluorescence intensity. (a) Representative flow cytometry plot displaying differences in expression of T-bet and Eomesodermin (Eomes) in rectosigmoid CD8+ TRM between a chronically HIV-1-infected participant not on ART (far left), a chronically HIV-1-infected participant on ART (second from left), and a seronegative participant (second from right). T-bet FMO control is displayed for comparison (far right). (b) Differences in the frequency of EomesHigh and EomesNeg rectosigmoid CD8+ TRM across HIV-1-disease status. Horizontal bars represent medians, whiskers represent interquartile ranges. (c) Representative flow cytometry plot displaying differences in CD103 fluorescence intensity between EomesHigh and EomesNeg rectosigmoid CD8+ TRM. (d) Difference in Eomes expression between CD103High and CD103Low rectosigmoid CD8+ TRM cells in a combined group of all study participants. Vertical bars represent interquartile ranges, horizontal bars represent medians, whiskers represent standard error of the mean. Co-expression analysis was generated using SPICE software. Level of significance is indicated with asterisks as follows: * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001.
Rectosigmoid EomesHigh CD8+ T-cells were primarily single positive for CD69 (CD103−CD69+S1PR1− median 47.4%); however, a notable fraction displayed the canonical tissue-resident phenotype (CD103+CD69+S1PR1−, median 26.6%)(Supplementary Figure 6b–c). To determine whether high Eomes expression observed in the CD103+CD69+S1PR1− subset was associated with chronic HIV-1 infection, we compared Eomes expression in CD8+ TRM and rTEFF cells across HIV-1 disease status (Figure 4a–b). Similar to the total rectosigmoid CD8+ T-cell population, tissue-resident CD8+ T-cells were primarily T-betLow/NegEomesNeg; however, HIV-1+ participants not on ART had higher percentages of EomesHigh tissue-resident CD8+ T-cells compared to ART-treated and seronegative participants. Unlike Eomes, no differences in T-bet expression were observed between groups (Figure 4). Within the TRM subset, viremic individuals not on ART displayed the highest median frequency of EomesHigh cells; however within rTEFF, only HIV-1 controllers displayed significantly greater Eomes expression compared to ART-treated and seronegative groups (Figure 4a–b, Supplementary Figure 7a). The majority of EomesHigh tissue-resident CD8+ T-cells were CD103Low; in contrast, EomesNeg cells were primarily CD103High (Figure 4c–d, Supplementary Figure 7b). Together, these data demonstrate that rectosigmoid tissue-resident CD8+ T-cells are primarily CD103High T-betLow/NegEomesNeg; however, in untreated HIV-1 infection, a substantial population of CD103Low EomesHigh tissue-resident CD8+ T-cells emerges.
DISCUSSION
Several studies have demonstrated the disparity in phenotype and function between CD8+ T-cells isolated from gastrointestinal mucosa and peripheral blood25, 26, 27. As the gut is an important site of HIV-1 pathogenesis, these findings highlight the importance of understanding the distinct properties of mucosal immune responses to HIV-15. With the discovery of tissue-resident CD8+ T-cells, numerous studies have eloquently described the tissue-specific mechanisms that shape the tissue-resident population as well as the capacity of tissue-resident CD8+ T-cells to protect the host against mucosal pathogens9, 10. Missing from this body of knowledge, however, is an understanding of the role of tissue-resident CD8+ T-cells in fighting HIV-1 infection in the gastrointestinal mucosa, a site of viral transmission and pathogenesis5, 37. Thus, the aim of this study was to investigate the abundance of tissue-resident CD8+ T-cells in human rectosigmoid mucosa and the contribution of tissue-resident CD8+ T-cells to the HIV-1-specific immune response during chronic infection.
The majority of CD8+ T-cells in human rectosigmoid mucosa were CD103+CD69+S1PR1−, with a T-betLowEomesNeg transcriptional profile reminiscent of tissue-resident CD8+ T-cells described in the literature10. This subset was negligible in blood, further evidence of the tissue-resident nature of these cells. As effector memory cells predominate in mucosal tissues and play an important role in secondary immune responses, most tissue-resident work has focused on memory T-cells31. Indeed, the majority of rectosigmoid effector memory CD8+ T-cells were CD103+CD69+S1PR1−, expanding on previous work demonstrating an abundance of effector memory CD8+ T-cells expressing CD69 and CD103 in jejunum, ileum, and colon of human cadavers28, 31. Additionally, the majority of effector CD8+ T-cells in rectosigmoid mucosa also displayed tissue-residency. Although the origin of this putative resident effector population, termed rTEFF, could not be definitively determined, one interpretation is that rTEFF are memory precursor effector cells (MPEC) transitioning into TRM. In murine skin and intestine, TRM develop from KRLG1Neg MPECs12, 36. In an oral Listeria monocytogenes murine model, intestinal intraepithelial KLRG1Neg MPECs were CD103+CD69+ and formed rapidly compared to spleen or lung MPECs in a TGF-β-dependent manner36. Interestingly, we observed that although expression of KLRG1 was relatively low on rectosigmoid rTEFF and TRM compared to blood CD8+ T-cells, rTEFF displayed significantly lower expression of KLRG1 compared to TRM cells (these authors, unpublished data). Alternatively, rTEFF may represent a mature tissue-resident population distinct from TRM. Longitudinal studies designed to track the appearance and longevity of pathogen-specific rTEFF may help elucidate the origin and role of rTEFF in mucosal immunity.
Previous studies reported robust polyfunctional Gag-specific CD8+ T-cells in rectosigmoid mucosa of controllers, identifying these cells as a possible correlate of immune control25, 26, 40. However, it remains unclear whether recent emigrants from blood or resident populations mediate these responses. We detected Gag-specific responses in both TRM and rTEFF populations, demonstrating that tissue-resident T-cells contribute to the HIV-1-specific immune response. Although this study did not have the statistical power to discern differences between controllers and viremic untreated individuals, the strongest median polyfunctional TRM and rTEFF responses were detected in controllers, suggesting tissue-resident CD8+ T-cells might play a role in controlling HIV-1 in rectosigmoid mucosa. Paradoxically, although controllers displayed the highest median percentages of polyfunctional Gag-specific tissue-resident CD8+ T-cell responses, these responses constituted a smaller proportion of the total rectosigmoid Gag-specific CD8+ T-cell response in controllers compared to viremic participants not on ART. Instead, in controllers, the majority of rectosigmoid Gag-specific polyfunctional CD8+ T-cells were single positive for CD69. CD69 is an early activation marker up-regulated on tissue-resident precursor cells after entering the tissue. Expression of CD69 occurs early in tissue-resident development, preceding CD103 expression, and aids in tissue-retention by repressing expression of the tissue egress receptor S1PR112, 13, 14, 41. Tissue-resident CD8+ T-cells have been shown to initiate immune responses, mediated through cytokine and chemokine secretion, leading to recruitment of circulating immune cells to the site of infection22, 23. Accordingly, one interpretation of these data is that individuals with more robust Gag-specific tissue-resident CD8+ T-cell responses (i.e., HIV-1 controllers) might in turn experience greater recruitment of HIV-1-specific CD8+ T-cells from circulation compared to individuals with weaker CD8+ tissue-resident responses. This enhanced recruitment of cells lacking the tissue-resident phenotype would dilute the proportion of Gag-specific cells in rectosigmoid mucosa with a tissue-resident phenotype, consistent with our observations. Clearly, further studies, likely involving nonhuman primate models, will be required to fully elucidate the role of tissue-resident CD8+ T-cells in the total mucosal anti-HIV-1 immune response.
Within both TRM and rTEFF populations, we observed two subsets of tissue-resident CD8+ T-cells that could be differentiated by CD103 fluorescence intensity. The CD103High subset resembled canonical tissue-resident CD8+ T-cells, consisting mainly of TEM/TEFF cells with a T-betLowEomesNeg profile. CD103High cells were enriched in epithelia and revealed a strong positive correlation with the percentage of rectosigmoid CD4+ T-cells. In contrast, the CD103Low subset was mainly composed of less-differentiated CD8+ T-cells expressing CD27, and was more abundant in lamina propria compared to CD103High cells. HIV-1+ individuals not on ART had lower percentages of CD103High but higher percentages of EomesHigh tissue-resident CD8+ T-cells compared to ART-treated participants and seronegative controls. Interestingly, both Gag-specific and EomesHigh tissue-resident CD8+ T-cells were primarily CD103Low. Collectively, these observations reveal heterogeneity in the tissue-resident CD8+ T-cell population that may be exacerbated during chronic untreated HIV-1 infection.
Three main interpretations, not mutually exclusive, emerged to explain the observed HIV-1-associated alterations in the tissue-resident CD8+ T-cell population. First, gut epithelial cells express E-cadherin and are a constitutive source of TGF-β, a prominent driver of tissue-resident development6, 7, 12, 16, 36, 42, 43. Certain subsets of CD4+ T-cells also produce TGF-β and CD4+ T-cells have been shown to aid development of TRM in the lung38, 44. Accordingly, loss of epithelial barrier function and/or CD4+ T-cells during HIV-1 infection may result in a reduction of gut TGF-β and E-cadherin, ultimately leading to a reduction in an accumulation and/or development of CD103High tissue-resident CD8+ T-cells. Second, chronic antigen exposure can affect CD103 expression. In murine lymphocytic choriomeningitis virus (LCMV) infection, chronic in vivo exposure to antigen in combination with TGF-β reduced CD103 expression on antigen-specific CD8+ T-cells, leading the authors to propose CD103 as a marker for resting versus recently stimulated TRM7. Consistent with this finding, we observed Gag-specific tissue-resident CD8+ T-cells to be primarily CD103Low whereas tissue-resident CD8+ T-cells responding to SEB were primarily CD103High. It seems unlikely that the significant reduction in the percentage of CD103High tissue-resident CD8+ T-cells observed in HIV-1+ individuals not on ART would be due solely to CD103 down-regulation on HIV-1-specific cells. However, the pool of rectosigmoid tissue-resident CD8+ T-cells undergoing activation-induced CD103 down-regulation might be broadened in individuals not on ART due to ‘bystander’ activation as a result of HIV-1-associated inflammation37. Third, the HIV-1-associated inflammatory state in individuals not on ART might lead to greater recruitment of CD8+ T-cells from circulation. An influx of blood CD8+ T-cells into rectosigmoid mucosa would reduce the proportion of CD103High cells in the total rectosigmoid CD8+ T-cell population and increase proportions of CD103Low/Neg cells as recent immigrants mature into CD103High tissue-residents. In support of this interpretation, a large fraction of CD103Low tissue-resident CD8+ T-cells displayed a less differentiated memory phenotype characterized by CD27 expression.
Again, these interpretations are likely not mutually exclusive. The CD103Low tissue-resident CD8+ T-cell subset may be heterogeneous, composed both of recent immigrants developing into tissue-resident CD8+ T-cells and activated, mature tissue-resident CD8+ T-cells. Interestingly, within the CD103Low subset, we observed heterogeneity in Eomes expression. Unlike CD103High cells, which displayed a homogenous T-betLowEomesNeg phenotype, the CD103Low subset contained cells expressing high, low, or no Eomes. Notably, although CD103Low cells were primarily EomesNeg, EomesHigh tissue-resident CD8+ T-cells were almost exclusively CD103Low. Murine skin and brain tissue-resident CD8+ T-cells severely down-regulate Eomes; thus, detecting high Eomes expression within this population was surprising12, 15, 16. However, high Eomes expression has been associated with a ‘terminally exhausted’ phenotype when co-expressed with the inhibitory receptor programmed cell death-1 (PD-1)45. As the frequency of EomesHigh tissue-resident CD8+ T-cells was greatest in HIV-1+ individuals not on ART, we surmised that the high Eomes expression in rectosigmoid tissue-resident CD8+ T-cells likely represents activated and/or exhausted cells. The data presented here do not contradict previous findings, but rather suggest that Eomes expression may vary during chronic infection and that tissue-resident CD8+ T-cells may be susceptible to exhaustion.
To our knowledge, this is the first report examining the characteristics and functionality of tissue-resident CD8+ T-cells in rectosigmoid mucosa during HIV-1 infection. As the gastrointestinal mucosa is an important site of HIV-1 transmission and pathogenesis1, the long-lived tissue resident T-cell population can potentially be exploited in the development of vaccines and immunotherapies designed to prevent and/or eradicate HIV-1 infection. Recent successes in HIV-1 vaccine development have lent support to the concept that antigen-specific CD8+ T-cells, positioned near the sites of mucosal viral entry, can play critical roles in preventing and/or clearing infection4, 18, 39. However, more work will be required to fully elucidate the pathways driving induction and maintenance of these populations, and to understand the relative contributions of both tissue-resident and recirculating memory T-cell subsets to host immunity.
METHODS
Participants and Sample Collection
HIV-1 positive and seronegative participants were enrolled through the SCOPE study at San Francisco General Hospital. Participants were categorized by plasma viral load (VL) and antiretroviral therapy (ART): Controllers (C) with VL consistently <2,000 copies/mL without ART; Viremic (V) subjects with VL ≥ 2,000 copies/mL without ART; ART treated (Tx) participants with VL < 40 copies/mL; and HIV-1 seronegatives (SN). Seronegative participants were healthy volunteers from whom samples were collected for research purposes only. Written informed consent for phlebotomy and rectosigmoid biopsy was obtained through protocols approved by the Committee on Human Subjects Research, University of California, San Francisco [Protocols #10-01218, 10-00263 and 10-01330]. Exclusion criteria included active sexually transmitted infections other than HIV-1; inflammatory bowel disease or other known inflammatory conditions affecting the gastrointestinal tract; colorectal cancer or other malignancies; and severe anemia or blood clotting disorders.
Twenty to 40 ml of blood was collected by sterile venipuncture into tubes containing EDTA. Twenty-four to 30 rectosigmoid biopsies were obtained by flexible sigmoidoscopy at 10–30cm from the anal verge2. Biopsies were immediately stored in R-15 [RPMI-1640 supplemented with fetal calf serum (15%), penicillin (100U/mL), streptomycin (100 mg/mL), and glutamine (2 mM)]. Samples were transported at room temperature to Davis, CA and processed immediately upon arrival.
Blood and Tissue Processing
Peripheral blood mononuclear cells (PBMCs) were isolated from blood using Ficoll-Paque™ (Pfizer-Pharmacia, New York, NY) and rested overnight in R-15 at 37°C, 5% CO2. Rectosigmoid mononuclear cells were isolated from biopsies as described46, 47. Briefly, biopsies were washed in R-15 and subjected to shaking incubation in 25mg/mL Liberase® DL (Roche, Indianapolis, IN) at 37°C for 30 minutes, then passed through a 16-gauge blunt end needle to mechanically disrupt tissue. Disrupted tissue was passed through a sterile 70μm cell strainer to collect cells. Undigested tissue was again incubated in Liberase and the process repeated until all tissue was digested. Isolated cells were washed three times in R-15 and rested overnight at 37°C, 5% CO2 in R-15 with 200x Zozyn (Pfizer-Pharmacia).
Antibodies and Peptide Pools
The following monoclonal antibodies were used in flow cytometry: CD8 (SK1: APC-H7), CCR7 (3D12: PE-Cy7), CD69 (L78: PE), IFN-γ (B27: PE-Cy7), MIP-1β (D21-1351: PerCP-Cy5.5), from BD Biosciences (San Jose, CA); CD103 (Ber-ACT8: Ax488), TNF-α (MAb11: BV605), IL-2 (MQ1-17H12: BV650), CD107a (H4A3: BV711), CD4 (RPA-TA: BV570, BV605), CD45RO (UCHL1: BV785), CD27 (O323: BV650), CD3 (UCHT-1: Ax700), and T-bet (4B10: BV711) from Biolegend (San Diego, CA). Unlabeled CD28 (L293), and CD49d (L25) were from BD Pharmingen (San Diego, CA). Eomesodermin (WD1928: PE-eFluor610) and S1PR1 (SW4GYPP: eFluor660) were from ThermoFisher (Waltham, MA). The HIV-1 Gag peptide pool (p55, HXB2 sequence) consisted of 15-mers with an 11 amino acid overlap (BD Biosciences). Staphylococcal enterotoxin B (SEB) was from Sigma Aldrich.
Antigen Stimulation and Intracellular Cytokine Staining (ICS)
Surface and intracellular staining was performed as described25 on freshly isolated blood and rectosigmoid mononuclear cells rested overnight at 37°C, 5% CO2. For stimulation assays, cells were incubated at 2×106 cells per 200μL R-15 for 6h in the presence of CD28 (1μg/mL) and CD49d (1μg/mL), anti-CD107a, 1μM GolgiStop™ (BD Biosciences), brefeldin A (5μg/mL, Sigma Aldrich) and the appropriate antigens: Gag-peptide pool (3.5μg/mL), Staphylococcal enterotoxin B (5μg/mL), or media containing DMSO (peptide solvent) as negative control. Cells were incubated for 10 minutes in PBS with 0.5mM EDTA prior to staining for surface markers and viability (Aqua Dead cell stain kit, Invitrogen) for 20 minutes at room temperature. Cells were fixed in 4% paraformaldehyde (PFA) and permeabilized using FACS Perm 2 (BD Biosciences) prior to intracellular staining for CD3, IFN-γ, MIP-1β, TNF-α, and IL-2. The Transcription Factor Buffer Set (BD Biosciences) was substituted for 4% PFA and FACS Perm 2 when staining for transcription factors. In such cases, cells were fixed with Transcription Factor Fix/Perm Buffer for 40 minutes at 4°C, washed twice in Transcription Factor Perm/Wash Buffer, and stained with antibodies for 40 minutes at 4°C in Transcription Factor Perm/Wash. For all protocols, after staining, cells were re-suspended in 1% PFA and stored at 4°C in the dark until analysis within 24h.
Data Acquisition and Analysis
Flow cytometry data were acquired with an LSR II cytometer and FACSDiva™ software (Becton Dickinson Immuno-cytometry Systems). Analysis was performed using Flowjo software (Flowjo LLC, Ashland, OR). Fluorescence minus one (FMO) samples and known negative populations were used to aid in gating (Supplementary Figures 1a, 6b, 8). The distinction between CD103 negative, low and high populations was made visually as shown in Supplementary Figure 8. Tissue resident responses were identified using Boolean gating within the live, CD3+, CD8+, CD45RO+, CD103+CD69+S1PR1− subset for TRM or live, CD3+, CD8+, CD45RO−, CD103+CD69+S1PR1− subset for rTEFF. An in-house statistical algorithm was used to determine whether antigen-specific responses differed significantly from the negative control25. For responses deemed statistically significant, net responses were then calculated by subtracting negative control values from antigen-specific responses. SPICE software was used to visualize multifunctional T-cell populations48. Statistical analyses were performed with GraphPad Prism V.6 (Graphpad Software, San Diego, CA) or SPICE. Non-parametric statistical tests included Wilcoxon matched-pairs signed rank test for paired samples, Mann-Whitney test for unpaired samples, Spearman correlation and linear regression analysis.
In Situ Tetramer Staining and Immunohistochemistry
Fresh biopsy specimens were shipped in complete media on ice overnight to the University of Minnesota. Biopsies were cut into 2 to 4 sections using a scalpel and stained with MHC-tetramers, CD103, and CD8 antibodies as previously described49, 50. Red background from MHC tetramer staining was used for morphological identification of intestinal crypts. Biotinylated MHC-class I monomers were loaded with peptides (National Institute of Health Tetramer Core Facility, Emory University, Atlanta GA) and converted to MHC-class I tetramers. HLA-B*57 molecules were loaded with HIV-1 Gag IW9 (ISPRTLNAW) or QW9 (QASQEVKNW) peptides. HLA-B*07 molecules were loaded with Nef TL10 (TPNGSIFTTL) peptides. An irrelevant peptide MART (ELAGIGILTV) from melanoma protein was used as negative control. Fresh tissue sections were incubated with MHC-class I tetramers (0.5 μg/ml), mouse-anti-human CD103 Abs (2.5 μg/ml, Biolegend), and rat-anti-human CD8 Abs (2 μg/ml, Acris). For secondary incubations, sections were incubated with rabbit-anti-FITC Abs (0.5 μg/mL, BioDesign, Saco, ME). For tertiary incubations, sections were incubated with Cy3-conjugated goat-anti-rabbit Abs (0.3 μg/mL, Jackson ImmunoResearch Laboratories), Alexa 488-conjugated goat-anti-mouse Abs (0.75 μg/mL, Molecular Probes), and Cy5-conjugated goat-anti-rat Abs (0.3 μg/mL, Jackson ImmunoResearch Laboratories). Sections stained with secondary antibodies alone were used as negative controls for CD103 and CD8 staining. Sections were imaged using an Olympus FluoView 1000 microscope. Confocal z-series were collected from ~3 μm from the surface of the section to 30–45 μm into the tissue. Montage images of multiple 800 × 800 pixels were created and used for analysis.
Quantitative Image Analysis
Anatomical compartments of rectosigmoid mucosa, crypts and intraepithelial lymphocytes were identified morphologically by increasing the red background from MHC tetramers to allow identification of goblet cells. The ~10 μm area directly adjacent was defined as the border. Surrounding areas with abundant CD8+ T cells and diffuse red background were identified as lamina propria. Areas that were ambiguous in structure were not included. To determine levels of CD103 expression, CD8+ T cells with no detectable CD103 staining above background were scored as CD103 negative, cells with CD103 staining 2–5× greater than background were scored as CD103low, cells with CD103 staining 6× or greater than background were scored as CD103high.
Supplementary Material
Acknowledgments
The authors thank Rebecca Hoh, Montha Pao, Monika Deswal and the clinical staff at San Francisco General Hospital for their assistance with participant recruitment and sample collection. We thank the participants for their willingness to contribute to this study. The SCOPE cohort was supported by the Delaney AIDS Research Enterprise (DARE; AI096109 and AI127966), NIAID (K24 AI069994), and the UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763). We also thank Dr. Alex Khoruts, University of Minnesota, for assistance with identification of anatomical compartments in rectosigmoid tissue samples. This work was supported by NIH/NIAID R01-AI057020 to BLS. The UC Davis Flow Cytometry Shared Resource Laboratory (FCSR) was supported by grants from the National Institutes of Health: NCI P30 CA0933730, and NIH NCRR C06-RR12088, S10-RR12964, S10-RR026825 and S10-OD018223-01A1; and the James B. Pendleton Charitable Trust. We thank the FCSR staff members Ms. Bridget McLaughlin and Mr. Jonathan Van Dyke, for assistance with flow cytometry.
Footnotes
The authors declare no conflicts of interest that could be perceived to bias this work.
Supplementary Material is linked to the online version of the paper at http://www.nature.com/mi
AUTHOR CONTRIBUTIONS
Project concept and direction: BLS with consultation from PS, PWH and SGD. Characterization of participant cohort and procurement of clinical samples: MS, PWH, SGD. Laboratory assays: BEK (flow cytometry) with assistance from AG; SL (in situ tetramer staining). Data analysis: BEK, SL, PS, BLS. Manuscript writing and editing: BEK, BLS. All authors read and approved the manuscript.
DISCLOSURE
The authors report no conflicts of interest that could be perceived to bias this work.
References
- 1.Hatano H, et al. Comparison of HIV DNA and RNA in gut-associated lymphoid tissue of HIV-infected controllers and noncontrollers. AIDS. 2013;27:2255–2260. doi: 10.1097/QAD.0b013e328362692f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shacklett BL, Ferre AL. Mucosal immunity in HIV controllers: the right place at the right time. Curr Opin HIV AIDS. 2011;6:202–207. doi: 10.1097/COH.0b013e3283453e2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345:169–174. doi: 10.1126/science.1255512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shacklett BL, Anton PA. HIV Infection and Gut Mucosal Immune Function: Updates on Pathogenesis with Implications for Management and Intervention. Curr Infect Dis Rep. 2010;12:19–27. doi: 10.1007/s11908-009-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 7.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–435. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Revs Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay LK, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 13.Skon CN, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay LK, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 15.Wakim LM, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. 2012;189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay LK, et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity. 2015;43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, et al. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. J Virol. 2011;85:11007–11015. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbone FR. Tissue-Resident Memory T Cells and Fixed Immune Surveillance in Nonlymphoid Organs. J Immunol. 2015;195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature. 2013;497:494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 22.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol. 2015;16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Critchfield JW, et al. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One. 2008;3:e3577. doi: 10.1371/journal.pone.0003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferre AL, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiniry BE, et al. Predominance of weakly cytotoxic, T-betLowEomesNeg CD8+ T-cells in human gastrointestinal mucosa: implications for HIV infection. Mucosal Immunol. 2016;10:1008–1020. doi: 10.1038/mi.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth JS, et al. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Front Immunol. 2014;5:294. doi: 10.3389/fimmu.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shacklett BL, et al. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol. 2003;77:5621–5631. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thome JJ, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamann D, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackay CR. Dual personality of memory T cells. Nature. 1999;401:659–660. doi: 10.1038/44309. [DOI] [PubMed] [Google Scholar]
- 34.Cepek KL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 35.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–568. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 36.Sheridan BS, et al. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol. 2015;36:22–30. doi: 10.1016/j.coi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Laidlaw BJ, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen SG, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferre AL, et al. Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J Virol. 2010;84:10354–10365. doi: 10.1128/JVI.00803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SL, Mackay LK, Gebhardt T. Distinct recirculation potential of CD69+CD103− and CD103+ thymic memory CD8+ T cells. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.60. [DOI] [PubMed] [Google Scholar]
- 42.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Revs Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 43.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh SA, Li MO. TGF-beta: guardian of T cell function. J Immunol. 2013;191:3973–3979. doi: 10.4049/jimmunol.1301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shacklett BL, Critchfield JW, Lemongello D. Isolating mucosal lymphocytes from biopsy tissue for cellular immunology assays. Methods Mol Biol. 2009;485:347–356. doi: 10.1007/978-1-59745-170-3_23. [DOI] [PubMed] [Google Scholar]
- 47.Shacklett BL, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 48.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–617. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- 50.Skinner PJ, Haase AT. In situ staining using MHC class I tetramers. Curr Protoc Immunol. 2005;Chapter 17(Unit 17.4) doi: 10.1002/0471142735.im1704s64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.