Abstract
Background
Immunotherapy is a firmly established pillar in the treatment of cancer, alongside the traditional approaches of surgery, radiotherapy, and chemotherapy. Like every treatment, also cancer immunotherapy causes a diverse spectrum of side effects, collectively referred to as immune-related adverse events.
Objective
This review will examine the main forms of immunotherapy, the proposed mechanism(s) of action, and the incidence of thyroid dysfunctions.
Methods
A comprehensive MEDLINE search was performed for articles published up to March 30, 2017.
Results
Following the pioneering efforts with administration of cytokines such as IL-2 and IFN-g, which caused a broad spectrum of thyroid dysfunctions (ranging in incidence from 1 to 50%), current cancer immunotherapy strategies comprise immune checkpoint inhibitors, oncolytic viruses, adoptive T-cell transfer, and cancer vaccines. Oncolytic viruses, adoptive T-cell transfer, and cancer vaccines cause thyroid dysfunctions only rarely. In contrast, immune checkpoint blockers (such as anti-CTLA-4, anti-PD-1, anti-PD-L1) are associated with a high risk of thyroid autoimmunity. This risk is highest for anti-PD-1 and increases further when a combination of checkpoint inhibitors is used.
Conclusions
Cancer patients treated with monoclonal antibodies that block immune checkpoint inhibitors are at risk of developing thyroid dysfunctions. Their thyroid status should be assessed at baseline and periodically after initiation of the immunotherapy.
Keywords: Thyroid dysfunctions, Endocrine adverse events, Cancer immunotherapy, Immune checkpoint inhibitors
Definition and brief history of cancer immunotherapy
Cancer immunotherapy can be defined as the administration of humoral and/or cell-based agents that exploit the immune system of the patient to fight his/her own cancer. The idea of using the immune system to treat tumors is not novel. It can be traced back to 1893 when William Bradley Coley (1862–1936), a bone surgeon at the New York Cancer Hospital, published a paper entitled “The treatment of malignant tumors by repeated inoculation of erysipelas: with a report of 10 original cases” [1]. Having been stunned by the death of one of his patients affected by sarcoma, he searched the literature for treatments he could have used to save the patient. He found some case reports describing amelioration of sarcoma after Streptococcus pyogenes infection. Thus, he injected a series of sarcoma patients with S. pyogenes and noted a reduction in tumor burden in some of them. He continued his studies releasing several versions of a bacterial cocktail, that became known as the “Coley’s toxin” [2], featuring additional strains of bacteria and chemical modifications, such as heat inactivation to make the extract safer. His findings, however, could not be replicated by others and therefore were met with skepticism and quickly abandoned. More than 3 decades passed until Raymond Pearl (1879–1940), a professor of Biology and Biostatistics at the Johns Hopkins Schools of Medicine and Public Health, revisited the association between infections and cancer. In 1929 he reviewed the first 7500 autopsies of the Johns Hopkins Hospital, performed between May 1889 and May 1923, and identified 816 cases affected by some form of malignant neoplasm (carcinomatous or sarcomatous). He then randomly selected 816 controls matched for age, sex, and race among the remaining 6684 autopsies where no neoplasia was noted, and compared the prevalence of tuberculosis in the two groups. Tuberculosis was significantly less prevalent in cases (54 of 816, 7%) than in controls (133 of 816, 16%), leading him to hypothesize that having tuberculosis protected against the development of neoplasia and to suggest that tuberculin therapy could be used to treat recurrent cancer [3]. Pearl’s hypothesis was experimentally tested at the Memorial Sloan-Kettering hospital by Old, Clarke, and Benacerraf in 1959. The authors used a model where cancer is induced by implanting S-180 sarcoma cells, a line derived from Swiss Webster mice, under the skin of mice of the same MHC haplotype. In this model, about 90% of the mice die of aggressive tumor within 2–5 weeks after the injection. The authors pre-treated mice with intravenous injections of either Bacillus Calmette-Guérin (BCG) or control medium, and then implanted them with the S-180 cells. There was no difference in tumor development between BCG and medium when the S-180 cells were implanted just 1 day after the intravenous injection. However, if the implantation was done 7 or more days after the injection, BCG pre-treated mice had a much smaller incidence of tumors than control mice [4]. This study laid the foundation for human studies, the first of which was published by Morales, Eidinger, and Bruce [5]. The authors injected BCG (120 mg weekly for 6 weeks) directly into the bladder of 9 patients with superficial bladder cancer, five treated for prevention of recurrence and four for residual tumor. They noted that the injection induced a classic granulomatous reaction and significantly reduced the number of recurrences. Nowadays, BCG injection remains the standard of care for treating low grade (non muscle invasive) urothelial carcinoma, where it reduces the odds of recurrence by 70%, through a mechanism that remains to be fully elucidated [6].
Despite being used in the clinics since 1976, cancer immunotherapy has captivated the medical community and general public only in the last few years, following the introduction of immune checkpoint inhibitors. These drugs showed for the first time that the survival curve of cancer patients could be altered not only by a significant, albeit modest, shift to the right, but also by the appearance of a right tail, that is by the fact that a small subset of patients was actually cured [7]. Curing cancer requires that all malignant cells are killed without harming the patient: harnessing the immune system is theoretically a more effective way to achieve this goal than the traditional types of treatment (surgery, radiation, and chemotherapy). Like every intervention, however, cancer immunotherapy is associated with a broad spectrum of side effects, collectively referred to as immune-related adverse events. This review will focus on the thyroid dysfunctions that develop after use of cancer immunotherapy.
Mechanisms of action and classification of cancer immunotherapy
There are several forms of cancer immunotherapy that have different mechanisms of action but, in the end, use a final common pathway: the recognition of unique tumor antigens by the patient’s own lymphoid cells. These unique tumor antigens arise randomly from the many somatic mutations that are found in cancer cells [8]. The majority of cancer mutations are single base substitutions, most often missense mutations that change the amino acid sequence of the encoded protein. Mutations have therefore the potential to create novel antigens or epitopes, that are seen as foreign rather than self by the patient’s lymphocytes. There is a large variation in the load of somatic mutations found in different cancers. Some cancers, like the ones whose pathogenesis is influenced by the environment (UV radiation for melanoma and smoking for lung cancer) or by genetic defects in the DNA repair genes, have more than 100 non-synonymous mutations per tumor; whereas other cancers, such as pediatric medulloblastomas, have the fewest [8]. It is predicted that the greater the mutation load, and thus the random formation of novel tumor antigens, the greater the response to cancer immunotherapy will be.
Cancer immunotherapy has been classified according to several criteria [9]. A useful scheme is based on the type of immune response: humoral versus cell-based, that primarily mediates the anti-tumor effect. Humoral forms of cancer immunotherapy include the administration of recombinant cytokines or recombinant monoclonal antibodies directed against molecules involved in immune regulation, the so-called immune checkpoints. Cell-based immunotherapies comprise the administration of oncolytic viruses, engineered T cells, or cancer vaccines. We will review the thyroid dysfunctions that have been reported following administration of each of these five forms of cancer immunotherapy, giving particular emphasis on checkpoint inhibitors, as nowadays they are the ones most commonly used (Table 1).
Table 1.
Summary of the prevalence of thyroid abnormalities secondary to cancer immunotherapy
| Treatment type | Prevalence of: | ||||
|---|---|---|---|---|---|
|
|
|||||
| Thyroid dysfunction not otherwise specified | Hypothyroidism | Hyperthyroidism (including transient subclinical hyperthyroidism) | Destructive thyrotoxicosis | Graves disease | |
| Cytokines | |||||
| IL-2 | 22% | 15–40% | 19% | NR | NR |
| IFNs | 1–50% | 2–3% | NR | ||
| Anti-CTLA-4 | 23% | 4–15% | 3% | NR | NR |
| Anti-PD-1 | 39% | 9–40% | 1–13% | 12% | NR |
| Anti-PD-L1 | 7–21% | 7–21% | 10% | NR | NR |
| Combination of anti-CTLA-4 + anti-PD-1 or anti-CTLA-4 + anti-PD-L1 | 50% | 2–27% | 22–30% | NR | NR |
| Oncolytic viruses | NR | NR | NR | NR | NR |
| Adoptive T-cell transfer | NR | NR | NR | NR | NR |
| Cancer vaccines (alone or in combination with IL-2 or adjuvant) | 0–25% | 4–11% | 11–24% | NR | NR |
NR not reported
Materials and methods
We searched the Medline database for English articles published up to March 30, 2017 using the following search terms: “thyroid dysfunction”, “hypothyroidism”, “hyperthyroidism”, “thyroiditis” and “endocrine Immune-related adverse events” in association with “cancer immunotherapy”, “interleukin”, “interferon”, “immune checkpoint inhibitors”, “oncolytic virus”, “adoptive T-cell transfer”, “cancer vaccine”, and “combination immunotherapy”. References from the selected articles were also reviewed. Figures were created with Microsoft PowerPoint.
Thyroid dysfunctions following cytokine administration
Interleukin-2 (IL-2) is produced by T lymphocytes and acts upon T lymphocytes themselves to promote their differentiation and proliferation, being in fact originally known as T-cell growth factor [10]. IL-2 also acts on natural killer cells to boost their effector functions, such as targeting and destruction of cancer cells [11]. Based on these properties, IL-2 has been used for the treatment of metastatic melanoma and renal cell carcinoma [12]. This type of treatment, however, is associated with a significant load of adverse events. Among them, thyroid dysfunction, mainly hypothyroidism, is common, as was initially reported by Atkins [13] and later confirmed by several authors [14-16]. Hypothyroidism occurs at an incidence of 15–40% [13, 17, 18] and, in a minority of cases, can be preceded by a transient period of hyperthyroidism [19-21]. Its pathogenesis, likely multi-factorial, includes autoimmune mechanisms, given that the functional alterations are associated with the appearance of thyroid antibodies [22]. In a cohort of 34 melanoma patients, 7 (21%) developed hypothyroidism, and in 5 of those it was associated with newly found microsomal and thyroglobulin antibodies.
Interferons (IFNs) are a family of small molecules initially reported in the late 1950s for their ability to “interfere” with viral replication [23], but later found endowed with numerous additional functions [24], such as immune modulation and anti-cancer properties [25]. IFNs have been used with good success to treat a variety of conditions, ranging from autoimmune diseases such as multiple sclerosis [26, 27], infectious diseases such as hepatitis [28], to cancer [29]. Despite their efficacy, IFNs induce several adverse events, including fever, leukopenia, neuropsychiatric disorders, and a broad spectrum of thyroid dysfunctions [30]. These include autoimmune hypothyroidism, the most common abnormality found in about 20% of cases [31, 32], destructive thyrotoxicosis in 2–3% of the patients [33, 34], and, more rarely, Graves disease [35, 36]. Following the original description of hypothyroidism [37], numerous papers have confirmed these findings [38, 39], reporting a prevalence of thyroid dysfunction ranging from 1 to 35% [40].
IFN administration causes thyroid abnormalities via several mechanisms (Fig. 1). It can induce the ectopic expression of MHC class II molecules (normally found on dendritic cells, macrophages, and B lymphocytes) on thyroid epithelial cells [41]. This expression is thought to enhance presentation of thyroid antigens to self-reactive lymphocytes and consequently initiate or promote autoimmunity. More recently, attention has focused on changes in the various lymphoid subsets. Soldevila and colleagues studied 30 melanoma patients treated with high-dose interferon alpha and reported the appearance of thyroiditis in 9 (30%) of them. At the time of diagnosis, cases with thyroiditis showed a higher percentage of peripheral blood lymphocytes than thyroiditis-free controls, accompanied by increases in natural killer lymphocytes, and transitional B cells [42]. These systemic immune alterations may explain the clinical observation that interferon therapy causes a variety of autoimmune phenomena in addition to thyroiditis, such as vitiligo and vasculitis [43].
Fig. 1.

a Anti-viral effects of interferons b The mechanism by which interferon causes autoimmune disease with a thyroid cell as an example
Despite the initial enthusiasm, cytokine-based cancer immunotherapy has been largely abandoned due to the appearance of more effective alternatives.
Thyroid dysfunctions following administration of monoclonal antibodies
Monoclonal antibodies can be used to directly target and destroy the tumor cells or to target immune molecules activating the immune system of the patient that in turn destroys the tumor. The direct approach to monoclonal antibody therapy requires that the target of the antibody is expressed predominantly on the tumor cell and the antibody mediating the cell cytotoxicity is coupled to a toxin, drug, or radioactive isotopes. An example is trastuzumab—an antibody that targets the HER-2/neu receptor. This receptor is overexpressed in about 25% of patients with breast cancer, which is associated with poor prognosis. Trastuzumab blocks the binding of the natural ligand to HER-2/neu and ultimately downregulates its expression. Another example is rituximab—a monoclonal antibody directed against the pan B-cell molecule CD20. Binding of rituximab to its antigen induces cell- mediated cytotoxicity and apoptosis of the B cell, features that have made it successful for the treatment of non-Hodgkin lymphomas.
The revolution in cancer immunotherapy, however, is undoubtedly due to the use of monoclonal antibodies that target and block molecules that are critical for immune regulation, so called immune checkpoint receptors. These checkpoints, such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and PD ligand 1 (PD-L1), normally produce inhibitory signals that dampen T-cell function. When the checkpoints are blocked, T cells remain activated and unleash more potent effector functions, ultimately leading to the destruction of cancer cells. The downside of this phenomenon is that the unleashed T cells will also recognize normal self-antigens, in addition to tumor antigens, and thus potentially cause a broad range of immune-mediated adverse events. Following the introduction of ipilimumab and tremelimumab, monoclonal antibodies that target CTLA-4, this class of drugs has expanded to include pembrolizumab, nivolumab, pidilizumab, and BGB-A317, which block PD-1, durvalumab, atezolizumab, avelumab, MDX-1105, blockers of PD-L1, and AMP-224, a PDL2-IgG1 fusion protein.
Numerous adverse events have been reported after administration of immune checkpoint inhibitors, mainly affecting skin, gastro-intestinal tract, and endocrine glands. A now “classical” endocrine adverse event following CTLA-4 blockade is hypophysitis, and similarly typical is the development of thyroid autoimmunity after PD-1/PD-L1 pathway inhibitors [44, 45]. It is important, however, to emphasize that the reporting of these adverse events is often published by oncologists who may not be familiar with the diagnosis of diseases they do not commonly see, such as endocrine dysfunction or cardiovascular complications. Therefore, an ascertainment bias is likely to be found in the current literature. For example, a recent meta-analysis found no differences in the occurrence of thyroid dysfunctions in trials with anti-CTLA-4 and anti-PD-1 antibodies, despite the fact that CTLA-4 blockade has been typically associated with hypophysitis and PD-1 blockade with thyroiditis [46].
Incidence, timing pattern, and management of various thyroid disorders upon treatment with anti-CTLA-4, anti-PD-1 and anti-PD-Ll immune checkpoint inhibitors have been comprehensively reviewed by several groups [44, 46-54]. Three of the published reports are of special interest [46, 47, 51]. Abdel-Rahman assessed the relative risk of hypothyroidism and hyperthyroidism secondary to anti-CTLA-4 (ipilimumab, tremelimumab) or anti-PD-1 (nivolumab, pembrolizumab) therapy using ten randomized controlled studies and comparing the incidence of these adverse events between cases assigned to checkpoint inhibitor treatment and controls assigned to the standard-of-care treatments. Based on a sample of 3278 patients, they found a relative risk of 8.3 for hypothyroidism (4.7–14.6 95% confidence interval), and 5.5 for hyperthyroidism (1.3–22.5 95% confidence interval) [46]. Bertrand and colleagues calculated incidence of hypothyroidism and hyperthyroidism upon anti-CTLA-4 treatment (ipilimumab, tremelimumab) based on 22 clinical trials (phase II, phase I, prospective observational studies, and compassionate use trial) published as of October 2013. They reported a hypo- and/or hyperthyroidism incidence of up to 5.6% in a total of 1265 patients (with only 1 study overlapping between the two meta-analyses) [47]. Systematic review and meta-analysis on thyroid dysfunction secondary to the immune checkpoint blockade by Osorio et al. included 35 clinical trials (phase I–III), comprising a total of 7318 patients, published up until May 2016 (14 of 35 studies were also included in at least one of the above-mentioned studies). A range of various treatments was used in the selected trials, i.e., anti-CTLA-4 (ipilimumab, tremelimumab), anti-PD-1 (nivolumab, pembrolizumab, lambrolizumab), anti-PD-L1 (atezolizumab, durvalumab), in combination or as monotherapy [51]. Incidence of thyroid dysfunction was significantly higher in melanoma patients treated with anti-PD-1 (7.5%; CI 6.6–8.6) than in those treated with anti-CTLA-4 antibody (3.6%; 1.7–7.4%), while no autoimmune thyroid adverse events were observed upon treatment with anti-PD-L1 agents. Rate of thyroid dysfunction following anti-PD-1 treatment was similar among patients with different types of cancer (melanoma, nonsmall-cell lung cancer and renal cell carcinoma) [51].
Thyroid dysfunction in cancer patients enrolled in clinical trials is often unnoticed because it can be asymptomatic or overshadowed by the symptoms of an advanced or progressing cancer [51]. To get a better understanding of the development of the thyroid dysfunction after treatment with ipilimumab [52, 55], pembrolizumab [48, 49, 51, 56] or nivolumab [57, 58], investigators measured serum levels of thyroid stimulating hormone (TSH), free thyroxine (FT4), and/or triiodothyronine (T3) at baseline and during the treatment, as well as thyroperoxidase and thyroglobulin antibodies [48, 49, 51]. For example, Osorio et al. reported that 10 of 48 (21%) patients who were euthyroid at baseline developed hypothyroidism during pembrolizumab treatment requiring thyroid hormone replacement. Of them, six developed an early and transient elevation of thyroid hormones before the onset of hypothyroidism, and 8 showed de novo appearance of thyroid autoantibodies [51]. Hypo- and/or hyperthyroidism develop relatively early during immune checkpoint inhibitor therapy, with a median onset of 10.7 or 9.1 weeks after initiation of nivolumab [59] or after 2–3 ipilimumab doses [54], but can occur at any time during treatment [60]. Based on these findings, thyroid status (as assessed by TSH and free T4 measurement) should be assessed at baseline and periodically following immune checkpoint inhibitors [60, 61].
The mechanism(s) underlying thyroid dysfunction after immune checkpoint inhibitors (Fig. 2) remain to be elucidated, similar to other non-thyroidal adverse events. The CTLA-4 pathway is crucial for the development, maintenance, and function of regulatory T cells (Tregs), thus suggesting that inhibition of Tregs contributes to the development of these adverse events. Indeed, in mice Tregs ameliorate disease in a model of experimental autoimmune thyroiditis, specifically through a CTLA-4-dependent mechanism [62]. A similar role on Tregs is exerted by the PD-1/PD-L1 pathway, considering that both PD-1 and PD-L1 are expressed on resting Tregs [63]. PD-1/PD-L1 blockade could induce thyroiditis by diminishing Treg function and at the same time enhancing effector functions. Treatment with PD-1 blockade, however, did not affect the levels of peripheral blood Tregs [64]. It remains to be elucidated whether development of thyroiditis upon immune checkpoint blockade in humans is associated with diminished number and/or function of Tregs
Fig. 2.

a Blockade of PD-1 on T cells as well as b blockade of PD-L1 on cancer cells promotes T-cell activation
Another possible mechanism of anti-PD1/PD1-L1-induced thyroid toxicity involves a direct binding of the injected monoclonal antibody to the thyroid cell, as it has been suggested for hypophysitis secondary to CTLA-4 blockade [65]. Indeed, PD-L1 and PD-L2 have been recently detected, both at the mRNA and protein level, in normal thyroid glands [58]. This indicates a possible role of the interaction between PD-1-expressing lymphocytes and PD-L-expressing thyrocytes in protecting the thyroid gland from autoimmunity. A disruption of this interaction, as induced by administration of PD-1 or PD-L1 antibodies, could therefore lead to infiltration of the thyroid with autoreactive T and B lymphocytes, ultimately causing thyroiditis.
Thyroid dysfunctions following injection of oncolytic viruses
The injection of an oncolytic virus into a tumor site has been approved by the FDA in 2015 for the treatment of melanoma. This form of immunotherapy exploits the ability of the virus to target the cancer cells, replicate and ultimately kill them (direct cytotoxicity) [66]. It also induces an activation of the host immune system through the release of tumor antigens that contributes to tumor eradication (indirect effect) [66, 67]. The approved oncolytic virus, talimogene laherparepvec (T-Vec, Imlygic™), is a herpes simplex virus type 1 combined with granulocyte/macrophage colony stimulating factor (GM-CSF) to recruit myeloid cells to the injection site and boost the antigen-presenting capabilities [68]. The response rate is around 60% for local melanoma lesions, 30% for non-injected distant sites, and 15% for visceral metastasis [69]. The most commonly reported adverse events are systemic symptoms such as fever, fatigue, nausea, and reactions at the injection site [66, 70].
Treatment with oncolytic viruses is associated with a very low rate of thyroid dysfunctions. In a phase III study evaluating the safety and efficacy of T-Vec, a new-onset thyroid dysfunction was reported only in one of the 292 patients and considered to be unrelated to talimogene [71]. No other study has reported thyroid disease after treatment with T-Vec. A low incidence of thyroid adverse events was reported also when T-Vec was combined with other forms of cancer immunotherapy, for example, with immune checkpoint inhibitors, or with checkpoint inhibitors and a third form of treatment (triple therapy) [72], confirming its overall safety. Notably, clinical trials published as of March 2017, evaluating safety and efficacy of other oncolytic viruses, report no incidence of thyroid dysfunctions [73-75]. Other immune-mediated adverse events, however, do occur after administration of oncolytic viruses, including glomerulonephritis, vasculitis, pneumonitis, psoriasis, and vitiligo [68-70].
Thyroid dysfunctions following adoptive T-cell transfer
The term “adoptive immunity” (not to be confused with adaptive immunity) originally referred to the transfer of lymphocytes from a donor to a normal syngeneic recipient. It has now been expanded to describe the transfer of lymphocytes that have been isolated from a donor (such as a cancer patient), selected and expanded in vitro to large numbers, and then injected back into the same donor, a transfer that is therefore not “adoptive” in the original sense of the word (“The choosing and making that to be one’s own which originally was not so”). The adoptive transfer of T cells includes several steps (Fig. 3), which can currently be performed only in specialized centres [76]. After surgical removal of the patient’s tumor, tumor infiltrating lymphocytes are isolated and expanded in culture medium containing IL-2 [77, 78]. The expanded lymphocytes are then tested for recognition of tumor antigens by a variety of approaches, including culture with recombinant minigenes synthesized after sequencing of the patient’s tumor exomes [79]. Lymphocytes can also be transfected with retroviral vectors encoding T-cell receptors specific for certain tumor antigens. These expanded tumor-specific lymphocytes are then transferred back to the patient who in the meantime underwent a lympho-depleting strategy, such as the administration of immunosuppressive drugs (cyclophosphamide and fludarabine) or total body irradiation. In 101 patients with metastatic melanoma, 24 experienced complete remission and 23 an ongoing response [80]. The mechanism of action of adoptive T-cell transfer therapy likely relies on the ability of the expanded T cells to recognize the novel tumor antigens that have randomly originated by somatic mutations. These mutations confer specificity to the immune response since they are not found in the normal tissue counterpart. This form of therapy—although costly and labor- intensive—is appealing due to the fact that injected T cells that are directly responsible for the anti-tumor response, and can confer immunological memory and have thus the potential to be curative with a single injection [81].
Fig. 3.
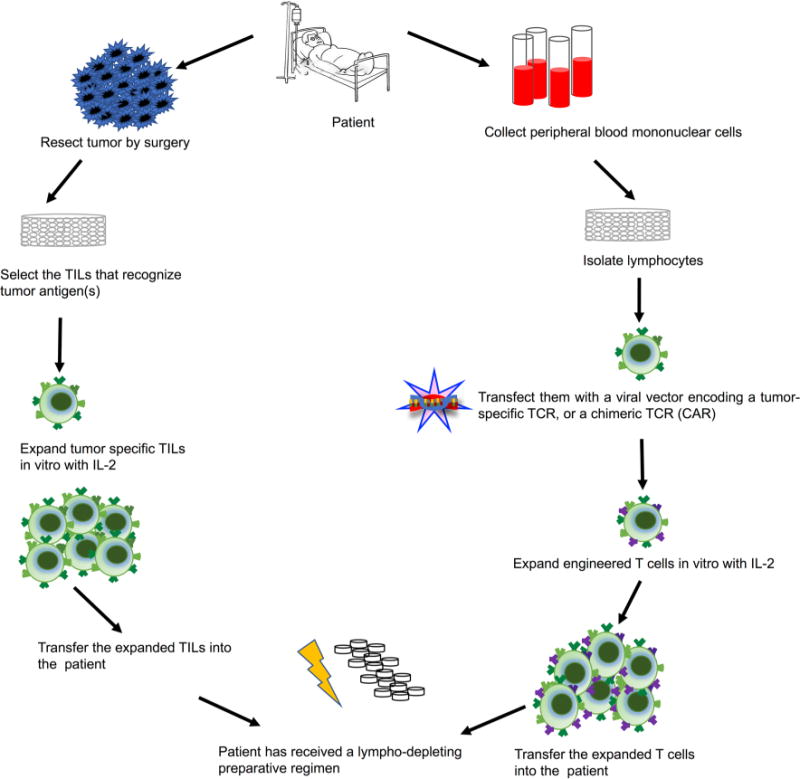
Steps involved in adoptive T-cell transfer therapies
A newer variation of adoptive cancer immunotherapy uses retroviruses to introduce a chimeric antigen receptor (CAR) in T cells isolated from the cancer patient. The transmembrane and intracellular domains of this chimera are those of a typical T-cell receptor, so they can provide the necessary signals for activation and co-stimulation. The extracellular domain is involved in a target binding, and may for example, be directed at the pan B-cell antigen CD19, to target malignant B cells. As in the more traditional adoptive immunotherapy, CAR T cells are expanded in vitro and then injected back into the patient (Fig. 3). This approach has been mainly used for haematological malignancies, such as acute lymphocytic leukemia and diffuse large B-cell lymphoma.
The adverse events associated with adoptive T-cell transfer therapy comprise systemic infections secondary to the treatment of the patient with immunosuppression, acute cytokine toxicity (“cytokine storm”), off-target toxicities, and autoimmune manifestations. These include severe rashes followed by vitiligo, uveitis with vision impairment, and hearing loss [82, 83]. Thus far, no endocrine adverse events, including thyroid dysfunction, have been described, although experience with this form of cancer immunotherapy is limited.
Thyroid dysfunction following cancer vaccines
Cancer vaccination is the injection of tumor cells or purified tumor antigens with the goal of enhancing the response of the patient’s immune system against his/her cancer (Fig. 4). The rationale for this approach is that cancers are known to be poorly immunogenic due to a variety of mechanisms that allow them to escape immunosurveillance. Several manipulations are required to make cancer vaccines safe, effective, and accessible to a large number of patients. Cancer cells to be injected are irradiated to prevent their replication in the host. They are then administered in a context that makes them more immunogenic, i.e., they are either transfected to express GM-CSF or injected with a by-stander cell line that expresses GM-CSF, to recruit myeloid cells to the injection site and enhance antigen presentation [84]. They can be also combined with inactivated bacteria, such as Bacillus Calmette-Guerin, Corynebacterium parvum, or Listeria monocytogenes [85], to provide an adjuvant signal via stimulation of the Toll-like receptor pathways. Finally, they can be modified to overexpress a protein enriched in the tumor cells, such as prostatic acid phosphatase.
Fig. 4.

Possible mechanisms of action of cancer vaccines
The ability of the immune system to recognize and kill the cancer cells depends upon the recognition of a particular combination of tumor antigens and MHC molecules, a combination that is therefore unique to each patient. Consequently, one approach is to surgically remove the tumor from the patient, isolate and modify the cancer cells, and then inject them back in that particular patient (autologous vaccine). Another approach is to use the cancer cells from a patient, strip them of their surface MHC molecules, and then inject in other patients who have the same type of cancer (allogeneic vaccine). In addition to injection of cancer cells, investigators are also exploring the idea of injecting cancer antigens that are either uniquely expressed by the tumor, such as the cancer-testis antigen NY-ESO, or preferentially by the tumor, such as the heat shock glycoprotein 96, to induce T-cell responses directed specifically against this target (a commercial product based on this idea is Vitespen used for renal cell carcinoma and melanoma) [86].
Cancer vaccines can be classified as prophylactic or therapeutic. Prophylactic cancer vaccines are based on the notion that many cancers are associated with viral infections, thus leading to the hypothesis that preventing these infections would reduce the risk of developing cancer. In 2005 the FDA approved the use in healthy women of Gardasil, a combination of recombinant human papilloma virus type 6, 11, 16, and 18 to prevent cervical cancer. Similarly, vaccination with Recombivax HB is used to prevent hepatitis B and the associated hepatocellular carcinoma. On the contrary, therapeutic cancer vaccines are the ones used in patients who already have been diagnosed with cancer. Examples include Sipuleucel-T for treatment of asymptomatic or minimally symptomatic metastatic, castration-resistant prostate cancer [87] and GVAX prostate, pancreas, colon, lung, or melanoma for the treatment of the respective cancers. Overall, therapeutic cancer vaccines have thus far failed to provide a meaningful survival benefit [88].
Thyroid dysfunctions have been reported after cancer vaccines. In a phase I clinical study, nine HLA-A*0201 positive patients with high-risk epithelial ovarian cancer received the cancer-testis antigen NY-ESO-1 and the immune adjuvant Montanide ISA-51 every 3 weeks for five vaccinations. One patient (11%) developed hypothyroidism requiring levothyroxine replacement. During the routine follow-up, 3 months after completion of vaccination regime one patient, who at the end of the study did not show any unusual toxicity, developed a subclinical hyperthyroidism which resolved without therapeutic intervention [89]. Interestingly, the patient who developed hypothyroidism had the highest immune response seen in the study and a prolonged complete clinical remission. Recently a case of severe hyperthyroidism after NY-ESO-1 vaccination has been reported [90]. The patient had a synovial sarcoma of the right infratemporal fossa treated first with intensity modulated radiotherapy to the head and the neck, followed by chemotherapy. Two years later she received two cycles of five vaccinations of NY-ESO-1 combined with the immune adjuvant Poly-ICLC emulsified in Montanide ISA-51. One month after the beginning of immunotherapy she developed a mild hyperthyroidism caused by Graves’ disease. She was first treated with a beta-blocker and L-carnitine with clinical improvement, but then the hyperthyroidism progressively worsened requiring a total thyroidectomy [91]. The mechanism proposed by the authors is molecular mimicry between NY-ESO-1 and thyroid autoantigens.
Chianese-Bullock et al. reviewed the occurrence of thyroid autoimmunity in 55 melanoma patients treated with low-dose of IL-2 in conjunction with a vaccine comprising peptide-pulsed dendritic cells, autologous tumor cells with GM-CSF in Montanide ISA-51, or synthetic peptides with GM-CSF in Montanide ISA-51. Fourteen patients (25%) developed thyroid abnormalities, including 2 cases of hypothyroidism and 13 of hyperthyroidism. The majority of these patients became euthyroid within several weeks after cessation of IL-2 treatment [92].
In a pilot study of 15 prostate cancer patients who were treated with E75 (immunogenic peptide from the HER2/neu protein) and Flt3 ligand adjuvant (a growth and differentiation factor for dendritic cells), two (13.3%) developed clinically significant hypothyroidism requiring thyroid hormone replacement therapy. However, both subjects were found to have anti-thyroid antibodies prior to treatment, consistent with pre-existing autoimmune thyroiditis [93]. The topic of pre-existing thyroid autoimmunity as a risk factor for development of thyroid toxicity following cancer vaccine has been investigated by De Remigis et al. [94]. The authors reported that GVAX induced the development of thyroglobulin antibodies independently of the underlying cancer (81% in prostate cancer, 75% colon cancer, and 76% pancreatic cancer) and the co-administration of ipilimumab (75% in GVAX only and 78% in GVAX plus ipilimumab). Exclusion of the patients who already had thyroid antibodies at baseline did not change the overall findings. Development of autoantibodies (antinuclear, thyroglobulin, thyroperoxidase, mitochondrial antibody, and cardiolipin antibodies) has also been reported in melanoma patients treated with a vaccine comprised of melanoma-specific antigen (the ganglioside GM-2 coupled to keyhole limpet hemocyanin) with or without high-dose interferon-α2b as an adjuvant, GMK plus sequential HDI, or GMK alone [95]. In both studies, no data on the clinical manifestations of thyroid autoimmunity were available.
Despite these few reports on thyroid dysfunctions following cancer vaccine treatment, phase III clinical trials have shown that the approved commercial cancer vaccines are overall safe, well tolerated, and associated with limited adverse events [96, 97]. Thyroid dysfunctions were reported only in 1 of 215 (0.46%) patients with stage IV melanoma [98], and in 1 of 48 (2.08%) patients with advanced renal cell carcinoma after treatment with Vitespen [99].
Thyroid dysfunction following combination therapy
The combination of different forms of immunotherapy is often considered necessary to induce a more robust and sustained anti-tumor immune response, although it can also increase the burden of adverse events. An example of combination therapy is, the use CTLA-4 and PD-1 blocking anti-bodies. Since CTLA-4 and PD-1 inhibit T-cell activation via different pathways, blocking these two inhibitory pathways is predicted to yield a synergistic effect. Dual blockade of CTLA-4 and PD-1 has been investigated in several clinical trials in patients with advanced melanoma (Supplemental Table S4 in [48]). Other ongoing trials are being conducted in renal cell carcinoma and non-small cell lung cancer with promising results. In October 2015 the combination ipilimumab + nivolumab received the FDA approval as a first line treatment of metastatic melanoma [100]. The expected high response rate with the combination treatment was indeed associated with more numerous endocrine adverse events. In particular, thyroid dysfunctions (mostly grade 1 or 2) occurred in 25% (78 of 313) of patients treated with the combination therapy but only in 13% (40 of 313) or 5% (16 of 311) of those treated with nivolumab alone or ipilimumab alone, respectively (Supplemental Table S3 in [100]).
Nivolumab plus ipilimumab therapy also demonstrated favorable anti-tumor activity and durable responses in patients with metastatic renal cell carcinoma. In this population, hypothyroidism (grade 1 or 2) occurred in 19% (9 of 47) or 28% (13 of 47) of patients treated with 3 mg/kg nivolumab plus 1 mg/kg ipilimumab or 1 mg/kg nivolumab plus 3 mg/kg ipilimumab, respectively [101]. Thyroid dysfunctions occurred at similar frequencies in patients with recurrent small cell lung cancer treated with 3 mg/kg nivolumab plus 1 mg/kg ipilimumab (7 of 54, 13%) or 1 mg/kg nivolumab plus 3 mg/kg ipilimumab (17 of 61, 28%). It was significantly less common (5 of 98, 5%), in patients treated with nivolumab (3 mg/kg) alone [102]. Overall, thyroid dysfunctions are common in patients treated with a combination therapy but rarely of high (grade 3 or 4) toxicity that requires treatment withdrawal or results in fatal events. Clinicians should nevertheless be aware that patients treated with combination therapy are significantly more prone to adverse events and thus be ready to diagnose and treat them.
Another type of combination therapy involves the coupling of immune checkpoint inhibitors with cancer vaccines. This combination does not seem to be associated with an increased load of immune-related adverse events [103]. In a phase I dose escalation trial, patients with metastatic, castration-resistant, prostate cancer were treated with ipilimumab and a poxviral-based vaccine targeting prostate-specific antigen (PSA). Hypothyroidism (grade 2) was diagnosed in 4 of 30 patients (13%) at the higher ipilimumab dose [104].
Gibney et al. used nivolumab (1, 3, or 10 mg/kg) with a multi-peptide vaccine (gp 100, MART-1, and NY-ESO-1) emulsified in Montanide ISA 51 VG to treat patients with advanced melanoma. Thyroiditis with hypothyroidism (grade 2) developed in about a fifth of the patients and was successfully managed with hormone replacement therapy [105]. The same group analyzed the safety and efficacy of nivolumab (3 mg/kg) with or without a multi-peptide vaccine in 92 ipilimumab-refractory melanoma patients. They reported thyroid dysfunctions (hypothyroidism and hyperthyroidism) in 8 of 61 (13%) patients in the nivolumab only arm and in 9 of 31 (29%) patients in the nivolumab plus cancer vaccine arm [106]. Recently, Freeman-Keller et al. [59] analyzed data from two melanoma trials using nivolumab with or without a multi-peptide cancer vaccine. One study featured patients with resected disease and the other patients with unresectable disease. Thyroid dysfunctions (grade 2) were noted in 7 of 33 (21%) patients in the resected cohort and 12 of 115 (8%) patients in the unresectable cohort. These dysfunctions were not associated with statistical differences in overall survival.
Conclusion
In conclusion, this review offers a glimpse on the immunological approaches that are currently used to treat cancer and the thyroid dysfunctions that can develop in response to them. The review also highlights the complexity of the patient’s immune response, which prompts the emergence of new and diversified forms of immunotherapy.
Acknowledgments
Funding The study was supported by NIH Grant RO1 CA-194042 to PC.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent No informed consent.
References
- 1.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 2.Bickels J, Kollender Y, Merinsky O, Meller I. Coley’s toxin: historical perspective. Isr Med Assoc J. 2002;4(6):471–472. [PubMed] [Google Scholar]
- 3.Pearl R. Cancer and tuberculosis. Am J Hygiene. 1929;9(1):97–159. [Google Scholar]
- 4.Old LJ, Clarke DA, Benacerraf B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature. 1959;184(Suppl 5):291–292. doi: 10.1038/184291a0. [DOI] [PubMed] [Google Scholar]
- 5.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 6.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol. 2014;11(3):153–162. doi: 10.1038/nrurol.2014.15. https://doi.org/10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. https://doi.org/10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. doi: 10.1126/science.1235122. https://doi.org/10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado JP, Agostinis P, Apte RN, Aranda F, Ayyoub M, Beckhove P, Blay JY, Bracci L, Caignard A, Castelli C, Cavallo F, Celis E, Cerundolo V, Clayton A, Colombo MP, Coussens L, Dhodapkar MV, Eggermont AM, Fearon DT, Fridman WH, Fucikova J, Gabrilovich DI, Galon J, Garg A, Ghiringhelli F, Giaccone G, Gilboa E, Gnjatic S, Hoos A, Hosmalin A, Jager D, Kalinski P, Karre K, Kepp O, Kiessling R, Kirkwood JM, Klein E, Knuth A, Lewis CE, Liblau R, Lotze MT, Lugli E, Mach JP, Mattei F, Mavilio D, Melero I, Melief CJ, Mittendorf EA, Moretta L, Odunsi A, Okada H, Palucka AK, Peter ME, Pienta KJ, Porgador A, Prendergast GC, Rabinovich GA, Restifo NP, Rizvi N, Sautes-Fridman C, Schreiber H, Seliger B, Shiku H, Silva-Santos B, Smyth MJ, Speiser DE, Spisek R, Srivastava PK, Talmadge JE, Tartour E, Van Der Burg SH, Van Den Eynde BJ, Vile R, Wagner H, Weber JS, Whiteside TL, Wolchok JD, Zitvogel L, Zou W, Kroemer G. Classification of current anticancer immunotherapies. Oncotarget. 2014;5(24):12472–12508. doi: 10.18632/oncotarget.2998. https://doi.org/10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotze MTCA, Chang A, Seipp CA, Simpson C, Vetto JT, Rosenberg SA. High-dose recombinant interleukin-2 in the treatment of patients with disseminated cancer. JAMA. 1986;256:3117–3124. [PubMed] [Google Scholar]
- 11.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. https://doi.org/10.1056/nejm198512053132327. [DOI] [PubMed] [Google Scholar]
- 13.Atkins MBMJ, Parkinson DR, Gould JA, Berkman EM, Kaplan M. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318:1557–1563. doi: 10.1056/NEJM198806163182401. [DOI] [PubMed] [Google Scholar]
- 14.Kruit WHBR, Goey SH, Jansen RL, Eggermont AM, Batchelor D, Schmitz PI, Stoter G. Interleukin-2-induced thyroid dysfunction is correlated with treatment duration but not with tumor response. J Clin Oncol. 1993;11:921–924. doi: 10.1200/JCO.1993.11.5.921. [DOI] [PubMed] [Google Scholar]
- 15.Tartour ESM, Dorval T, Baudin E, Fridman WH. Endocrine involvement in immunotherapy. Ann Endocrinol. 1995;56:143–148. [PubMed] [Google Scholar]
- 16.Vassilopoulou-Sellin RSA, Dexeus FH, Theriault RL, Pololoff DA. Acute thyroid dysfunction (thyroiditis) after therapy with interleukin-2. Horm Metab Res. 1992;24:434–438. doi: 10.1055/s-2007-1003353. [DOI] [PubMed] [Google Scholar]
- 17.Tartour E, Schlumberger M, Dorval T, Baudin E, Fridman WH. Endocrine involvement in immunotherapy. Ann Endocrinol (Paris) 1995;56(2):143–148. [PubMed] [Google Scholar]
- 18.Vassilopoulou-Sellin R, Sella A, Dexeus FH, Theriault RL, Pololoff DA. Acute thyroid dysfunction (thyroiditis) after therapy with interleukin-2. Horm Metab Res. 1992;24(9):434–438. doi: 10.1055/s-2007-1003353. https://doi.org/10.1055/s-2007-1003353. [DOI] [PubMed] [Google Scholar]
- 19.Fentiman IS, Balkwill FR, Thomas BS, Russell MJ, Todd I, Bottazzo GF. An autoimmune aetiology for hypothyroidism following interferon therapy for breast cancer. Eur J Cancer Clin Oncol. 1988;24(8):1299–1303. doi: 10.1016/0277-5379(88)90219-2. [DOI] [PubMed] [Google Scholar]
- 20.Lowndes SA, Asher R, Middleton MR. Thyrotoxicosis with pegylated interferon alfa-2b. Arch Dermatol. 2010;146(11):1273–1275. doi: 10.1001/archdermatol.2010.306. https://doi.org/10.1001/archdermatol.2010.306. [DOI] [PubMed] [Google Scholar]
- 21.Scalzo S, Gengaro A, Boccoli G, Masciulli R, Giannella G, Salvo G, Marolla P, Carlini P, Massimini G, Holdener EE, et al. Primary hypothyroidism associated with interleukin-2 and interferon alpha-2 therapy of melanoma and renal carcinoma. Eur J Cancer. 1990;26(11–12):1152–1156. doi: 10.1016/0277-5379(90)90275-x. [DOI] [PubMed] [Google Scholar]
- 22.Atkins MB, Mier JW, Parkinson DR, Gould JA, Berkman EM, Kaplan MM. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med. 1988;318(24):1557–1563. doi: 10.1056/NEJM198806163182401. https://doi.org/10.1056/nejm198806163182401. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 24.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, Walter MR, Nagabhushan TL, Trotta PP, Pestka S. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58(12):2489–2499. [PubMed] [Google Scholar]
- 25.Baron S, Tyring SK, Fleischmann WR, Jr, Coppenhaver DH, Niesel DW, Klimpel GR, Stanton GJ, Hughes TK. The interferons: mechanisms of action and clinical applications. JAMA. 1991;266(10):1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 26.Bhargava P, Newsome SD. An update on the evidence base for peginterferon beta-1a in the treatment of relapsing-remitting multiple sclerosis. Ther Adv Neurol Disord. 2016;9(6):483–490. doi: 10.1177/1756285616656296. https://doi.org/10.1177/1756285616656296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotondi M, Oliviero A, Profice P, Mone CM, Biondi B, Del Buono A, Mazziotti G, Sinisi AM, Bellastella A, Carella C. Occurrence of thyroid autoimmunity and dysfunction throughout a nine-month follow-up in patients undergoing interferon-beta therapy for multiple sclerosis. J Endocrinol Invest. 1998;21(11):748–752. doi: 10.1007/BF03348040. [DOI] [PubMed] [Google Scholar]
- 28.Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: toward a new classification. Hepatology. 2006;43(4):661–672. doi: 10.1002/hep.21146. https://doi.org/10.1002/hep.21146. [DOI] [PubMed] [Google Scholar]
- 29.Agarwala SS, Kirkwood JM. Update on adjuvant interferon therapy for high-risk melanoma. Oncology. 2002;16(9):1177–1187. (discussion 1190–1172, 1197) [PubMed] [Google Scholar]
- 30.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6(1):34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 31.Mazziotti G, Sorvillo F, Stornaiuolo G, Rotondi M, Morisco F, Ruberto M, Cioffi M, Amato G, Caporaso N, Gaeta GB, Carella C. Temporal relationship between the appearance of thyroid autoantibodies and development of destructive thyroiditis in patients undergoing treatment with two different type-1 interferons for HCV-related chronic hepatitis: a prospective study. J Endocrinol Invest. 2002;25(7):624–630. doi: 10.1007/BF03345087. https://doi.org/10.1007/BF03345087. [DOI] [PubMed] [Google Scholar]
- 32.Prummel MF, Laurberg P. Interferon-alpha and autoimmune thyroid disease. Thyroid. 2003;13(6):547–551. doi: 10.1089/105072503322238809. https://doi.org/10.1089/105072503322238809. [DOI] [PubMed] [Google Scholar]
- 33.Csaki AC, Blum M. Thyrotoxicosis after interferonalpha therapy. Thyroid. 2000;10(1):101. doi: 10.1089/thy.2000.10.101. https://doi.org/10.1089/thy.2000.10.101. [DOI] [PubMed] [Google Scholar]
- 34.Wong V, Fu AX, George J, Cheung NW. Thyrotoxicosis induced by alpha-interferon therapy in chronic viral hepatitis. Clin Endocrinol (Oxf) 2002;56(6):793–798. doi: 10.1046/j.1365-2265.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- 35.Braga-Basaria M, Basaria S. Interferon-alpha-induced transient severe hypothyroidism in a patient with Graves’ disease. J Endocrinol Invest. 2003;26(3):261–264. doi: 10.1007/BF03345167. https://doi.org/10.1007/BF03345167. [DOI] [PubMed] [Google Scholar]
- 36.Rotondi M, Mazziotti G, Biondi B, Manganella G, Del Buono AD, Montella P, Di Cristofaro M, Di Iorio G, Amato G, Carella C. Long-term treatment with interferon-beta therapy for multiple sclerosis and occurrence of Grave’s disease. J Endocrinol Invest. 2000;23(5):321–324. doi: 10.1007/BF03343730. [DOI] [PubMed] [Google Scholar]
- 37.Fentiman IS, Thomas BS, Balkwill FR, Rubens RD, Hayward JL. Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet. 1985;1(8438):1166. doi: 10.1016/s0140-6736(85)92475-4. [DOI] [PubMed] [Google Scholar]
- 38.Carella C, Mazziotti G, Amato G, Braverman LE, Roti E. Clinical review 169: interferon-alpha-related thyroid disease: pathophysiological, epidemiological, and clinical aspects. J Clin Endocrinol Metab. 2004;89(8):3656–3661. doi: 10.1210/jc.2004-0627. https://doi.org/10.1210/jc.2004-0627. [DOI] [PubMed] [Google Scholar]
- 39.Ward DL, Bing-You RG. Autoimmune thyroid dysfunction induced by interferon-alpha treatment for chronic hepatitis C: screening and monitoring recommendations. Endocr Pract. 2001;7(1):52–58. doi: 10.4158/EP.7.1.52. https://doi.org/10.4158/EP.7.1.52. [DOI] [PubMed] [Google Scholar]
- 40.Koh LK, Greenspan FS, Yeo PP. Interferon-alpha induced thyroid dysfunction: three clinical presentations and a review of the literature. Thyroid. 1997;7(6):891–896. doi: 10.1089/thy.1997.7.891. https://doi.org/10.1089/thy.1997.7.891. [DOI] [PubMed] [Google Scholar]
- 41.Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- 42.Soldevila B, Alonso N, Martinez-Arconada MJ, Granada ML, Boada A, Vallejois V, Fraile M, Fernàndez-Sanmartin MA, Pujol-Borrel R, Puig-Domingo M, Sanmarti A, Martinez-Càceres Regulatory T cells and other lymphocyte subpopulations in patients with melanoma developing interferon-induced thyroiditis during high-dose interferon-alfa 2b treatment. Clin Endocrinol. 2013;78:621–628. doi: 10.1111/cen.12036. [DOI] [PubMed] [Google Scholar]
- 43.Palacios-Alvarez I, Roman-Curto C, Mir-Bonafe JM, Canueto J, Usero-Barcena T, Fernandez-Lopez E. Autoimmune response as a side effect of treatment with interferon-alpha in melanoma: does this have prognostic implications? Int J Dermatol. 2015;54(3):e91–e93. doi: 10.1111/ijd.12698. https://doi.org/10.1111/ijd.12698. [DOI] [PubMed] [Google Scholar]
- 44.Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, Gore M, Larkin J. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 2017;86(4):614–620. doi: 10.1111/cen.13297. https://doi.org/10.1111/cen.13297. [DOI] [PubMed] [Google Scholar]
- 45.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. https://doi.org/10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta-analysis. Future Oncol. 2016;12(3):413–425. doi: 10.2217/fon.15.222. https://doi.org/10.2217/fon.15.222. [DOI] [PubMed] [Google Scholar]
- 47.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. https://doi.org/10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101(11):4431–4439. doi: 10.1210/jc.2016-2300. https://doi.org/10.1210/jc.2016-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, Dietz AB, Ryder M. Pembrolizumab-induced thyroiditis. Comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017 doi: 10.1210/jc.2017-00448. https://doi.org/10.1210/jc.2017-00448. [DOI] [PMC free article] [PubMed]
- 50.Gonzalez-Rodriguez E, Rodriguez-Abreu D. Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist. 2016;21(7):804–816. doi: 10.1634/theoncologist.2015-0509. https://doi.org/10.1634/theoncologist.2015-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, Merghoub T, Rudin CM, Fish S, Hellmann MD. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–589. doi: 10.1093/annonc/mdw640. https://doi.org/10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(2):371–381. doi: 10.1530/ERC-13-0499. https://doi.org/10.1530/erc-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torino F, Barnabei A, Paragliola R, Baldelli R, Appetecchia M, Corsello SM. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid. 2013;23(11):1345–1366. doi: 10.1089/thy.2013.0241. https://doi.org/10.1089/thy.2013.0241. [DOI] [PubMed] [Google Scholar]
- 54.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. https://doi.org/10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164(2):303–307. doi: 10.1530/EJE-10-0833. https://doi.org/10.1530/eje-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alhusseini M, Samantray J. Hypothyroidism in cancer patients on immune checkpoint inhibitors with anti-PD1 agents: insights on underlying mechanisms. Exp Clin Endocrinol Diabetes. 2017;125(4):267–269. doi: 10.1055/s-0042-119528. https://doi.org/10.1055/s-0042-119528. [DOI] [PubMed] [Google Scholar]
- 57.Narita T, Oiso N, Taketomo Y, Okahashi K, Yamauchi K, Sato M, Uchida S, Matsuda H, Kawada A. Serological aggravation of autoimmune thyroid disease in two cases receiving nivolumab. J Dermatol. 2016;43(2):210–214. doi: 10.1111/1346-8138.13028. https://doi.org/10.1111/1346-8138.13028. [DOI] [PubMed] [Google Scholar]
- 58.Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, Hirota K, Ueda Y, Kanai Y, Yamashita Y, Kondo E, Sone M, Yasoda A, Inagaki N. Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid. 2017;27(7):894–901. doi: 10.1089/thy.2016.0562. https://doi.org/10.1089/thy.2016.0562. [DOI] [PubMed] [Google Scholar]
- 59.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136. https://doi.org/10.1158/1078-0432.ccr-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raedler LA. Keytruda (pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8:96–100. Spec Feature. [PMC free article] [PubMed] [Google Scholar]
- 61.Raedler LA. Opdivo (nivolumab): second PD-1 inhibitor receives FDA approval for unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8:180–183. Spec Feature. [PMC free article] [PubMed] [Google Scholar]
- 62.Morris GP, Brown NK, Kong YC. Naturally-existing CD4(+)CD25(+)Foxp3(+) regulatory T cells are required for tolerance to experimental autoimmune thyroiditis induced by either exogenous or endogenous autoantigen. J Autoimmun. 2009;33(1):68–76. doi: 10.1016/j.jaut.2009.03.010. https://doi.org/10.1016/j.jaut.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. https://doi.org/10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, Seja E, Kivork C, Siebert J, Kaplan-Lefko P, Wang X, Chmielowski B, Glaspy JA, Tumeh PC, Chodon T, Pe’er D, Comin-Anduix B. PD-1 blockade expands intratumoral memory T Cells. Cancer Immunol Res. 2016;4(3):194–203. doi: 10.1158/2326-6066.CIR-15-0210. https://doi.org/10.1158/2326-6066.cir-15-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra245. doi: 10.1126/scitranslmed.3008002. https://doi.org/10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 66.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–662. doi: 10.1038/nrd4663. https://doi.org/10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mavani HJ, Wick JY. Oncology’s trojan horse: using viruses to battle cancer. Consult Pharm. 2016;31(12):676–684. doi: 10.4140/TCP.n.2016.676. https://doi.org/10.4140/TCP.n.2016.676. [DOI] [PubMed] [Google Scholar]
- 68.Greig SL. Talimogene Laherparepvec: first global approval. Drugs. 2016;76(1):147–154. doi: 10.1007/s40265-015-0522-7. https://doi.org/10.1007/s40265-015-0522-7. [DOI] [PubMed] [Google Scholar]
- 69.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH, Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. https://doi.org/10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 70.Harrington KJ, Andtbacka RH, Collichio F, Downey G, Chen L, Szabo Z, Kaufman HL. Efficacy and safety of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in patients with stage IIIB/C and IVM1a melanoma: subanalysis of the phase III OPTiM trial. OncoTargets Ther. 2016;9:7081–7093. doi: 10.2147/OTT.S115245. https://doi.org/10.2147/ott.s115245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.FDA. Talimogene laherparepvec BLA 125518 pharmacovigilance plan: review memorandum. 2014. [Google Scholar]
- 72.Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, Kaufman HL, Andtbacka RH. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB–IV melanoma. J Clin Oncol. 2016;34(22):2619–2626. doi: 10.1200/JCO.2016.67.1529. https://doi.org/10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107(10):1373–1379. doi: 10.1111/cas.13027. https://doi.org/10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, Moon A, Mun JH, Sommermann EM, Avidal LM, Patt R, Pelusio A, Burke J, Hwang TH, Kirn D, Park YS. Phase 1b trial of biweekly intravenous Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol Ther. 2015;23(9):1532–1540. doi: 10.1038/mt.2015.109. https://doi.org/10.1038/mt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong J, Sachdev E, Mita AC, Mita MM. Clinical development of reovirus for cancer therapy: an oncolytic virus with immune-mediated antitumor activity. World J Methodol. 2016;6(1):25–42. doi: 10.5662/wjm.v6.i1.25. https://doi.org/10.5662/wjm.v6.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hinrichs CSRS. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, Samuels Y, Rosenberg SA. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19(6):747–752. doi: 10.1038/nm.3161. https://doi.org/10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran ETS, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4 + T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parkhurst M, Gros A, Pasetto A, Prickett T, Crystal JS, Robbins P, Rosenberg SA. Isolation of T-cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on CD137 expression. Clin Cancer Res. 2017;23(10):2491–2505. doi: 10.1158/1078-0432.CCR-16-2680. https://doi.org/10.1158/1078-0432.CCR-16-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goff SLDM, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, Klebanoff CA, Hughes MS, Restifo NP, Langhan MM, Shelton TE, Lu L, Kwong ML, Ilyas S, Klemen ND, Payabyab EC, Morton KE, Toomey MA, Steinberg SM, White DE, Rosenberg SA. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34:2389–2397. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg SAPB, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 82.Seaman BJGE, Brewer CC, Zalewski CK, King KA, Rudy S, Van Waes C, Morgan RA, Dudley ME, Yang JC, Rosenberg SA, Kim HJ. Audiovestibular dysfunction associated with adoptive cell immunotherapy for melanoma. Otolaryngol Head Neck Surg. 2012;147:744–749. doi: 10.1177/0194599812448356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeh SKN, Kerkar SP, Heller CK, Palmer DC, Johnson LA, Li Z, Bishop RJ, Wong WT, Sherry RM, Yang JC, Dudley ME, Restifo NP, Rosenberg SA, Nussenblatt RB. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology. 2009;116:981–989. doi: 10.1016/j.ophtha.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, Giedlin M, Louis JL, Sugar EA, Pons A, Cox AL, Levine J, Murphy AL, Illei P, Dubensky TW, Jr, Eiden JE, Jaffee EM, Laheru DA. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18(3):858–868. doi: 10.1158/1078-0432.CCR-11-2121. https://doi.org/10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wood CG, Mulders P. Vitespen: a preclinical and clinical review. Future Oncol. 2009;5(6):763–774. doi: 10.2217/fon.09.46. https://doi.org/10.2217/fon.09.46. [DOI] [PubMed] [Google Scholar]
- 87.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. https://doi.org/10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 88.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–524. doi: 10.1038/nrclinonc.2014.111. https://doi.org/10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 89.Diefenbach CS, Gnjatic S, Sabbatini P, Aghajanian C, Hensley ML, Spriggs DR, Iasonos A, Lee H, Dupont B, Pezzulli S, Jungbluth AA, Old LJ, Dupont J. Safety and immunogenicity study of NY-ESO-1b peptide and montanide ISA-51 vaccination of patients with epithelial ovarian cancer in high-risk first remission. Clin Cancer Res. 2008;14(9):2740–2748. doi: 10.1158/1078-0432.CCR-07-4619. https://doi.org/10.1158/1078-0432.ccr-07-4619. [DOI] [PubMed] [Google Scholar]
- 90.Vita R, Guarneri F, Agah R, Benvenga S. Autoimmune thyroid disease elicited by NY-ESO-1 vaccination. Thyroid. 2014;24(2):390–394. doi: 10.1089/thy.2013.0170. https://doi.org/10.1089/thy.2013.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chee R, Agah R, Vita R, Benvenga S. L-carnitine treatment in a seriously ill cancer patient with severe hyperthyroidism. Hormones (Athens) 2014;13(3):407–412. doi: 10.14310/horm.2002.1494. https://doi.org/10.14310/horm.2002.1494. [DOI] [PubMed] [Google Scholar]
- 92.Chianese-Bullock KA, Woodson EM, Tao H, Boerner SA, Smolkin M, Grosh WW, Neese PY, Merrill P, Petroni GR, Slingluff CL., Jr Autoimmune toxicities associated with the administration of antitumor vaccines and low-dose interleukin-2. J Immunother. 2005;28(4):412–419. doi: 10.1097/01.cji.0000171314.00924.2b. [DOI] [PubMed] [Google Scholar]
- 93.McNeel DG, Knutson KL, Schiffman K, Davis DR, Caron D, Disis ML. Pilot study of an HLA-A2 peptide vaccine using flt3 ligand as a systemic vaccine adjuvant. J Clin Immunol. 2003;23(1):62–72. doi: 10.1023/a:1021904432489. [DOI] [PubMed] [Google Scholar]
- 94.De Remigis A, de Gruijl TD, Uram JN, Tzou SC, Iwama S, Talor MV, Armstrong TD, Santegoets SJ, Slovin SF, Zheng L, Laheru DA, Jaffee EM, Gerritsen WR, van den Eertwegh AJ, Le DT, Caturegli P. Development of thyroglobulin antibodies after GVAX immunotherapy is associated with prolonged survival. Int J Cancer. 2015;136(1):127–137. doi: 10.1002/ijc.28973. https://doi.org/10.1002/ijc.28973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tarhini AA, Shin D, Lee SJ, Stuckert J, Sander CA, Kirkwood JM. Serologic evidence of autoimmunity in E2696 and E1694 patients with high-risk melanoma treated with adjuvant interferon alfa. Melanoma Res. 2014;24(2):150–157. doi: 10.1097/CMR.0000000000000050. https://doi.org/10.1097/cmr.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall SJ, Klotz L, Pantuck AJ, George DJ, Whitmore JB, Frohlich MW, Sims RB. Integrated safety data from 4 randomized, double-blind, controlled trials of autologous cellular immunotherapy with sipuleucel-T in patients with prostate cancer. J Urol. 2011;186(3):877–881. doi: 10.1016/j.juro.2011.04.070. https://doi.org/10.1016/j.juro.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, Yu H, Dong S, Zhong Y, Hu S. Recognizing and managing on toxicities in cancer immunotherapy. Tumour Biol. 2017;39(3):1010428317694542. doi: 10.1177/1010428317694542. https://doi.org/10.1177/1010428317694542. [DOI] [PubMed] [Google Scholar]
- 98.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, Yuh L, Gupta R, Srivastava PK. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26(6):955–962. doi: 10.1200/JCO.2007.11.9941. https://doi.org/10.1200/jco.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 99.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, Isakov L, Flanigan R, Figlin R, Gupta R, Escudier B, Group CRS An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372(9633):145–154. doi: 10.1016/S0140-6736(08)60697-2. https://doi.org/10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 100.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. https://doi.org/10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA, Kollmannsberger C, Heng DYC, Spratlin J, McHenry MB, Amin A. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.72.1985. https://doi.org/10.1200/jco.2016.72.1985. [DOI] [PMC free article] [PubMed]
- 102.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, Shepherd FA, Laurie SA, Geese WJ, Agrawal S, Young TC, Li X, Antonia SJ. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. https://doi.org/10.1016/s1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McDermott D, Haanen J, Chen TT, Lorigan P, O’Day S, Investigators MDX Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20) Ann Oncol. 2013;24(10):2694–2698. doi: 10.1093/annonc/mdt291. https://doi.org/10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 104.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, Dahut WL, Schlom J, Gulley JL. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. https://doi.org/10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, Eysmans C, Richards A, Schell MJ, Fisher KJ, Horak CE, Inzunza HD, Yu B, Martinez AJ, Younos I, Weber JS. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21(4):712–720. doi: 10.1158/1078-0432.CCR-14-2468. https://doi.org/10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, Kroeger J, Richards A, McCormick L, Moberg V, Cronin H, Zhao X, Schell M, Chen YA. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. 2016;4(4):345–353. doi: 10.1158/2326-6066.CIR-15-0193. https://doi.org/10.1158/2326-6066.CIR-15-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]


