Abstract
Introduction
The melanocortin system is a primordial and critical system for survival, involved in a wide variety of physiological functions. It includes melanocortin receptors (MCRs) and melanotropin ligands (MCLs). MCRs are important drug targets that can regulate several key physiological processes. Extensive efforts have been made to develop peptide and peptidomimetics targeting melanocortin receptors including MC1R, MC3R, MC4R and MC5R. Most research is focused on developing potent and selective melanotropins. However, developing bioavailable melanotropins remains challenging.
Areas covered
Herein, the authors summarize promising strategies for developing bioavailable MCLs by using cyclized N-methylated melanotropins, and using cyclotide and tetrapeptide as templates. They discuss their unique advantages in oral availability and targeting MCRs in the central nervous system or in peripheral tissues. Finally, they discuss the observed differences in thepharmacology of MCRs between in vitro and in vivo tests.
Expert opinion
N-methylated cyclized melanotropins have great potential to become bioavailable drugs targeting MCRs in the brain, while MCR-grafted cyclotides tend to target MCRs in peripheral tissue. A better understanding of the biased signaling process is a new challenge and opportunity for the future discovery of bioavailable MCLs.
Keywords: N-methylated peptide, cyclotide, bioavailable peptide, cyclized peptide
1. Introduction
The melanocortin receptors (MCRs) are a family of five G protein-coupled receptors that regulate several key physiological processes, and are thus important drug targets. MC1R is mainly expressed on skin melanocytes. Upon sun exposure, MC1R is activated, which leads to melanin production and skin pigmentation [1]. Skin pigmentation not only draws attention from the cosmetic industry, but is also considered an effective way to shield ultraviolet radiation from the sun for skin cancer prevention [2]. The MC1R is expressed on various cells of the immune system such as cytotoxic T cells and dendritic cells, where it exerts anti-inflammatory and immunomodulatory effects [3,4]. MC1R is overexpressed on most of the melanoma cells, which makes it the identification tag for melanoma imaging and therapy [5,6]. The MC2R is expressed in the adrenal cortex, where it regulates the production of glucocorticoids. The MC3R and MC4R are distributed in the hypothalamus and are both valuable drug targets for treating obesity. The MC3R knockout mice exhibit moderate obesity with increased weight and adipose mass, suggesting a role of the MC3R on regulating energy homeostasis [7]. The MC4R regulates body weight through controlling feeding behavior. Loss of function in MC4R leads to hyperphagia, while the MC4R activation leads to inhibition of food intake [7]. The MC4R agonist is also targeted for treating male and female sexual dysfunction [8]. Moreover, Alzheimer transgenic mice showed neurogenesis and cognitive recovery through the MC4R activation [9]. It has been suggested that the MC4R agonists can also treat autism and schizophrenia through activation of the central oxytocin system [10]. The MC5R is expressed at high level on sebaceous and exocrine glands where it regulates the secretion of sebum and a variety of exocrine [11]. The MC5R is also required for antigen-presenting cells to modulate immune response [12] (Table 1).
Table 1.
The major expression sites and pharmacological interests of melanocortin receptors.
| Receptor | Site of expression | Pharmacological interests |
|---|---|---|
| MC1R | Skin melanocytes, immune system | Cosmetic purpose, skin cancer prevention, anti-inflammation, melanoma |
| MC2R | Adrenal cortex | Glucose metabolism, anti-inflammation |
| MC3R | Hypothalamus | Obesity |
| MC4R | Hypothalamus | Obesity, sex dysfunction, Alzheimer’s disease, autism, schizophrenia |
| MC5R | Exocrine glands, immune system | Immune response modulation |
Melanotropin ligand (MCL) development targeting the melanocortin system is mainly based on the peptide sequence of endogenous agonists called melanocyte stimulating hormones (MSHs). There are three MSH peptides: α-MSH, β-MSH, and γ-MSH. They can activate MC1R, MC3R, MC4R, and MC5R to trigger cAMP production without much selectivity. The MC2R is a special member of MCRs that can only be activated by adrenocorticotropic hormone [13], and thus would not be affected by peptide drugs based on MSHs. NDP-α-MSH (MT-I), the first generation of peptide drug targeting the melanocortin system, was developed using α-MSH as a template. To improve stability, Met4 was replaced with Nle4 to avoid oxidation. Phe7 was mutated to D-Phe7, which was found to improve both potency and stability [14]. NDP-α-MSH can effectively induce skin pigmentation and is approved in Europe and Australia to treat erythropoietic protoporphyria [15]. The core sequence His-Phe-Arg-Trp, which is shared by all MSHs, was determined as the minimal active sequence for MCR activation [16]. The Melanotan II (MT-II), the second-generation peptide drug, was developed by using lactam bridge to cyclize the His-D-Phe-Arg-Trp pharmacophore [17] (Table 2).
Table 2.
Development of peptide drugs targeting melanocortin receptors based on their endogenous agonists.
| Sequence | |
|---|---|
| Endogenous agonists | |
| α-MSH | Ac-Ser1-Tyr2-Ser3-Met4-Glu5-His6-Phe7-Arg8-Trp9-Gly10- Lys11-Pro12-Val13-NH2 |
| β-MSH | Ala1-Glu2-Lys3-Lys4-Asp5-Glu6-Gly7-Pro8-Tyr9-Arg10- Met11-Glu12-His13-Phe14-Arg15-Trp16-Gly17-Ser18- Pro19-Pro20-Lys21-Asp22-OH |
| γ-MSH | Tyr1-Val2-Met3-Gly4-His5-Phe6-Arg7-Trp8-Asp9-Arg10- Phe11-Gly12-OH |
| Peptide drug | |
| NDP-α-MSH (MT-I) | Ac-Ser1-Tyr2-Ser3-Nle4-Glu5-His6-D-Phe7-Arg8-Trp9- Gly10-Lys11-Pro12-Val13-NH2 |
| Melanotan II (MT-II) | Ac-Nle4-cyclo[Asp5-His6-D-Phe7-Arg8-Trp9-Lys10]-NH2 |
| SHU9119 | Ac-Nle4-cyclo[Asp5-His6-D-Nal(2′)7-Arg8-Trp9-Lys10]-NH2 |
| N(Me)MT-II /N(Me) SHU9119 | Ac-Nle4-cyclo[Asp5-(NMe)His6-D-Phe/D-Nal(2′)7-(NMe) Arg8-(NMe)Trp9-Lys10]-NH2 |
During the last decade, extensive efforts have been made to develop peptide drugs targeting the melanocortin system. Selectivity has been mainly addressed to modulate certain biological process without interfering with others. However, only a few of them went to the market or clinical trials. The major barrier is delivery. First, it is critical to have the right design strategy that can have the peptide drug delivered to certain region of the body where the desired biological process is regulated. The MC3R and MC4R are mostly expressed in the central nervous system, and thus need to be modulated by peptides with the ability to penetrate the blood–brain barrier. The MC1R and MC5R are mainly expressed in the peripheral tissue, so that it is safer to modulate them with peptides that cannot get into the brain. Second, another major barrier for peptide drugs to be accepted by the market is the oral bioavailability. Since most peptides are easily degraded in the gut, they are mostly administrated by injections, which is strongly disfavored by patients. In this review article, we will discuss using cyclotides, cyclic peptides, and constrained tetrapeptides as melanotropin ligands. We will discuss their unique advantages for targeting certain subtype of MCRs. The oral bioavailability is also a major focus of our review. Moreover, we will discuss the emerging field of studying biased signaling on MCRs and how it will affect the drug design process.
2. Cyclized melanotropins for targeting MCRs in central nervous system
After the discovery of NDP-α-MSH, a conjecture was made that the D-Phe7 would stabilize a reverse-turn conformation through L/D configuration, which could be responsible for the improved stability and potency on MCRs. To further enforce the reverse-turn like structure, various cyclized peptides including MT-II/SHU9119 were developed. SHU9119 is the first antagonist developed. In the previous decade, numerous linkers and constrained amino acids have been applied to improve the potency and selectivity of the cyclized melanotropins. Our recent review article summarized the design and structure activity relationship for cyclized peptides targeting MCRs [18–24].
Compared to MT-I, which has limited ability to pass through the blood–brain barrier [25], cyclized peptides have their unique advantage of passing through the blood–brain barrier and exert direct actions in the central nervous system. In addition, cyclized peptides exhibit increased stability. The pro-erectile function of the cyclic peptide of MT-II was found to be mediated by MCRs in the brain and spinal cord [26]. More direct evidence found that 8 h post-intravenous administration of 10 mg/kg backbone cyclized peptide BL3020-1 in mice, a brain concentration of 107 ng/mg, is able to be achieved [27]. Moreover, BL3020-1 also showed oral bioavailability due to increased membrane permeability [27].
Thus, cyclized peptides are ideal for targeting MC3R and MC4R that are mainly expressed in the brain. Table 3 summarized the studies of biological stability and bioavailability of melanotropin peptides from endogenous α-MSH to different generations of synthetic melanotropins which have been widely used for research and clinical trials.
Table 3.
Pharmacology and bioavailability of peptide drugs targeting melanocortin receptors.
| Peptide drug | Pharmacology and Bioavailability |
|---|---|
| α-MSH | Endogenous agonist. Biologically unstable; T½– minutes |
| MT-I | Universal agonist. Biologically more stable; T½= 2–4 h |
| Does not cross the blood–brain barrier (BBB) | |
| MT-II | Universal agonist. Biologically stable; T½= 1–2 days |
| Crosses BBB | |
| SHU9119 | Antagonist – MC3R and MC4R, agonist – MC1R and MC5R |
| Biologically stable | |
| N(Me)MT-II/N(Me) SHU9119 | Selective agonist/antagonist of MC1R, MC3R, MC4R, MC5R [28,29] |
| Biological stable, T½ >1 week | |
| Cell permeable | |
| Cyclotides (SFTI, kalata B1) | Thermally stable/orally available |
2.1. N-methylation of cyclized melanotropins
Lack of stability and bioavailability of melanotropin ligands for the MCR subtypes is still the most difficult hurdle for their application in medicine. To ameliorate the bioavailable selective agonists and antagonists for MCRs, conformational modulation in peptide backbone that is imparted by N-methyl steric constraints, mono and multiply N-methylated analogs have been applied. Recent studies suggest that N-methylation on cyclized peptides can improve the oral bioavailability [30]. The increased intestinal permeability was shown to be related to the existence of cis-peptide bonds in cyclized peptides, which is stabilized by N-methylation [31]. Moreover, N-methylation can enhance potency and selectivity to receptors through introducing conformational constrains and prevention of hydrogen bonds [32]. As an example, one work from the Kessler group, the study on cyclic N-methylated somatostatin analogs related to the Veber–Hirschmann peptide generated a library of 30 compounds with varying degrees of methylation of the secondary amides contained in the starting macrocycle [33]. Extensive in vitro evaluation showed that specific methylation of D-Trp8, Lys9, and Phe11 gave rise to a large enhancement in membrane permeability in a Caco-2 cell monolayer model, while other methylated derivatives gave no enhancement. This compound also displayed reasonable oral bioavailability in rat. The optimized peptide with regard to cell permeability was also the peptide most efficiently cyclized using standard peptide coupling conditions. The authors suggested that the linear precursor peptide exhibits a dynamic structure in solution while undergoing some preorganization to bring the N and C termini into close proximity to improve the efficiency of the cyclization reaction. The optimized macrocycle showed no degradation after extensive incubation in rat serum and was stable in simulated gut media. Progression of the lead analog to in vivo studies showed an oral bioavailability of F = 9% and a volume of distribution (VD) of 3.7 L/kg, with an elimination half-life of 74 min. The relatively high oral bioavailability, and in particular the ability to cross the gut wall, was not fully explained and demonstrates the complexity of the pharmacokinetic profile. The high VD suggests that the compound can distribute effectively into tissues and move out of the plasma compartment [33].
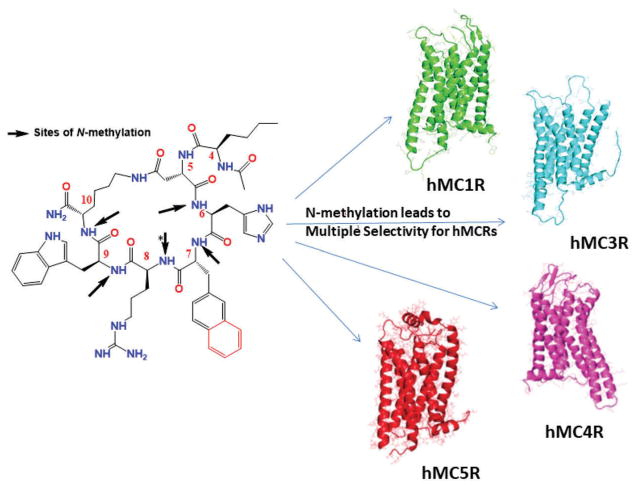
The latest approach of N-methylated MSH studies was investigated on cyclized MTII/SH9119 templates Ac-Nle4-c [Asp5, D-Phe7/Nal(2′)7, Lys10]-α-MSH(4-10)-NH2 [28,29]. Sixty-four peptides from mono and multiply N-methylated analogs were investigated, and it was found that the activity and selectivity profile induced by N-methylation is very different for the Ac-Nle4-c[Asp5, D-Nal(2′)7, Lys10]-NH2 compared to a similar approach in Ac-Nle-c[Asp5, D-Phe7, Lys10]-α-MSH(4-10) NH2. This is striking as both stem peptides differ only in the exchange of one amino acid (D-Nal(2′)7 instead of D-Phe7 in Ac-Nle-c[Asp5, D-Phe7, Lys10]-α-MSH(4-10)NH2. Both of the systematic N-methylation of Ac-Nle4-c[Asp5, D-Phe7/Nal(2′)7, Lys10]-α-MSH(4-10)NH2 led to the super potent selective agonist of the hMC1R based on binding affinities [28,29]. One of the peptides from the Ac-Nle-c[Asp5, D-Phe7, Lys10]-α-MSH(4-10)NH2 series, (Ac-Nle-c[Asp-(NMe)-His-D-Phe/D-Nal(2′)-(NMe) Arg-(NMe)Trp-Lys]-α-MSH(4-10)NH2), only activates MC1R (EC50 = 13 nM) but no other MCR subtypes [28]. However, the systematic N-methylation of Ac-Nle4-c[Asp5, D-Nal(2′)7, Lys10]-NH2 led to multiple selective agonists and antagonist of the MCRs (Figure 1). Apart from this, the newly discovered peptide Ac-Nle-c[Asp-(NMe)-His-D-Nal(2′)-Arg-(NMe)Trp-(NMe) Lys]-α-MSH(4-10)NH2 is a universal antagonist of the hMCR system, which could be important to modulate the endogenous agonist MSH in the melanocortin system [29]. However, N-methylation may also reduce the biological activity of MCLs depending on the sequence of the template peptide. The research with N-methylation on the BL3020-1 template showed reduced biological activity and selectivity on MCRs [34]. In this regard, the ring size also is critical important to the potency and selectivity for the design of selective and potent melanotropins. Our latest studies demonstrated that aromatic linker or bulky linker could play an important role for the hMCRs selectivity. Due to the ability to penetrate the blood–brain barrier, cyclized peptides are especially important for targeting the MC3R and the MC4R, which are mainly expressed in the brain. Future work is still required to develop cyclized peptides with improved selectivity and oral bioavailability. N-methylation remains to be a promising method to enhance receptor selectivity and membrane penetration of cyclized peptides.
Figure 1.
Multiple N-methylation of N(Me)MT-II/N(Me)SHU9119 led to multiple selective hMCRs ligands.
3. Using cyclotides template
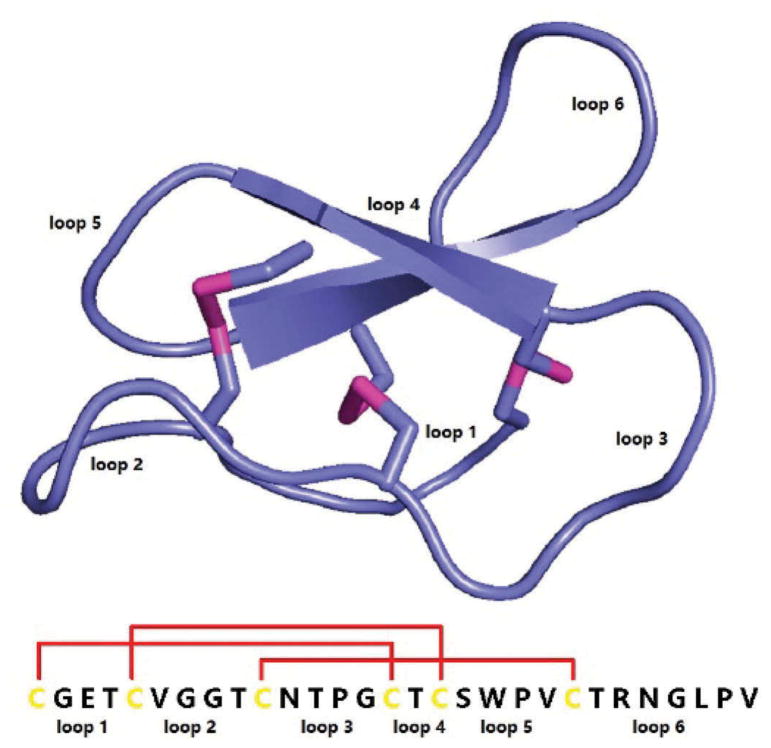
Cyclotides are small cyclic peptides with intramolecular disulfide bonds knots. The first cyclotide was isolated after the observation that African women use tea from the leaves of the plant Oldenlandia affinis to facilitate childbirth [35]. The peptide, named kalata B1, was further characterized as head-to-tail cyclized peptide with three pairs of disulfide bonds (Figure 2). Kalata B1 was found to have exceptional stability to thermal, chemical, or enzymatic degradation [37]. Moreover, an in vivo study confirmed the oral bioavailability of kalata B1-derived peptides [38]. Many peptide epitopes have been grafted into cyclotide templates in an attempt to improve stability and oral availability of peptide drugs [39].
Figure 2.
Structure and sequence of kalata B1 (PDB ID: 1NB1[36]).
In a recent study, the melanotropin pharmacophore His-Phe/D-Phe-Arg-Trp was grafted into the loop 6 of kalata B1 scaffold [40]. The most potent grafted peptide, kalata B1 (GHFRWG; 23–28), was found to selectively bind to and activate MC4R. The Ki for MC4R was 29 nM, which exhibited 107-fold and 314-fold selectivity to MC4R over MC1R and MC5R, respectively, with no detectable interactions with MC3R. In functional assay, the EC50 of the most potent peptide was only 580 nM, which is 150-fold less potent than α-MSH. The lower receptor potency could be due to nonideal presentation of the pharmacophore in the kalata B1 template.
Cyclotide templates can provide stability and oral bioavailability to peptide drugs targeting the MCRs, which are extremely valuable. Moreover, a pharmacokinetics study showed that the cyclotide Momordica cochinchinensis trypsin inhibitor-II has no brain uptake [41]. Thus, cyclotide templates are ideal for developing MC1R/MC5R ligands that only hit target receptors in peripheral tissues without interfering with brain function. Future directions will involve optimizing the cyclotide scaffold for better pharmacophore presentation as well as screening for improved pharmacophore sequence that can provide further selectivity.
4. Constrained tetrapeptides
Since the minimal active sequence His-Phe-Arg-Trp was discovered, extensive structure–activity studies have been carried out on tetrapeptides. Compared to other design strategies, tetrapeptides have their unique advantages. The synthesis of tetrapeptides is relatively easy, and thus would have low manufacturing costs compared to cyclized peptides or cyclotides. Due to the short sequence and a lack of enzymatic cleavage sites, modified tetrapeptides could be relatively stable in serum. Some short peptides such as tripeptides and tetrapeptides are also shown to be orally available [42–44]. Oral administration of the synthetic dermorphin tetrapeptide showed almost the same antinociceptive potency as morphine, which suggests that the tetrapeptide can pass through the blood–brain barrier and modulate opioid receptors in the brain [44]. Nevertheless, the lack of conformational constrains of tetrapeptides often leads to low potency to MCR modulation. The tetrapeptide sequence Ac-His-D-Phe-Arg-Trp-NH2 is 32–2000 times less potent than NDP-α-MSH in activation of different MCR subtypes from mice [45].
One way to increase potency and selectivity of tetrapeptides is to optimize the amino acid side chain groups to better fit into the binding pocket. Numerous research was performed in analyzing MCR activation by tetrapeptides that have substitutions at each position of the His-D-Phe-Arg-Trp template [46–49]. His position was found to tolerate substitutions from aromatic residues such as Phe and Trp, whereas these substitutions lead to selectivity toward the MC4R [46]. The D configuration at the D-Phe position was found to be critically important for potency [47]. By introducing a bulky substitution such as D-Nal(2′) or (pI) D-Phe, antagonist activity was observed for the MC3R and/or MC4R[47]. Most substitutions at the Arg position lead to significant loss in potency [48]. Trp can be replaced by other aromatic residues such as Nal(2′) and Phe [49].
Another strategy to improve potency and selectivity is to introduce local constraints to the tetrapeptide template to enforce the turn configuration. To generate the MC4R selective tetrapeptides, the side chain constrained amino acid Tic was used to replace His [50]. The tetrapeptide Tic-(pOMe) D-Phe-Arg-Trp-NH2 was found to be selective partial agonist at MC4R with EC50 of 17 nM. Further in vivo study showed 24% reduction in food intake with rats intracerebroventricularly administrated with this constrained tetrapeptide. Another research used the constrained amino acids Aia, Aba, and Ata to replace His, which led to discovery of three MC4R selective agonists and one MC5R selective antagonist [51]. Two of the MC4R selective agonists were able to reach EC50 of 0.3 nM, which is even more potent than MT-II. Introduction of Tic to the Trp position was also shown to improve selectivity of tetrapeptides for MC1R [49].
Tetrapeptides will continue to be a hot area of research, not only because of their unique small molecule-like properties, but also because it can provide valuable information that can be further used in the cyclized peptide and cyclotide design. Future work is required to further improve potency and selectivity for MCRs as well as to test the oral bioavailability and blood–brain barrier penetration of tetrapeptides.
5. Potential biased signaling events on MCRs and their influence in drug design process
Currently, all drug screening efforts for MCR agonists are based on an assumption that the only physiologically relevant signaling pathway following MCR activation is the activation of GSα, which further leads to cAMP production. However, some animal studies and clinical trial results clearly contradict with this assumption. For example, both feeding behavior and erectile function are controlled by MC4R in the brain. Clinical trial data confirmed that MT-II can cause both appetite loss and spontaneous penile erection [52]. However, the MC4R selective small molecule THIQ was shown to only increase erectile activity [53] but has no effect on food intake [54]. This evidence suggests that the MC4R may regulate feeding behavior and erectile function in different manner. A plausible explanation was provided with an in vivo study suggesting that suppression of food intake is mediated by Gqα and G11α, which further activate PLC/Ca2+ signaling pathway [55]. MT-II was shown to still inhibit food intake in mice lacking GSα in the paraventricular nucleus of the hypothalamus, whereas the effect was lost in mice deficient of Gqα and G11α [55]. Similar biased signaling event was suggested for MC1R. A small molecule drug AP1189 was shown to cause ERK1/2 phosphorylation and intracellular Ca2+ mobilization but not cAMP production through MC1R and MC3R [56]. While AP1189 was found to promote anti-inflammatory responses through MC1R and MC3R, it did not show any skin pigmentation effect.
The major challenge with current studies on biased signaling of MCRs is that the results are highly dependent on the cellular context. NDP-α-MSH was shown to activate the Gqα/PLC/Ca2+ signaling pathway in immortalized GT1-1 cells [57]. However, the same effect cannot be observed in the highly related cell line GT1-7 [58]. In an in vivo study, MT-II-induced activation of ERK1/2 was found to be abolished by the cAMP inhibitor Rp-cAMPs [59]. However, NDP-α-MSH-induced ERK1/ 2 activation was shown to be insensitive to PKA inhibitor but sensitive to PI3K blockage in the MC4R overexpressing CHO-K1 cells [60]. These discrepancies require more research and careful examination to come up with plausible model for potential biased signaling events on MCRs. Moreover, to establish a valid assay system that distinguishes different signaling pathways remains challenging.
Potential biased signaling can bring both challenge and opportunity to drug development targeting MCRs. Great efforts are required to eventually elucidate the signaling pathway that regulates each physiological process. Proper assay systems need to be established to screen for compounds that can regulate certain signaling pathway through certain MCR subtype. New structure–activity relationships need to be built. Nevertheless, all these efforts will bring us the opportunity to distinguish and selectively regulate those physiological processes that are controlled by the same MCR subtype.
6. Conclusion
MCRs are valuable drug targets for a wide variety of diseases from melanoma to obesity. During the last decades, peptide-and peptidomimetics-based drug design and discovery have been advanced based on selectivity and potency. However, few of melanotropins are on the market due to the main barriers of bioavailability or oral availability. This review introduced several useful strategies to develop bioavailable melanotropins. Cyclized peptides have the ability to pass the blood–brain barrier, and thus are suitable for targeting MC3R and MC4R in the brain. Increasing lipophilicity of cyclized peptides with N-methylations can effectively improve selectivity and oral availability. Cyclotides have exceptional oral availability and are suitable to target MC1R and MC5R in peripheral tissues due to the inability to pass through the blood–brain barrier. Tetrapeptide derivatives have favorable druggability features and have promising membrane penetration properties upon further modifications. More importantly than these design strategies, we want to obtain a complete understanding of the biased signaling of MCRs. Since previous drug screening efforts have only considered cAMP as the consequence of MCR activation, new structure–activity relationship studies may be required to further our understanding on the biased signaling of melanocortin system.
7. Expert opinion
Despite the fact that MCRs regulate certain physiological properties that are extremely attractive to pharmaceutical companies, the development of peptide drugs targeting MCRs is severely hindered by the lack of oral bioavailability. The recent approaches using N-methylated cyclized peptides, cyclotides, and constrained tetrapeptides to improve the bioavailability are promising. However, to reach the ultimate goal of providing selective and orally available peptide agonists and antagonists for each MCR, more efforts with each design strategy are required.
Among these design strategies, N-methylated cyclized peptides have the potential to pass the blood–brain barrier and are thus important to target MCRs in the brain. It is worth to mention that these compounds will also be available to MCRs in the peripheral tissues. Thus, selectivity toward certain receptor type is required to eliminate side effects. The future direction for cyclized peptide development is to further enhance selectivity and membrane permeability. N-methylation appears to be a good solution to improve both properties; for tetrapeptide development, it will be critical to enhance selectivity, potency as well as to validate the oral bioavailability and blood–brain barrier penetration.
Since tetrapeptides have similar size and properties as small molecule drugs, many pharmacological rules such as Lipinski’s rule of five may also apply for tetrapeptide design, which could provide valuable instructions for the drug design process. Apart from optimizations on side chain groups, further improvements may also happen on the backbone of tetrapeptide to make azapeptides or peptoids, which would further adapt small molecule properties. Moreover, further research is needed to understand the oral availability and blood–brain barrier penetration of currently developed tetrapeptides targeting MCRs.
Due to the oral availability and thermostability, cyclotide grafted drugs keep the top interests for the development of nutraceutical drug. Different cyclotide templates will be tested to find the best template for melanocortin drug development. However, the major disadvantage for cyclotides is the relatively high synthetic costs. Some key technical hurdles to the development of cycloid-based therapeutics will need to be addressed in the near future. First, the synthesis and purification will need to be optimized to lower the cost. This might be achieved by either chemical synthesis or molecular biology techniques (recombinant peptide expression). Second, modifications will need to be revised for enhancing membrane permeability without compromising biologically active peptide conformations.
Regardless of the importance of oral bioavailability, the downstream regulation of the drug candidate is even more important. Numerous studies demonstrate that in vivo studies do NOT always match the pharmacology data, which means that the biological theory needs to be improved. The failure of THIQ to reduce food intake always reminds us that without a precise biological model, tremendous efforts in drug design and screening process could be in vain. Even though emerging evidence suggests the coexistence of Gqα/PLC/Ca2+ signaling pathway, Gsα/AC/cAMP signaling pathway, and ERK1/2 pathway downstream of MCR activation, it still requires more research to elucidate the relationship between these pathways and come up with valid assay systems to screen for biased ligands. However, the biased signaling event on MCRs would also provide opportunities to differentially regulate physiological processes that are regulated by the same receptor, such as food intake and erectile dysfunction. New structure–activity relationship studies will need to be done to provide peptide drugs that can target certain signaling pathway of different MCRs. Overall, these studies highlight the fact that the ability of chemists and molecular pharmacologists to design and synthesize peptides to improve structure–bioavailability will be extremely valuable in developing the next generation of peptide-based drug leads.
Article highlights.
Melanocortin receptors are drug targets that regulate important physiological processes such as skin pigmentation, energy homeostasis, feeding behavior, sex function and neuroprotection.
Even though the endogenous ligands are peptides, the development of peptide drugs targeting MC1R, MC3R, MC4R and MC5R is hindered by the lack of oral bioavailability.
Cyclized melanotropin is a useful strategy for developing MCRs ligands due to their ability to penetrate the blood-brain-barrier. A promising way to improve oral bioavailability for cyclized peptide is to introduce N-methylation.
Cyclotide templates are ideal for developing MCRs ligands due to oral bioavailability and thermos-stability.
Constrained tetrapeptides have great potential to have membrane penetration that can result in oral bioavailability and blood-brain-barrier penetration, even though further studies are required to provide improved potency and selectivity.
This box summarizes key points contained in the article.
Acknowledgments
Funding
This work is supported by National Institutes of Health grant GM108040.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445(7130):843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 2.Pawelek JM. Approaches to increasing skin melanin with MSH analogs and synthetic melanins. Pigment Cell Res. 2001;14(3):155–160. doi: 10.1034/j.1600-0749.2001.140304.x. [DOI] [PubMed] [Google Scholar]
- 3.Loser K, Brzoska T, Oji V, et al. The neuropeptide alpha-melanocyte-stimulating hormone is critically involved in the development of cytotoxic CD8+ T cells in mice and humans. PloS One. 2010;5(2):e8958. doi: 10.1371/journal.pone.0008958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auriemma M, Brzoska T, Klenner L, et al. α-MSH-stimulated tolerogenic dendritic cells induce functional regulatory T cells and ameliorate ongoing skin inflammation. J Invest Dermatol. 2012;132(7):1814–1824. doi: 10.1038/jid.2012.59. [DOI] [PubMed] [Google Scholar]
- 5.Ren G, Liu S, Liu H, et al. Radiofluorinated rhenium cyclized α-MSH analogues for PET imaging of melanocortin receptor 1. Bioconjug Chem. 2010;21(12):2355–2360. doi: 10.1021/bc100391a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai M, Liu Z, Qu H, et al. Utilize conjugated melanotropins for the earlier diagnosis and treatment of melanoma. Eur J Pharmacol. 2011;660(1):188–193. doi: 10.1016/j.ejphar.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 8.King SH, Mayorov AV, Balse-Srinivasan P, et al. Melanocortin receptors, melanotropic peptides and penile erection. Curr Top Med Chem. 2007;7(11):1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 9.Giuliani D, Neri L, Canalini F, et al. NDP-α-MSH induces intense neurogenesis and cognitive recovery in Alzheimer transgenic mice through activation of melanocortin MC4 receptors. Mol Cell Neurosci. 2015;67:13–21. doi: 10.1016/j.mcn.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Modi ME, Inoue K, Barrett CE, et al. Melanocortin receptor agonists facilitate oxytocin-dependent partner preference formation in the prairie vole. Neuropsychopharmacol. 2015;40(8):1856–1865. doi: 10.1038/npp.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Kelly MA, Opitz-Araya X, et al. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91(6):789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 12.Lee DJ, Taylor AW. Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. J Immunol. 2013;191(8):4103–4111. doi: 10.4049/jimmunol.1300182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridmanis D, Roga A, Klovins J. ACTH receptor (MC2R) specificity: what do we know about underlying molecular mechanisms? Front Endocrinol (Lausanne) 2017;8:13. doi: 10.3389/fendo.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer TK, Sanfilippo PJ, Hruby VJ, Hadley ME, et al. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A. 1980;77(10):5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373(1):48–59. doi: 10.1056/NEJMoa1411481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hruby VJ, Wilkes BC, Hadley ME, et al. Alpha-melanotropin: the minimal active sequence in the frog skin bioassay. J Med Chem. 1987;30(11):2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 17.Al-Obeidi F, Castrucci AM, Hadley ME, et al. Potent and prolonged acting cyclic lactam analogues of alpha-melanotropin: design based on molecular dynamics. J Med Chem. 1989;32(12):2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 18.Cai M, Hruby VJ. Design of cyclized selective melanotropins. Biopolymers. 2016;106:876–883. doi: 10.1002/bip.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hruby VJ, Cai MY, Nyberg J, et al. Approaches to the rational design of selective melanocortin receptor antagonists. Expert Opin Drug Dis. 2011;6(5):543–557. doi: 10.1517/17460441.2011.565743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai M, Nyberg J, Hruby VJ. Melanotropins as drugs for the treatment of obesity and other feeding disorders: potential and problems. Curr Top Med Chem. 2009;9(6):554–563. doi: 10.2174/156802609788897817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai M, Hruby VJ. The melanocortin receptor system: a target for multiple degenerative diseases. Curr Protein Pept Sci. 2016;17(5):488–496. doi: 10.2174/1389203717666160226145330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hruby VJ, Cai M. Design of peptide and peptidomimetic ligands with novel pharmacological activity profiles. Annu Rev Pharmacol Toxicol. 2013;53:557–580. doi: 10.1146/annurev-pharmtox-010510-100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hruby VJ, Cai M, Cain JP, et al. Design, synthesis and biological evaluation of ligands selective for the melanocortin-3 receptor. Curr Top Med Chem. 2007;7(11):1107–1119. doi: 10.2174/156802607780906645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hruby VJ. Designing peptide receptor agonists and antagonists. Nat Rev Drug Discov. 2002;1(11):847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 25.Vaidyanathan G, Zalutsky MR. Fluorine-18-labeled [Nle4,D-Phe7]-alpha-MSH, an alpha-melanocyte stimulating hormone analogue. Nucl Med Biol. 1997;24(2):171–178. doi: 10.1016/s0969-8051(96)00211-9. [DOI] [PubMed] [Google Scholar]
- 26.Wessells H, Hruby VJ, Hackett J, et al. AC-NLE-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 induces penile erection via brain and spinal melanocortin receptors. Neuroscience. 2003;118(3):755–762. doi: 10.1016/s0306-4522(02)00866-7. [DOI] [PubMed] [Google Scholar]
- 27.Hess S, Linde Y, Ovadia O, et al. Backbone cyclic peptidomimetic melanocortin-4 receptor agonist as a novel orally administrated drug lead for treating obesity. J Med Chem. 2008;51(4):1026–1034. doi: 10.1021/jm701093y. [DOI] [PubMed] [Google Scholar]
- 28••.Doedens L, Opperer F, Cai M, et al. Multiple N-methylation of MT-II backbone amide bonds leads to melanocortin receptor subtype hMC1R selectivity: pharmacological and conformational studies. J Am Chem Soc. 2010;132(23):8115–8128. doi: 10.1021/ja101428m. This manuscript provides an example of N-methylation on cyclized melanotropin with enhanced selectivity toward MC1R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai M, Marelli UK, Bao J, et al. Systematic backbone conformational constraints on a cyclic melanotropin ligand leads to highly selective ligands for multiple melanocortin receptors. J Med Chem. 2015;58(16):6359–6367. doi: 10.1021/acs.jmedchem.5b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Beck JG, Chatterjee J, Laufer B, et al. Intestinal permeability of cyclic peptides: common key backbone motifs identified. J Am Chem Soc. 2012;134(29):12125–12133. doi: 10.1021/ja303200d. This manuscript reveals key backbone motifs for cyclized peptides to be orally available. [DOI] [PubMed] [Google Scholar]
- 31.Marelli UK, Ovadia O, Frank AO, et al. Cis-peptide bonds: a key for intestinal permeability of peptides? Chemistry. 2015;21(43):15148–15152. doi: 10.1002/chem.201501600. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee J, Rechenmacher F, Kessler H. N-methylation of peptides and proteins: an important element for modulating biological functions. Angew Chem Int Ed Engl. 2013;52(1):254–269. doi: 10.1002/anie.201205674. [DOI] [PubMed] [Google Scholar]
- 33.Biron E, Chatterjee J, Ovadia O, et al. Improving oral bioavailability of peptides by multiple N-methylation: somatostatin analogues. Angewandte Chemie. 2008;47(14):2595–2599. doi: 10.1002/anie.200705797. [DOI] [PubMed] [Google Scholar]
- 34.Linde Y, Ovadia O, Safrai E, et al. Structure–activity relationship and metabolic stability studies of backbone cyclization and N-methylation of melanocortin peptides. Biopolymers. 2008;90(5):671–682. doi: 10.1002/bip.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gran L, Sandberg F, Sletten K. Oldenlandia affinis (R&S) DC. A plant containing uteroactive peptides used in African traditional medicine. J Ethnopharmacol. 2000;70(3):197–203. doi: 10.1016/s0378-8741(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 36.Rosengren KJ, Daly NL, Plan MR, et al. Twists, knots, and rings in proteins. Structural definition of the cyclotide framework. J Biol Chem. 2003;278(10):8606–8616. doi: 10.1074/jbc.M211147200. [DOI] [PubMed] [Google Scholar]
- 37.Colgrave ML, Craik DJ. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry. 2004;43(20):5965–5975. doi: 10.1021/bi049711q. [DOI] [PubMed] [Google Scholar]
- 38.Thell K, Hellinger R, Sahin E, et al. Oral activity of a nature-derived cyclic peptide for the treatment of multiple sclerosis. Proc Natl Acad Sci U S A. 2016;113(15):3960–3965. doi: 10.1073/pnas.1519960113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Poth AG, Chan LY, Craik DJ. Cyclotides as grafting frameworks for protein engineering and drug design applications. Biopolymers. 2013;100(5):480–491. doi: 10.1002/bip.22284. This manuscript explains how to graft peptide pharmacophores into cyclotide templates. [DOI] [PubMed] [Google Scholar]
- 40•.Eliasen R, Daly NL, Wulff BS, et al. Design, synthesis, structural and functional characterization of novel melanocortin agonists based on the cyclotide kalata B1. J Biol Chem. 2012;287(48):40493–40501. doi: 10.1074/jbc.M112.395442. This manuscript provides an example of grafting melanotropin pharmacophore into cyclotide template for enhanced oral availability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CK, Stalmans S, de Spiegeleer B, et al. Biodistribution of the cyclotide MCoTI-II, a cyclic disulfide-rich peptide drug scaffold. J Pept Sci. 2016;22(5):305–310. doi: 10.1002/psc.2862. [DOI] [PubMed] [Google Scholar]
- 42.Tang L, Sakai Y, Ueda Y, et al. Effects of oral administration of tripeptides derived from type I collagen (collagen tripeptide) on atherosclerosis development in hypercholesterolemic rabbits. J Biosci Bioeng. 2015;119(5):558–563. doi: 10.1016/j.jbiosc.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Lin H, Tullman R, et al. Absorption and disposition of a tripeptoid and a tetrapeptide in the rat. Biopharm Drug Dispos. 1999;20(2):69–75. doi: 10.1002/(sici)1099-081x(199903)20:2<69::aid-bdd153>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Chaki K, Sakurada S, Sakurada T, et al. Comparison of the anti-nociceptive effects of new [D-Arg2]-dermorphin tetrapeptide analogs and morphine in mice. Pharmacol Biochem Behav. 1988;31(2):439–444. doi: 10.1016/0091-3057(88)90371-1. [DOI] [PubMed] [Google Scholar]
- 45.Haskell-Luevano C, Holder JR, Monck EK, et al. Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. J Med Chem. 2001;44(13):2247–2252. doi: 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- 46.Holder JR, Bauzo RM, Xiang Z, et al. Structure–activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH(2) at the mouse melanocortin receptors. 1. Modifications at the His position. J Med Chem. 2002;45(13):2801–2810. doi: 10.1021/jm0104872. [DOI] [PubMed] [Google Scholar]
- 47.Holder JR, Bauzo RM, Xiang Z, et al. Structure–activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH(2) at the mouse melanocortin receptors: part 2 modifications at the Phe position. J Med Chem. 2002;45(14):3073–3081. doi: 10.1021/jm010524p. [DOI] [PubMed] [Google Scholar]
- 48.Holder JR, Xiang Z, Bauzo RM, et al. Structure–activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. Part 3: modifications at the Arg position. Peptides. 2003;24(1):73–82. doi: 10.1016/s0196-9781(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 49.Holder JR, Xiang Z, Bauzo RM, et al. Structure–activity relationships of the melanocortin tetrapeptide Ac-His-D-Phe-Arg-Trp-NH2 at the mouse melanocortin receptors. 4. Modifications at the Trp position. J Med Chem. 2002;45(26):5736–5744. doi: 10.1021/jm020296e. [DOI] [PubMed] [Google Scholar]
- 50.Ye Z, MacNeil T, Weinberg DH, et al. Structure–activity relationship of linear tetrapeptides Tic-DPhe-Arg-Trp-NH2 at the human melanocortin-4 receptor and effects on feeding behaviors in rat. Peptides. 2005;26(10):2017–2025. doi: 10.1016/j.peptides.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 51••.Van Der Poorten O, Fehér K, Buysse K, et al. Azepinone-containing tetrapeptide analogues of melanotropin lead to selective hMC4R agonists and hMC5R antagonist. ACS Med Chem Lett. 2015;6(2):192–197. doi: 10.1021/ml500436s. This manuscript provides an example of structurally constraining the tetrapeptide pharmacophore to reach subnanomolar efficacy for melanocortin receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorr RT, Lines R, Levine N, et al. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996;58(20):1777–1784. doi: 10.1016/0024-3205(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 53.Martin WJ, McGowan E, Cashen DE, et al. Activation of melanocortin MC(4) receptors increases erectile activity in rats ex copula. Eur J Pharmacol. 2002;454(1):71–79. doi: 10.1016/s0014-2999(02)02479-2. [DOI] [PubMed] [Google Scholar]
- 54.Muceniece R, Zvejniece L, Vilskersts R, et al. Functional evaluation of THIQ, a melanocortin 4 receptor agonist, in models of food intake and inflammation. Basic Clin Pharmacol Toxicol. 2007;101(6):416–420. doi: 10.1111/j.1742-7843.2007.00133.x. [DOI] [PubMed] [Google Scholar]
- 55••.Li YQ, Shrestha Y, Pandey M, et al. G(q/11)α and G(s)α mediate distinct physiological responses to central melanocortins. J Clin Invest. 2016;126(1):40–49. doi: 10.1172/JCI76348. This manuscript provides evidence that strongly supports the existence of biased signaling events on melanocortin receptors with physiological significance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montero-Melendez T, Gobbetti T, Cooray SN, et al. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J Immunol. 2015;194(7):3381–3388. doi: 10.4049/jimmunol.1402645. [DOI] [PubMed] [Google Scholar]
- 57.Newman EA, Chai BX, Zhang W, Mulholland MW, et al. Activation of the melanocortin-4 receptor mobilizes intracellular free calcium in immortalized hypothalamic neurons. J Surg Res. 2006;132(2):201–207. doi: 10.1016/j.jss.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Buch TR, Heling D, Damm E, Breit A, et al. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. J Biol Chem. 2009;284(39):26411–26420. doi: 10.1074/jbc.M109.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146(9):3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 60.Vongs A, Lynn NM, Rosenblum CI. Activation of MAP kinase by MC4-R through PI3 kinase. Regul Pept. 2004;120(1–3):113–118. doi: 10.1016/j.regpep.2004.02.018. [DOI] [PubMed] [Google Scholar]