Abstract
Retinoic acid (RA), a dietary vitamin A metabolite, is crucial in maintaining intestinal homeostasis. RA acts on intestinal leukocytes to modulate their lineage commitment and function. Although the role of RA has been characterized in immune cells, whether intestinal epithelial cells (IECs) rely on RA signaling to exert their immune-regulatory function has not been examined. Here we demonstrate that lack of retinoic acid receptor alpha (RARα) signaling in IECs results in deregulated epithelial lineage specification, leading to increased numbers of goblet cells and Paneth cells. Mechanistically, lack or RARα resulted in increased KLF4+ goblet cell precursors in the distal bowel, whereas RA treatment inhibited klf4 expression and goblet cell differentiation in zebrafish. These changes in secretory cells are associated with increased reg3g, reduced luminal bacterial detection and an underdeveloped intestinal immune system, as evidenced by an almost complete absence of lymphoid follicles and gut resident mononuclear phagocytes. This underdeveloped intestinal immune system shows a decreased ability to clear infection with Citrobacter rodentium. Collectively, our findings indicate that epithelial cell-intrinsic RARα signaling is critical to the global development of the intestinal immune system.
Keywords: Intestinal epithelial cell, retinoic acid, RARα, intestinal immunity, dendritic cells, isolated lymphoid follicles
Introduction
Vitamin A, through its metabolite retinoic acid (RA), plays a key role in embryogenesis, determination of cell lineage, and fate commitment in multiple cell types beyond its well-known role in the maintenance of the retinal epithelium 1. In the intestine, vitamin A plays an important role in intestinal immune homeostasis 2,3. For example, RA metabolism within dendritic cells was shown necessary and sufficient to induce gut homing molecules on T lymphocytes, paving the way toward characterization of the role of vitamin A in intestinal immunity 4. These studies were followed by reports showing that RA induces gut tropism on B cells 5, T helper subsets 6, myeloid precursors, and innate lymphoid cells (ILCs) 7,8. In addition, RA modulates the generation of Foxp3+ regulatory T cells 9–11, Th17 cells 9, IgA class switching in B cells 5,12, intestinal and systemic dendritic cell development 13 and the generation and function of group 3 ILCs (ILC3s), in which RA signaling induces the transcription of IL-22, one of the functional hallmarks of ILC3s 8,14,15,16. Not surprisingly, vitamin A deficiency results in altered intestinal immune homeostasis and immune responses upon intestinal challenges 17,18, which can eventually lead to colorectal cancer progression 19,20.
RA mediates its effects by binding to nuclear receptors: RA receptors (RARs -α, -β and -γ) and retinoic X receptors (RXRs -α, -β, and -γ). All-trans-retinoic acid binds RAR(s), which then dimerize(s) with RXR(s) to activate transcription 1. Importantly, unliganded RARs act as transcriptional repressors through the recruitment of cofactors that promote chromatin compaction. Ligand binding leads to a conformational change and recruitment of transcriptional coactivators. Importantly, both transcriptional activation and repression via RARs have been implicated in the regulation of cell fate 21.
Vitamin A absorption takes place in the small intestine where intestinal epithelial cells (IECs) directly absorb and metabolize pro-retinoid carotenoids into various retinoids 22. The intestinal epithelium is a dynamic system with constant turnover. Intestinal stem cells that reside at the bottom of the crypt feed the intestinal gland with proliferative progenitors that, after several rounds of division, stop proliferating and terminally differentiate into two distinct linages; absorptive and secretory. Absorptive cells or enterocytes, are responsible for the absorption of nutrients and they represent the more abundant cell type in the small intestine. The secretory lineage gives rise to goblet cells (mucus secretors), enteroendocrine (hormone producers), and Paneth cells (producers of antimicrobial agents and lysozyme) 23. Both goblet cells and enteroendocrine cells populate the villi whereas Paneth cells stay at the bottom of the crypt playing critical roles in maintaining homeostasis between the host immune system and the microbiota 24,25. Although much is known regarding pro-retinoid metabolism within IECs, little is known about retinoid signaling in these cells and whether this signaling contributes to the maintenance of intestinal immune homeostasis.
In the present study, we specifically targeted the RARα isoform in murine IECs to determine whether epithelial intrinsic RARα signaling participates in the establishment of intestinal mucosal homeostasis. Deletion of RARα within the IEC compartment revealed altered differentiation and function of the epithelial barrier, resulting in a dramatic increase in the numbers of goblet cells and Paneth cells as well as a decrease in the number of enteroendocrine cells. This observation was paralleled by changes in microbiota composition and microbial-epithelial interactions. Furthermore, we found decreased innate lymphoid cells (ILCs) and isolated lymphoid follicles in the colon. Interestingly, mice with deletion of RARα in the IEC compartment showed reduced clearance of the attaching and effacing bacteria C. rodentium. Collectively, these studies indicate that RARα signaling in IECs controls epithelial cell differentiation and function, which broadly impacts the composition and function of the intestinal mucosal immune system.
Result
Deletion of RARα within intestinal epithelial cells results in dysregulated epithelial lineage commitment
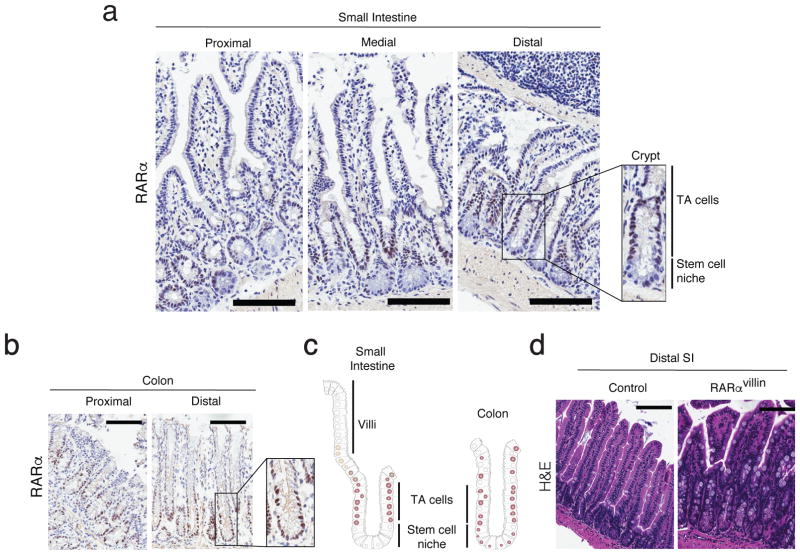
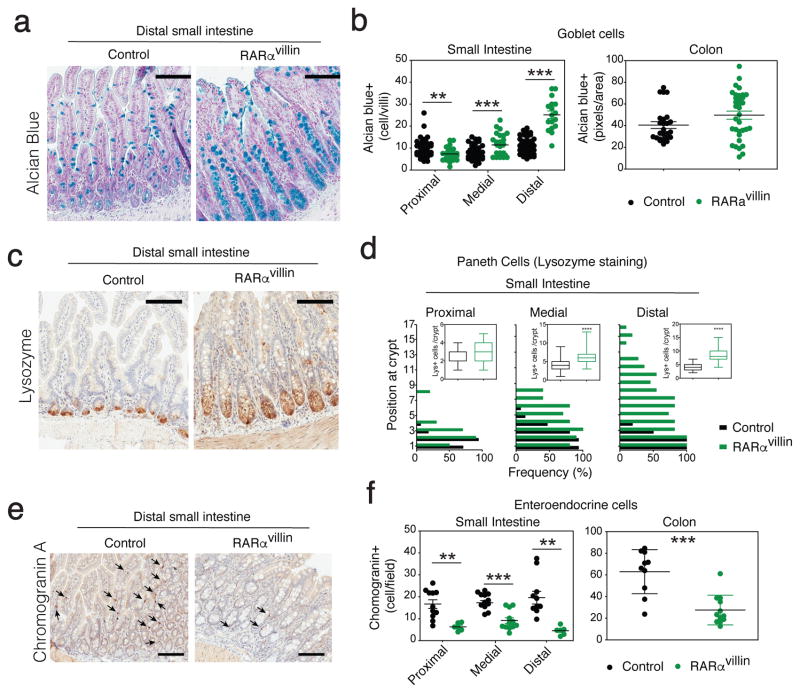
Although RA signals through different retinoic acid receptor (RAR) isoforms, deletion of RARα has been shown sufficient to impair RA signaling within the immune system 19,20,26. Hence, we analyzed the pattern of RARα expression within the intestinal mucosa. We observed that RARα expression in the small intestine follows a gradient in the intestinal gland, being absent at the bottom of the crypt (stem cell niche), expressed in the early progenitors of the transient amplifying (TA) zone and then decreasing as cells migrate to the top of the villi. In contrast, colonic crypts express RARα both in cells within the stem cell niche and the transient amplifying compartment (Figure 1a–c). To investigate the role of RARα signaling in IECs in vivo, we examined the effects of conditional deletion of RARα within the IEC compartment on intestinal immune composition and function. Mice with conditional targeting of the RARα locus (RARαf/f) were crossed with cre-expressing mice to enable the specific deletion of RARα in IECs (RARαf/f x Villin-cre). Examination of Cre recombinase expression in the small intestine confirmed villin-driven expression starting in the upper crypts towards the top of the villi, whereas the bottom of the crypts and lamina propria remained negative (Supplementary figure S1a). This is in agreement with Cre expression in Villin-cre mice described by Gumucio et al 27. The resulting RARαvillin mice showed partial depletion of RARα in progenitor cells (TA cells), which varies along the intestinal tract (Supplementary figure S1b). RARαvillin mice were born in normal Mendelian ratios and were grossly normal. We found no differences in body weight or in longevity as monitored for 2 years (data not shown). Examination of intestinal H&E staining suggested differences in epithelial cellular composition (Figure 1d). We therefore next examined whether RARα deficiency resulted in altered patterns of epithelial differentiation. We found that RARα deficiency in IECs resulted in increased numbers of goblet cells and Paneth cells within the most distal segments of the small intestine in RARαvillin mice compared to their control counterparts (Figure 2a–d). By contrast, we observed decreased numbers of enteroendocrine cells in RARαvillin mice compared to controls (Figure 2e, f). Aberrant proportions on secretory cell lineages suggest an altered homeostasis in the intestinal crypt after RARα depletion.
Figure 1.
RARα expression in intestinal epithelial cells from the small intestine and colon. (a–b) Frozen sections from the proximal, medial and distal small intestine (a) and proximal and distal colon (b) were stainied for RARα. Onsets show a digital magnification of the crypt within the respective boxes. (c) Cartoon showing the RARα expression pattern through the crypt-villi axis (Small intestine) or crypt (colon). One representative figure out of three experiments. (d) H&E staining of distal small intestine sections of control and RARαvillin mice. One representative figure out of three experiments. TA: transit amplifying. Scale bars 100uM
Figure 2.
RARα controls epithelial homeostasis. (a–b) Mucins-containing goblet cells were stained with Alcian Blue and their number per villus determined. (c–d) Paneth cells were immunostained with anti-lysozyme and their number per villus and position along the crypt-villus axis determined (n = 7–19 villus/mouse). (e–f) Enteroendocrine cells were immunostained with anti-Chromogranin A and their number per villus determined (n = 7–12 villus/mouse). Data in (a–b) are representative of three mice/genotype. **P < 0.01; *** P < 0.005; Student’s t-test. Error bars represent SEM in all panels. Scale bars 100uM
In order to gain further mechanistic insights, we analyzed both proliferative capacity and apoptosis in IECs lacking RARα. We observed significant differences exclusively in the proximal small intestine, whereas the colon as well as medial and distal small intestinal segments showed similar numbers of cleaved-caspase 3+ apoptotic cells in RARαvillin compared to control animals (Supplementary figure S2a), suggesting that apoptosis is unlikely to account for the increase in secretory IECs observed in RARαvillin mice. By contrast, the number of Ki-67+ proliferating cells was significantly enlarged in RARαvillin mice showing the most pronounced differences between control and RARαvillin mice within the distal rather than proximal small intestine (Supplementary figure S2b, c). Similarly, differences were most prominent in the distal colon (Supplementary figure S2d, e). Changes in the proliferative compartment and cell differentiation did not result in altered crypt length throughout the small intestine of RARαvillin mice (Supplementary figure S2f). Altogether, these data suggest that RARα signaling in the TA compartment controls proliferation and secretory cell lineage commitment in late progenitors.
RA-RARα axis modulate the expression of the goblet cell master regulator KLF4
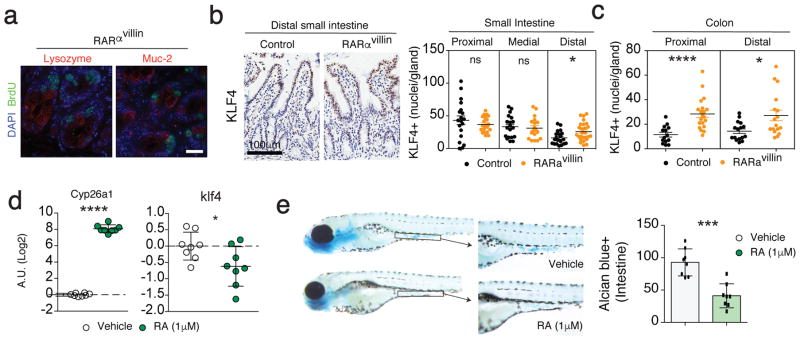
We next assessed the possibility that RARα controlled proliferation in differentiated secretory cells. To this end, mice were injected with BrdU, which is incorporated in proliferating cells and we co-stained for BrdU together with Lyzozyme (Paneth cells) and Muc2 (Goblet cells). We were not able to detect colocalization between proliferating BrdU+ cells and either Paneth or goblet cells (Figure 3a). We next assessed whether secretory cell hyperplasia was associated with altered stem cell homeostasis in the absence of RARα. Positive cells for olfactomedin-4 (OLFM4), a robust intestinal stem cells marker 28,29 was comparable between RARαvillin and control animals (Supplementary figure S3a, b), suggesting that increased secretory cell numbers were not associated with expansion of the stem cell pool at the bottom of the crypt. Although we cannot completely exclude that RA might modulate stem cell functions, our data suggest that RA-RAR signaling does not modulate stem cell homeostasis, which is in agreement with RARα expression in TA cells (Figure 1a).
Figure 3.
RARα modulates differentiation within the secretory branch through KLF4. (a) Proliferative cells were identified by BrdU incorporation in parallel with lysozyme or muc-2 to detect paneth cells (left) and goblet cells (right), respectively. (b–c) KLF4 expression was measured in the distal small intestine (b) and colon (c) by immunohistochemistry and number of positive nuclei were counted per intestinal gland. 7–10 crypts were counted per intestine section in 2 mice per genotype. (d) RT-qPCR analysis of RAR target gene cyp26a1 and the transcription factor klf4 in zebrafish embryos treated with either vehicle or 1μM RA from 72 hours post-fertilization (hpf) till 108 hpf. Each dot represents a pool of 20 embryos. The mRNA expression was normalized to that of ef1α. (e) Whole-mount alcian blue staining of zebrafish embryos treated with either vehicle or 1μM RA from 72 hpf till 108 hpf (images). The graph represent quantification of alcian blue positive cells per intestine (n=8 per group). *P < 0.05; *** P < 0.005; **** P < 0.001 Student’s t-test. Error bars represent SEM. Scale bar; 20 μm (a), 100 μm (b)
The transcription factor Krüppel-like factor 4 (KLF4) has been shown to be a master regulator of terminal goblet cell differentiation 30,31. We then addressed if KLF4-expressing cells (goblet cell precursors) were altered in RARαvillin mice. Staining of KLF4 in the small and large bowel showed significantly increased KLF4+ cells in the distal small intestine and colon (Figure 3b, c). To investigate whether RA is sufficient to regulate klf4 expression, we took advantage of the zebrafish system, in which RAR signaling and the mechanisms controlling goblet cell differentiation appear to be highly conserved when compared to mammals 32. As expected, 72 hours post fertilization (hpf) embryos exposed to 1μM RA for 36 hours showed increased levels of the RAR target gene, cyp26a1 (Figure 3d). Treatment with RA resulted in decreased klf4 transcript levels compared to untreated embryos (Figure 3d), which was associated with decreased goblet cell numbers as seen by Alcian blue staining (Figure 3e), suggesting that the RA-RARα axis negatively modulates klf4 expression, which might impact goblet cell differentiation.
Conditional RARα deletion in IECs results in reg3γ overexpression and microbial dysbiosis
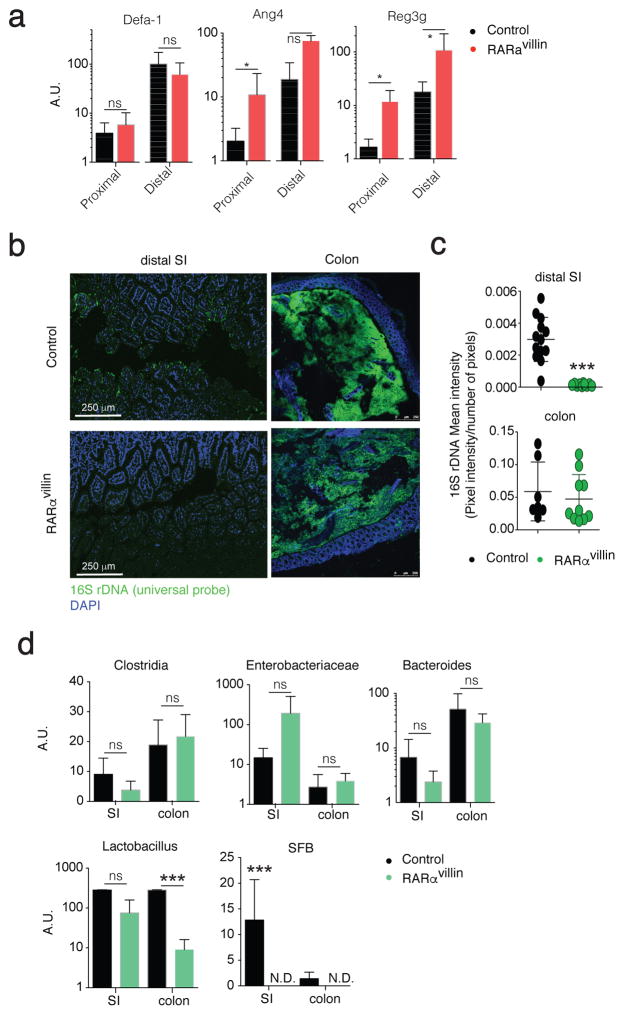
Paneth cells and goblet cells are responsible for the production and secretion of a variety of antimicrobial peptides (AMPs), such as Reg3γ to maintain a gap between the microbiota and the epithelial layer 33,34. Our results showing increased numbers of Paneth cells and goblet cells, particularly in the distal small intestine, suggested possible differences in the AMP levels and composition of the microbiota. We therefore analyzed a panel of AMPs produced by Paneth cells. Although we did not detected changes in defa-rs1 we observed significantly higher ang4 transcript levels in the proximal small intestine whereas reg3g transcript levels were higher in both proximal and distal small intestine, from RARαvillin mice compared to controls (Figure 4a). Since Reg3γ-deficiency results in increased bacterial colonization at the ileal epithelial cell surface 34, we investigated the presence of luminal commensal bacteria close to the epithelium using fluorescence in situ hybridization. In agreement with higher Reg3γ levels, we observed decreased luminal commensal bacteria in the ileum of RARαvillin mice compared to control counterparts (Figure 4b, c). Analysis in the colon showed similar bacterial loads between RARαvillin and control mice. Next, we analyzed the relative abundance of different bacterial genera in the small intestine and colon of RARαvillin and control mice using qPCR. Interestingly, whereas Clostridia, enterobacteriaceae, bacteroides and lactobacillus did not differ in the small intestine luminal content between RARαvillin mice and control mice, we found significant differences in lactobacillus in the colon and in segmented filamentous bacteria (SFB) in both the ileum and colon of RARαvillin mice compared to control mice (Figure 4d). Altogether, these data suggest that RARα deficiency in the intestinal epithelium results in increased Reg3γ levels, which might alter bacterial colonization at the ileal epithelial surface.
Figure 4.
Dysbiosis in RARαvillin mice. (a) qPCR analysis from FACS-sorted epithelial cells (CD45negEpCAM+) obtained from the proximal or distal small intestine of either control or RARαvillin mice. Data shows transcript levels as arbitrary units (A.U.) respect to hprt (n = 3 mice). (b) Fluorescence in situ hybridization of universal 16S ribosomal RNA in DAPI-stained ileal tissues from control and RARαvillin mice. Original magnification, 10X. One representative image of 3–6 images/mouse (n = 2 mice). (c) 16S rDNA mean intensities (sum of pixel intensities/number of pixels) are reported. Scale bar, 10 μm. (d) qPCR analysis shows arbitrary units (A.U.) of lactobacillus, enterobacterae (entero), bacteroides, clostridia and segmented filamentous bacteria (SFB) relative to universal 16S levels. Bacterial DNA was isolated from luminal stool obtained from the small intestine (SI) or colon (n = 3; 2 experiments). *P < 0.05; *** P < 0.005; ns, non-significant; N.D., non-detected; Student’s t-test. Results are shown as mean ± SEM in all panels. Scale bar; 250 μm (b)
Retinoic acid receptor alpha in IECs is required for proper intestinal immune homeostasis
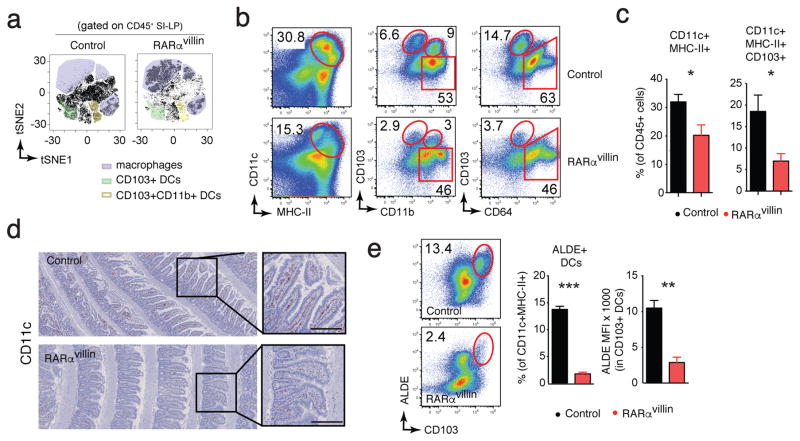
IECs and the microbiota provide inputs that help shape the composition and function of the intestinal immune system 35,36. Since our data showed that mice lacking RARα in IECs displayed alterations within IECs and the microbiota, we next assessed whether loss of RARα in IECs impacts the intestinal immune compartment by examining whether the immune composition of the lamina propria in RARαvillin mice was altered. We first performed an unsupervised analysis of flow cytometry data using t-distributed stochastic neighbor embedding (tSNE) 37. CD45+DAPIneg lamina propria cells were exported, concatenated, and displayed in a single tSNE contour plot in which clusters defining macrophages and DC subpopulations were determined based on specific markers (Supplementary figure S2). Comparison between samples, in which number of events were kept constant, showed an overall relative decrease in DC subpopulations in the RARαvillin compared to control mice (Figure 5a). Similarly, two-dimensional dot plots revealed that the mononuclear phagocytic compartment, defined as CD11chiMHC-IIhi, was decreased by almost one-third in RARαvillin mice compared to control mice (Figure 5b, c). Within mononuclear phagocytic cells, dendritic cells (defined as CD103+) and macrophages (defined as CD64+CD103−) were decreased 3-fold and 0.5-fold in RARαvillin mice compared to control mice, respectively (Figure 5b, c). Since analysis by flow cytometry requires enzymatic digestion to generate cell suspensions, and therefore can result in genotype-dependent damage to sensitive cell types, we confirmed our findings through immunohistochemistry. Immunohistochemical staining in small intestinal “Swiss rolls” confirmed decreased numbers of CD11c+ cells in RARαvillin mice compared to control mice (Figure 5d), further supporting the observations obtained by flow cytometry.
Figure 5.
Dendritic cell numbers are decreased in RARαvillin mice. Cell suspensions from small intestinal lamina propria were analyzed by FACS. (a) Analysis of single live CD45+ events from the flow cytometry data of SI lamina propria isolated form control or RARαvillin mice using the t-distributed stochastic linear embedding (tSNE) algorithm (one representative analysis of two) (b) Representative dot plots showing total dendritic cells (left column) and subsets defined by CD103 and CD11b expression (middle column) as well as macrophages defined by CD64 (right column). (c) Quantification of the frequencies of dendritic cells (DCs) and the CD103+ subset. (n = 4; 2 experiments) (d) Expression of CD11c in the small intestine was determined by immunohistochemistry. Representative images of 6 images/mouse (n = 3 mice). (e) Representative dot plot and quantification of RA-producing dendritic cells in the small intestine lamina propria determined by ALDEFLUOR (ALDE) (n = 4; 2 experiments). *P < 0.05; **P < 0.01; *** P < 0.005; Student’s t-test. Results are shown as mean ± SEM in all panels. Scale bar; 100 μm (d)
Intestinal CD103+ dendritic cells possess the ability to metabolize vitamin A into RA 38, and are thus capable of efficiently inducing Foxp3+ T regulatory cells, gut tropism on T and B cells, and IL-22 production by ILC3s 39. The ability to metabolize RA requires the activity of retinaldehyde dehydrogenases, which can be monitored using the ALDEFLUOR (ALDE) assay 40. Whereas almost 14% of CD11c+MHC-II+ cells were CD103hiALDE+ in the small intestine of control mice, we found a significantly lower proportion (only ~3%) of CD103hi ALDE+ dendritic cells in RARαvillin mice (Figure 5e). In addition, mean fluorescence intensity analysis revealed significantly lower ALDE activity per CD103+ dendritic cell in cells from RARαvillin mice compared to their control counterparts (Figure 5e). Collectively, these results suggest that RA signaling in IECs is crucial for the development of intestinal dendritic cells that have the ability to produce RA.
RARαvillin mice have reduced numbers of isolated lymphoid follicles
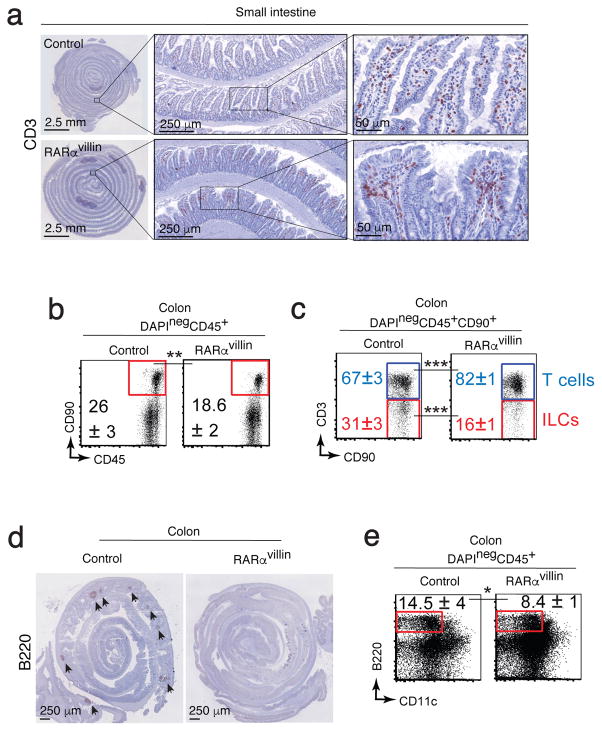
Since reduction in intestinal dendritic cell numbers as well as defects in RA production by CD103hi dendritic cells could result in decreased numbers of T cells in the intestine 41, we next compared the presence of lymphocytes in the small intestine. Immunohistochemistry staining with CD3, revealed that T cells are present throughout the small intestine lamina propria in both RARαvillin and control mice (Figure 6a), suggesting that decreased numbers of ALDE+ dendritic cells in RARαvillin mice do not result in reduced T cell intestinal recruitment. Staining with CD90 allowed us to analyze the presence of innate lymphoid cells (CD90+CD3−), presumably lymphoid tissue inducer (LTis) cells, since they clustered in cryptopatches (Supplementary figure S3a), defined by presence of CD11c+ but not for CD3+ cells (Supplementary figure S2b). No differences were evident in the number of cryptopatches between RARαvillin and control mice (Supplementary figure S3c); however, colonic cryptopatches appeared to be larger in RARαvillin compared to control mice (Supplementary figure S2b). Despite an apparent enlargement of cryptopatches, the frequencies of CD90+ cells within the colon lamina propria were significantly reduced in RARαvillin compared to control mice (Figure 6b). These CD90+ cells were mainly composed of T cells (CD3+) rather than ILCs (CD3neg) as seen by decreased frequencies of CD3neg cells within the CD90+ compartment in RARαvillin compared to control mice (Figure 6c). Colonic cryptopatches can develop into mature isolated lymphoid follicles, which are composed mainly of B cells 42. Staining with B220 to detect mature lymphoid follicles revealed a lack of these structures in RARαvillin mice (Figure 6d). In addition, the colon lamina propria from RARαvillin mice showed decreased frequencies of B220+ B cells compared to control (Figure 6e), indicating that B cell numbers and isolated lymphoid follicle structures were decreased in mice lacking RARα signaling specifically in IECs, likely due to defects in the transition from cryptopatches to isolated lymphoid follicles. Altogether, these data demonstrate that RARα signaling in intestinal epithelial cells is critical to the maintenance of intestinal immune homeostasis.
Figure 6.
RARα deficiency results in altered intestinal immune development. (a) Expression of CD3 in the small intestine was determined by immunohistochemistry. Representative image of 6 images/mouse (n = 3 mice). (b–c) Representative dot plots showing total CD90+ cells (b) and T cells and ILCs within the CD90+ compartment (c) (n = 3–4; 2 experiments). (d) Colon Swiss rolls showing immunohistochemistry for B220 in control and RARαvillin mice. Data are representative of three mice/genotype. (e) Colon cell suspension staining for B220 and CD11c reveals decreased B cells in RARαvillin mice (n = 3; 2 experiments). *P < 0.05; **P < 0.01; *** P < 0.005; Student’s t-test. Results are shown as mean ± SD in all panels. Scale bars as indicated.
RARα deficiency in IECs confers increased susceptibility to C. rodentium infection
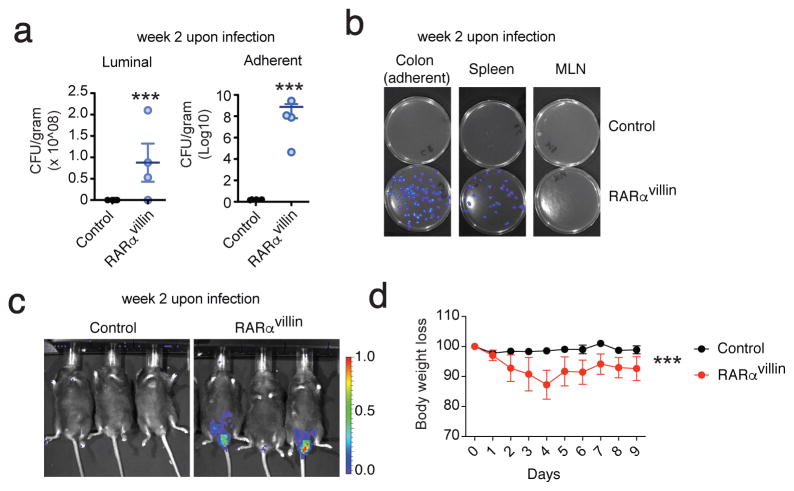
We further explored the ability of RARα signaling in IECs to regulate intestinal immunity during infection. C. rodentium is a well-studied gram-negative mouse intestinal pathogen related to adherent invasive E. coli, a cause of serious human infection that is associated with intestinal lesions in Crohn’s disease 43. Murine infection with C. rodentium results in a distal colitis that is generally cleared 2–3 weeks post infection by immunocompetent mice. Clearance requires both innate and adaptive immunity with a particularly important role for dendritic cells and ILCs 44. Given the profound impact of RARα signaling deficiency in IECs upon the composition of epithelial, myeloid, and isolated lymphoid follicle compartments, we hypothesized that RARαvillin mice would differ from control littermates in their response to C. rodentium infection. Indeed, RARαvillin mice were slower to clear the infection relative to their control littermates as determined by whole-body bioluminescence, stool CFU, and bacterial translocation to spleen and mesenteric lymph nodes (Figure 7a–c) and lost more weight during C. rodentium infection (Figure 7d). Collectively, our results place RARα signaling within the epithelial cell compartment as critical in controlling intestinal immunity (Figure 8).
Figure 7.
RARα deficiency in the epithelial compartment results in defective clearance of C. rodentium. (a) CFU from control and RARαvillin mice infected with C. rodentium. (n = 4; 2 experiments) (b). Bioluminescence of cultured stool samples (colon), spleen, and MLN cell suspensions. (c) Bioluminescence of whole animal. (d) Body weight loss curves from control and RARαvillin mice infected with C. rodentium (2 x109 CFU). (n = 5; 2 experiments *** P < 0.005; Two-way repeated measures ANOVA with Bonferroni post-test (a). Student’s t-test (c).
Figure 8.
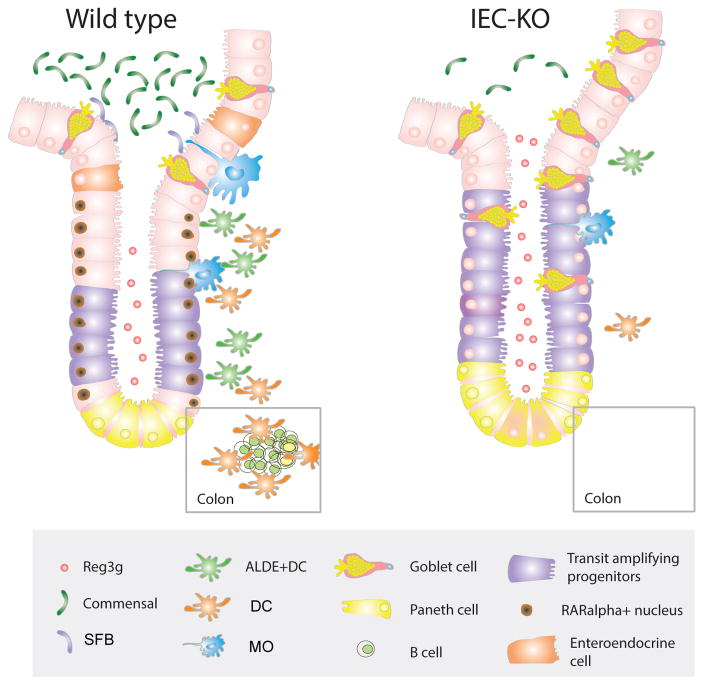
RARα signaling on IECs regulates intestinal secretory cell differentiation and immunological fitness. Scheme showing the proposed model in which RARα expression in proliferating cells (transit amplifying progenitors) restrict secretory cell differentiation and Reg3g expression which is associated to proper microbiota composition and immune cell development.
Discussion
RA is known to be critical for proper immune function in the intestine. However, much of the research in this context has centered on immune cells, with the question of whether RA signaling in IECs contributes to immune homeostasis in the gut remaining largely unexplored. In the present study, we describe RARα-dependent epithelial cellular linage commitment and resultant changes in epithelial function, linking these alterations to several important changes in the immune compartment, highlighting a previously unrecognized role for intestinal epithelial retinoid signaling in the regulation of intestinal immune homeostasis.
Examination of intestinal H&E stained sections from these mice suggested differences in the numbers of secretory epithelial cells. Indeed, defective RARαvillin signaling resulted in a marked increase in the numbers of goblet cells and changes in the distribution of Paneth cells along the crypt-villus axis as well as decreased numbers of enteroendocrine cells. These findings suggest that RARα plays a critical role in cell fate determination in the intestine, in keeping with its well-known role as a morphogen. The intestinal epithelium is continuously turning over. The stem cells in the base of the crypt divide to give rise to cellular progenitors with cell fate-determination in the intestine under the control of various signaling pathways including Wnt, BMP, EGF and Notch signaling. The Notch pathway plays a critical role in intestinal epithelial cell fate by regulating the choice of absorptive versus secretory lineages. Hyperactivated Notch signaling expands the proliferative zone and suppresses secretory lineage commitment 45. This is due to Hes1 (Notch-dependent transcription factor) inhibition of Math1 (also known as Atoh1), a determinant of secretory lineage differentiation46. Importantly, inducible deletion of Math1 in the mouse intestine results in the almost complete absence of all secretory epithelial cell types including goblet cells, Paneth cells, and enteroendocrine cells 47. Our RARαvillin mice showed no differences in Hes1 activity compared to controls (data not shown).
Given the phenotype observed in our RARαvillin mice, with increased goblet and Paneth cells but decreased enteroendocrine suggests that RARα could be participating in the determination of cellular fate within the secretory branch, but after secretory progenitor commitment. Further differentiation of the secretory lineage into goblet, enteroendocrine, and Paneth cells requires other factors like Klf4, Neurogenin3 and beta-catenin, respectively 30. Klf4 is a transcription factor that plays a determinant role inhibiting intestinal cell proliferation and regulates goblet cell maturation 30. Interestingly, Klf4 is under direct regulation of RARα 48 and Klf4 depletion specifically in intestinal epithelial cells leads to increased transient amplifying compartment, increased number and altered positioning of Paneth cells along the crypt-villus axis 49, similarly to RARαvillin mice.
We observed an upregulation of Klf4 in RARαvillin intestinal crypts, particularly in distal small intestine and colon, and retinoid treatment of zebrafish enhanced goblet cell differentiation. Therefore, RARα could be placed downstream of the master regulator of secretory cells, Math1, but upstream Klf4 in the hierarchical regulation of intestinal epithelial cell differentiation.
Paneth cells and goblet cells are secretory epithelial cells that play a critical role in the maintenance of microbial epithelial homeostasis through the production and release of antimicrobial peptides and mucins. These products limit the direct interaction between the intestinal epithelium and the stem cell niche (crypts of Lieberkuhn) and the microbiota. It is possible that RA signaling impacts AMP secretion/activity, such as has been demonstrated in skin, and resulting in changes in stool microbial composition and bacterial proximity to the epithelial surface 50. Whether RA induces AMP through direct binding of RAR to their cis-regulatory elements needs further investigation. Previous reports have demonstrated a critical role for bacterial products (i.e., pathogen-associated molecular patterns) and their receptors in the development of the intestinal immune system 51. In fact, diminished bacterial sensing by the intestinal immune system leads to an underdeveloped immune system manifested by decreases in mononuclear phagocytic cells and reduction in isolated lymphoid follicle numbers and size 52. This prompted us to speculate that changes in the microbiota composition might contribute to a defect in intestinal immune development in RARαvillin mice. By contrast, RA controls the function of LTis beginning in the fetal stages in a microbiota-independent fashion. Importantly, low maternal levels of RA results in underdeveloped secondary lymphoid structures in the offspring, including isolated lymphoid follicles and Peyer’s patches, defects that are not reversed by the addition of dietary vitamin A after birth 14. Our data showing absence of isolated lymphoid follicles in RARαvillin mice compared to control mice suggest that RARα signaling in epithelial cells is not completely required to induce the formation of maternal-driven secondary lymphoid organs. Indeed, the accumulation of CD90+CD3neg LTi-like cells in colonic cryptopatches rather suggests a defect in the transition from cryptopatches (containing LTis) toward fully mature isolated lymphoid follicles (containing B cells) in RARαvillin mice. The precise mechanism by which RARα signaling in IECs controls the maturation of isolated lymphoid follicles will be the subject of future studies.
Interestingly, despite profound differences in epithelial cellular composition between RARαvillin and control mice, we did not observe any major differences in intestinal paracellular permeability using FITC-dextran (data not shown). This finding is in agreement with previous in vitro observation that various RAR ligands do not affect permeability of Caco-2 intestinal epithelial monolayers 53. In contrast, Baltes et al. have shown an increase in intestinal permeability in response to RA using these same cells. It would therefore be expected that defective RA signaling would result in decreased permeability, which would not be detected by our assay given the poor permeability of FITC-dextran at baseline 54.
In considering how RARα may regulate cell fate, it is important to survey the roles of RARα as both a transcriptional activator and repressor. In the absence of ligand, RARα is known to occupy RA responsive elements (RAREs) while coupled to transcriptional repressors such as NCOR. On the other hand, when ligand-bound, RAR activates transcription through the release of repressors and recruitment of coactivators such as histone deacetylases. It is therefore possible that the changes observed within the epithelial compartment are in part due to de-repression of otherwise silenced promoters in RARαvillin mice rather than absence of RA-dependent signaling. Similarly, one must be cautious when comparing our in vivo results with studies examining RA-dependent signaling via the use of RARα agonists. Nevertheless, RA supplementation was recently shown to suppress intestinal mucus production in a zebrafish model of enterocolitis, in keeping with our observed increase in goblet cell numbers in RARαvillin mice 55.
In conclusion, we have shown that RARα signaling in the intestinal epithelia influences the cellular linage composition in the intestinal barrier, which is associated with dramatic alterations in intestinal immune homeostasis and protective responses against bacterial infection (Figure 8). The identification of this dominant role of RARα signaling within IECs in modulating intestinal immune homeostasis raises new and interesting questions regarding the cell-intrinsic roles of RA in settings of nutritional deprivation and RA supplementation.
Experimental procedures
Animal husbandry
RARαf/f have been previously described 56. RARαf/f mice were crossed with villin-cre mice to generate RARαvillin mice. Mice were in a C57/BL/6 background. All mice were used between 8 and 12 weeks of age. Where indicated, mice were intraperitoneally administered 2.4 mg BrdU 2h prior euthanasia. Mice were maintained under specific pathogen-free conditions and handled according to protocols approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Zebrafish (Danio rerio) embryos were obtained from natural spawnings and raised at 28.5 °C in E3 water supplemented with methylene blue and HEPES (10 mM). Embryos were exposed to all-trans Retinoic Acid (RA) from 72 to 108 hpf in groups of 20 embryos in a volume of 5 ml. The exposure medium was renewed at 96 hpf. All analyses were performed at 108 hpf. RA (Cat. No. R2625, Sigma-Aldrich) was dissolved in DMSO and diluted to working concentrations with E3 water.
Fluorescence in situ hybridization
Small intestinal tissues (distal ileum) were prepared for analysis by fixation in Carnoy’s fixative followed by embedding in paraffin and sectioning to 5 μm. Processing and hybridizations to a universal bacterial probe (100 μM) directed against the 16S rRNA gene ([Alexa488]-GCTGCCTCCCGTAGGAGT-[Alexa488]) were carried out using DAPI as a counterstain 34. Tissues were visualized using a CHGR Leika SP5 microscope and captured using LASAF software. Pictures were taken under 10X magnification and analyzed in an automated manner using the open-source CellProfiler software. Mean intensities (the sum of pixel intensities/number of pixels) are reported.
IEC and lamina propria isolation
Colon and small intestine lamina propria cells were isolated as previously described18 with slight modification. Briefly, intestines were harvested and placed in ice-cold HBSS. After removal of residual mesenteric fat tissue, Peyer’s patches were excised (in the case of small intestine dissection) and the intestines were opened longitudinally. Tissues were then washed in ice-cold HBSS and cut into 1-cm pieces. After 3 more washes in HBSS, tissues were incubated twice in 20 mL of serum-free media with 5 mM EDTA and 0.145 mg/ml DTT at 37°C at 250 rpm for 30 min. The supernatant was harvested by filtration through a 100-μm cell strainer and centrifugation at 500 × g; the pellet, consisting of the IEC fraction, was resuspended in MACS buffer. Tissue pieces were washed 3 times with 10 mL serum-free media with 2 mM EDTA. Small intestine pieces were next digested in serum-free media containing Liberase TL (0.1 mg/ml, Roche) at 37°C at 250 rpm for 45 min. Cells were washed and passed through a 70-μm cell strainer. Cells were resuspended in 4.5 ml of 44% Percoll. 2.3 mL of 67% Percoll were then underlaid. Percoll gradient separation was performed by centrifugation for 20 min at 600 × g at room temperature. Lymphoid fractions were collected at the interphase of the Percoll gradient, washed once, and resuspended in FACS buffer (1% FBS DPBS) or culture medium.
Flow cytometry
Single cell suspensions were incubated for 10 min at room temperature with Fc blocking (CD16/32) antibody (eBioscience) prior to staining with fluorochrome-conjugated antibodies against mixtures of the following antigens: CD90.2, CD45, CD3e, CD11c, CD11b, GR1, MHCII, CD103, CD4, CD8α, CD8β, TCR-γδ, B220 (all from Biolegend). DAPI or Live/Dead Fixable Blue Dead Cell Stain Kit (Invitrogen) were used to exclude dead cells. Multiparameter analysis was performed on a LSR II (BD) and bi-dimensional dot plots were generated using FlowJo software (Tree Star) whereas t-SNE high dimensional plots using Cytobank 37.
Quantitative real-time PCR
Distal small intestine (ileum) and proximal colon (colon) tissues were harvested and stored in RNAlater (Ambion). RNA was extracted from homogenized tissues using the RNeasy Kit (Qiagen). Total RNA was isolated from pools of 20 zebrafish embryos using TRIzol LS reagent (Invitrogen), according to the manufacturer’s protocol. RNA samples were reverse-transcribed using the iScript cDNA Synthesis kit (Bio-Rad). Using the iQ SYBR Green Supermix (Bio-Rad) for quantitative PCR, mRNA levels were determined using the iCycler with iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). Reaction conditions consisted of 40 cycles of PCR with 58°C or 59°C annealing temperature. Mouse primers sequences have been previously described and are as follows57: Clostridia F-ACT CCT ACG GGA GGC AGC, Clostridia R-GCT TCT TAG TCA GGT ACC GTC AT; SFB F-GAC GCT GAG GCA TGA GAG CAT, SFB R-GAC GGC ACG GAT TGT TAT TCA, Lactobacillus F- AGC AGT AGG GAA TCT TCC A, Lactobacillus F- CGC CAC TGG TGT TCY TCC ATA TA; Bacteroidetes F- GGT TCT GAG AGG AGG TCC C, Bacteroidetes R- GCT GCC TCC CGT AGG AGT; Enterobacteriaceae F- GTG CCA GCM GCC GCG GTA A, Enterobacteriaceae R- GCC TCA AGG GCA CAA CCT CCA AG, Muc2 F-AGG GCT CGG AAC TCC AGA AA, Muc2 R- CCA GGG AAT CGG TAG ACA TCG, Chga F- CCC ACT GCA GCA TCC AGT T, Chga R-AGT CCG ACT GAC CAT CAT CTT TC, Lys1 F-AGA ATG CCT GTG GGA TCA AT, Lys1 R- CTG GGA CAG ATC TCG GTT TT, Lgr5 F-CCT ACT CGA AGA CTT ACC CAG T, Lgr5 R- GCA TTG GGG TGA ATG ATA GCA, Dhrs3 F- GCG CTG GTA GTG TTC CCT C, Dhrs3 R-GGT GTT GAC ATG CTG GGA CTT, Gapdh F- CAT GGC CTT CCG TGT TCC TA, Gapdh R-GCG GCA CGT CAG ATC CA. Zebrafish primers sequences are as follows: klf4 F- CGG TTC AAG ATG GAA GGA GGA, klf4 F – CTG TTG AAG CAA GCT GTT CG, ef1a F- ACC TAC CCT CCT CTT GGT CG, ef1a R- GGA ACG GTG TGA TTG AGG GAA, cyp26a1 F- GAT GCT CTG GAG CAC TAC ATT C, cyp26a1 R- GTT CTT GCT CGT CCG TCT TTA T.
Immunohistochemistry
Small intestines and colons were flushed with PBS prior to fixation in 10% neutral buffered formalin and paraffin embedding. 4μm sections were de-waxed and rehydrated prior to heat-mediated antigen retrieval in citrate buffer (ph 6). Anti-chromogranin A (Abcam ab15160, 1:3000), anti-RARα (Santa Cruz biotechnologies, 1:500), anti-Cre-recombinase (Abcam, 1:125), anti-cleaved caspase 3 (Cell signaling technologies, 1:400), anti-OLFM4 (Cell signaling technologies, 1:400), anti KLF4 (R&D Systems, 1:200) and anti-Ki67 (Biolegend, 16A8, 1:50) antibodies were used for immunohistochemistry. Borg decloaker solution was used for antigen retrieval prior anti-Lysozyme antibody incubation (Thermo Ab-1 RB-372-A1, 1:2000) for Paneth cells detection. Samples were hematoxylin counterstained before mounting. For goblet cell staining, slides were incubated in 1% Alcian Blue/3% acetic acid solution for five minutes and counterstained with nuclear fast red. Pictures taken under 10X magnification were blindly quantified using ImageJ software.
For whole organ analysis, small intestines and colons were prepared as Swiss rolls before fixation. Tissues were placed in cassettes and submerged in OCT compound (Tissue Tek), and submerged in isopentane cooled with liquid nitrogen and stored at −80C. 5 μm sections were then stained using the following antibodies: CD90.2 (BD Bioscience, clone 53-2.1, 1:50), CD3 (BD Bioscience, clone 17A2, 1:50), B220 (BD Bioscience, clone RA3-6B2, 1:50) and CD11c (BD Bioscience, clone HL3, 1:50).
Zebrafish alcian Blue staining
Whole-mount alcian blue staining of zebrafish embryos was performed as previously described (Oehlers et al., 2013). Larvae were mounted in glycerol and imaged using a Leica M165 FC stereomicroscope equipped with a Leica DFC450 C digital camera. Quantification of positive cells from the middle segment of the intestinal tract to the anus per intestine was performed manually.
Immunofluorescence
Paraffin embedded sections were dewaxed and rehydrated following regular IHC procedures. Blocking was done in 10% goat serum IF buffer −0.1% BSA, 0.2% Triton, 0.5% Tween20 in PBS. Primary antibodies were incubated at 4°C overnight (Anti-BrdU -BD Pharmingen 1:200-, anti-Lysozyme -Thermo Ab-1 RB-372-A1 1:2000-, anti-mucin 2 -Santa Cruz Biotechnology sc-15334 1:200-). Fluorescence labeled secondary antibodies were used at 1:2000 dilution in IF buffer (anti-mouse AF488 and anti-rabbit AF555). DAPI was used for DNA counterstain before imaging using a DeltaVision confocal microscope.
Citrobacter rodentium infection
Bioluminescent C. rodentium strain ICC180 (derived from DBS100) was used for all inoculations (provided by Dr. Gad Frankel, Imperial College London) 58. C. rodentium was grown overnight in Luria-Bertani broth with shaking at 37°C in the presence of 50 μg/ml kanamycin. The following morning, cultures were diluted to OD = 1 followed by an additional 5 hours of growth. Bacterial density was confirmed by dilution plating. 8–12 week old sex-matched mice were inoculated by oral gavage with 2×109 CFU resuspended in 200 μl PBS. For quantification of bacterial shedding and spleen translocation, stool pellets and spleens from individual mice were weighed, homogenized in PBS, and plated in serial dilutions on LB agar containing 50 μg/ml kanamycin. Mice were sacrificed on day14 post infection.
Bioluminescence imaging
Mice were anesthetized with isoflurane and placed in a supine position in a custom-built chamber for imaging with an IVIS-100 system and Living Image Software (Xenogen). Whole-body images were taken over 1 to 3 min 4, 7 10 and 14 days post infection. Luminescence emitted from the same gate in individual mice was quantified as counts per second and pseudocolor images were generated representing light intensity.
Supplementary Material
Acknowledgments
We thank Natalia Nedelsky for editorial assistance and members of the Villablanca lab for helpful comments. This work was supported in part by an Alberta Innovates-Health Solutions Fellowship, 201100215 to H.B.J., the Samana Cay MGH Research Scholar Fund and R01-AI084880 to M.J.P., the Helmsley Trust and N.I.H. grants DK097485, DK062432, and DK086502 to R.J.X. and funding from Swedish Research Council VR grant K2015-68X-22765-01-6 and Wallenberg Academy Fellow (WAF) program to E.J.V.
Footnotes
Disclosure: The authors declare no competing financial interests.
Author contributions: H.B.J., L.S.L., O.E.D., S.D., J.D.C., and E.J.V. designed, performed experiments and analyzed the data. M.B.Y., M.J.P., J.R.M., and Y.B. analyzed the data and provided feedback. R.J.X. and E.J.V. conceived the study, designed experiments, analyzed the data, and wrote the manuscript.
References
- 1.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 2.Czarnewski P, Das S, Parigi SM, Villablanca EJ. Retinoic Acid and Its Role in Modulating Intestinal Innate Immunity. Nutrients. 2017;9 doi: 10.3390/nu9010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erkelens MN, Mebius RE. Retinoic Acid and Immune Homeostasis: A Balancing Act. Trends Immunol. 2017 doi: 10.1016/j.it.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 6.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 7.Villablanca EJ, et al. beta7 integrins are required to give rise to intestinal mononuclear phagocytes with tolerogenic potential. Gut. 2014;63:1431–1440. doi: 10.1136/gutjnl-2013-305386. [DOI] [PubMed] [Google Scholar]
- 8.Kim MH, Taparowsky EJ, Kim CH. Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut. Immunity. 2015;43:107–119. doi: 10.1016/j.immuni.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 10.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokuyama Y, Tokuyama H. Retinoids as Ig Isotype-Switch Modulators. Cellular immunology. 1996;170:230–234. [PubMed] [Google Scholar]
- 13.Klebanoff CA, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med. 2013;210:1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goverse G, et al. Vitamin A Controls the Presence of RORgamma+ Innate Lymphoid Cells and Lymphoid Tissue in the Small Intestine. J Immunol. 2016;196:5148–5155. doi: 10.4049/jimmunol.1501106. [DOI] [PubMed] [Google Scholar]
- 16.Spencer SP, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassani B, Villablanca EJ, De Calisto J, Wang S, Mora JR. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol Aspects Med. 2012;33:63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villablanca EJ, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141:176–185. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya N, et al. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8+ T Cell-Mediated Immunity in Colorectal Cancer. Immunity. 2016 doi: 10.1016/j.immuni.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall JA, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. S1074-7613(11)00081-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weston AD, Blumberg B, Underhill TM. Active repression by unliganded retinoid receptors in development: less is sometimes more. J Cell Biol. 2003;161:223–228. doi: 10.1083/jcb.200211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3:63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu TC, et al. Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight. 2016;1:e86907. doi: 10.1172/jci.insight.86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. nrmicro2546 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Brown CC, et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42:499–511. doi: 10.1016/j.immuni.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 28.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Grover PK, Hardingham JE, Cummins AG. Stem cell marker olfactomedin 4: critical appraisal of its characteristics and role in tumorigenesis. Cancer Metastasis Rev. 2010;29:761–775. doi: 10.1007/s10555-010-9262-z. [DOI] [PubMed] [Google Scholar]
- 30.Yu T, et al. Kruppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS One. 2012;7:e32492. doi: 10.1371/journal.pone.0032492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz JP, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brugman S. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol. 2016;64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8:712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliev ID, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 36.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Kotecha N, Krutzik PO, Irish JM. Web-based analysis and publication of flow cytometry experiments. Curr Protoc Cytom. 2010;Chapter 10(Unit10):17. doi: 10.1002/0471142956.cy1017s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson-Lindbom B, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parigi SM, Eldh M, Larssen P, Gabrielsson S, Villablanca EJ. Breast Milk and Solid Food Shaping Intestinal Immunity. Front Immunol. 2015;6:415. doi: 10.3389/fimmu.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokota A, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luda KM, et al. IRF8 Transcription-Factor-Dependent Classical Dendritic Cells Are Essential for Intestinal T Cell Homeostasis. Immunity. 2016;44:860–874. doi: 10.1016/j.immuni.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Taylor RT, Lugering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173:7183–7189. doi: 10.4049/jimmunol.173.12.7183. [DOI] [PubMed] [Google Scholar]
- 43.Darfeuille-Michaud A, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology. 1998;115:1405–1413. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 44.Collins JW, et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12:612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- 45.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 47.van Es JH, de Geest N, van de Born M, Clevers H, Hassan BA. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nature communications. 2010;1:18. doi: 10.1038/ncomms1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi JH, Zheng B, Chen S, Ma GY, Wen JK. Retinoic acid receptor alpha mediates all-trans-retinoic acid-induced Klf4 gene expression by regulating Klf4 promoter activity in vascular smooth muscle cells. J Biol Chem. 2012;287:10799–10811. doi: 10.1074/jbc.M111.321836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol. 2011;349:310–320. doi: 10.1016/j.ydbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–529. doi: 10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- 51.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009;2:478–485. doi: 10.1038/mi.2009.114. [DOI] [PubMed] [Google Scholar]
- 53.Wojtal KA, et al. The effects of vitamin A on cells of innate immunity in vitro. Toxicol In Vitro. 2013;27:1525–1532. doi: 10.1016/j.tiv.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Baltes S, Nau H, Lampen A. All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev Growth Differ. 2004;46:503–514. doi: 10.1111/j.1440-169x.2004.00765.x. [DOI] [PubMed] [Google Scholar]
- 55.Oehlers SH, Flores MV, Hall CJ, Crosier KE, Crosier PS. Retinoic acid suppresses intestinal mucus production and exacerbates experimental enterocolitis. Disease models & mechanisms. 2012;5:457–467. doi: 10.1242/dmm.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapellier B, et al. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- 57.Bouskra D, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. nature07450 [pii] [DOI] [PubMed] [Google Scholar]
- 58.Wiles S, Robertson BD, Frankel G, Kerton A. Bioluminescent monitoring of in vivo colonization and clearance dynamics by light-emitting bacteria. Methods Mol Biol. 2009;574:137–153. doi: 10.1007/978-1-60327-321-3_12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.