Abstract
Objective
To determine the racial disparities in severe sepsis hospitalizations and outcomes in United States academic medical center-affiliated hospitals.
Design
Retrospective analysis of sepsis hospitalizations.
Setting
United States Academic medical center-affiliated hospitals participating in Vizient Consortium from 2012 to 2014.
Patients
Sepsis hospitalizations using International Classification of Diseases, Ninth Revision discharge diagnoses codes defined by the Angus method.
Interventions
None
Measurements and Main Results
We compared rates of sepsis hospitalization, ICU admission, organ dysfunction and hospital mortality between blacks and whites. We repeated the analyses stratified by community-acquired, healthcare-associated, and hospital-acquired sepsis subtypes. Of 10,244,780 hospitalizations in our cohort, 1,114,386 (10.9%) had sepsis. Sepsis subtypes included community-acquired sepsis (61.8%), healthcare-associated sepsis (23.8%), and hospital-acquired sepsis (14.4%). While the proportion of discharges with sepsis was lower for blacks than whites (106.72 vs 109.43 per 1,000 hospitalizations, p<0.001), the proportion of black sepsis hospitalizations was higher for individuals >30 years old. Blacks exhibited lower adjusted sepsis hospital mortality than whites (OR 0.85; 95 % CI: 0.84–0.86). The adjusted odds of hospital mortality following community-acquired, healthcare-associated, and hospital-acquired sepsis were lower for blacks than whites.
Conclusion
In this current series of hospital discharges at United States academic medical center-affiliated hospitals, blacks exhibited lower adjusted rates of sepsis hospitalizations and mortality than whites
Keywords: Sepsis, Racial Disparity, Epidemiology, Hospital-Acquired, Community-Acquired, Healthcare-associated
INTRODUCTION
Sepsis, the syndrome of life-threatening organ dysfunction resulting from a dysregulated host response to infection, is a major public health problem in the United States (US), accounting for over 750,000 hospital admissions, 850,000 emergency department (ED) visits, and contributing to 200,000 deaths annually.(1–4) Racial disparities in healthcare are important, suggesting potential gaps in the identification of disease or delivery of care.(2) Prior epidemiological studies have shown that incidence of age- and sex-standardized population rates of sepsis are higher in blacks than whites, attributing these disparities to differences in biological susceptibility, and higher rates of infection as well as organ dysfunction in the black population.(5, 6)
Prior studies using hospital discharge data to evaluate sepsis racial disparities have important limitations. The data for these efforts originated from a limited number of states and may not reflect national patterns. These studies also used older datasets dating back to 2000 and may not accurately reflect current patterns of sepsis care, which may have evolved with the promotion of international sepsis care guidelines. (7) These studies also could not discern important sepsis phenotypes such as community-acquired (CAS), healthcare associated (HCAS), and hospital acquired sepsis (HAS), which have different origins, courses of care and outcomes.(8)
The Vizient Consortium Clinical Database/Resource Manager (CDB/RM) provides contemporary data on patients hospitalized at academic medical centers-affiliated hospitals across the US. In this study, we sought to characterize racial disparities in sepsis hospitalizations and outcomes in the Vizient Consortium.
MATERIALS AND METHODS
Study Design
We conducted a retrospective analysis using hospital discharge data from the Vizient Consortium Clinical Database/Resource Manager (CDB/RM).(9) The study received approval from the Institutional Review Board of the University of Alabama at Birmingham.
Data Description
Vizient is a collaboration of academic medical centers and their affiliated hospitals from 42 states in the US.(9) This consortium of 120 not-for-profit academic medical centers and nearly 300 affiliated hospitals aims to improve institutional, clinical, operational, and financial performance. As part of quality comparison, and improvement initiatives, Vizient maintains hospital discharge data set (the CDM/RM), encompassing information on all discharges among consortium hospitals voluntarily contributing data. The CDB/RM includes all elements of the standard UB-04 hospital discharge data set, including patient demographics, discharge diagnoses, procedures, and outcomes. A UB-04 data set stems from a standardized uniform billing form approved by Center for Medicare and Medicaid services for hospitals to submit bills to third party payers.(10) As Vizient Consortium extrapolate data based on hospital billing related procedures from hospital discharge data, we do not have patient-related care information, such as laboratory values and vital signs. The Vizient CDB/RM has been used in a range of scientific studies and quality improvement initiatives. (8, 11–13)
Case Selection
The selected study participants were patients hospitalized with sepsis from January 1, 2012 through December 31, 2014. We excluded patients aged <18 years, patients with reported age >120 years, prisoners, transferred patients, law enforcement patients, hospice patients, and patients with unknown hospital discharge status, missing race information, missing diagnosis, or with a race other than black or white (Figure 1). We did not exclude participants who were re-hospitalized for sepsis.
Figure 1.
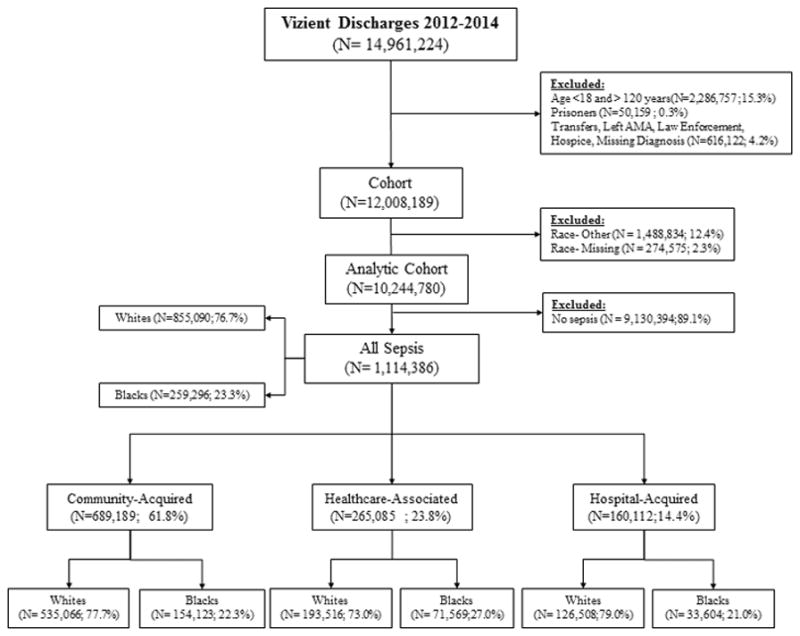
Study Population
Using the taxonomy of Angus, et al., we defined sepsis as hospitalizations with International Classification of Diseases, 9th revision (ICD-9) discharge diagnoses for both a serious infection and organ dysfunction.(1) We expanded the Angus definition to include diagnosis codes for pulmonary organ dysfunction: 518.8 (respiratory failure), 786.03 (apnea), and 799.1 (respiratory arrest). We included hospitalizations with discharge codes 995.92 (severe sepsis) and 785.52 (septic shock) as sepsis. We additionally selected patients with discharges with code 995.91 if it was associated with a corresponding organ dysfunction code. This approach has been shown to have modest sensitivity, but high specificity, modest to high positive predictive value, and high negative predictive value.(14, 15) In contrast, approaches limited to explicit codes have been shown to have very low sensitivities.(14, 15) Although the recent Sepsis-3 consensus guidelines suggest revised definitions for sepsis and severe sepsis, we opted to use the Angus approach because the Sepsis-3 definitions cannot be operationalized with conventional discharge datasets.(16) Furthermore, we wished to facilitate direct comparison with prior discharge-diagnosis-based assessments of sepsis racial disparities.(17)
Hospital-acquired infections have different mechanisms, microbial patterns, treatment strategies and outcomes than community-acquired infections.(8) In addition, infections acquired in healthcare settings such as long-term care facilities and nursing homes have etiological features distinct from community-acquired infections.(18) Prior studies could not distinguish diagnoses present on hospital admission from those developing at later points of hospitalization and thus could not differentiate community-acquired sepsis from hospital-acquired sepsis.(5, 6) A unique feature of the Vizient CDB/RM is the presence of “present at admission” flags for each discharge diagnosis. Following our prior strategy, we categorized cases with serious infection and organ dysfunction concurrently present on admission as community-acquired sepsis (CAS).(8) We categorized sepsis cases with both infection and organ dysfunction not present at admission as hospital-acquired sepsis (HAS). Furthermore, using the patient origin data field, we classified CAS cases originating from a nursing home, those on dialysis at the time of hospitalization, and those admitted to the hospital in the prior 30 days as health care-associated sepsis (HCAS).
Outcomes
The primary outcomes were sepsis hospitalization and hospital mortality. Hospital mortality was obtained using discharge status codes. Prior studies have shown that discharge disposition as coded in the CDB/RM have high concordance with patient-level case audits.(19) Additional outcomes of interest were number of organ dysfunction, and intensive care unit (ICU) admission at any time of hospitalization.
Covariates
Other examined variables included age, gender, and insurance pay type, weighted Charlson comorbidity index, ICU length of stay, 3M All Patient Refined Diagnosis Related Group (APR-DRG) risk of mortality and severity of illness at admission. We calculated weighted Charlson scores using secondary diagnostic codes and categorized the scores as 0, 1–2, 3–4 and >5.(8, 20) The APR severity of illness and risk of mortality are determined by the 3M APR-DRG Grouper System developed by 3M Health Information Systems (Saint Paul, MN), and assigns a category of risk (minor, moderate, major, or extreme) in a disease-specific manner based on the underlying problem and complicating or comorbid conditions.(21)
Data Analysis
We identified the proportion of sepsis discharge hospitalizations for each race category (blacks and whites). We also determined age-stratified sepsis hospitalizations for each race group. Using chi-squared and t-tests, as appropriate, we compared patient characteristics between blacks and white sepsis patients, including age, gender, insurance type, admission type, APR-DRG risk of mortality, APR-DRG severity of illness, ICU admission, length of hospital stay and hospital mortality. To evaluate the association between race and sepsis mortality, we fitted a multivariable random intercept regression model with hospital mortality as the primary outcome and race as the main exposure. We fitted hospital as a random intercept in order to account for the clustering of patients within each hospital. Model 1 assesses association between sepsis mortality and race adjusting for the hospital clustering. Model 2 includes Model 1 and further adjustment for age, gender, year, and risk of mortality at admission. We repeated all analyses stratified by sepsis subtypes (CAS, HCAS, and HAS). We further repeated these analysis for sepsis and sepsis sub-types for each race groups (18–30, 31–40, 41–50, 51–60, 61–70, 71–80, more than 80 [in years]). Considering that not all sepsis cases are high acuity, we conducted sensitivity analyses and replicated the main associations in those who were admitted to ICU, and those who had more than two organ dysfunctions. We conducted all the analyses using Stata 14.1 (Stata, Inc. College Station, Texas).
RESULTS
During 2012–2014 there were 14,961,224 hospitalizations among 249 hospitals. We included 10,244,780 in the analysis. (Figure 1) Among these hospitalizations, there were 1,114,386 sepsis cases (108.8 cases per 1,000 hospitalizations; 95% CI: 108.6–109.0), including 259,296 blacks (106.7 per 1,000; 95% CI 106.3–107.1), and 855,090 whites (109.4 per 1,000; 95% CI 109.2–10.9.6). Sepsis subtypes included: CAS 61.8%, HCAS 23.8% and HAS 14.4%. (Table 1)
TABLE 1.
Association between sepsis hospitalizations and race across sepsis subtypes
| Sepsis Subtype | White Sepsis (Cases per 1,000 Hospitalizations) | Black Sepsis (Cases per 1,000 Hospitalizations) | Odds Ratio* (95%CI) |
|---|---|---|---|
| All Sepsis | 109.43 | 106.72 | 0.97 (0.96–0.98) |
| Community-Acquired Sepsis | 68.53 | 63.43 | 0.87 (0.86–0.88) |
| Healthcare-Associated Sepsis | 24.76 | 29.46 | 1.30 (1.29–1.32) |
| Hospital-Acquired Sepsis | 16.13 | 13.83 | 0.86 (0.85–0.87) |
Sepsis hospitalizations were slightly lower for blacks than whites (106.72 vs. 109.43 per 1,000 hospitalizations; OR 0.97; 95% CI: 0.96–0.98; p<0.001). However, upon stratification by age decile, the proportion of hospitalizations for sepsis was higher for blacks aged >30 years old. (Supplemental Figure 1) Similarly, while CAS was less common in blacks than whites (63.43 vs. 68.53 per 1,000; OR 0.87; 95% CI: 0.86–0.88; p<0.001), CAS hospitalization was more common for blacks aged >30 years old. HCAS was more common in blacks than whites (29.46 vs 24.76 per 1,000; OR 1.30; 95% CI: 1.29–1.32 p<0.001); this association persisted for all age deciles. HAS was less common in blacks than whites (18.78 vs. 20.50 per 1,000; OR 0.86; 95% CI: 0.85–0.87 p<0.001); on age-stratification, HAS hospitalization rates was higher for blacks aged ≤50 years old.
Black individuals hospitalized for sepsis were younger than whites. (Supplemental Table 1) The most frequent type of insurance for both blacks and whites was Medicare. Blacks were more likely to be on Medicaid. Charlson comorbidity scores were higher for blacks than whites. Among all sepsis subtypes, the number of organ dysfunctions was similar for whites and blacks. (Supplemental Table 2) Similar proportions of whites and blacks had multiple organ dysfunctions (≥2 organ dysfunctions; 22.38 vs 23.62%). Blacks were more likely to have renal dysfunction, while whites were more likely to have respiratory, cardiovascular, and hematologic dysfunction. Similar patterns of organ dysfunctions by race were observed for each sepsis subtype (CAS, HCAS, HAS). For CAS, blacks were less likely to have major or extreme risks of mortality. Approximately 40% of all sepsis discharges were admitted to the ICU at some point during hospitalization, and this was similar between blacks and whites for all sepsis subtypes.
Sepsis hospital mortalities were: all sepsis 12.3 %, CAS 10.1 %, HCAS 13.2 %, and HAS 20.7%. Mortality was lower for blacks than whites in all sepsis subtypes after adjusting for age, gender, risk of mortality, and year in random-intercept models: all sepsis OR 0.85 (95% CI: 0.84–0.86); CAS 0.85(0.83–0.87); HCAS 0.91 (0.88–0.94); HAS 0.94(0.91–0.98). (Supplemental Table 3, Figure 2). When further stratified by age groups, adjusted measures of association indicated lower odds of mortality for blacks for all sepsis and CAS in the older age groups. (Supplemental Tables 4 and 5) However, these associations were generally attenuated for HCAS and HAS. (Supplemental Tables 6 and 7) Among those who were admitted to intensive care unit, the adjusted associations were significant for all sepsis [OR 0.96(95% CI 0.94–0.97)], and CAS [0.88(0.86–0.90)] but not for HCAS [0.97(0.94–1.01)] and HAS 1.05(1.00–1.09)]. CAS was the only significant subtype for those who had more than 2 organ dysfunction. (Supplemental Table 8)
Figure 2.
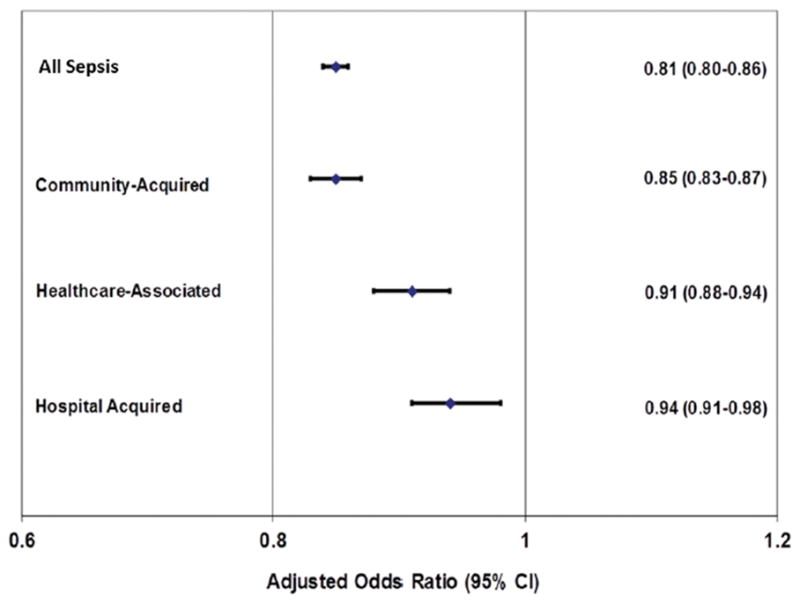
Adjusted odds ratio between sepsis mortality and race derived using mixed-effects method
DISCUSSION
In these data from the Vizient Consortium, there was a higher proportion of sepsis hospital discharges among whites than blacks. However, these associations varied by age strata and sepsis subtypes, with older blacks exhibiting higher sepsis rates than older white patients. Hospitalization characteristics were largely similar between blacks and whites. The adjusted odds of sepsis hospital mortality were lower for blacks than whites across sepsis subtypes.
Our finding of higher sepsis mortality in whites than blacks contrasts with prior studies. Using 2000 and 2005 hospital discharge data from seven states, Barnato, et al. and Mayr, et al. identified higher age-standardized sepsis incidence and case fatality rates for blacks compared with whites, attributing these differences to variations in hospital characteristics, socio-economic status, infections rates, and risk of organ dysfunction.(5, 6) Using statewide data, the authors assumed that all severe sepsis cases occurring in these communities were captured and produced population-based estimates of sepsis incidence and fatality. Our analysis differs in that the Vizient Consortium does not contain all hospitals in a given community. We view our paper not as a measure of the total community extent of sepsis, but as an indicator of the characteristics of sepsis patients presenting to this national hospital consortium.
Several observations support the validity of our findings. The Vizient CDB/RM contains national data reflecting a geographically diverse set of hospitalizations from the nation’s leading academic medical center-affiliated hospitals; while not nationally representative, these data arise from hospitals across the country, providing a more representative sample as compared with prior estimates which were produced using data from single or several states. Our findings are consistent with other hospital-based data sets. For example, Sandoval, et al. also observed lower sepsis mortality in racial minorities compared to whites in acute-care, non-federal California hospitals.(22) Similar to prior studies using National Inpatient Sample and National Hospital Discharge Survey, we also observed few racial differences in the number of comorbidities and organ dysfunctions. (23, 24) Interestingly, these prior studies did not find any racial differences in sepsis related mortality rates. Our data are also more contemporary relative to prior reports. Over the last ten years, international efforts have sought to improve the recognition and treatment of sepsis, which may have increased both the rates of sepsis documentation and survival.(25) Sepsis documentation practices have also evolved, potentially influencing observed racial differences.(26)
In contrast to prior efforts, we were able to stratify the analysis by sepsis subtype, highlighting important observations. For example, while CAS and HAS were less common among blacks as compared with whites, HCAS rates were higher for blacks. The higher HCAS rates in blacks may be explained by higher rates of infections among nursing home patients as well as the higher rates of hospital readmission.(27) However, HCAS was more common in younger blacks and older whites, suggesting the presence of additional factors driving these patterns. Our observations underscore the likely differences in epidemiology, etiology, course and outcomes between sepsis subtypes. These explanations are further supported by our sensitivity analyses where HAS-related mortality was slightly higher among those who had more than 2 organ dysfunctions, underscoring potential etiological factors driving these disparities Additional studies are needed to elucidate the influence of these factors in driving racial differences in sepsis outcomes.
There are several important limitations in our study. We identified severe sepsis using the Angus taxonomy, which assumes a connection between a documented infection and organ dysfunction. However, it is impossible to verify that the two entities are in fact related in given patients. The Angus strategy may also be impacted by coding practices. However, the Angus approach has been widely used in epidemiologic studies including prior studies using Vizient Database. (12, 13, 15, 28) Although the use of Angus algorithm to identify these sepsis phenotypes in administrative databases is a point of debate, this approach is still most reasonable and has shown to have high specificity, modest to high positive predictive values, and high negative predictive value over other approaches compared to chart review.(14, 15) While the chart review is the gold standard approach for accurate assessments of sepsis subtypes, it is not feasible in this extensive dataset of Vizient Consortium.
Hospital discharge data are influenced by recall and documentation bias and may be driven by factors unrelated to clinical care. While we used a large, geographically diverse administrative database, the Vizient CDB/RM data are not nationally representative. This prevented us from estimating age and gender standardized sepsis rates similar to prior studies. Our data do not include community hospitals unaffiliated with academic medical centers. We did not examine neighborhood factors that may have confounded the associations between race, sepsis care, and outcomes.(29)
We studied in-hospital mortality as we did not have access to longer term outcomes. While we used 3M APR-DRG risk of mortality for risk adjustment, this measure was not designed specifically for sepsis patients. Our observations may also be driven by differences in age, geographic location, hospital characteristics, hospital variation, and organ dysfunction.(5, 6) While there could be racial differences in sepsis care, we could not assess these variations within this dataset.
CONCLUSION
In conclusion, in these contemporary data from US academic medical center-affiliated hospitals, blacks exhibited lower odds of sepsis hospitalizations and adjusted sepsis death than whites.
Supplementary Material
Supplemental Figure 1: Age-Stratified Sepsis Hospitalizations by sepsis subtypes
Acknowledgments
FINANCIAL SUPPORT
H.E.W was supported by the National Institute of Nursing Research [R01-NR012726] and J.P.D was supported by the National Institute of General Medical Sciences [F31-GM122180] of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
We thank Dr Samuel Hohmann PhD, Vizient Consortium, for his assistance in reviewing the manuscript
Footnotes
CONFLICTS OF INTEREST
Dr. Wang, Dr. Chaudhary, and Mr. Donnelly do not report any related conflicts of interest.
AUTHOR CONTRIBUTIONS
H.E.W, N.S.C, and J.P.D conceived the study. H.E.W obtained funding. N.S.C and J.P.D conducted the analysis. N.S.C drafted the manuscript and all authors contributed to its critical review. H.E.W assumes overall responsibility for the paper.
Copyright form disclosure: Dr. Donnelly’s institution received funding from the National Institutes of Health (NIH)/National Institute of General Medical Sciences, and he received support for article research from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Critical care medicine. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Wang HE, Jones AR, Donnelly JP. Revised National Estimates of Emergency Department Visits for Sepsis in the United States. Critical Care Medicine. 2017;45(9):1443–1449. doi: 10.1097/CCM.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnato AE, Alexander SL, Linde-Zwirble WT, et al. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. American journal of respiratory and critical care medicine. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayr FB, Yende S, Linde-Zwirble WT, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. Jama. 2010;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive care medicine. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the university healthsystem consortium. Critical care medicine. 2015;43(9):1945–1951. doi: 10.1097/CCM.0000000000001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vizient. 2017 [cited 2017 February 9] Available from: https://www.vizientinc.com/
- 10.Center for Medicare and Medicaid Services, Department of Health and Human Services. CMS Manual System. 2006. [Google Scholar]
- 11.Davenport DL, Holsapple CW, Conigliaro J. Assessing surgical quality using administrative and clinical data sets: a direct comparison of the University Health System Consortium Clinical Database and the National Surgical Quality Improvement Program data set. American Journal of Medical Quality. 2009 doi: 10.1177/1062860609339936. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly JP, Hohmann SF, Wang HE. Unplanned Readmissions After Hospitalization for Severe Sepsis at Academic Medical Center–Affiliated Hospitals. Critical care medicine. 2015;43(9):1916–1927. doi: 10.1097/CCM.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HE, Donnelly JP, Shapiro NI, et al. Hospital variations in severe sepsis mortality. American Journal of Medical Quality. 2014 doi: 10.1177/1062860614534461. 1062860614534461. [DOI] [PubMed] [Google Scholar]
- 14.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52(6):e39–43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C, Dantes R, Epstein L, et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009–2014. JAMA. 2017;318(13):1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA: the journal of the American Medical Association. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnato AE, Alexander SL, Linde-Zwirble WT, et al. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. American journal of respiratory and critical care medicine. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care–associated pneumonia: results from a large US database of culture-positive pneumonia. CHEST Journal. 2005;128(6):3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 19.Sutton JM, Hayes AJ, Wilson GC, et al. Validation of the University Health System Consortium administrative dataset: concordance and discordance with patient-level institutional data. journal of surgical research. 2014;190(2):484–490. doi: 10.1016/j.jss.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. Journal of clinical epidemiology. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 21.Averill RF, Goldfield N, Hughes JS, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0. Methodology Overview: 3M Health Information Systems. 2003 [Google Scholar]
- 22.Sandoval E, Chang DW. Association Between Race and Case Fatality Rate in Hospitalizations for Sepsis. Journal of racial and ethnic health disparities. 2016;3(4):625–634. doi: 10.1007/s40615-015-0181-0. [DOI] [PubMed] [Google Scholar]
- 23.Bime C, Poongkunran C, Borgstrom M, et al. Racial Differences in Mortality from Severe Acute Respiratory Failure in the United States, 2008–2012. Annals of the American Thoracic Society. 2016;13(12):2184–2189. doi: 10.1513/AnnalsATS.201605-359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esper AM, Moss M, Lewis CA, et al. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Critical care medicine. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 26.Gohil SK, Cao C, Phelan M, et al. Impact of Policies on the Rise in Sepsis Incidence, 2000–2010. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;62(6):695–703. doi: 10.1093/cid/civ1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginde AA, Moss M, Shapiro NI, et al. Impact of older age and nursing home residence on clinical outcomes of US emergency department visits for severe sepsis. Journal of critical care. 2013;28(5):606–611. doi: 10.1016/j.jcrc.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189(5):548–555. doi: 10.1164/rccm.201311-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooke CR, Kahn JM. Deconstructing racial and ethnic disparities in critical care. Critical care medicine. 2010;38(3):978–980. doi: 10.1097/CCM.0b013e3181cc15d3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Age-Stratified Sepsis Hospitalizations by sepsis subtypes


