Abstract
The risk of cancer due to PCB exposure in humans is highly debated. In eastern Slovakia, high exposure of the population to organochlorines (especially PCBs) was associated with various disease and disorder pathways, viz., endocrine disruption, metabolic disorder & diabetes, and cancer, thereby disturbing several cellular processes, including protein synthesis, stress response, and apoptosis. We have evaluated a Slovak cohort (45-month children, at lower and higher levels of PCB exposure from the environment) for disease and disorder development to develop early disease cancer bio-markers that could shed new light on possible mechanisms for the genesis of cancers under such chemical exposures, and identify potential avenues for prevention.
Microarray studies of global gene expression were conducted from the 45-month-old children on the Affymetrix platform followed by Ingenuity Pathway Analysis (IPA®) to associate the affected genes with their mechanistic pathways. High-throughput qRT-PCR TaqMan low-density array (TLDA) was performed to further validate the selected genes on the whole blood cells of the most highly exposed children from the study cohort (n = 71).
TP53, MYC, BCL2, and LRP12 differential gene expressions suggested strong relationships between potential future tumor promotion and PCB exposure in Slovak children. The IPA analysis further detected the most important signaling pathways, including molecular mechanism of cancers, prostate cancer signaling, ovarian cancer signaling, P53 signaling, oncostatin M signaling, and their respective functions (viz., prostate cancer, breast cancer, progression of tumor, growth of tumor, and non-Hodgkin's disease). The results suggest that PCB exposures, even at the early age of these children, may have lifelong consequences for the future development of chronic diseases.
Keywords: Polychlorinated biphenyls (PCBs), Exposure, Future cancer incidence, Gene expression, Pathways
Introduction
Polychlorinated biphenyls (PCBs) are man-made synthetic chemical mixtures containing multiple isomers at different degrees of chlorination (with 209 possible congeners), which has been widely used for the industrial purpose over the last few decades. Since its ban in production during 1979 in the USA and other developed countries, due to its improper disposal, they remain persistent and ubiquitous in environment as contaminants. Humans are exposed to these complex chemicals that are routinely found in samples of human and animal tissues. The prolonged exposures to PCBs have been associated with the development of different diseases and disorder development, viz., reproductive (Plísková et al. 2005), neurological (Park et al. 2009, 2010), and endocrine effects (Rádiková et al. 2008), as well as hearing impairments (Trnovec et al. 2010), metabolic disorders including diabetes (Langer et al. 2014; Ghosh et al. 2013, 2014, 2015; Silverstone et al. 2012; Lee et al. 2011, 2012; Ukropec et al. 2010), cardiovascular diseases (Goncharov et al. 2010; Sergeev and Carpenter 2010), and cancers (Haslam et al. 2016; Parada et al. 2016; Leng et al. 2016, Pavuk et al. 2003, 2004; Bencko et al. 2009; IARC 2016 [IARC Monographs, vol. 107, 2016, available online at http://monographs.iarc.fr/ENG/Monographs/vol107/mono107.pdf]).
There is considerable debate in the scientific literature regarding the carcinogenicity of PCBs (Faroon et al. 2001; Laden et al. 2002; Golden et al. 2003, 2009). The United States Environmental Protection Agency (U.S. EPA) classified this class of compounds as probably carcinogenic to humans (http://www.epa.gov/epawaste/hazard/tsd/pcbs/pubs/effects.htm), and the International Agency for Research on Cancer has classified it as a group 1 carcinogen (IARC 2013, 2016). Other federal agencies that have assessed the issue have similar findings (NTP 2006, 2011, 2016; Shields 2006). Nonetheless, it is widely acknowledged that the data supporting these conclusions are based on epidemiological information and some of the strongest evidence for PCBs and cancer comes from population based studies. However, the limited numbers of occupationally exposed subjects, inconsistencies and shortcomings in the methods of assessing by measuring the exposures made the assessment difficult (Carpenter 2011; Knerr and Schrenk 2006; Golden and Kimbrough 2009). This is also highly complex due to variability of mixtures of PCB congeners across studies and the inherent difficulties of ascribing health effects to specific compounds (Hopf et al. 2009).
PCBs have been deemed to be probable carcinogens by the Environmental Protection Agency, and exposure to high levels of PCBs has been consistently linked to increased risk of non-Hodgkin lymphoma (NHL) (Freeman and Kohles 2012). PCB congeners have been associated with increased risk of adult NHL in cohort and case-control studies (Colt et al. 2005; De Roos et al. 2005; Engel et al. 2007a, 2007b). Significant positive trends in acute lymphocytic leukemia (ALL) risk were apparent with increasing concentrations of PCB congeners 118, 138, and 153, and the association with the PCBs was stronger among non-Hispanic whites than among Hispanics despite similar distributions of PCB levels among controls in each racial/ethnic group (Ward et al. 2009). The findings suggest that PCBs, which are considered human carcinogens and cause perturbations of the immune system, may represent a previously unrecognized risk factor for childhood leukemia. Detection of any PCB congener in the dust conferred a twofold increased risk of ALL while studying a population-based case-control study in 35 counties in northern and central California in 2001–2006. A preliminary study has also indicated that plasma levels of PCBs might pose a risk of cutaneous malignant melanoma (Gallagher et al. 2011).
Our pioneering recent gene expression studies in a unique, prospectively studied Slovak cohort (Ghosh et al. 2015) have revealed associations with disease and disorder development that are in accord with various reported epidemiological studies (Ghosh et al. 2013, 2014, 2015; Mitra et al. 2012, Dutta et al. 2008, 2012). These results indicated that certain genes are over-expressed at higher exposure levels. Even with the strong epidemiological evidence, mentioned above, the direct evidence through molecular transcriptional changes towards carcinogenicity upon PCB exposure in such exposed population is limited. In this study, we hypothesized that long-term low exposure to PCBS is associated with increased risk of cancer development, as reflected in specific gene alterations and their mechanistic pathways. In turn, these patterns (genes in the pathways) could be markers to detect the possibilities of disease early. To that end, epidemiological data (45-month-old Slovak exposed children) with special emphasis on differential gene expression and pathway analysis by IPA informed our insights into the underlying gene expression changes that may pose an early sign of developing cancer in later life under such an exposure scenario of children.
Methods
The study was undertaken with the prior approval by the Howard University Institutional Review Board (IRB-07-GSAS-30). Subjects for this study principally belong to a well-defined cohort of mother-and-children pairs, originally enrolled in the “Slovak PCB Effects on Early Child Development Study” between 2001 and 2004 (Hertz-Picciotto et al. 2003, Sonneborn et al. 2008). Biospecimens (placenta, maternal blood, and cord blood) were collected from mothers and corresponding children at delivery along with the detailed questionnaires. Repeated follow-up information along with the collection of blood was obtained at 6, 16, and 45 months of age following prior Institutional Review Board approval at Slovak Medical University. Details of the recruitment and characterization of this cohort have also been described elsewhere (Park et al. 2010). Shortly, mothers with children were recruited from the Michalovce District area, highly contaminated by PCB from a chemical manufacturing plant and from the Svidnik/Stropkov District, with lower environmental PCB contamination and situated approximately 70 km north from Michalovce. We selected 71 subjects (male = 30, female = 41) solely based on their blood PCB concentrations at the age of 45 months (with the average blood PCB-153 levels 210 ng/g of lipid) from earlier studies (Ghosh et al. 2013, 2014, 2015, Dutta et al. 2012, Mitra et al. 2012), including all who had attained 45 months of age.
The control subjects (n = 5; males n = 2, females n = 3) were from the Stropkov/Svidnik area, and they followed all the same procedures as the high-exposure subjects. In reality, due to the legacy of widespread environmental contamination of PCBs in Slovakia, it is practically impossible to find subjects who are not exposed to any extraneous chemicals in their life course, whatever the age we chose.
The blood samples were collected by the trained nurses and/or by certified phlebotomist under the supervision of the medical team from Slovak Medical University. Blood was collected into a PAXgene blood RNA tube (IVD), which were brought to Bratislava and then ultimately shipped to collaborators lab at USA.
RNA isolation and cDNA synthesis
The RNA samples were prepared from the whole blood of the children who attained 45 months of age in this cohort and were processed as previously described (Ghosh et al. 2013, 2015). RNA extraction was performed according to the manufacturer's instruction using PAXgene Blood RNA kit (Cat # 762164, PreAnlytiX GmbH, Germany) and TRIzol® Plus RNA Purification System (Invitrogen California CA), respectively, followed by cDNA synthesis with High-capacity cDNA Reverse Transcription Kits (Part # 4387406; Applied Biosystems, CA, USA) (See Ghosh et al. 2013, 2014, 2015 for detailed procedural description). The RNA was stored at − 80 °C. The cDNAs were stored in − 15 to – 25 °C, if not used immediately (within 24 h), or stored in 2 to 8 °C.
Analysis towards the identification of cellular processes and pathways involved by ingenuity pathway analysis (IPA).
For this study, we used our previously published data, along with the “Minimum Information About a Microarray Experiment” (MIAME) compliant data that has been submitted to the Gene Expression Omnibus (GEO) database. The datasets used in this paper can be accessed from the following GEO links: GSE22868 (45 month, Dutta et al. 2012), GSE22668 (Mixed PCB, Ghosh et al. 2015), GSE22667 (PCB-153, Ghosh et al. 2013), GSE22632 (PCB-138, Ghosh et al. 2013), and GSE32420 (0 month, new cohort), from the children's cord blood from a recruitment under a new cohort study, initiated in Slovakia.
Datasets containing gene identifiers and corresponding expression values (fold change) were uploaded into IPA software (Ingenuity® Systems, www.ingenuity.com). Each gene identifier was plotted to its similar gene entity in the Ingenuity Pathways Knowledge Base. We engaged the information in the Ingenuity Knowledge Base (Genes Only) as a reference set that consider both direct and indirect relationships. The molecules were included as and/or the relationships only. To advance our pathway analysis, we incorporated additional 527 gene transcripts from IPA knowledge base to our results. We used the data sources from ingenuity expert findings and used the “Core Analysis” function to interpret the data in the perspective of biological processes, pathways, and networks. Differentially expressed gene identifiers were defined as value parameters for analysis and identified the relationship between gene expression alterations and related changes in bio-functions under the subcategories of molecular and cellular functions, physiological system development and function, and disease and disorders. Genes differentially expressed with p < 0.05 were overlaid onto global molecular networks developed from information contained in the knowledge base. Networks were then algorithmically generated based on their connectivity. Networks were “named” on the most prevalent functional group(s) present. Canonical pathway (CP) analysis identified function specific genes significantly present within the networks.
High-throughput TaqMan® low-density array (TLDA)
To identify altered gene expression, we used predesigned TLDA cards (Applied Biosystems®, CA) to examine the expression of four genes of interest as identified in the methods described above (including IPA knowledge base) to examine in our exposed Slovak population through a small population validation study. The TaqMan low-density array (TLDA) procedures are depicted in details that can be found in Ghosh et al. (2015).
TLDA data analysis
The TLDA data were analyzed by SDS Ver. 2.4 software (ABI, CA). Threshold cycle (Ct) data for all target genes and control gene 18s RNA were used to calculate ΔCt values [ΔCt = Ct (target gene) – Ct (18s RNA)]. Then, ΔΔCt values were calculated by subtracting the calibrator (control) from the ΔCt values of each target. To visualize and further expression analysis, the data were exported in plate centric format to DataAssist V2.0 (ABI, CA) which allowed us to inspect the status of a gene(s) in the respective groups of lower and higher PCB exposures.
Results
Differential expression of genes with 45-month Slovak children
The Human Genome U133 Plus 2.0 chip that we used for the microarray gene expression analysis used the Affymetrix array platform and probe sets. The microarray data were filtered and normalized by PLIER (http://www.affymetrix.com/support/technical/technotes/plier_technote.pdf) (Seo and Hoffman 2006), and a subsequent statistical analysis was performed using the Partek Genomics Suite™ analysis tool. Differential expression was compared using unpaired t test statistics. From the 54,675 probe sets, for the Slovak population of 45-month-old children, we selected the top 2163 genes that were differentially expressed (both up-/down-regulated) and determined to be statistically significant using the t test, with FDR of p < 0.05). Out of these 2163, about 47% of the genes were up-regulated and 53% were down-regulated, when compared to control (See Supplemental Table 1). This also corroborates the results of our previous studies where most the genes were primarily down-regulated (Ghosh et al. 2011, 2015; De et al. 2010, Dutta et al. 2012; Mitra et al. 2012).
The effects of the differentially expressed genes on overall biological processes in Slovak PCB-exposed children
The list of biological effects associated with exposure to PCBs in Slovak subjects can be found in two levels: network level (Table 1) and bio-functions level (Fig. 1). Analysis of the genes identified above revealed six significant genetic networks (score ≥ 20.0), as listed in Table 1. This score indicates the likelihood that the assembly of a set of focus genes in a network could be explained by random chance alone. The database attributed general cellular functions to each network which are determined by interrogating the Ingenuity Pathway Knowledge base for relationships between the genes in the network and the cellular functions they impact. The top-scoring networks among disease and disorder include the following: cancer, organismal injury and abnormalities, immune cell trafficking, inflammatory response, neurological disease, connective tissue disorder, immunological disease, inflammatory disease, and tumor morphology (Fig. 1; panel B). Among the genes in the molecular and cellular functions network, gene expression, cell cycle, cell-to-cell signaling and interaction, cell death & survival, cell morphology, cellular growth & proliferation, cell function & maintenance, RNA post-translational modification, and cell signaling function were revealed (Fig. 1; panel C). In the physiological system development and functions network, notable functions were organismal development, cell-mediated immune response, immune cell trafficking, organ morphology, tissue morphology, tissue development, connective tissue development, hematological function, lymphoid tissue structure development, organ development, and skeletal/muscular system development & function (Fig. 1, panel A).
Table 1.
High-scoring networks (score > 20) identified by Ingenuity Pathway Analysis® in PCB-exposed Slovak children (age 45 months). The top six of 24 networks are represented here
| Network ID | Genes in network | Score | Focus molecules | Functions |
|---|---|---|---|---|
| 1 | ADAM10, ALCAM, APP, ARPC2, ATF1, BACE1, BAX, BCAS2, BCL2, BCL2L11, BECN1, CAV3, CDK2, CREB1, CREBBP, DPH5, E2F2, EGLN1, EP300, FKBP8, GHITM, GRIN2A, HES6, HNRNPM, HSPH1, IGFBP5, ITPR3, LAPTM4B, LATS1, LNX2, LRPPRC, LYN, MAPK8, MDM2, NDUFB5, NDUFB8, NEDD9, NFATC4, NOS1, NUDT16L1, PIK3C3, PMAIP1, PSEN1, PSMA1, PTEN, PTGS2, RASSF3, RBL2, REV1, RHBDD2, RHEB, RNF146, RSF1, SEC22B, SKP2, SMARCA2, SMARCE1, SNAP23, SOD1, STX6, TMED9,TMOD3,TNKS2,TNPO2, TOPBP1, TPD52, TRMT10C, WHSC1, YWHAQ, YWHAZ. | 63 | 70 | Cell death & survival cellular development cellular growth & proliferation |
| 2 | AMIGO2, ANP32B, APOA5, ATG5, ATG12, ATP1B1, BACH1, BCL2L14, BRWD1, C1QTNF7, CAPN2, CAST, CCAR1, CDC42EP3, CLEC7A, CORO1C, CSNK1A1, CXCR4, CYLD, DAD1, DAPK3, DDX28, DNAJC10, DUSP22, EMP1, FADD, GID4, GLIPR1, GPBP1, HAVCR2, HIRA, HUS1B, IFT122, ING3, JADE1, LGALS8, MAP4K3, MTHFSD, NCAPH2, NCOA1, NLK, NR3C1, PAWR, PAX3, PIK3R3, PLAGL1, PPID, PPP3CA, PPP3R1, RANBP9, RMND5A, RTN4, SDPR, SERTAD2, SON, SPTBN1, STRN, SYNCRIP, THAP1, THRA, THRB, TM2D3, TNFAIP8, TNFRSF10D, TNFRSF12A, TNFSF4, TP53BP2, WDR3, WDR26, ZAK | 63 | 70 | Cell death & survival inflammatory disease ophthalmic disease |
| 3 | ADAM9, ADAM15, ANKRD12, BCAR1, BIRC2, BLZF1, CCNH, CDC42, CDK8, CDK19, CHCHD6, CNR2, CRK, CSF1, CSGALNACT2, CTBP2, CXCL12, DCAF13, DIDO1, DISC1, DSE, DVL1, ERBB3, EVI5, F12, FRA10AC1, FYB, GAS1, GMFG, GOPC, GSR, GTF2B, GTF2H2, ITGAV, ITGB1, ITGB4, ITPR2, JRK, KRTAP1–3, LCP2, LIMS1, MAPK1, MED13, MMP14, MMP28, NFRKB, NRG1, OLFML2A, PDE4D, PLA2G10, PLEC, PNN, PTK2, PTP4A2, RAB21, RANBP2, RUNX2, SENP6, SETD2, SMAD2, SNIP1, SNW1, SORBS3, SP100, SUMO1, U2AF2, UBE2I, WIPF1, ZFYVE16, ZNF143 | 63 | 70 | Cell-to-cell signaling & interaction, cellular movement, cell morphology |
| 4 | ABCC3, ABCG1, AGT, AHR, ARNT, ATF3, ATM, ATR, CDCP1, CDK12, CLDN7, CTR9, CTSC, CYP2A6 (includes others), DYNC1H1, DYNC1I2, EDN1, EFNA4, FAAP100, FANCA, FANCM, FOXA2, FUT11, GCLM, GRSF1, HNF4A, HNRNPD, HSP90AA1, IL22, IL37, IL18RAP, IL1A, IL6R, IRS2, JAG2, KHSRP, KLF6, KRT10, MUC5B, MYO5A, NFE2L2, NIPBL, NPC1L1, OAT, PLPP6, PQLC3, PRKCB, PTGES, RAB10, RAB14, RAB11A, RAB39B, RNF168, RP2, SOX10, SREBF1, SREK1IP1, SUCLG1, SYF2, TADA2B, TAF5L, SUCLG1, SYF2, TADA2B, TAF5L, TNFRSF9, TOP3A, TP53INP1, TTN, TXN, UGT1A7 (includes others), USP29, XPA, ZCCHC6 | 63 | 70 | Cell-to-cell signaling & interaction, hematological system development & function, immune cell trafficking |
| 5 | AHCTF1, APC, ARFGEF1, ARID4A, AXIN1, BAZIB, BLOC156, BUB3, CBX1, CBX3, CDK5R1, CETN2, CITED2, CRBN, CUL4A, DAB2, DDA1, DYNC111, ETV6, F2R, FL11, H2AFY, HDAC2 HDAC9, HMG20B, IER5, IKZF1, ING1, ITGA2B, let-7, NACC2, NCOR1, NUP98, NUP107, NUP133, NUP153, NUP160, PAK2, PDSSA, PHF21A, PLA2G16, PREX1, PRKAR2, RAD21, RAD23B, RAE1, RCOR1, RNY, RUNX1T1, SAP30, SESN3, SMARCA5, SMARCAD1, SNX1, SNX2, SNX5, SNX6, SPDEF, SSB, STAG1, STAG2, STX4, STXBP3, TBX3, TFAP2A, TPR, TROVE2, VEGFB, VPS26A, YYI | 34 | 54 | Gene expression, post-translational modification, cellular movement cellular morphology |
| 6 | ANGEL1, ANP32A, AP5M1, CARD8, CASP8, CCNA2, CEP97, CHAMP18, DDX46, GCNT2, GMEB1, HIVEP3, KBTBD7, MBD2, MGME1, MKKS, MLK2, NEIL3, NF1B, NR1D2, NUPRI, OSER1, PCTP, PCYOX1, PEX10, PEX12, PMP22, PTGS3, RFTN2, RFX5, SIGMAR1, SLC16A6, ST3GA12, THG1L, UCA1, UEVLD, VANGL1, WDR76, ZBTB34, ZPR1 | 23 | 33 | Developmental disorder, hereditary disorder, metabolic disease |
The genes found to be differentially regulated in our experiments and the number of such genes displayed in the “focus molecules” column have been highlighted in bold print that meet the criteria cutoff and/or filter criteria and were mapped to its corresponding gene object in IPA knowledge base (bold up-regulated, regular font down-regulated). The score is generated using a p value calculation. This score indicates the likelihood that the assembly of a set of focus genes in a network could be explained by random chance alone. The database attributed general cellular functions to each network which are determined by interrogating the Ingenuity Pathway Knowledge base for relationships between the genes in the network and the cellular functions they impact
Fig. 1.
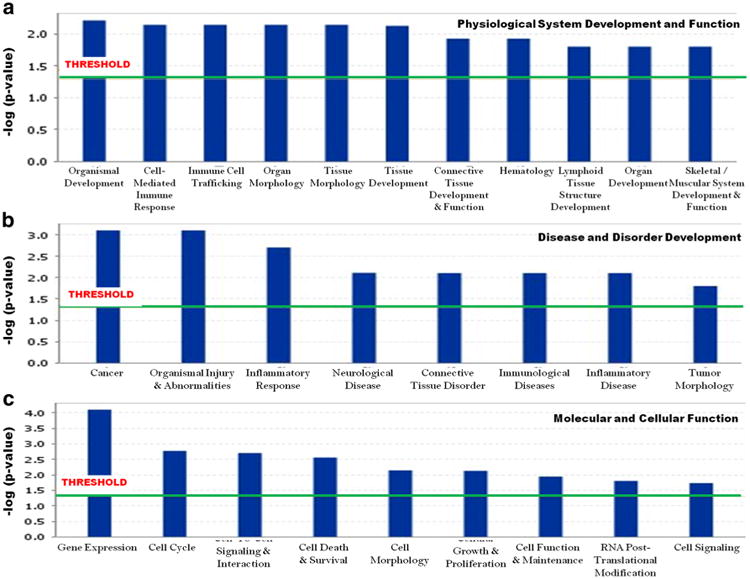
The key (top-scoring) bio-functions in developing toxicities with the differentially expressed gene set in 45-month-old children of Slovakia was obtained through IPA analysis. Exposure to PCBs was associated with physiological system development and functions (a), disease and disorder development (b), and molecular and cellular functions (c). The most statistically significant bio-functions that were identified in the IPA Tox analysis are listed here according to their p value (− log). The threshold line corresponds to a p value of 0.05
The canonical pathways and GO enrichment of biological processes in Slovak PCB-exposed children
In the CP analysis, we chose to build the pathways connecting the top six networks (networks 1–6, score ≥ 20, Table 1). The top eleven (11) cancer-related CPs primarily revealed through this approach were as follows: p53 signaling, chronic myeloid leukemia signaling, thyroid cancer signaling, apoptosis signaling, endometrial cancer signaling, xenobiotic metabolism signaling, prostate cancer signaling, ovarian cancer signaling, oncostatin M signaling, molecular mechanisms of cancers, and colorectal cancer metastasis signaling (Fig. 2); along with their related function (Fx), e.g., progression of tumor, growth of tumor, non-Hodgkin's disease, breast cancer, and prostate cancer that were identified as differentially expressed in 45-month children were integrated into computational generated networks based on evidence stored in the IPA knowledge base indicating relevance of this network. Further in-depth analysis also identified some important pathways, viz., aryl hydrocarbon signaling, G-protein coupled signaling, cell-cycle: G2/M DNA damage checkpoint regulation, and NF-kB signaling, (Fig. 3), which are in accord with our previous investigations (Ghosh et al. 2010, 2011, 2013, 2014, 2015, Mitra et al. 2012, Dutta et al. 2012).
Fig. 2.
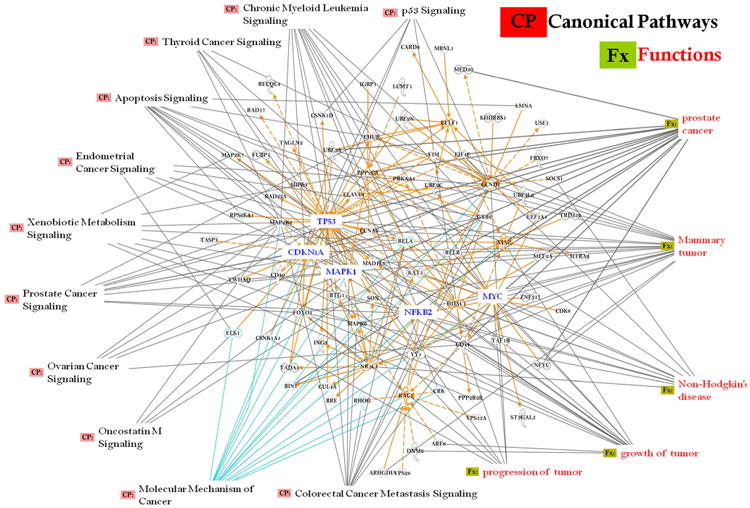
Connectivity of differentially expressed genes in the important cancer signaling pathways in the exposed children are shown according to gene expression levels (with ≥ 1.5-fold change, t test, p < 0.05). Genes in the top six networks were allowed to grow our pathway with the direct/ indirect relationship from the IPA knowledge base using the most stringent filter, i.e., based on the data from our human study. Solid interconnecting lines show the genes that are directly connected, and the dotted lines signify the indirect connection between the genes and cellular functions. Canonical functions (signaling) that are highly represented are shown within the box (CP) along with their related function (Fx): they were identified as differentially expressed in our experimental results and in the results from the 45-month-old children
Fig. 3.
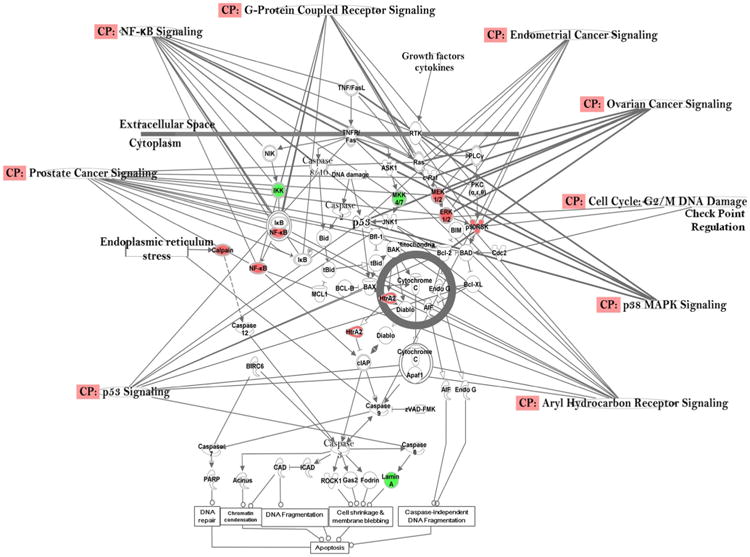
Differentially expressed genes in the important cancer-relevant signaling pathways and their connectivity in the 45-month-old PCB exposed population in Slovakia, showing genes with significant effects on expression levels (with ≥ 1.5-fold change, t test, p < 0.05) with some downstream effects, e.g., DNA repair, DNA fragmentation, chromatin condensation, cell shrinkage & membrane blebbing, caspase-independent DNA fragmentation, and apoptosis. Geometric figures in red denote up-regulated genes and those in green indicate down-regulation. Canonical pathways (functions/signaling; CP) viz., p53 signaling, prostate cancer signaling, NF-kB signaling, G-protein coupled receptor signaling, endometrial cancer signaling, ovarian cancer signaling, cell cycle: G2/M DNA damage checkpoint regulation, p38 MAPK signaling, and aryl hydrocarbon receptor signaling that are highly represented are shown within the box. Genes in uncolored notes were not identified as differentially expressed in our experiment
Population validation of selected genes through TLDA
High-throughput quantitative real-time PCR (q-PCR by TLDA in ABI platform) confirmed the PCB-associated altered expression of our four signature genes under population validation (n = 71). All four genes were well-amplified (either up-/down-regulated, Table 2, Fig. 4).
Table 2. Differential expression of four genes of interest through relative quantification (ΔΔCt) that selected for high-throughput TLDA card design and their corresponding probe sets (pre-designed and validated, from ABI, CA) in small set population validation study.
| Gene name (probe sets) | Descriptions/functions | Population results (n = 71)a |
|---|---|---|
| LPR12 (Hs00257526_s1) | Low-density lipoprotein–related protein 12; candidate tumor suppressor gene | −0.67(n = 52, 94%) +0.30 (n = 3, 5.4%) |
| MYC (Hs00153408_m1) | Proto-oncogene, cell cycle progression, apoptosis, and transformation/transcription of specific target gene | − 0.73 (n = 52, 98%) +0.10 (n = 1, 1.8%) +0.80 (n = 16, 24%) |
| TP53 (Hs01034249_m1) | Tumor protein p53 | − 0.40 (n = 51, 71%) +0.28 (n = 15, 21%) |
| BCL2 (Hs99999018_m1) | B cell lymphoma 2 family regulator protein | − 4.86 (n = 14, 20%) +2.45 (n = 56, 78%) |
Number (n) in parenthesis is the total number of subjects where such changes were observed. % calculation was made only among the subjects with amplification under this validation platform
Data represented as ΔΔ Ct changes (relative quantification) with down-regulation (−)/up-regulation (+)
Fig. 4.
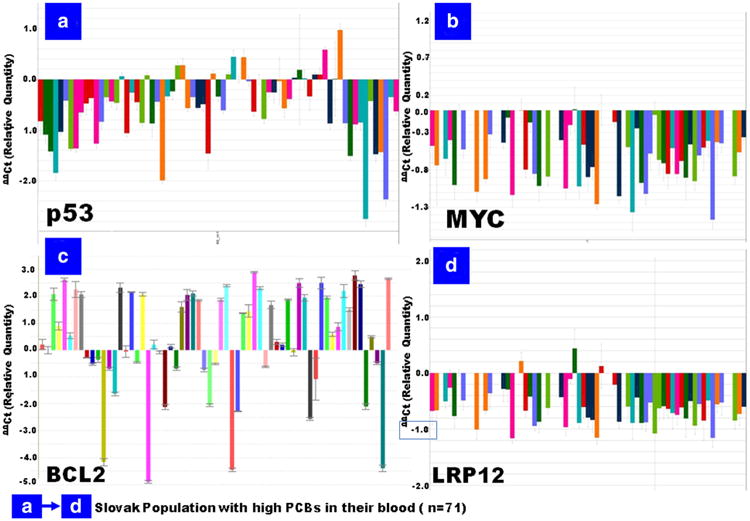
Quantitative real-time PCR (qRT-PCR) validation of the selected three cancer-related genes of interest by TaqMan low-density array (TLDA) in ABI platform (7900HT fast real-time PCR system) after analysis by SDS RQ Manager Version 1.2.1 (ΔΔCt). The panels a–d (with the respective genes) represent the relative quantification of the genes in the small population validation study (the population with highest level of PCBs in their blood; n = 71). The relative quantification is calculated in contrast to calibrator samples, i.e., the subjects with no/background PCBs exposures in the population
We found four genes that were well-annotated in the connectivity analysis (Fig. 2) and in the important cancer-relevant signaling pathways (Fig. 3). Those are TP53 MYC, BCL2, and LRP12. TP53 gene results (Fig. 3) were consistent with the human studies that provided prior suggestive evidence that PCBs are carcinogenic (ATSDR 2001, Faroon et al. 2001, Laden et al. 2002). The signature genes, TP53, MYC, BCL2, and LRP12, which we presume could serve as putative biomarkers under such an exposure scenario, were further validated in the PCB-exposed human population for their consistency (Fig. 4, panels A–D). The genes TP53 MYC and LRP12 were mostly down-regulated and were well-corroborated with the population samples (Fig. 4, panels A, B, C, and D respectively). The BCL2 gene has come up with either up- and/or down-regulated in the population study (Slovak children) (Fig. 4).
Discussion
During the last decade, with the advent of genomics and advances in molecular biology, there has been increasing interest in the use of panels of genes and their pathways as biomarkers in epidemiological research to enhance toxic exposure assessment. Our group has identified several such genes of interest that were significantly associated with PCB exposure within the specific disease pathways. We observed that several important cancer-related genes were affected. In our gene expression studies, we identified TP53, MYC, BCL2, and possibly LRP12 as potentially important signature biomarkers for the future disease risk assessment in such populations.
We observed that the p53 gene (a tumor suppressor gene) was down-regulated in the experimental model and had a similar pattern of dysregulation in the majority of human subjects in our study (Fig. 4, panel A). In one of our prior investigations, we also found that p53 was down-regulated over a short exposure period, showing loss of cell viability and apoptosis, but was up-regulated over a chronic exposure of 12-weeks period (Ghosh et al. 2007), which corroborates our present observation concerning the involvement of cell cycle and cell death pathways in the molecular and cellular functions (Fig. 1C). The central role of p53 as a regulator of the cell cycle and a key tumor suppressor gene is well-established in human cancer research (e.g., Dang 1999).
Another gene of importance in carcinogenesis is LRP12, which is part of the LDLR superfamily and mediates signal transduction (Qing et al. 1999; Battle et al. 2003). Some researchers such as Garnis and colleagues (Garnis et al. 2004) have published evidence of an oncogenic role for this gene in certain oral cancers. Our epidemiological investigations on LRP12 corroborated those prior finding (Fig. 4, panel D). The LRP12 gene, on which we might be first group to be reporting in relation to PCB exposure, has an expression pattern that might possibly be related to tumor morphology and cancer development under such exposure scenario (Fig. 1, panel B). However, the information on that gene is limited. Some researchers have reported about the over-expression of LRP12 in association with oral cancer and suggested that it may act as an oncogene (Garnis et al. 2004). Clearly, more work is needed to confirm such speculation. We note that, based on gene expression patterns in our study population, one of the top CPs was lipid metabolism that may reflect the influences of PCB's extremely lipophilic profiles.
The BCL2 gene has been implicated in a number of cancers, including melanoma, breast, prostate, and lung carcinomas (Yip and Reed 2008). In our observation, BCL2 appeared to be one of the key molecules affected by PCBs. The down-regulation of the BCL2 gene in our experimental study suggested that high PCB exposure can favor the apoptotic pathway, which is in agreement with our previous results with an in vitro model (Ghosh et al. 2007, 2010). The over-expression of BCl2 (78% in our epidemiological setting) can be substantiated by our prior observation, where the expression of BCL2 also demonstrated reverse expression, which was up-regulated in the chronically exposed human liver cell line (HepG2) and complemented the anti-apoptotic nature, thereby maintaining continuous growth (Ghosh et al. 2007). The over-expression of the anti-apoptotic BCl-2 protein in lymphocytes alone does not cause cancer. But simultaneous over-expression of BCl-2 and the proto-oncogene myc may produce aggressive B cell malignancies including lymphoma (Otake et al. 2007).
Epidemiological evidence of association between exposures to PCBs and increased cancer risks has come from diverse occupational populations. For example, dose-related increased risks for liver cancer, cholangiocarcinoma (bile duct cancer), various gastrointestinal malignancies, lymphoma, and leukemia have been reported among workers who were employed in manufacturing capacitors in several countries (Gustavsson and Hogstedt 1997; Mallin et al. 2004; Prince et al. 2006a, b). Some studies detected no increase in cancer incidence among similarly exposed workers. One consistent association in the occupational epidemiology literature has been the occurrence of NHL among workers exposed to PCBs (Rothman et al. 1997; Colt et al. 2005, 2009; De Roos et al. 2005; Engel et al. 2007a, b; Spinelli et al. 2007; Bertrand et al. 2010, Laden et al. 2010) and was recently summarized by Freeman and Kohles (2012). There is also at least one report (Ng et al. 2010) documenting an interaction between occupational PCB exposure and a SNP of the aryl hydrocarbon receptor gene (IVS1 + 4640 G/A), suggesting an underlying genetic susceptibility for NHL.
The regression analysis of BCL2 gene expression showed that 76% of the variation observed seen in 45-month children of Slovakia can be explained by the sum of their PCBs load (ng/g lipid) in their blood. This finding could paved the way for the future of development of qPCR gene expression-based exposure profiling, through large population validation, which could be helpful for defining a subgroup at higher risk for future disease (Fig. 5).
Fig. 5.
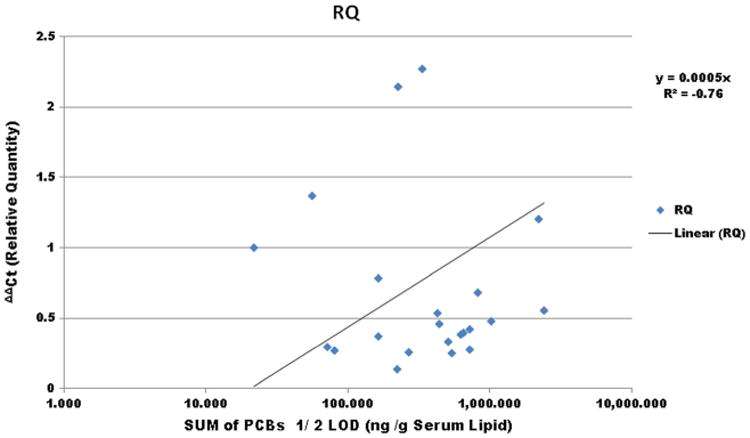
The regression analysis of BCL2 gene expression (qRT-PCR, ΔΔCt (relative quantity)) when plotted against sum of PCBs in blood (ng/g serum lipid) depicts that 76% (R2, coefficient of determination = 0.76) of the variance seen in gene expression can be explained by the sum of PCBs load (ng/g lipid, 1/2 LOD) in their blood, with a few outliers (19%). The correlation coefficient is 0.66
Our pathway analysis (by IPA®) poses a significant overview of the underlying biological processes that suggest that these children may have higher cancer risk in the later part of their lives. The important cancer signaling pathways altered in the 45-month-old exposed children included p53 signaling, prostate cancer signaling, endometrial cancer signaling, ovarian cancer signaling, colorectal cancer metastasis, molecular mechanism of cancers, oncostatin M signaling, xenobiotic metabolism signaling, thyroid cancer signaling, and chronic myeloid-leukemia signaling, with a strong connectivity to the focus molecules of TP53, MAPK1, MYC, NFKβ2, and CDKN1A (p21). Furthermore, there were direct and indirect connections between these genes and cellular functions, e.g., progression of tumor, growth of tumor, non-Hodgkin's disease, breast cancer, and prostate cancer (Fig. 2). The downstream effect effects, e.g., DNArepair, DNA fragmentation, chromatin condensation, cell shrinkage & membrane blebbing, and caspase-independent DNA fragmentation can lead ultimately to apoptosis and other cellular instability (Fig. 3). Apoptosis, a cell-suicide program that is executed mainly by caspases, is critical for maintaining tissue homeostasis, and impaired apoptosis is now recognized to be a key step in tumorigenesis. Whether a cell should live or die is largely determined by the Bcl-2 family of anti- and pro-apoptotic regulators (Cory et al. 2003).
In our study, we observed the down-regulation of MAPK1, which could represent the dysregulation of an important, environmentally sensitive network that regulates cell growth (Huang et al. 2008). MAPK1 is part of the MAP kinase family, with widespread effects on cell growth and proliferation, inflammation, the regulation of transcription, and ontological development (Voong et al. 2008).
Risk of breast cancer was found by another group of researchers to be positively associated with heavy PCB congeners, age, postmenopausal status, family history of breast cancer, and living close to an industrial facility: that study showed an association between heavy and potentially estrogenic PCB congeners and breast cancer risk (Recio-Vega et al. 2011). In another study defining the human exposure to polyhalogenated hydrocarbons and incidence of selected malignancies in analyzing the incidence of selected malignancies in two populations exposed to polychlorinated hydrocarbons, Bencko et al. 2009 opined that neither PCBs nor TCDDs/Fs appear to contribute to the observed significantly lower incidence of breast and prostate cancer in the Michalovce District and lower bladder cancer incidence in Uherske Hradiste District of Slovakia. However, anti-estrogenic and anti-androgenic properties have been described for hydroxylated and methylsulfonyl PCB metabolites. These properties could contribute to a mechanism through which these metabolites might modulate the development of breast, prostate, and bladder cancer, which is also corroborated and exhibited well in our pathway analysis (Fig. 2).
In a study that investigated a population-based cohort of 32,496 Swedish men aged 45–79 years (1988–2011) along with experimental studies about dietary PCBs in relation to prostate cancer incidences, there was a suggestion of a role of non-dioxin-like PCB153 in the development of high-grade and fatal prostate cancer (Ali et al. 2016). PCB 153, the major contributor of the PCB load (ΣPCBs) in our studies subjects, may also pose a possible future risk of developing prostate cancer, as revealed through our pathway analysis (Figs. 2 and 3).
Our investigation of differential gene expression status in the 45-months old exposed Slovak children exposed to PCBs revealed that the highest number of genes was involved in molecular mechanisms of cancer (N = 335), along with substantial number of genes involved in the majority of cancer signaling pathways (Fig. 6). In all cases, most of these genes do not overlap with the IPA knowledgebase, which suggests that PCB exposure creates a discreet gene expression signature in specific pathways that might lead to development of diseases and disorders later in life.
Fig. 6.
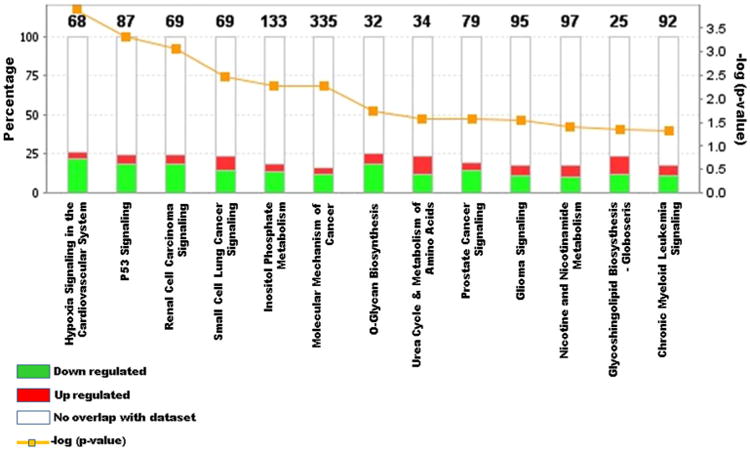
Differential gene expression status in the 45-month-old exposed Slovak children is shown as the analyzed IPA core comparison, where major molecular mechanism and signaling pathways are represented with the percent of genes involved in either up (red) and/or down-regulation (green), relative to the number of no overlapping genes (white), along with their − (log) p values. Molecular mechanisms of cancer showed the highest number of genes involved (335). In all cases, the majority of impacted genes does not match with the known disease and disorders in the IPA knowledgebase, which might suggest that the PCB exposure influences a discreet gene expression signature in specific pathways leading to development of disease and disorder in such exposure scenario in the later part of life
Finally, we observed an interesting finding when we ran the comparison analysis over the IPA data analysis and then choose the “disease & disorder comparison” among all of our datasets. In all exposed groups (this 45-month cohort and the new cohort children) and our earlier experiential exposure scenarios, it is suggested that there may be future increased cancer risks in such highly exposed children in their life course where the threshold value (the ratio of number of relevant genes/proteins in dataset divided by the total number of the genes/proteins in the respective pathway in the IPA knowledge base) is consistently at or above 1.5 (Fig. 7).
Fig. 7.
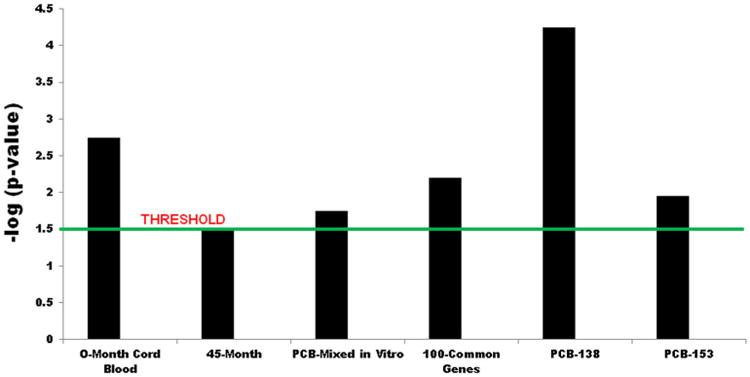
Disease & disorder comparison (cancer) under different experimental and Slovak population conditions by IPA® analysis. The gene sets from the study were filtered, uploaded, and run through in the IPA comparative data analysis module. The important disease and disorder genes that are represented here were at or above the threshold value (corresponding to a p value of 0.05). Fischer's exact test was used to calculate a p value determining the probability of disease assigned to the dataset
Accumulating all the above results together, the pathway analysis studies showed that there are significant similarities in functionality, pathway, and disease & disorder development. The possibilities of cancer are well-represented among the results and are comparable in disease and disorders function over IPA analysis in the epidemiological setting. Our results are in agreement with the reported epidemiological studies on PCB exposure. Our techniques become a useful tool for toxicity evaluation and will empower us to study the process of development of diseases towards understanding the potential health risk.
In conclusion, although it is recognized that many common complex diseases are a result of multiple genetic and environmental risk factors, studies of gene-environment interaction remain a challenge and have had limited success to date. It is necessary to continue to develop biomarkers, so that, prediction of long-term effect of chemical exposures can be made well before the development of disease. These might also facilitate the development of more effective therapy for vulnerable groups. It would be difficult to follow humans for decades to see if they develop diseases based on what they were exposed to before birth Some of them may be transgenerational. Even seemingly minor exposures during early development can lead to functional deficits and increased disease risks later in life. For this, we recommend longitudinal studies to follow the health of exposed populations to better establish the relationship between exposure and disease. Given that many disorders arise during fetal development from due to disruptions in the developmental dynamics but still poorly understood interplay of genes and environment, prevention may have to occur decades before a symptom even appears. Our investigation could provide robust biomarkers that can provide an indicator for highly vulnerable groups upon PCB exposure. The results have important implications regarding cancer screening and other cancer prevention strategies for implementation in exposed populations. Effort is underway to bridge this knowledge gap in our laboratory.
Supplementary Material
Acknowledgments
This work received support from U.S. National Institutes of Health grants # R01-CA96525. Thanks to Prof. Gray Harris, Dean of the Graduate School, Howard University for continuing supplemental support to this research initiative. Thanks are also due to the Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) and Dr. Annapurni Jayam Trouth, MD of Howard University for their assistance with the blood collection from healthy donors, as per approved HU IRB # IRB-07- GSAS-30. The contents of this report are solely the responsibility of the authors.
Funding information: This study is supported by the 1UO1ES016127-01 from the National Institute of Environmental Health Sciences (NIEHS/ NIH), the European Commission through the 7FP project OBELIX (No. 227391), Ministry of Health, Slovak Republic through projects 2007/07-SZU-03, 2012/41-SZU-05, and 2012/47-SZU-11, Slovak Research and Development Agency through projects APVV-0571-12 and APVV-0444-11, the project “Center of Excellence of Environmental Health,” ITMS No. 26240120033, based on the supporting Operational Research and Development Program financed from the European Regional Development Fund, 5G12MD007597-25 (NIMHD, PI: Southerland), and from the R200174 grant from Howard University
Glossary
- U.S. EPA
United States Environmental Protection Agency
- GEO
Gene Expression Omnibus (database)
- HEPG2
liver hepatocellular carcinoma cell line (human)
- IPA®
Ingenuity Pathway Analysis
- MIAME
Minimum Information About a Microarray Experiment
- NHL
non-Hodgkin lymphoma
- OCs
organochlorine compounds
- PCBs
polychlorinated biphenyls
- POPs
persistent organic pollutants
- qRT-PCR
quantitative real-time polymerases chain reaction
- TLDA
TaqMan low-density array
Footnotes
Presented as part at the 9th International PCB Workshop in Kobe, Japan 9–16 October, 2016 as Poster.
Electronic supplementary material: The online version of this article (https://doi.org/10.1007/s11356-017-0149-1) contains supplementary material, which is available to authorized users.
Compliance with ethical standards: Conflict of interest: The authors declare that they have no conflict of interest.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for polychlorinated biphenyls. US Department of Health & Human Services, Public Health Service; Washington DC: 2001. [Google Scholar]
- Ali I, Julin B, Glynn A, Högberg J, Berglund M, Johansson JE, Andersson SO, Andrén O, Giovannucci E, Wolk A, Stenius U, Åkesson A. Exposure to polychlorinated biphenyls and prostate cancer: population-based prospective cohort and experimental studies. Carcinogenesis. 2016;37:1144–1151. doi: 10.1093/carcin/bgw105. [DOI] [PubMed] [Google Scholar]
- Battle MA, Maher VM, McCormick JJ. ST7 is a novel low-density lipoprotein receptor-related protein (LRP) with a cytoplasmic tail that interacts with proteins related to signal transduction pathways. Biochemistry. 2003;42:7270–7282. doi: 10.1021/bi034081y. [DOI] [PubMed] [Google Scholar]
- Bencko V, Rames J, Ondrusova M, Plesko I, Jurickova L, Trnovec T. Human exposure to polyhalogenated hydrocarbons and incidence of selected malignancies—central European experience. Neoplasma. 2009;56:353–357. doi: 10.4149/neo_2009_04_353. [DOI] [PubMed] [Google Scholar]
- Bertrand KA, Spiegelman D, Aster JC, Altshul LM, Korrick SA, Rodig SJ, Zhang SM, Kurth T, Laden F. Plasma organochlorine levels and risk of non-Hodgkin lymphoma in a cohort of men. Epidemiology. 2010;21:172–180. doi: 10.1097/EDE.0b013e3181cb610b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO. Health effects of persistent organic pollutants: the challenge for the Pacific Basin and for the world. Rev Environ Health. 2011;26:61–69. doi: 10.1515/reveh.2011.009. [DOI] [PubMed] [Google Scholar]
- Colt JS, Severson RK, Lubin J, Rothman N, Camann D, Davis S, Cerhan JR, Cozen W, Hartge P. Organochlorines in carpet dust and non-Hodgkin lymphoma. Epidemiology. 2005;16:516–525. doi: 10.1097/01.ede.0000164811.25760.f1. [DOI] [PubMed] [Google Scholar]
- Colt JS, Rothman N, Severson RK, Hartge P, Cerhan JR, Chatterjee N, Cozen W, Morton LM, De Roos AJ, Davis S, Chanock S, Wang SS. Organochlorine exposure, immune gene variation, and risk of non-Hodgkin lymphoma. Blood. 2009;113:1899–1905. doi: 10.1182/blood-2008-04-153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S, Huang CSD, Jerry M, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Dang CV. c-Myc target genes involved in cell growth, apoptosis and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos AJ, Hartge P, Lubin JH, Colt JS, Davis S, Cerhan JR, Severson RK, Cozen W, Patterson DG, Jr, Needham LL, Rothman N. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin's lymphoma. Cancer Res. 2005;65:11214–11226. doi: 10.1158/0008-5472.CAN-05-1755. [DOI] [PubMed] [Google Scholar]
- De S, Ghosh S, Chatterjee R, Chen YQ, Moses L, Kesari A, Hoffman EP, Dutta SK. PCB congener specific oxidative stress response by microarray analysis using human liver cell line. Environ Int. 2010;36:907–917. doi: 10.1016/j.envint.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SK, Ghosh S, De S, Hoffman EP. CYP1A1 and MT1K are congener specific biomarker genes for liver diseases induced by PCBs. Environ Toxicol Pharmacol. 2008;25:218–221. doi: 10.1016/j.etap.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Dutta SK, Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP. Differential GeneExpression and Functional Analysis of PCBexposedChildren: Understanding Disease andDisorder Development. Environ Int. 2012;40:143–154. doi: 10.1016/j.envint.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LS, Laden F, Andersen A, Strickland PT, Blair A, Needham LL, Barr DB, Wolff MS, Helzlsouer K, Hunter DJ, Lan Q, Cantor KP, Comstock GW, Brock JW, Bush D, Hoover RN, Rothman N. Polychlorinated biphenyl levels in peripheral blood and non-Hodgkin's lymphoma: a report from three cohorts. Cancer Res. 2007a;67:5545–5552. doi: 10.1158/0008-5472.CAN-06-3906. [DOI] [PubMed] [Google Scholar]
- Engel LS, Lan Q, Rothman N. Polychlorinated biphenyls and non-Hodgkin lymphoma. Cancer Epidemiol Biomark Prev. 2007b;16:373–376. doi: 10.1158/1055-9965.EPI-07-0055. [DOI] [PubMed] [Google Scholar]
- Faroon OM, Keith S, Jones D, De Rosa C. Carcinogenic effects of polychlorinated biphenyls. Toxicol Ind Health. 2001;17:41–62. doi: 10.1191/0748233701th098oa. [DOI] [PubMed] [Google Scholar]
- Freeman MD, Kohles SS. Plasma levels of polychlorinated biphenyls, non-Hodgkin lymphoma, and causation. J Environ Public Health. 2012;2012:258981. doi: 10.1155/2012/258981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher RP, Macarthur AC, Lee TK, Weber JP, Leblanc A, Mark Elwood J, Borugian M, Abanto Z, Spinelli JJ. Plasma levels of polychlorinated biphenyls and risk of cutaneous malignant melanoma: a preliminary study. Int J Cancer. 2011;128:1872–1880. doi: 10.1002/ijc.25503. [DOI] [PubMed] [Google Scholar]
- Garnis C, Coe BP, Zhang L, Rosin MP, Lam WL. Over expression of LRP12, a gene contained within an 8q22 amplicon identified by high-resolution array CGH analysis of oral squamous cell carcinomas. Oncogene. 2004;23:2582–2586. doi: 10.1038/sj.onc.1207367. [DOI] [PubMed] [Google Scholar]
- Ghosh S, De S, Dutta SK. Altered protein expressions in chronic PCB-153-induced human liver (Hep G2) cells. Int J Toxicol. 2007;26:203–212. doi: 10.1080/10915810701352648. [DOI] [PubMed] [Google Scholar]
- Ghosh S, De S, Chen Y, Sutton DC, Ayorinde FO, Dutta SK. Polychlorinated biphenyls (PCB-153) and (PCB-77) absorption in human liver (Hep G2) and kidney (HK2) cells in vitro: PCB levels and cell death. Environ Int. 2010;36(8):893–900. doi: 10.1016/j.envint.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Zang S, Mitra PS, Ghimbovschi S, Hoffman EP, Dutta SK. Global gene expression and ingenuity biological functions analysis on PCBs 153 and 138 induced human PBMC in vitro reveals differential mode (s) of action in developing toxicities. Environ Int. 2011;37:838–857. doi: 10.1016/j.envint.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Trnovec T, Palkovicova L, Hoffman EP, Washington K, Dutta SK. Status of LEPR gene in PCB-exposed population: a quick look. Int J Hum Genet. 2013;13:27–32. doi: 10.1080/09723757.2013.11886193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Palkovicova L, Trnovec T, Loffredo CA, Washington K, Mitra PS, Dutta SK. Biomarkers linking PCB exposure and obesity. Curr Pharma Biotechnol. 2014;15:1058–1068. doi: 10.2174/1389201015666141122203509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Mitra PS, Loffredo CA, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Zang S, Hoffman EP, Dutta SK. Molecular expression and biological pathways analysis of human equivalence PCBs-exposure in vitro reveals disease and disorder development akin to human consequences. Environ Res. 2015;138C:202–216. doi: 10.1016/j.envres.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden R, Kimbrough R. Weight of evidence evaluation of potential human cancer risks from exposure to polychlorinated biphenyls: an update based on studies published since 2003. Crit Rev Toxicol. 2009;39:299–331. doi: 10.1080/10408440802291521. [DOI] [PubMed] [Google Scholar]
- Golden R, Doull J, Waddell W, Mandel J. Potential human cancer risks from exposure to PCBs: a tale of two evaluations. Crit Rev Toxicol. 2003;33:543–580. [PubMed] [Google Scholar]
- Goncharov A, Bloom M, Pavuk M, Birman I, Carpenter DO. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens. 2010;28:2053–2060. doi: 10.1097/HJH.0b013e32833c5f3e. [DOI] [PubMed] [Google Scholar]
- Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Indust Med. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Haslam A, Robb SW, Bonner MR, Lindblad W, Allegra J, Shen Y, Vena JE. Polychlorinated biphenyls and omega-3 fatty acid exposure from fish consumption, and thyroid cancer among New York anglers. J Environ Sci (China) 2016;41:270–277. doi: 10.1016/j.jes.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kočan A, Charles MJ, Čižnar P, Langer P, Sovčikova E, James R. PCBs and early childhood development in Slovakia: study design and background. Fresenius Environ Bull. 2003;12:208–214. [Google Scholar]
- Hopf NB, Waters MA, Ruder AM. Cumulative exposure estimates for polychlorinated biphenyls using a job-exposure matrix. Chemosphere. 2009;76:185–193. doi: 10.1016/j.chemosphere.2009.03.058. [DOI] [PubMed] [Google Scholar]
- Huang C, Liu LY, Li ZF, Wang P, Ni L, Song LP, et al. Effects of small interfering RNAs targeting MAPK1 on gene expression profile in HeLa cells as revealed by microarray analysis. Cell Biol Int. 2008;32:1081–1090. doi: 10.1016/j.cellbi.2008.04.019. [DOI] [PubMed] [Google Scholar]
- IARC. Bitumens and bitumen emissions, and some N- and S- heterocyclic polycyclic aromatic hydrocarbons, vol 107. WHO press, World Health Organization; Geneva: 2013. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- IARC. Polychlorinated biphenyls and polybrominated biphenyls, vol 107. WHO Press, World Health Organization; Geneva: 2016. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- Knerr S, Schrenk D. Carcinogenicity of “non-dioxin like” polychlorinated biphenyls. Crit Rev Toxicol. 2006;36:663–694. doi: 10.1080/10408440600845304. [DOI] [PubMed] [Google Scholar]
- Laden F, Ishibe N, Hankinson SE, Wolff MS, Gertig DM, Hunter DJ, Kelsey KT. Polychlorinated biphenyls, cytochrome P450 1A1, and breast cancer risk in the nurses' health study. Cancer Epidemiol Biomark Prev. 2002;11:1560–1565. [PubMed] [Google Scholar]
- Laden F, Bertrand KA, Altshul L, Aster JC, Korrick SA, Sagiv SK. Plasma organochlorine levels and risk of non-Hodgkin lymphoma in the Nurses' Health Study. Cancer Epidemiol Biomark Prev. 2010;19:1381–1384. doi: 10.1158/1055-9965.EPI-10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P, Ukropec J, Kocan A, Drobna B, Radikova Z, Huckova M, Imrich R, Gasperikova D, Klimes I, Trnovec T. Obesogenic and diabetogenic impact of high organochlorine levels (HCB, p, p′-DDE, PCBs) on inhabitants in the highly polluted Eastern Slovakia. Endocr Regul. 2014;48:17–24. doi: 10.4149/endo_2014_01_17. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34(8):1778–1784. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lind L, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind PM. Associations of persistent organic pollutants with abdominal obesity in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Environ Int. 2012;40:170–178. doi: 10.1016/j.envint.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Leng L, Li J, Luo XM, Kim JY, Li YM, Guo XM, Chen X, Yang QY, Li G, Tang NJ. Polychlorinated biphenyls and breast cancer: a congener-specific meta-analysis. Environ Int. 2016;88:133–141. doi: 10.1016/j.envint.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Mallin K, McCann K, D'Aloisio A, Freels S, Piorkowski J, Dimos J, Persky V. Cohort mortality study of capacitor manufacturing workers, 1944-2000. J Occup Environ Med. 2004;46:565–576. doi: 10.1097/01.jom.0000128156.24767.12. [DOI] [PubMed] [Google Scholar]
- Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP, Dutta SK. Analysis of the toxicogenomic effects of exposure to persistent organic pollutants (POPs) in Slovakian girls: correlations between gene expression and disease risk. Environ Int. 2012;39:188–199. doi: 10.1016/j.envint.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program (NTP) NTP Technical Report Series No 530. Research Triangle Park, NC: National Toxicology. Program; 2006. Toxicology and carcinogenesis studies of a binary mixture of 3 3′,4, 4′, 5-pentachlorobiphenyl (PCB 126) (CAS no. 57465-28-8) and 2, 2′,4,4′, 5, 5′-hexachlorobiphenyl (PCB 153) (CAS no. 35065-27-1) in female Harlan Sprague-Dawley rats (gavage studies) p. 264. NIH Publication No. 06-4466. [PubMed] [Google Scholar]
- National Toxicology Program (NTP) NTP 12th report on carcinogens. Rep Carcinog. 2011;12:iii–499. [PubMed] [Google Scholar]
- National Toxicology Program (NTP) 14th repost on carcinogens. U.S. Department of Health and Human Services Public Health Service National Toxicology Program; 2016. https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html. [Google Scholar]
- Ng CH, Janoo-Gilani R, Sipahimalani P, Gallagher RP, Gascoyne RD, Connors JM, Weber JP, Lai AS, Leach S, Le ND, Brooks-Wilson AR, Spinelli JJ. Interaction between organochlorines and the AHR gene, and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2010;21:11–22. doi: 10.1007/s10552-009-9429-5. [DOI] [PubMed] [Google Scholar]
- Otake Y, Soundararajan S, Sengupta TK, Kio EA, Smith JC, Pineda-Roman M, Stuart RK, Spicer EK, Fernandes DJ. Over expression of nucleolin in chronic lymphocyticleukemia cells induces stabilization of bcl2 mRNA. Blood. 2007;109:3069–3075. doi: 10.1182/blood-2006-08-043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada H, Jr, Wolff MS, Engel LS, Eng SM, Khankari NK, Neugut AI, Teitelbaum SL, Gammon MD. Polychlorinated biphenyls and their association with survival following breast cancer. Eur J Cancer. 2016;56:21–30. doi: 10.1016/j.ejca.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Park JS, Sovcikova E, Kocan A, Linderholm L, Bergman A, Trnovec T, Hertz-Picciotto I. Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in eastern Slovakia. Environ Health Perspect. 2009;117:1600–1606. doi: 10.1289/ehp.0900611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Environ Health. 2010;9:51. doi: 10.1186/1476-069X-9-51. https://doi.org/10.1186/1476-069X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, Lynch CF, Kocan A, Petrik J, Chovancova J. Case-control study of PCBs, other organochlorines and breast cancer in Eastern Slovakia. J Expo Anal Environ Epidemiol. 2003;13:267–275. doi: 10.1038/sj.jea.7500277. [DOI] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, Lynch CF, Schecter A, Petrik J, Chovancova J, Kocan A. Environmental exposure to PCBs and cancer incidence in eastern Slovakia. Chemosphere. 2004;54:1509–1520. doi: 10.1016/j.chemosphere.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Plísková M, Vondrácek J, Canton RF, Nera J, Kocan A, Petrík J, et al. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ Health Perspect. 2005;113:1277–1284. doi: 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MM, Hein MJ, Ruder AM, Waters MA, Laber PA, Whelan EA. Update: cohort mortality study of workers highly exposed to polychlorinated biphenyls (PCBs) during the manufacture of electrical capacitors, 1940-1998. Environ Health. 2006a;5:13. doi: 10.1186/1476-069X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MM, Ruder AM, Hein MJ, Waters MA, Whelan EA, Nilsen N, Ward EM, Schnorr TM, Laber PA, Davis-King KE. Mortality and exposure response among 14, 458 electrical capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Environ Health Perspect. 2006b;114:1508–1514. doi: 10.1289/ehp.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing J, Wei D, Maher VM, McCormick JJ. Cloning and characterization of a novel gene encoding a putative transmembrane protein with altered expression in some human transformed and tumor-derived cell lines. Oncogene. 1999;18:335–342. doi: 10.1038/sj.onc.1202290. [DOI] [PubMed] [Google Scholar]
- Rádiková Z, Tajtáková M, Kocan A, Trnovec T, Seböková E, Klimes I, Langer P. Possible effects of environmental nitrates and toxic organochlorines on human thyroid in highly polluted areas in Slovakia. Thyroid. 2008;18:353–362. doi: 10.1089/thy.2007.0182. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Velazco-Rodriguez V, Ocampo-Gómez G, Hernandez-Gonzalez S, Ruiz-Flores P, Lopez-Marquez F. Serum levels of polychlorinated biphenyls in Mexican women and breast cancer risk. J Appl Toxicol. 2011;31:270–278. doi: 10.1002/jat.1672. [DOI] [PubMed] [Google Scholar]
- Rothman N, Cantor KP, Blair A, Bush D, Brock JW, Helzlsouer K, Zahm SH, Needham LL, Pearson GR, Hoover RN, Comstock GW, Strickland PT. A nested case-control study of non-Hodgkin lymphoma and serum organochlorine residues. Lancet. 1997;350:240–244. doi: 10.1016/S0140-6736(97)02088-6. [DOI] [PubMed] [Google Scholar]
- Seo J, Hoffman EP. Probe set algorithms: is there a rational best bet? BMC Bioinform. 2006;7:395. doi: 10.1186/1471-2105-7-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeev AV, Carpenter DO. Exposure to persistent organic pollutants increases hospitalization rates for myocardial infarction with comorbid hypertension. Prim Prev Insights. 2010;2:1–9. doi: 10.4137/PPRI.S4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields PG. Understanding population and individual risk assessment: the case of polychlorinated biphenyls. Cancer Epidemiol Biomarkers Prev. 2006;15(5):830–839. doi: 10.1158/1055-9965.EPI-06-0222. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Batell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120:727–732. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Petrik J, Kocan A, Palkovicova L, Trnovec T, Nguyen D, Hertz-Picciotto I. Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birth weight. Paediatr Perinat Epidemiol. 2008;22:202–213. doi: 10.1111/j.1365-3016.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- Spinelli JJ, Ng CH, Weber JP, Connors JM, Gascoyne RD, Lai AS, Brooks-Wilson AR, Le ND, Berry BR, Gallagher RP. Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer. 2007;121:2767–2775. doi: 10.1002/ijc.23005. [DOI] [PubMed] [Google Scholar]
- Trnovec T, Sovcíková E, Pavlovcinová G, Jakubíková J, Jusko TA, Husták M, Jurecková D, Palkovicová L, Kocan A, Drobná B, Lancz K, Wimmerová S. Serum PCB concentrations and cochlear function in 12-year-old children. Environ Sci Technol. 2010;44:2884–2889. doi: 10.1021/es901918h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec J, Radikova Z, Huckova M, Koska J, Kocan A, Sebokova E, Drobna B, Trnovec T, Susienkova K, Labudova V, Gasperikova D, Langer P, Klimes I. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia. 2010;53:899–906. doi: 10.1007/s00125-010-1683-2. [DOI] [PubMed] [Google Scholar]
- Voong LN, Slater AR, Kratovac S, Cressman DE. Mitogen-activated protein kinase ERK1/2 regulates the class II transactivator. J Biol Chem. 2008;283:9031–9039. doi: 10.1074/jbc.M706487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH, Colt JS, Metayer C, Gunier RB, Lubin J, Crouse V, Nishioka MG, Reynolds P, Buffler PA. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect. 2009;117:1007–1013. doi: 10.1289/ehp.0900583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


