Abstract
Tobacco smoking, driven by the addictive properties of nicotine, continues to be a worldwide health problem. Based on the well-established role of glutamatergic neurotransmission in drug addiction, novel medication development strategies seek to halt nicotine consumption and prevent relapse to tobacco smoking by modulating glutamate transmission. The presynaptic inhibitory metabotropic glutamate receptors (mGluR) 2/3 are key autoreceptors on glutamatergic terminals that maintain glutamate homeostasis. Accumulating evidence suggests the critical role of mGluR2/3 in different aspects of nicotine addiction, including acquisition and maintenance of nicotine taking, nicotine withdrawal, and persistent nicotine seeking even after prolonged abstinence. The involvement of mGluR2/3 in other neuropsychiatric diseases, such as anxiety, depression, schizophrenia, Alzheimer’s disease, Parkinson’s disease and pain, provides convincing evidence suggesting that mGluR2/3 may provide an effective therapeutic approach for comorbidity of smoking and these disorders. This focused review article highlights that mGluR2/3 provide a promising target in the search for smoking cessation medication with novel mechanisms of actions that differ from those of currently FDA-approved pharmacotherapies.
Keywords: metabotropic glutamate receptor 2/3, nicotine, reward, relapse, withdrawal, addiction
Tobacco smoking causes major global health problems including lung cancer and cardiovascular disease, resulting in approximately one-tenth of all worldwide premature adult deaths (1).The development of novel more effective smoking cessation treatments is essential, because currently available therapies remain largely ineffective in the majority of people that attempt to quit (2, 3). Recent studies on the neurophysiological mechanisms involved in drug addiction highlight potential targets for the development of effective medications. The well-established role of glutamatergic transmission in the acquisition, development and expression of dependence to different drugs of abuse (4–6) provides converging evidence indicating that modulation of glutamate neurotransmission could provide a promising way to intervene in the development of drug addiction at different points along the causal chain.
Inhibition of glutamatergic transmission through various means has been found to attenuates nicotine-induced behavioral effects in measures of the reinforcing and motivational effects, reward enhancement, and reinstatement of nicotine-seeking (4, 6–14). However, approaches using direct ionotropic glutamate receptor (iGluRs) antagonists exhibit serious limitations, including neurotoxicity and severe adverse behavioral effects (15). Metabotropic glutamate receptors (mGluRs) are functionally slow-acting modulators of glutamate transmission, and targeting these receptors may therefore entail less risk of unwanted side effects (16). Group II (mGluR2/3) receptors are the key autoreceptors maintaining glutamate homeostasis (17, 18). Accumulating studies demonstrated that activation of mGluR2/3, specifically mGluR2, inhibits the rewarding and motivational effects of multiple drugs of abuse, such as cocaine, morphine, heroin, nicotine and alcohol, with an acceptable side-effect profile (18–23). This review article summarizes our current knowledge of mGluR2/3 in nicotine addiction supporting therapeutic interventions targeting these receptors for smoking cessation.
NEUROBIOLOGY OF MGLUR2/3
Protein and mRNA expression studies reveal mGluR2/3 to be distributed in brain regions and circuits associated with neuropsychiatric disorders, including drug addiction. High density of mGluR2/3 has been identified in brain areas related to processing of reward, motivated behaviors, learning, memory and emotion, including the nucleus accumbens (NAc), dorsal striatum, hippocampus, amygdala, prefrontal cortex (PFC), thalamus, and olfactory bulb (24–26). Although the distributions of mGluR2 and mGluR3 are overlapping in many brain regions, mGluR3 has broader distributions than mGluR2. Moreover, the cellular locations of these two receptor subtypes are different; mGluR2 is extensively expressed on presynaptic axon terminals, whereas mGluR3 is located on both presynaptic and postsynaptic elements as well as on glia cells (24–26).
mGluR2/3 performs a modulatory role on synaptic neurotransmission by coupling to Gi/o proteins which negatively regulate adenylyl cyclase activities and directly regulates ion channels and other downstream signaling partners via liberation of Gβγ subunits (27). mGluR2/3 controls neurotransmitter release through different mechanisms including inhibition of presynaptic Ca+ channels, activation of presynaptic K+ channels, or direct interference with vesicular release (27). They function as autoreceptors to regulate the release of glutamate and as heteroreceptors to regulate the release of γ-aminobutyric acid (GABA) and dopamine (28). Several electrophysiological studies support this conclusion. Specifically, the mGluR2/3 agonists L-CCG1 and (1S,3S)-ACPD increased paired pulse facilitation, decreased miniature excitatory post synaptic currents (EPSCs) frequency without affecting the amplitude in rat NAc slices (29), suggesting mGluR2/3 activation decreases glutamate release from the presynaptic terminal. In addition, the mGluR2/3 agonists DCG IV and APDC increased the paired pulse ratio and the coefficient of variation of the EPSCs in both excitatory and inhibitory neurons in mouse thalamocortical slices, and this effect was blocked by the mGluR2/3 antagonist LY341495 (30). Moreover, DCG IV and L-CCG1 decreased EPSCs in rat subthalamic nucleus slices, and this effect was reversed by LY341495 (31).
Consistent with these in vitro electrophysiological findings, in vivo microdialysis studies found that intra-NAc infusion of APDC reduced extracellular glutamate levels in the NAc (32). Intra-NAc infusion of the agonists LY379268, DCG-IV, and LY354740 reduced extracellular dopamine levels in the NAc (28, 33, 34). Interestingly, several lines of evidence suggest that mGluR2/3 activity regulates basal glutamate and dopamine levels. Specifically, intra-PFC infusion of the antagonist LY341495 augmented basal glutamate levels not only in the PFC itself, but also in the downstream projection areas including the NAc and ventral tegmental area (VTA) (35, 36). Similarly, intra-NAc of LY341495 increased basal NAc glutamate levels (32). Furthermore, intra-NAc of another mGluR2/3 antagonist MGS0039 increased extracellular NAc dopamine levels (34). Altogether, these data suggest mGluR2/3 activation may provide an important negative feedback mechanism to prevent excessive glutamate and dopamine neurotransmission in the mesocorticolimbic system implicated in the pathophysiology of neuropsychiatric disorders, including substance use disorders. Given the well-established role of these neurotransmitters in mediating nicotine reward, withdrawal, and seeking behavior (4–6), pharmacological interventions directed at mGluR2/3 may have therapeutic potential in the treatment of nicotine addiction through an action on several underlying mechanisms.
MGLUR2/3 AND NICOTINE REWARD
Nicotine addiction is characterized by the persistence of compulsive nicotine taking, the emergence of aversive withdrawal symptoms upon abstinence from smoking (e.g., anhedonia, anxiety, craving and irritability), and relapse to tobacco smoking even after periods of abstinence (37). Tobacco smoking or nicotine administration produces mild euphoria, increased arousal, relaxation and decreased fatigue in humans. The rewarding effects and conditioned reinforcement of nicotine drive the acquisition and maintenance of tobacco use (38). Animal studies demonstrate that mGluR2/3 activation attenuated nicotine reinforcement. Specifically, systemic administration of the agonist LY379268 decreased nicotine self-administration in rats (14) and squirrel monkeys (39), suggesting that mGluR2/3 activation inhibits the primary reinforcing effects of nicotine. These effects are at least partially medicated by mGluR2/3 in the mesolimbic pathway because local injection of LY379268 into the VTA and NAc produced similar inhibitory effects (14). These effects appear dose dependent: 3 mg/kg LY379268 decreased both nicotine and food taking, suggesting nonspecific locomotor effects or cognitive impairment at high dose. However, 1 mg/kg LY379268 selectively decreased nicotine but not food taking, suggesting that low dose of LY379268 specifically inhibits the reinforcing effects of nicotine but not those of a natural reinforcer. Similar effects were observed with the mGluR2 positive allosteric modulators (PAM), which differ from full agonists by binding to the sites distinct from the orthosteric binding site and potentiate the potency and maximal efficacy of agonists, such as endogenously released glutamate. Specifically, the mGluR2 PAM AZD8418 and AZD8529 inhibited nicotine self-administration but had no effect on food-maintained responding in rats (40). AZD8529 also inhibited nicotine and, to a lesser extent, food self-administration in squirrel monkeys (41). Furthermore, another mGluR2 PAM from different class (i.e., a benzothiazolone derivative) attenuated nicotine self-administration in rats (42). Altogether, these results highlight the important role of mGluR2/3 in the mesolimbic pathway in nicotine reinforcement and suggest that mGluR2 activation alone may be sufficient to produce a therapeutic effect. Moreover, selective mGluR2 ligands may produce fewer unwanted side effects than the ligands targeting both receptors.
The agonist LY314582 blocked nicotine-induced decreases in intracranial self-stimulation (ICSS) thresholds. However, LY314582 itself also elevated ICSS thresholds (43). Similarly, the mGluR2 PAM BINA elevated ICSS thresholds (44). Although its effect on nicotine-enhanced brain reward is unknown, BINA blocked cocaine-induced decrease in ICSS thresholds (44). These findings indicate that mGluR2/3 activation attenuates nicotine-enhanced brain reward function. However, this action is most likely due to the intrinsic aversive effect of this compound, rather than directly reversing the reward-enhancing effects of nicotine.
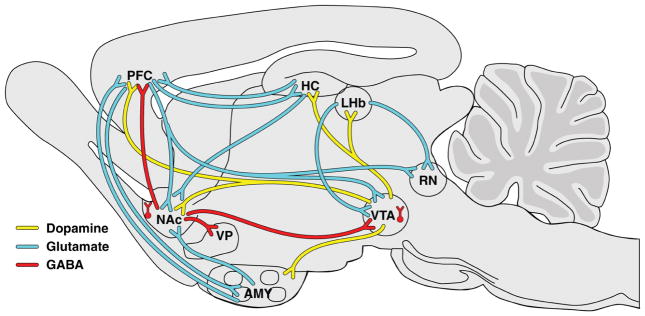
The mesocorticolimbic dopamine pathway that originates in the VTA and projects to the NAc and PFC play a key role in mediating the reinforcing and motivational properties of nicotine (Figure 1) (45–47). Nicotine augments the firing frequency of VTA dopamine neurons through complex interactions among acetylcholine, glutamate, GABA and dopamine, leading to increased dopamine in the NAc (6, 48–53). The mechanisms of these interactions have been described in detail by Markou’s group previously (6). Among them, two mechanisms are most relevant to the topic addressed in this review. First, nicotine directly activates dopamine neurons by binding to α4β2-containing nicotinic acetylcholine receptors (nAChRs) (48, 54, 55). Second, nicotine indirectly activates dopamine neurons by enhancing presynaptic glutamate release via binding to α7 nAChRs on glutamate terminals (56–58) and by promoting the induction of NMDA-dependent long-term potentiation at excitatory synapses (59). Glutamatergic afferents from the PFC, lateral hypothalamus, ventral pallidum and amygdala regulate VTA dopamine cell activity (60). Based on this interplay of effects, nicotine-enhanced glutamate neurotransmission, at least in part, mediates the reinforcing and incentive motivational properties of tobacco smoke or acute nicotine administration (8, 61, 62). In vivo microdialysis studies found that the mGluR2/3 agonist LY379268 and the mGluR2 PAM AZD8529 attenuated nicotine-induced increases in NAc dopamine levels in nicotine-naive and nicotine-experienced animals (41, 63). Thus, it appears that pretreatment with mGluR2/3 agonists trigger presynaptic negative feedback on glutamatergic neurotransmission, preventing further release of nicotine-induced glutamate and dopamine, therefore blocking the reinforcing effects of nicotine.
Figure 1.
Glutamate, GABA and dopamine interactions that are involved in the development of nicotine dependence. Nicotine binds to excitatory nicotinic acetylcholine receptors (not shown in figure) that are located throughout the brain as auto- or heteroreceptors at presynaptic terminals that regulate the release of several neurotransmitters, including dopamine, glutamate and γ-aminobutyric acid (GABA). The mesolimbic dopaminergic neurons (depicted as yellow lines) mediate the reinforcing effects of several drugs of abuse including nicotine. These dopaminergic neurons originate in the ventral tegmental area and project to several limbic and cortical regions, including the nucleus accumbens, prefrontal cortex, amygdala, hippocampus, and habenula. The activity of these dopaminergic neurons is regulated by reciprocal glutamatergic (excitatory; depicted as blue) and GABAergic (inhibitory; depicted as red lines) projections that originate from the aforementioned cortical and limbic brain regions. AMY, amygdala; LHb, lateral habenula; HC, hippocampus; NAc, nucleus accumbens; PFC, prefrontal cortex; RN, raphe nucleus; VP, ventral pallidum; VTA, ventral tegmental area. [(6), with permission with Neuropharmacology]
MGLUR2/3 AND NICOTINE WITHDRAWAL
In humans, nicotine withdrawal is associated with negative affective symptoms, such as anxiety, depression and irritability (37). In contrast to the somatic signs that are primarily peripherally mediated, the centrally-mediated negative affective withdrawal signs are hypothesized to critically contribute to the persistence of nicotine taking and relapse to smoking after abstinence (61, 64–68). Hence, amelioration of these affective signs of early nicotine withdrawal may prevent relapse to smoking when the urge to reinitiate nicotine use is the strongest (69–71).
In rodents, nicotine withdrawal is associated with aversive affective signs, such as anhedonia and anxiety-like behavior (74–478). However, these affective signs of nicotine withdrawal are difficult to detect reliably in most of the tests used to model such states, such as the forced swim, elevated plus maze and light-enhanced startle tests. (74). On the other hand, the anhedonic aspect of nicotine withdrawal (i.e., a reward deficit) has been reliably measured using the ICSS procedure. Since the first successful application of ICSS to assess the anhedonia during nicotine withdrawal by Dr. Markou two decades ago (77), several lines of evidence have suggested the potential involvement of glutamate receptors in the anhedonic-like signs of nicotine withdrawal using this procedure. Specifically, the AMPA/kainate receptor antagonist NBQX (9) and the mGluR5 antagonist MPEP (78) aggravated brain reward deficits associated with nicotine withdrawal. Importantly, the mGluR2/3 antagonist LY341495 reversed, while the agonist LY314582 exacerbated, the affective signs of nicotine withdrawal in rats (79). However, another mGluR2/3 agonist LY379268 had no significant effect on nicotine withdrawal-induced reward deficits (80).
Compared to the well-documented neuromechanisms underlying nicotine reward and reinstatement of nicotine seeking, the neuromechanisms underlying nicotine withdrawal are largely unknown. It is generally accepted that repeated drug administration results in cellular and molecular adaptations in the same systems that mediate the reinforcing effects of drugs (6, 81). Thus, glutamatergic neurotransmission within the mesocorticolimbic system is likely involved in the development of drug dependence, withdrawal, and expression of drug seeking (81, 82). Chronic nicotine exposure induced a downregulation in cysteine-glutamate exchange (83), increased expression of postsynaptic AMPA and NMDA receptor subunits (9, 84), a reduction in the glutamate transporter-1 and an enduring increase in dendritic spine head diameter (85). Strikingly, daily nicotine self-administration resulted in wide-spread downregulation of mGluR2/3 function in most cortical and limbic brain sites, including the PFC, NAc, VTA, amygdala, hippocampus and hypothalamus (14), indicating an impaired negative feedback control on glutamatergic terminals and suggesting the crucial role of mGluR2/3 in the development of nicotine addiction. These observed changes are likely to be adaptations developed to counteract the decreased glutamatergic tone (i.e., hypoglutamatergic state) upon abrupt cessation of nicotine administration (6, 21, 85–88). Altogether, these findings suggest that early nicotine withdrawal symptoms may be driven, in part, by these neuroadaptations in glutamatergic transmission (6, 21, 85, 88).
This hypoglutamate hypothesis may explain why compounds that decrease glutamate transmission (e.g., the AMPA/kainate receptor antagonist NBQX, the mGluR2 agonist LY314582 or the mGluR5 antagonist MPEP) worsened, while compounds that increase glutamate release (e.g., the mGluR2 antagonist LY341495) ameliorated, early nicotine withdrawal-induced reward deficits as indicated above. However, given that LY314582 or MPEP itself also elevated brain reward thresholds (43), it is most likely that the actions of mGluR modulation on early nicotine withdrawal-induced reward deficits are due to additive, rather than synergistic, effects on ICSS thresholds. It is interesting that brain reward deficit after nicotine removal is short lasting in animal studies, with an effect peaked at 24 hours after nicotine removal and returned back to baseline within 36 hours (79). The dynamic changes of glutamatergic neurotransmission in the brain during the course of nicotine withdrawal is unknown; how long do the hypoglutamate status and nicotine-induced receptor/transporter adaptations last and how does this hypoglutamate status contribute to early relapse? A better understanding of these questions will increase our knowledge of nicotine dependence.
It is noteworthy that while early nicotine withdrawal contributes to smoking relapse soon after quitting, the conditioned-craving induced by smoking-associated cues may be another important factor underlying relapse even after prolonged abstinence when baseline craving and early withdrawal symptoms have progressively subsided (89). Human studies have shown that smoking-cue-provoked cigarette craving time-dependently increases within the first 35 days of abstinence (89). We (90) and others (91) have assessed the impact of nicotine withdrawal periods on vulnerability to cue-induced nicotine seeking in rats. We found that cue-induced nicotine craving is long-lasting and persists even after 42 days of forced withdrawal in the home cages, with highest levels of nicotine-seeking responding around 7–21 days of withdrawal (90). The neurobiological mechanisms underlying incubation of nicotine craving are largely unknown. Increased Fos expression in different brain areas (e.g., amygdala, PFC, orbitofrontal cortex and NAc) were observed after 14 days of nicotine withdrawal (91). Due to the important role of glutamate system in nicotine withdrawal and nicotine seeking, assessment of the involvement of glutamate receptors including mGluR2/3 on incubation of nicotine craving may be warranted. Interestingly, recent studies by Caprioli et al show that the mGluR2 PAM AZD8529 attenuated incubation of methamphetamine craving (92).
MGLUR2/3 AND REINSTATEMENT OF NICOTINE SEEKING
High rate of relapse to smoking is a major hurdle to long-term quitting. Maintenance of tobacco smoking is largely driven by conditioned motivational effects of environmental stimuli (e.g., the sight and smell of a lit cigarette or contexts within which smoking occurs) previously associated with smoking (93, 94). These conditioned cues also play an important role in relapse to smoking in humans (95–97). Smokers report strong urges to smoke after being exposed to specific environments in which they used to smoke (98).
In animal studies, nicotine administration facilitates the formation of strong associations between environmental cues and nicotine. Specifically, conditioned stimuli (e.g., light or tone) paired with nicotine delivery enhanced acquisition and maintenance of nicotine self-administration (99, 100). Moreover, rats with contingent presentation of nicotine-associated cues during extinction training required longer periods to extinguish those behaviors compared with those animals not presented with such cues (100, 101). In addition, nicotine-seeking behavior can be reinstated by presenting response-contingent nicotine-paired stimuli (14, 102, 103).
The mGluR2/3 agonist LY379268 attenuated cue-induced reinstatement of nicotine seeking in rats (14) and blocked both nicotine priming-induced and cue-induced reinstatement of nicotine-seeking behavior in squirrel monkeys (39). Similarly, the mGluR2 PAMs AZD84188 and AZD8529 blocked cue-induced reinstatement of nicotine seeking in rats (40). Moreover, AZD8529 attenuated both nicotine priming- and cue-induced reinstatement of nicotine seeking in squirrel monkeys (47). Interestingly, pretreatment with N-acetylcysteine, a ligand that increases extrasynaptic levels of glutamate by stimulating cysteine-glutamate exchange, attenuated cue-induced reinstatement of nicotine seeking (104, 105). This effect was completely blocked by the mGluR2/3 antagonist LY341495, suggesting increased glutamatergic tone at presynaptic mGluR2/3 by N-acetylcysteine-induced glutamate release underlying the observed behavioral effect. Activation of mGluR2/3 has been shown to inhibit different forms of drug seeking reinstatement induced by conditioned cues/contexts associated with the self-administration of cocaine, heroin, alcohol, and methamphetamine (13, 47, 64, 106–109). Moreover, both the agonist LY379268 and the PAMs AZD84188 and AZD8529 also blocked cue-induced reinstatement of food seeking (14, 39, 47). Thus, there appears to be potent effects of mGluR2/3 activation on conditioned behavioral responses, regardless of the reinforcers. Again these studies suggest that mGluR2 activation alone may be sufficient to generate a therapeutic effect. In nicotine-experienced rats, LY379268 blocked nicotine-induced increases in NAc dopamine only when nicotine was self-administered in the presence of the cues/context previously associated with nicotine intake (63), further confirming the critical role of mGluR2/3 in conditioned responding. Considering the high rates of comorbidity between smoking and dependence to other drugs of abuse, the broad efficacy of mGluR2/3 in preventing relapse to wide and different classes of abused drugs is extremely promising for medication development for the treatment of individuals with poly-drug addiction.
Glutamate projections from the PFC to the NAc have been identified as the final common pathway driving relapse to drug seeking (5). Chronic nicotine-related neuroadaptations in glutamatergic neurotransmission, as indicated above, play a role in relapse vulnerability (5). After chronic nicotine self-administration, re-exposure to nicotine-associated cues increases extracellular glutamate levels that are superimposed on the hypoglutamatergic state associated with nicotine withdrawal (6, 85). This glutamate overflow state was associated with rapid, transient synaptic plasticity as illustrated by cue-induced increases in dendritic spine head diameter and augmentation of the ratio of AMPA to NMDA currents (85). Similar to the role of mGluR2/3 activation on nicotine reinforcement, pretreatment with agonists/PAMs triggers presynaptic negative feedback inhibition of glutamate neurotransmission, thus, preventing nicotine priming- or cue-induced release of glutamate and blocking the reinstatement of nicotine-seeking behavior.
MGLUR2/3 AND SMOKING-ASSOCIATED PSYCHIATRIC DISORDERS
As the primary excitatory neurotransmitter in the brain, glutamate is implicated in a wide range of brain functions. In addition to drug addiction, imbalances in glutamate neurotransmission have been implicated in the pathophysiology of different neurological and psychiatric disorders, such as anxiety disorders, major depression, schizophrenia, and chronic pain. As the key regulators of glutamate homeostasis, mGluR2/3 appear to be critically involved in these disorders and have been considered as promising pharmacological targets for the treatment of these conditions (110).
Specifically, mGluR2/3 agonists exhibited anxiolytic effects in animal models evaluating anxiety-like behaviors, such as the elevated plus maze, conflict drinking, four-plate test, stress-induced hyperthermia, light-dark box and fear potentiated startle tests; and in clinical studies in individuals with panic disorder and generalized anxiety disorder (110, 111). Interestingly, the antidepressant-like effects of mGluR2/3 was observed with both agonists and antagonists in a variety of rodent models of depression (110). While the negative feedback on presynaptic glutamate release is considered to contribute to the anti-depressant efficacy of mGluR2/3 activation, the modulation of neurochemical and molecular pathways underlying depression is considered to contribute to the anti-depressant efficacy of mGluR2/3 blockade (110). Regardless of the exact mechanisms, the intriguing findings of anxiolytic and anti-depressant profiles of mGluR2/3 modulators suggest that mGluR2/3 modulation may ameliorate the depression-like anhedonia and anxiety-like behavior of nicotine withdrawal, therefore facilitate smoking cessation. This notion is supported by the studies showing that the mGluR2/3 antagonist LY341495 reversed spontaneous nicotine withdrawal-precipitated brain reward deficit in rats (79).
Studies in animal models of schizophrenia showed that mGluR2/3 agonists reversed some schizophrenia-like symptoms induced by phencyclidine or amphetamine (e.g., blocked locomotor hyperactivity and stereotypy, improved sensorimotor gating and social novelty recognition, and ameliorated attention and working memory deficits) (112). Both an mGluR2/3 agonist and PAM have been assessed in phase II clinical trials in patients with schizophrenia. However, findings are inconclusive, with an initial report of significant improvement (113) not being replicated in more recent studies with other mGluR2 agonists or modulators (114, 115).
Extensive studies demonstrated that individuals with neuropsychiatric disorders are more likely to smoke, smoke more heavily, and are more likely to develop nicotine dependence and have lower abstinence rates than those without neuropsychiatric illness (116). Historically, individuals with major depressive disorder, generalized anxiety disorder, schizophrenia or other psychiatric diseases were not usually recruited in Phase 2 and 3 clinical trials assessing the efficacy of smoking cessation medications. The shared glutamate mechanisms underlying nicotine addiction and some neuropsychiatric disorders offer a new avenue for potential therapeutic intervention for smokers with these comorbidities. Modulation of glutamatergic neurotransmission through mGluR2/3 may not only aid smoking cessation, but also improve some of the core symptoms related to these disorders, thus, providing a novel treatment for these “difficult-to-treat” smokers.
MEDICATION DEVELOPMENT TARGETING MGLUR2/3
Due to the high level of conservation within the extracellular orthosteric binding site of mGluR2 and mGluR3 (117), all the well-validated compounds currently commercially available bind with similar affinity to both receptors, making it impossible to distinguish the function of each receptor with these pharmacological tools. In recent years, selective mGluR2 PAMs that act on less well-conserved allosteric receptor sites within transmembrane spanning domains have been synthesized (40, 41, 118). PAMs are devoid of substantial intrinsic activity in the absence of the orthosteric agonist. Instead, PAMs potentiate the potency and efficacy of full agonists, thus offering more physiological means to activate the receptors than agonists. Studies using these PAMs have increased our knowledge of the relative contribution of mGluR2 vs. mGluR3 in drug addiction and other neuropsychiatric disorders. As indicated above, while mGluR2/3 agonists inhibited the reinforcement of both drug and food (13, 14, 119), mGluR2 PAMs (e.g., AZD8418, AZD8529 and BINA) specifically inhibited nicotine or cocaine self-administration without affecting food-maintained responding (40, 44). Furthermore, chronic administration of the agonist LY379268 decreased nicotine self-administration only during the first week of the 14-day treatment period (14). However, the inhibitory effects of repeated AZD8529 treatment on nicotine self-administration persisted for 14 days, without the development of tolerance in rats (40). Similarly, no tolerance developed after repeated AZD8529 treatment for three consecutive sessions in nicotine self-administered monkeys (41). These findings suggest that compared with mGluR2/3 agonists, (i) selective activation of mGluR2 alone may produce specific effects on drug-related behaviors, but not inhibit the reinforcement of a nature reinforcer (i.e., food); and (ii) PAMs have better subtype selectivity and fewer side-effects, and are less likely to develop tolerance after chronic treatment. Hence, it seems that selective mGluR2 ligands, especial PAMs, may be more promising for medication development. Based on the innovative and promising preclinical studies of mGluR2/3 on nicotine dependence by Markou’s group and others, the National Institute in Drug Abuse initiated the first clinical trial assessing the efficacy of mGluR2/3 ligands on smoking cessation in 2015. This ongoing multi-center, randomized, phase II study (NCT02401022) examines the efficacy of AZD8529 (1.5 and 40 mg) in smoking cessation (120). Findings from this clinical trial would provide initial insight into the potential validity of mGluR2/3 as therapeutic target for smoking cessation aid.
In conclusion, significant progress has been made in understanding the involvement of mGluR2/3 in different aspects of nicotine addiction. Compelling evidence from preclinical and clinical studies suggests that mGluR2/3 may provide a promising target in the search for smoking cessation medication with novel mechanisms of actions that differ from those of currently FDA-approved pharmacotherapies.
Acknowledgments
This work was funded by R56DA011946 (AM) and R21DA040195 (XL) from the National Institute on Drug Abuse (NIDA), 20KT-0046 (XL) and 24RT-0034 (XL) from Tobacco-Related Disease Research Program (TRDRP), California, and a research contract from AstraZeneca (AM). Dr. Anthenelli’s contributions to this manuscript were supported, in part, by NIDA-VA CSP #s 1032 and 1033.
Footnotes
DISCLOSURES
Drs. Cross and Li report no biomedical financial interests or potential conflicts of interest. Dr. Anthenelli provides consulting services to Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clinical therapeutics. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 3.McNeil JJ, Piccenna L, Ioannides-Demos LL. Smoking cessation-recent advances. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2010;24:359–367. doi: 10.1007/s10557-010-6246-8. [DOI] [PubMed] [Google Scholar]
- 4.Markou A. Review. Neurobiology of nicotine dependence. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Semenova S, D'Souza MS, Stoker AK, Markou A. Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology. 2014;76(Pt B):554–565. doi: 10.1016/j.neuropharm.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 8.Markou A. Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biological psychiatry. 2007;61:17–22. doi: 10.1016/j.biopsych.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 9.Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 11.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 12.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. The Journal of pharmacology and experimental therapeutics. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 13.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisaga A, Popik P, Bespalov AY, Danysz W. Therapeutic potential of NMDA receptor antagonists in the treatment of alcohol and substance use disorders. Expert Opin Investig Drugs. 2000;9:2233–2248. doi: 10.1517/13543784.9.10.2233. [DOI] [PubMed] [Google Scholar]
- 16.Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 17.Conn PJ, Jones CK. Promise of mGluR2/3 activators in psychiatry. Neuropsychopharmacology. 2009;34:248–249. doi: 10.1038/npp.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. European journal of pharmacology. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Current drug abuse reviews. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lea PMt, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liechti ME, Markou A. Role of the Glutamatergic System in Nicotine Dependence: Implications for the Discovery and Development of New Pharmacological Smoking Cessation Therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- 22.Markou A. The role of metabotropic glutamate receptors in drug reward, motivation and dependence. Drug News Perspect. 2007;20:103–108. doi: 10.1358/dnp.2007.20.2.1083435. [DOI] [PubMed] [Google Scholar]
- 23.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacology & therapeutics. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 25.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 26.Gu G, Lorrain DS, Wei H, Cole RL, Zhang X, Daggett LP, et al. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: Implication in emotional responses and central disinhibition. Brain Res. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 27.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain research Brain research reviews. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 28.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- 29.Manzoni O, Michel JM, Bockaert J. Metabotropic glutamate receptors in the rat nucleus accumbens. Eur J Neurosci. 1997;9:1514–1523. doi: 10.1111/j.1460-9568.1997.tb01506.x. [DOI] [PubMed] [Google Scholar]
- 30.Mateo Z, Porter JT. Group II metabotropic glutamate receptors inhibit glutamate release at thalamocortical synapses in the developing somatosensory cortex. Neuroscience. 2007;146:1062–1072. doi: 10.1016/j.neuroscience.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen KZ, Johnson SW. Group II metabotropic glutamate receptor modulation of excitatory transmission in rat subthalamic nucleus. The Journal of physiology. 2003;553:489–496. doi: 10.1113/jphysiol.2003.052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. The Journal of pharmacology and experimental therapeutics. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 33.Greenslade RG, Mitchell SN. Selective action of (−)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell. Neuropharmacology. 2004;47:1–8. doi: 10.1016/j.neuropharm.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Karasawa J, Yoshimizu T, Chaki S. A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neuroscience letters. 2006;393:127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 35.Xie X, Steketee JD. Repeated exposure to cocaine alters the modulation of mesocorticolimbic glutamate transmission by medial prefrontal cortex Group II metabotropic glutamate receptors. Journal of neurochemistry. 2008;107:186–196. doi: 10.1111/j.1471-4159.2008.05593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. The Journal of pharmacology and experimental therapeutics. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 38.Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. The Journal of pharmacology and experimental therapeutics. 1985;234:1–12. [PubMed] [Google Scholar]
- 39.Justinova Z, Le Foll B, Redhi GH, Markou A, Goldberg SR. Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys. Psychopharmacology. 2016;233:1791–1800. doi: 10.1007/s00213-015-3994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, D'Souza MS, Nino AM, Doherty J, Cross A, Markou A. Attenuation of nicotine-taking and nicotine-seeking behavior by the mGlu2 receptor positive allosteric modulators AZD8418 and AZD8529 in rats. Psychopharmacology. 2016;233:1801–1814. doi: 10.1007/s00213-016-4220-2. [DOI] [PubMed] [Google Scholar]
- 41.Justinova Z, Panlilio LV, Secci ME, Redhi GH, Schindler CW, Cross AJ, et al. The Novel Metabotropic Glutamate Receptor 2 Positive Allosteric Modulator, AZD8529, Decreases Nicotine Self-Administration and Relapse in Squirrel Monkeys. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sidique S, Dhanya RP, Sheffler DJ, Nickols HH, Yang L, Dahl R, et al. Orally Active Metabotropic Glutamate Subtype 2 Receptor Positive Allosteric Modulators: Structure-Activity Relationships and Assessment in a Rat Model of Nicotine Dependence. J Med Chem. 2012;55:9434–9445. doi: 10.1021/jm3005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- 44.Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, et al. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handbook of experimental pharmacology. 2009:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- 47.D'Souza MS, AM . The “Stop” & “Go” of Nicotine Dependence: Role of GABA and glutamate. In: Pierce RC, Kenny PJ, editors. Addiction: A neurobiological perspective. New York: Cold Spring Harbor Press; 2012. pp. 251–268. [Google Scholar]
- 48.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 49.Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 51.Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–352. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 52.Lecca D, Cacciapaglia F, Valentini V, Gronli J, Spiga S, Di Chiara G. Preferential increase of extracellular dopamine in the rat nucleus accumbens shell as compared to that in the core during acquisition and maintenance of intravenous nicotine self-administration. Psychopharmacology. 2006;184:435–446. doi: 10.1007/s00213-005-0280-4. [DOI] [PubMed] [Google Scholar]
- 53.Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse (New York, NY. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- 54.Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. The Journal of pharmacology and experimental therapeutics. 2003;306:407–420. doi: 10.1124/jpet.103.049262. [DOI] [PubMed] [Google Scholar]
- 55.Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- 56.Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. The Journal of pharmacology and experimental therapeutics. 2000;294:458–465. [PubMed] [Google Scholar]
- 57.Schilstrom B, Nomikos GG, Nisell M, Hertel P, Svensson TH. N-methyl-D-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998;82:781–789. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- 58.Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse (New York, NY. 2000;35:129–136. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 59.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 60.Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends in pharmacological sciences. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 62.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 63.D'Souza MS, Liechti ME, Ramirez-Nino AM, Kuczenski R, Markou A. The metabotropic glutamate 2/3 receptor agonist LY379268 blocked nicotine-induced increases in nucleus accumbens shell dopamine only in the presence of a nicotine-associated context in rats. Neuropsychopharmacology. 2011;36:2111–2124. doi: 10.1038/npp.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Current drug abuse reviews. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine Tob Res. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- 67.Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine Tob Res. 2009;11:323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug Alcohol Depend. 2010;108:7–12. doi: 10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abrantes AM, Strong DR, Lejuez CW, Kahler CW, Carpenter LL, Price LH, et al. The role of negative affect in risk for early lapse among low distress tolerance smokers. Addictive behaviors. 2008;33:1394–1401. doi: 10.1016/j.addbeh.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, et al. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine Tob Res. 2009;11:493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, et al. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33:2131–2138. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engelmann JM, Radke AK, Gewirtz JC. Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacology. 2009;207:13–25. doi: 10.1007/s00213-009-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: neuroadaptation in human addiction. Biological psychiatry. 2010;68:719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 78.Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. European journal of pharmacology. 2007;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. The Journal of pharmacology and experimental therapeutics. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- 80.Liechti ME, Markou A. Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol. 2007;74:1299–1307. doi: 10.1016/j.bcp.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 81.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 82.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 83.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 88.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 89.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biological psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Markou A, Li J, Tse K, Li X. Cue-induced nicotine-seeking behavior after withdrawal with or without extinction in rats. Addict Biol. 2016 doi: 10.1111/adb.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Le AD. Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving. J Neurosci. 2016;36:8612–8623. doi: 10.1523/JNEUROSCI.1505-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 2 AZD8529 on Incubation of Methamphetamine Craving After Prolonged Voluntary Abstinence in a Rat Model. Biological psychiatry. 2015;78:463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 94.Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacol Biochem Behav. 1993;44:891–900. doi: 10.1016/0091-3057(93)90021-k. [DOI] [PubMed] [Google Scholar]
- 95.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 96.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- 98.Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- 99.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- 100.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 101.Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, et al. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- 102.LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology. 2006;184:417–425. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramirez-Nino AM, D'Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225:473–482. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moro F, Orru A, Marzo CM, Di Clemente A, Cervo L. mGluR2/3 mediates short-term control of nicotine-seeking by acute systemic N-acetylcysteine. Addict Biol. 2016 doi: 10.1111/adb.12443. [DOI] [PubMed] [Google Scholar]
- 106.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- 107.Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- 108.Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 109.Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, et al. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Golubeva AV, Moloney RD, O'Connor RM, Dinan TG, Cryan JF. Metabotropic Glutamate Receptors in Central Nervous System Diseases. Current drug targets. 2016;17:538–616. doi: 10.2174/1389450116666150316224011. [DOI] [PubMed] [Google Scholar]
- 111.Spooren W, Lesage A, Lavreysen H, Gasparini F, Steckler T. Metabotropic glutamate receptors: their therapeutic potential in anxiety. Curr Top Behav Neurosci. 2010;2:391–413. doi: 10.1007/7854_2010_36. [DOI] [PubMed] [Google Scholar]
- 112.Maksymetz J, Moran SP, Conn PJ. Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Molecular brain. 2017;10:15. doi: 10.1186/s13041-017-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature medicine. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 114.Litman RE, Smith MA, Doherty JJ, Cross A, Raines S, Gertsik L, et al. AZD8529, a positive allosteric modulator at the mGluR2 receptor, does not improve symptoms in schizophrenia: A proof of principle study. Schizophrenia research. 2016;172:152–157. doi: 10.1016/j.schres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, et al. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. Journal of clinical psychopharmacology. 2011;31:349–355. doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- 116.Lipari RN, SVH . The CBHSQ Report. Rockville (MD): 2017. Smoking and Mental Illness Among Adults in the United States. [PubMed] [Google Scholar]
- 117.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 118.Cid JM, Tresadern G, Duvey G, Lutjens R, Finn T, Rocher JP, et al. Discovery of 1-butyl-3-chloro-4-(4-phenyl-1-piperidinyl)-(1H)-pyridone (JNJ-40411813) a novel positive allosteric modulator of the metabotropic glutamate 2 receptor. J Med Chem. 2014;57:6495–6512. doi: 10.1021/jm500496m. [DOI] [PubMed] [Google Scholar]
- 119.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 120.Acri JB, Cross AJ, Skolnick P. From bench to bedside: mGluR2 positive allosteric modulators as medications to treat substance use disorders. Psychopharmacology. 2017;234:1347–1355. doi: 10.1007/s00213-016-4501-9. [DOI] [PubMed] [Google Scholar]