Abstract
Background
Globally, 49% of the estimated 1.8 million children living with HIV are accessing antiretroviral therapy (ART). There are limited data concerning long-term durability of first-line ART regimens and time to transition to second-line.
Methods
Children initiating their first ART regimen between 2–14 years of age and enrolled in one of 208 sites in 30 Asia-Pacific and African countries participating in the Pediatric International Epidemiology Databases to Evaluate AIDS consortium were included in this analysis. Outcomes of interest were: First-line ART failure (clinical, immunologic, or virologic), change to second-line, and attrition (death or loss to program [LTP]). Cumulative incidence was computed for first-line failure and second-line initiation, with attrition as a competing event.
Results
In 27,031 children, median age at ART initiation was 6.7 years. Median baseline CD4% for children ≤5 years was 13.2% and CD4 count for those >5 years was 258 cells/µl. Almost all (94.4%) initiated a non-nucleoside reverse transcriptase inhibitor (NNRTI); 5.3% a protease inhibitor (PI), and 0.3% a triple nucleoside (NRTI)–based regimen. At one year, 7.7% had failed and 14.4% had experienced attrition; by five years, the cumulative incidence was 25.9% and 29.4%, respectively. At one year after ART failure, 13.7% had transitioned to second-line and 11.2% had experience attrition; by five years, the cumulative incidence was 31.6% and 25.9%, respectively.
Conclusions
High rates of first-line failure and attrition were identified in children within five years after ART initiation. Of children meeting failure criteria, only one-third were transitioned to second-line ART within five years.
Keywords: HIV, children, ART, failure, first-line, second-line
Background
As of 2015, globally 1.8 million children were living with HIV, the vast majority of whom resided in low- and middle-income countries (LMIC).1 Of these children (ages 0–14), 49% were accessing treatment, ranging from 20% in West and Central Africa to >95% in Europe and North America.1,2 The effectiveness of combination antiretroviral therapy (ART) in children is undisputed, with 12-month viral suppression rates ranging from 49–83.3%.3–10 However, there are limited data from LMIC concerning the long-term durability of first-line ART regimens. Because children in LMIC HIV treatment programs tend to start treatment at older ages with significant immune compromise, and are often monitored in the absence of virologic data, estimates of first-line ART durability taken from high-income settings may not be generalizable to these programs.11, 9,12 Though targeted viral load (VL) testing is currently recommended to confirm suspected failure many public-sector programs still rely on clinical and immunologic criteria to detect therapeutic failure, and are likely to continue to do so in full or in part because of issues with access to equipment, reagents or availability of consistent specimen transport to reference laboratories.13 Within this context it is important to understand the cumulative incidence of failure and the time to transition to second-line ART.
The cumulative incidence of switch to second-line ART in children has been assessed in both clinical trials and cohort studies. However, studies vary with regard to the initial ART regimen, monitoring strategy and definitions for the switch to a second regimen. The proportion switched to second-line after five years on treatment in the EPPICC Cohort and the PENPACT Trial were 35% and 29%, respectively.4,6 In Asia, the proportions of switch to second-line have been reported at 22% and 17.6% in cohorts with median on ART observation periods of 4.5 years and 4.9 years, respectively.14,15 However, much lower rates of switch have been reported from sub-Saharan Africa, with one observational cohort from South Africa reporting a three-year estimated probability of switch of only 6.2%, and the ARROW trial reporting switch rates at approximately 2 years on ART of 5%–6%.10,16 With the exception of the ARROW Trial, which identified failure based on clinical or immunologic criteria, the above studies predominately or exclusively utilized VL criteria for failure. As the positive predictive value of the WHO’s immunologic criteria for failure has been estimated to range from 20.0 to 54.9% in children, there is a high likelihood that first-line failure is significantly under-diagnosed in studies and programs utilizing CD4 monitoring in the absence of VL.17 Of note, while the South African cohort only had a 6.2% cumulative incidence of switch, the cumulative incidence of virologic failure was 19.3%, thus suggesting that even in the face of virologic failure there may be issues with transitioning antiretroviral regimens.16
In light of the complex environment in which HIV-infected children in LMIC are being assessed for failure and transitioned to second-line regimens, the International Epidemiology Databases to Evaluate AIDS (IeDEA) consortium sought to explore the time to and factors associated with ART failure as well as change to second-line in children initiating ART between 2–14 years of age.
Methods
Study Design and Setting
This retrospective cohort study utilized de-identified patient-level and site-level data drawn from the IeDEA Pediatric Cohort. The IeDEA consortium is composed of seven regional data centers that collect, harmonize, and analyze data drawn from HIV care and treatment programs within their region. Data from five of the IeDEA Pediatric Cohorts (Asia-Pacific; East Africa; West Africa; Central Africa; Southern Africa) are represented in this analysis.18 The Central African clinics are represented twice as Central Africa 1.0 and Central Africa 2.0 because the clinics within the region differ between two time periods.
This analysis was approved, as part of East Africa IeDEA, by the Indiana University Institutional Review Board (IRB) as well as local, and where required national, regulatory bodies affiliated with the participating programs and regional data centers. The majority of participating sites and regulatory bodies do not require written informed consent for the use of de-identified routinely collected patient-level data.
Study Population
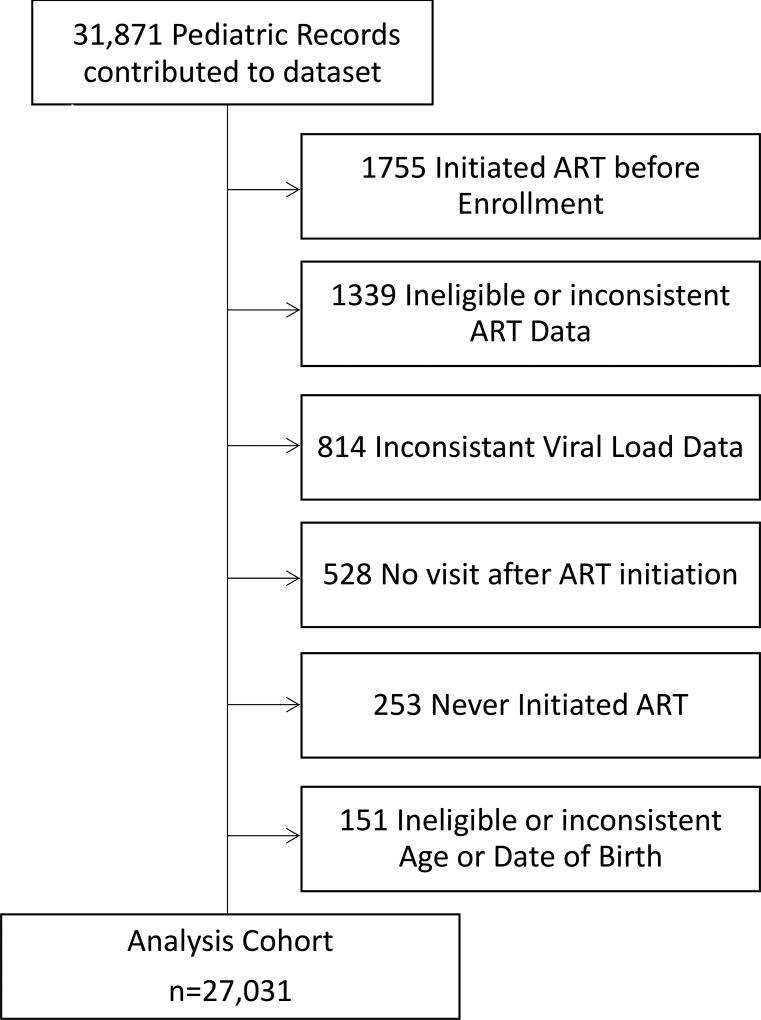
ART-naïve HIV-infected children enrolling in care prior to 10 years of age and initiating their first ART regimen with a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based, triple nucleoside reverse transcriptase inhibitor (NRTI)-based, or protease inhibitor (PI)-based three to four drug regimens between the ages of 2 and 14 years were included in this analysis. Children under 2 years were ineligible because the WHO clinical and immunologic criteria for failure were not well defined for this age group when the study was designed. Many HIV programs transition children from the pediatric to the adult clinic at 14 years and so 14 years was chosen as the upper age limit for inclusion. Children were excluded if they did not initiate a standard and consistent ART regimen, VL data were inconsistent (two VL tests on the same day with different values), there were no visits after ART initiation, or if their age was inconsistently documented (Figure 1).
Figure 1.
Cohort Composition
Data Collection and Management
Data were collected as part of routine clinical care via locally designed data collection instruments and then transcribed into the local electronic patient database. Laboratory studies, such as HIV VL and CD4 cell counts, were analyzed by local clinical laboratories utilizing local protocols and procedural standards. Vital status ascertainment was variable across sites with some sites having active tracing programs for those LTP and other sites relying on passive death reporting.
De-identified data were transferred from local programs to affiliated regional data centers harmonization and transfer to the East African Regional Data Center (EA-RDC) where a single analysis dataset was created. Data quality checks were incorporated into each step of the process. This analysis utilized patient-level data collected from 02/1994 to 02/2015 depending on region-specific data availability. Additional data were collected through two site-level surveys, which assessed programmatic factors such as recommended first and second-line ART regimens, antiretroviral availability, monitoring strategies (clinical, immunologic and/or virologic monitoring) and criteria for failure over the life of the program, as well as site characteristics such as facility type (public, academic, non-profit/private), location (urban, rural, in-between), population served (family [adults and children] or children only). The site-level data were assessed up to 2011–2012 for the majority of programs.
Analysis
The primary outcome of interest was the time to first-line ART failure. A minimum of 24 weeks on ART were required prior to being eligible for an assessment of failure. Clinical failure was defined as the appearance or reappearance of a WHO stage 3 or 4 condition. Immunologic failure was defined as the development or return to age-associated CD4 cell thresholds including an absolute CD4 count of less than 200 cells/µl or a CD4 percent of less than 10% for children between 2 and 5 years, as well as a CD4 count less than 100 cells/µl for children 5 years or older. Virologic failure was defined as a single VL measurement exceeding 5000 copies/ml, consistent with the WHO Guidelines when this analysis was designed.13
Throughout this analysis the decision hierarchy for failure assumed that virologic parameters superseded immunologic parameters which in turn superseded clinical parameters. The first VL measurement within 6 months after an immunologic or clinical failure event was used to determine the failure status. Likewise, if the first CD4 count within 2 months after a clinical failure did not meet immunologic criteria for failure, then the clinical failure was superseded by immunologic status. If a clinical failure event was documented and no immunologic or VL data were available then the patient was considered to have failed. An example of the decision analysis: If a patient had a WHO stage-4 event, a CD4 cell count less than 100 cells/µl but had an undetectable VL within 6 months after the clinical and immunologic failure events, in the absence of a change in ART regimen, they were not considered to have failed their ART regimen.
The secondary outcome of interest was the time from first-line ART failure to change to second-line ART, defined as a class change in the base component (e.g., NNRTI to PI or vice versa) and a change in at least one NRTI. Attrition (death or LTP) was used as a competing risk in both the time to first-line ART failure and time to change to second-line analyses. Death and LTP were viewed as the composite variable attrition in both analyses, because death is frequently under reported within sub-Saharan African ART programs and consequently may be misclassified as LTP.19 LTP was defined as no visit during the 6 months prior to database closure in the absence of documented death, transfer, or relocation outside of the clinic catchment.
Covariates assessed at ART initiation included sex, WHO stage, age, CD4 and weight-for-age Z-score (WAZ), facility location, facility type, population served, whether the site began providing ART before or after 2004, site virologic monitoring (confirmatory VL, routine VL, or no VL), and composition of the first-line regimen (NNRTI versus PI-based). WHO stage was converted into a binary variable WHO-stage1–2 versus 3–4. WAZ was defined using the 2000 CDC Growth Charts for ages 0 to <20 years.20 Sites that monitored VL every 6 –12 months were defined as routine VL sites while sites that used VL to confirm clinical or immunologic failure were defined as confirmatory VL sites. Neither the IeDEA region nor the country were included as factors in the proportional hazards model due to co-linearity with other variables (e.g., type of monitoring strategy).
Missing CD4 counts were imputed using a mixed-effects model with a spline in the time factor, where the outcome was the longitudinal CD4 count and the predictors included a random intercept, baseline age, sex, and the spline basis functions. The subject-specific effect was estimated from the above model, and it was used to estimate the corresponding subject’s missing CD4 cell count adjusting for age at ART initiation and sex. Missing WAZ and WHO stage at ART initiation were similarly imputed using a linear regression model, where the outcome was the WAZ at ART initiation or the WHO stage, and the predictors included sex, age and CD4 cell count at ART initiation. Children missing, at baseline, both CD4 count and WHO stage or missing both CD4 count and WAZ, as well as those with no CD4 data at any time during follow-up were excluded from the multivariable analysis but included in the univariate analysis. It should be noted that imputed quantities were used only in the multivariable models as risk factors of failure and switch to second line ART after failure but were not used to define treatment failure (clinical or immunologic).
Medians and interquartile ranges (IQR) for continuous variables and frequency percentages for categorical factors were calculated based on the observed data. Cumulative incidence was computed for first-line failure and second-line regimen initiation. Attrition was treated as a competing event as described earlier.
Patients not experiencing the outcome of interest or a competing event were censored on the database closure date. A cause-specific proportional hazards model was used to identify factors associated with each outcome. Hazard ratios were calculated for each covariate in the cause-specific proportional hazards model. Because of available data spanned more than two decades in some cases, there was the concern that some of the results of these analyses might not be generalizable to more recent calendar periods. To address this potential concern, we performed a sensitivity analysis where data were restricted to persons initiating ART during the most recent five years of available data (ART initiation no earlier than December 31, 2010). All analyses were implemented in R, Version 3.0.2. P-values less than 0.05 were considered significant.
Results
Data were contributed by 208 clinical sites from 30 countries. Of the 31,871 records received by the EA-RDC, 4,840 were not included in the analysis for one or more of the following reasons: ART-initiated prior to enrollment; never initiated ART; ART regimen was incomplete or inconsistent; age data were inconsistent; or there were insufficient visit data (Figure 1). Of the remaining 27,031 children, 52.8% were from Southern Africa, 27.8% East Africa, 7.2% Asia-Pacific, 6.4% West Africa, and 5.9% from Central Africa (Table 1). Children came predominately from clinics that were pediatric-specific (69.1%), publicly operated (59.4%), were located in between an urban and rural area (49.3%), and began operation prior to 2004 (62.9%).
Table 1.
Patient Demographics and Distribution by Site Characteristic
| Characteristic | N | (%) |
|---|---|---|
| Region | ||
| Asia-Pacific | 1935 | (7.2) |
| Central Africa 1.0 | 49 | (0.2) |
| Central Africa 2.0 | 1550 | (5.7) |
| East Africa | 7511 | (27.8) |
| Southern Africa | 14270 | (52.8) |
| West Africa | 1716 | (6.4) |
| Affiliation | ||
| Academic | 2225 | (8.2) |
| Public | 16055 | (59.4) |
| Non-profit | 8239 | (30.5) |
| Missing | 512 | (1.9) |
| Location | ||
| Urban | 12413 | (45.9) |
| Rural | 860 | (3.2) |
| In-between | 13321 | (49.3) |
| Missing | 437 | (1.6) |
| Type | ||
| Children only | 18685 | (69.1) |
| Family Clinics | 7088 | (26.2) |
| Missing | 1258 | (4.7) |
| Year Started Providing ART | ||
| Prior to 2004 | 16993 | (62.9) |
| 2004 and after | 10038 | (37.1) |
| Female | 13339 | (49.4) |
| Initial Regimen Type | ||
| NNRTI-based | 25512 | (94.4) |
| NRTI-based | 80 | (0.3) |
| PI-based | 1439 | (5.3) |
|
|
||
| Median | (IQR) | |
|
|
||
| Age at first visit | 6.1 | (3.5–9.1) |
| Age at ART start | 6.7 | (4.2–9.7) |
| CD4 count at ART start (cells/µl)* | 258 | (112–444)^ |
| CD4% at ART start‡ | 13.2 | (8.7–19.0)^ |
Patients >= 5 years old
Closest within 90 days prior and 7 days post ART initiation
Patients <5 years old
The proportion of clinics reporting use of routine VL monitoring increased from 7% for 2004 to 21.2% for 2011–2012. For 2004, 99.4% of clinics reported the use of an NNRTI, 6.6% a PI, and 3.0% a NRTI-based first-line regimen. For 2011–2012, 100% of clinics reported the use of an NNRTI, 47.5% a PI, and 1.0 % a NRTI-based first-line regimen. For 2004, 95.8% of clinics reported that they had access to second-line ART regimens while 99.0% reported that they had access for 2011–2012.
Among the 27,031 children included in this analysis, there were nearly equal proportions of male and female children. The median age at first visit was 6.1 years (IQR: 3.5–9.1) while the median age at ART initiation was 6.7 years (IQR: 4.2–9.7) (Table 1). At ART initiation, the median CD4 percent for children <5 years was 13.2% (IQR 8.7–19.0) and the median CD4 count for children ≥5 years was 258 cells/µl (IQR: 112–444). The vast majority (94.4%) of children initiated an NNRTI-, 5.3% a PI- and 0.3% a NRTI-based regimen.
During 19.5 months of median follow-up after ART initiation, 4,763 children were identified as having failed first-line ART with the mode of ascertainment for the earliest failure event being 32.4% by clinical, 22.2% by immunologic, and 45.4% by virologic criteria. During the same period 6,037 children experienced an attrition event. The cumulative incidence of any type of failure at one and five years after ART initiation was 7.7% (95% CI: 7.4–8.1) and 25.9% (95% CI: 25.6–27.0), respectively (Figure 2a). At one and five years after ART initiation, the cumulative incidence of attrition was 14.4% (95% CI: 14.1–15.-0) and 29.4% (95% CI: 29.1–30.5), respectively (Figure 2a).
Figure 2.
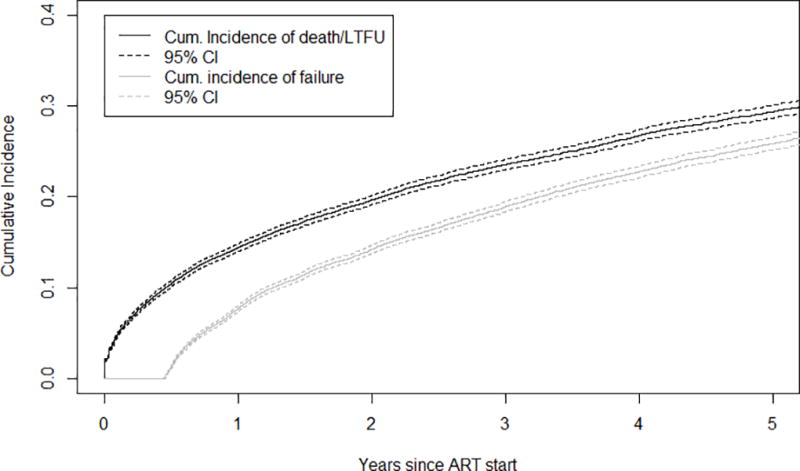
a. Cumulative Incidence of Failure and Death/Loss to program after ART-initiation
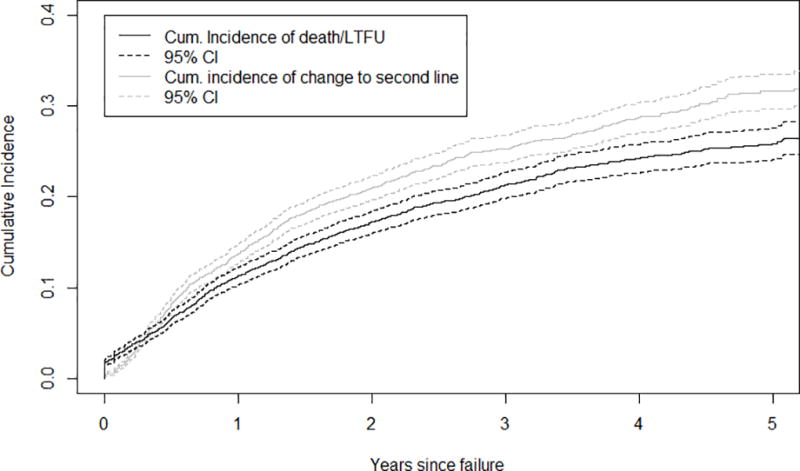
b. Cumulative Incidence of Change to second-line and Death/Loss to Program after a Failure Event
In an analysis involving the 22,257 children with available data, factors at ART initiation associated with failure or attrition, in the univariate analyses, are outlined in Table 2. In a multivariable analysis, the factors at ART-initiation that were associated with a lower hazard of any type of failure were higher WAZ, higher CD4 count, enrollment at a private clinic or a clinic starting ART provision after 2004, while a higher hazard of failure was associated with being male, having a higher WHO stage, initiating a PI-based regimen, or enrollment at an in-between clinic. A lower hazard of attrition was significantly associated with older age, higher WAZ, higher CD4 cell count, enrollment at a rural clinic, a family clinic, a clinic starting ART provision after 2004, or a clinic using any VL testing. Higher WHO stage and being enrolled at a private clinic were significantly associated with a higher hazard of attrition. In the analyses based on data from the most recent five years (sensitivity analyses described in the Methods, not shown), the results were virtually identical with those of the overall multivariable analyses presented above.
Table 2.
Cause-specific hazard ratios and 95% confidence intervals for factors associated with first line ART failure or attrition in a competing risk analysis
| Factor n = 22,257 |
Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| Failure | ||||||
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 1.092 | 1.031 – 1.156 | 0.003 | 1.100 | 1.034 – 1.169 | 0.002 |
| Age per year | 1.027 | 1.018 – 1.036 | <0.001 | 0.999 | 0.988 – 1.009 | 0.788 |
| WAZ per sd | 0.945 | 0.931 – 0.960 | <0.001 | 0.960 | 0.945 – 0.976 | <0.001 |
| CD4 count per 50 cells/µl | 0.963 | 0.958 – 0.968 | <0.001 | 0.960 | 0.954 – 0.965 | <0.001 |
| WHO per stage | 1.222 | 1.146 – 1.304 | <0.001 | 1.142 | 1.064 – 1.225 | <0.001 |
| Regimen | ||||||
| NNRTI | Ref | Ref | ||||
| PI | 1.090 | 0.964 – 1.232 | 0.169 | 1.482 | 1.284 – 1.712 | <0.001 |
| Clinic Type | ||||||
| Public | Ref | Ref | ||||
| Academic | 1.183 | 1.083 – 1.292 | <0.001 | 0.990 | 0.866 – 1.131 | 0.880 |
| Non-profit/Private | 0.562 | 0.520 – 0.607 | <0.001 | 0.375 | 0.320 – 0.440 | <0.001 |
| Clinic Location | ||||||
| Urban | Ref | Ref | ||||
| Rural | 1.235 | 1.061 – 1.438 | 0.006 | 1.090 | 0.826 – 1.113 | 0.318 |
| In-between | 0.868 | 0.818 – 0.921 | <0.001 | 1.121 | 1.039 – 1.208 | 0.003 |
| Clinic Type | ||||||
| Children only | Ref | Ref | ||||
| Family Clinic | 1.328 | 1.249 – 1.412 | <0.001 | 0.959 | 0.826 – 1.113 | 0.580 |
| Clinic Started Providing ART | ||||||
| Before 2004 | Ref | Ref | ||||
| 2004 or after | 1.091 | 1.029 – 1.157 | 0.003 | 0.880 | 0.802 – 0.965 | 0.007 |
| VL Availability | ||||||
| None | Ref | Ref | ||||
| Confirmatory | 1.300 | 1.101 – 1.536 | <0.001 | 1.638 | 1.349 – 1.989 | <0.001 |
| Routine | 1.391 | 1.175 – 1.647 | <0.001 | 1.078 | 0.878 – 1.323 | 0.475 |
| Death/Lost to Program | ||||||
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 1.002 | 0.952 – 1.054 | 0.941 | 0.990 | 0.935 – 1.048 | 0.727 |
| Age per year | 0.987 | 0.979 – 0.995 | 0.001 | 0.980 | 0.971 – 0.989 | <0.001 |
| WAZ per sd | 0.861 | 0.850 – 0.871 | <0.001 | 0.893 | 0.880 – 0.906 | <0.001 |
| CD4 count per 50 cells/µl | 0.990 | 0.986 – 0.994 | <0.001 | 0.991 | 0.987 – 0.995 | <0.001 |
| WHO per stage | 1.198 | 1.131 – 1.270 | <0.001 | 1.112 | 1.042 – 1.186 | 0.001 |
| Regimen | ||||||
| NNRTI | Ref | Ref | ||||
| PI | 1.023 | 0.915 – 1.144 | 0.689 | 1.008 | 0.868 – 1.171 | 0.918 |
| Clinic Type | ||||||
| Public | Ref | Ref | ||||
| Academic | 1.061 | 0.960 – 1.173 | 0.243 | 0.876 | 0.759 – 1.011 | 0.071 |
| Non-profit/Private | 2.056 | 1.949 – 2.169 | <0.001 | 1.431 | 1.264 – 1.621 | <0.001 |
| Clinic Location | ||||||
| Urban | Ref | Ref | ||||
| Rural | 0.627 | 0.508 – 0.773 | <0.001 | 0.626 | 0.493 – 0.796 | <0.001 |
| In-between | 1.520 | 1.442 – 1.602 | <0.001 | 1.033 | 0.953 – 1.119 | 0.435 |
| Clinic Type | ||||||
| Children only | Ref | Ref | ||||
| Family Clinic | 0.791 | 0.745 – 0.841 | <0.001 | 0.843 | 0.752 – 0.944 | 0.003 |
| Clinic Started Providing ART | ||||||
| Before 2004 | Ref | Ref | ||||
| 2004 or after | 0.684 | 0.647 – 0.723 | <0.001 | 0.874 | 0.792 – 0.964 | 0.007 |
| VL Availability | ||||||
| None | Ref | Ref | ||||
| Confirmatory | 0.691 | 0.625 – 0.764 | <0.001 | 0.577 | 0.506 – 0.658 | <0.001 |
| Routine | 0.442 | 0.397 – 0.492 | <0.001 | 0.364 | 0.310 – 0.426 | <0.001 |
The 4,763 patients identified with first-line ART failure were followed for a median of 14.3 months after a failure event during which 990 (20.8%) were transitioned to second-line and 833 (17.5%) had an attrition event. The cumulative incidence of switch to second-line ART for the entire cohort (irrespective of failure) at one and five years after ART initiation was 0.38% (95% CI: 0 – 1%) and 0.49% (95% CI: 0 – 1%), respectively. Among those with a documented failure event, the cumulative incidence of change to second-line at one and five years after failure was 13.7% (95% CI: 12.9–15.0) and 31.6% (95% CI: 30.9–32.4), respectively (Figure 2b). The cumulative incidence of attrition at one and five years after failure was 11.2% (95% CI: 10.5–12.4) and 25.9% (95% CI: 25.1–28.8), respectively (Figure 2b).
Among 4,120 children with available data, factors at ART initiation associated with change to second-line ART or attrition, in the univariate analyses, are outlined in Table 3. In a multivariable analysis, baseline factors that were associated with a lower hazard of change to second-line ART after failure included a higher CD4 cell count, PI-based first-line, and enrollment in a family clinic, an in-between clinic, or an academically affiliated clinic. Being male, older, and enrolled in a clinic with confirmatory VL testing was associated with a higher hazard of switch to second-line ART. Baseline factors that were associated with a lower hazard of attrition after first-line ART failure included higher WAZ, higher CD4 cell count, enrollment in a family clinic, or an academically affiliated clinic. Receiving care at a private clinic was significantly associated with a higher hazard of attrition after failure (Table 3). In the analyses based on data from the most recent five years (not shown), the results were virtually identical with those of the overall multivariable analyses presented above.
Table 3.
Cause-specific hazard ratios and 95% confidence intervals for factors associated with change to a second-line ART regimen or attrition in a competing risk analysis
| Factor n = 4,120 |
Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| Change to Second-line ART | ||||||
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 1.287 | 1.133 – 1.463 | <0.001 | 1.317 | 1.147 – 1.513 | <0.001 |
| Age per year | 1.089 | 1.069 – 1.110 | <0.001 | 1.043 | 1.020 – 1.067 | <0.001 |
| WAZ per sd | 0.971 | 0.938 – 1.006 | 0.101 | 0.999 | 0.961 – 1.039 | 0.969 |
| CD4 count per 50 cells/µl | 0.925 | 0.911 – 0.937 | <0.001 | 0.930 | 0.9176 – 0.946 | <0.001 |
| WHO per stage | 0.990 | 0.858 – 1.143 | 0.895 | 0.969 | 0.818 – 1.126 | 0.613 |
| Regimen | ||||||
| NNRTI | Ref | Ref | ||||
| PI | 0.387 | 0.260 – 0.576 | <0.001 | 0.355 | 0.221 – 0.572 | <0.001 |
| Clinic Type | ||||||
| Public | Ref | Ref | ||||
| Academic | 0.632 | 0.510 – 0.783 | <0.001 | 0.354 | 0.254 – 0.494 | <0.001 |
| Non-profit/Private | 1.149 | 0.964 – 1.369 | 0.121 | 0.839 | 0.594 – 1.186 | 0.321 |
| Clinic Location | ||||||
| Urban | Ref | Ref | ||||
| Rural | 0.553 | 0.361 – 0.847 | 0.006 | 0.709 | 0.443 – 1.136 | 0.152 |
| In-between | 0.889 | 0.780 – 1.012 | 0.076 | 0.839 | 0.705 – 0.997 | 0.046 |
| Clinic Type | ||||||
| Children only | Ref | Ref | ||||
| Family Clinic | 0.720 | 0.627 – 0.827 | <0.001 | 0.521 | 0.375 – 0.725 | <0.001 |
| Clinic Started Providing ART | ||||||
| Before 2004 | Ref | Ref | ||||
| 2004 or after | 0.782 | 0.687 – 0.891 | <0.001 | 1.009 | 0.826 – 1.231 | 0.932 |
| VL Availability | ||||||
| None | Ref | Ref | ||||
| Confirmatory | 1.321 | 0.825 – 2.118 | 0.244 | 1.767 | 0.687 – 1.274 | 0.040 |
| Routine | 1.851 | 1.150 – 2.964 | 0.010 | 1.604 | 0.886 – 2.902 | 0.119 |
| Death/ Lost to Program | ||||||
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 1.042 | 0.908 – 1.194 | 0.563 | 1.049 | 0.901 – 1.221 | 0.539 |
| Age per year | 1.024 | 1.003 – 1.044 | 0.023 | 0.996 | 0.970 – 1.020 | 0.732 |
| WAZ per sd | 0.907 | 0.874 – 0.941 | <0.001 | 0.921 | 0.885 – 0.959 | <0.001 |
| CD4 count per 50 cells/µl | 0.979 | 0.968 – 0.990 | <0.001 | 0.977 | 0.963 – 0.989 | 0.001 |
| WHO per stage | 0.980 | 0.836–1.149 | 0.803 | 0.900 | 0.756 – 1.071 | 0.236 |
| Regimen | ||||||
| NNRTI | Ref | Ref | ||||
| PI | 0.923 | 0.691 – 1.232 | 0.585 | 0.842 | 0.587 – 1.208 | 0.351 |
| Clinic Type | ||||||
| Public | Ref | Ref | ||||
| Academic | 0.799 | 0.642 – 0.995 | 0.045 | 0.532 | 0.374 – 0.757 | <0.001 |
| Non-profit/Private | 1.685 | 1.419 – 2.002 | <0.001 | 1.447 | 1.033 – 2.029 | 0.032 |
| Clinic Location | ||||||
| Urban | Ref | Ref | ||||
| Rural | 0.695 | 0.453 – 1.067 | 0.096 | 0.714 | 0.439 – 1.161 | 0.175 |
| In-between | 1.010 | 0.877 – 1.162 | 0.893 | 0.747 | 0.614 – 0.910 | 0.004 |
| Clinic Type | ||||||
| Children only | Ref | Ref | ||||
| Family Clinic | 0.766 | 0.659 – 0.890 | <0.001 | 0.714 | 0.494 – 0.942 | 0.039 |
| Clinic Started Providing ART | ||||||
| Before 2004 | Ref | Ref | ||||
| 2004 or after | 0.764 | 0.663 – 0.880 | <0.001 | 0.921 | 0.695 – 1.115 | 0.487 |
| VL Availability | ||||||
| None | Ref | Ref | ||||
| Confirmatory | 0.805 | 0.549 – 1.179 | 0.265 | 0.828 | 0.541 – 1.267 | 0.385 |
| Routine | 0.835 | 0.568 – 1.228 | 0.360 | 0.624 | 0.379 – 1.027 | 0.064 |
Discussion
This five-region analysis of data from sub-Saharan Africa and Asia demonstrated a high rate of first-line failure (25.9%) among children at five years after ART initiation. Data for comparison are limited, as most studies utilize the rate of switch to second-line or VL failure as the outcome measures when assessing first-line ART durability while our study utilizes a site-dependent composite failure variable that includes clinical, immunologic, and/or VL criteria. Though not directly comparable, our failure rates appear somewhat lower than the PENPACT trial, which reported a failure rate of 36% (VL ≥1,000 copies/mL) at a median of five years after ART initiation.21 Our results appear consistent with the ARROW Trial, which reported virologic failure (>400 copies/mL) in approximately 23% of children at a median of 3.7 years and somewhat higher than data from Soweto reporting virologic failure (confirmed VL >1000) in 16.3% of children at a median of 36 months.22
In the multivariable analysis, failure was associated with a PI-based regimen but not with routine VL testing. This finding is counter to our a priori assumptions. An additional exploratory analysis showed that the proportion of patients on a PI-based regimen with a VL was significantly greater than for individuals on an NNRTI-based regimen (p <0.001). Based on this finding, we posit that there is confounding by site, as the sites most likely to have routine VL testing, primarily in South Africa, are also have a higher proportion of children on PI-based first-line ART. Other factors that may have contributed to the finding of an association between PI-based first line regimen and failure include the possibility of co-linearity with age (in South Africa PI-based ART is initiated in children <3 years) and the use of unboosted PIs in the early phase of ART rollout in some countries. Additional factors associated with an increased hazard of failure were, not unexpectedly, related to the child’s disease severity at ART initiation, including higher WHO stage, lower WAZ and lower CD4 count. Results from the sensitivity analyses focusing on patients initiating ART during the most recent five years were identical to those involving the complete database. This suggests that the associations between various predictors and the cause-specific hazards of attrition or of treatment failure were generally consistent throughout the observation period and, more importantly, are still relevant in the current reality of HIV pediatric patient care.
At one year after first-line failure, the cumulative incidence of reaching one of the study endpoints of change to second-line ART (13.7%) or attrition (11.2%) was 24.9%. Thus 75.1% of children, one year after meeting criteria for first-line failure, were still in care and on a first-line regimen. The one-year rate of switch to second-line therapy in our analysis is significantly lower than that previously reported by the Southern Africa IeDEA region (38% within one-year of virologic failure).16 Though the data in this analysis are insufficient to explore factors contributing to a physician’s assessment of failure, our findings that a lower CD4 count was associated with an increased probability of switch and that a PI-based regimen was associated with a lower probability of switch, similar to the South African analysis, suggest that multiple factors impact a clinician’s decision to transition a patient to second-line ART.16 Delays in switch to second-line may be related to clinician concerns about the diagnostic accuracy of the failure criteria; the availability or effectiveness of subsequent ART regimens; as well as implementation of interventions to improve adherence.
The use of routine VL monitoring was not associated with a higher likelihood of switch after failure, but was associated with a trend toward a lower likelihood of attrition after failure. This finding persisted in the sensitivity analysis focusing on patients initiating ART during the most recent five years of data, thus the increasing use of routine VL in the most recent period did not modify the association between various predictors and the likelihood (hazard) of initiating second-line ART regimens. Since the endpoint for this analysis was the first failure event, in neither the initial analysis nor in the sensitivity analysis did we assess whether or not patients subsequently suppressed their VL without a regimen change. It is possible that a detectable VL triggered an adherence intervention that subsequently led to suppression, which obviated the need for a regimen change. In addition, academic and family-based clinics were less likely to transition patients to second-line, but also had lower hazards of attrition after failure compared to public or child-only clinics. This leads us to speculate that these clinics may implement adherence intervention strategies, triggered by identification of a failure event, prior to enacting a transition to second-line ART.
Because the data used in this analysis came from HIV clinical programs, there are a number of weaknesses in the study. Death was passively reported by most programs. Consequently, there is the potential for individuals who have died to be misclassified as being LTP.23 In addition, individuals who are LTP may be engaged in HIV care at another program. As such, the composite attrition variable (death or LTP) constitutes a sub-optimal summary of the patient experience after leaving a program. However, we anticipate that a significant proportion of patients classified as LTP either died or completely disengaged from care; given that both these events compete for the identification of failure, we believe that structuring the analysis in this way allows for the most conservative estimates given the limitations of these data. In addition, this analysis may under estimate loss to program as children who has no follow-up time on ART were excluded from this analysis however as retention was not the focus of this analysis, this does not impact the key findings of this paper. As noted previously, failure was identified as the first episode of meeting clinical, immunologic, or viral criteria, and interventions other than a change to second-line therapy may have occurred (e.g., adherence counseling, referral to a support group, assignment of a peer navigator, etc.). Unfortunately, alternative interventions could not be assessed in this analysis. In addition, missing data on clinical events and laboratory testing such as CD4 cell counts and VLs may have led to an underestimate of the cumulative risk of failure over time.
Despite these significant limitations, the strengths of this analysis are that the data are from a large geographically diverse cohort that is representative of the HIV-infected pediatric population receiving ART in sub-Saharan Africa and Asia. In addition, this is one of the few studies that has been able to assess the incidence of failure, based on the monitoring strategies utilized by the programs, and assess time from an indication of failure to transition to second-line ART.
In conclusion, based on this analysis, approximately a quarter of pediatric HIV patients in sub-Saharan Africa and Asia experience at least one failure event within five years of ART initiation. However, the rate of change to second-line ART regimens was low. Further studies are needed in order to understand how HIV programs healthcare providers assess and respond to treatment failure events.
Acknowledgments
Sources of Support: This work was supported through grants from the National Institutes of Health through the National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development under the following grants: East Africa IeDEA (U01AI069911), IeDEA Asia-Pacific (U01 AI069907), IeDEA West Africa (U01 AI069919), IeDEA Southern Africa (U01 AI069924), IeDEA Central Africa 1.0 (U01 AI069927), IeDEA Central Africa 2.0 (U01 AI096299). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We acknowledge the clinics and investigators within each program who contributed data to this analysis:
East Africa IeDEA:
Lameck Diero, Samuel Ayaya, AMPATH Plus, MOI University Eldoret, Kenya; Elizabeth Bukusi, Kenya Medical Research Institute (KEMRI), Kisumu, Kenya; John Ssali, Masaka Regional Referral Hospital, Masaka, Uganda; G.R. Somi, National AIDS Control Program (NACP) Dar es Salaam, Tanzania; Rita Elias Lyamuya, Morogoro Regional Hospital, Morogoro, Tanzania; Kapella Ngonyani, Tumbi Regional Hospital, Pwani, Tanzania; and Emanuel Lugina, Ocean Road Cancer Institute, Dar es Salaam, Tanzania.
IeDEA Asia-Pacific:
PS Ly*, and V Khol, National Centre for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; N Kumarasamy*, S Saghayam, and E Chandrasekaran, YRGCARE Medical Centre, CART CRS, Chennai, India; DK Wati*, D Vedaswari, and IY Malino, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati*, and D Muktiarti, Cipto Mangunkusumo – Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; SM Fong*, KJ Wong, and FB Daut, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff*†, and P Mohamad, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; KA Razali*, TJ Mohamed, and MR Drawis, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy*, and KC Chan, Penang Hospital, Penang, Malaysia; T Sudjaritruk*, V Sirisanthana, and L Aurpibul, Department of Pediatrics, Faculty of Medicine, Chiang Mai University and Research Institute for Health Sciences, Chiang Mai, Thailand; R Hansudewechakul*, P Ounchanum, S Denjanta, and A Kongponoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon*, P Kosalaraksa, P Tharnprisan, and T Udomphanit, Division of Infectious Diseases, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, PHPT-IRD UMI 174 (Institut de recherche pour le développement and Chiang Mai University), Chiang Mai, Thailand; T Puthanakit*, S Anugulruengkit, W Jantarabenjakul and R Nadsasarn, Department of Pediatrics, Faculty of Medicine and Research Unit in Pediatric and Infectious Diseases, Chulalongkorn University, Bangkok, Thailand; K Chokephaibulkit*‡, K Lapphra, W Phongsamart, and N Vanprapar, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; KH Truong*, QT Du, and TT Nguyen, Children’s Hospital 1, Ho Chi Minh City, Vietnam; VC Do*, TM Ha, and VT An Children’s Hospital 2, Ho Chi Minh City, Vietnam; LV Nguyen*, DTK Khu, and LT Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam; ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn*, JL Ross, and C Sethaputra, TREAT Asia/amfAR -- The Foundation for AIDS Research, Bangkok, Thailand; DA Cooper, MG Law*, and A Kariminia, The Kirby Institute, UNSW Australia, Sydney, Australia; (*Steering Committee members; † Current Steering Committee Chair; ‡ co-Chair).
West Africa IeDEA
Site investigators and cohorts: Adult cohorts: Marcel Djimon Zannou, CNHU, Cotonou, Benin; Armel Poda, CHU Souro Sanou, Bobo Dioulasso, Burkina Faso; Fred Stephen Sarfo, Komfo Anokeye Teaching Hospital, Kumasi, Ghana; Eugene Messou, ACONDA CePReF, Abidjan, Ivory Coast; Henri Chenal, CIRBA, Abidjan, Ivory Coast; Kla Albert Minga, CNTS, Abidjan, Ivory Coast; Emmanuel Bissagnene, & Aristophane Tanon, CHU Treichville, Ivory Coast; Moussa Seydi, CHU de Fann, Dakar, Senegal; Akessiwe Akouda Patassi, CHU Sylvanus Olympio, Lomé, Togo.Pediatric cohorts: Sikiratou Adouni Koumakpai-Adeothy, CNHU, Cotonou, Benin; Lorna Awo Renner, Korle Bu Hospital, Accra, Ghana; Sylvie Marie N’Gbeche, ACONDA CePReF, Abidjan, Ivory Coast; Clarisse Amani Bosse, ACONDA_MTCT+, Abidjan, Ivory Coast; Kouadio Kouakou, CIRBA, Abidjan, Ivory Coast; Madeleine Amorissani Folquet, CHU de Cocody, Abidjan, Ivory Coast; François Tanoh Eboua, CHU de Yopougon, Abidjan, Ivory Coast; Fatoumata Dicko Traore, Mariam Sylla, Hopital Gabriel Toure, Bamako, Mali; Elom Takassi, CHU Sylvanus Olympio, Lomé, Togo
Coordinating & data centers: François Dabis, Elise Arrive, Eric Balestre, Renaud Becquet, Charlotte Bernard, Shino Chassagne Arikawa, Alexandra Doring, Antoine Jaquet, Karen Malateste, Elodie Rabourdin, Thierry Tiendrebeogo : ADERA, Isped & Inserm U1219, Bordeaux, France.
Sophie Desmonde, Julie Jesson, Valeriane Leroy : Inserm U1027, Toulouse, France Didier Koumavi Ekouevi, Jean-Claude Azani, Patrick Coffié, Guy Gnepa, Christian Gerard Kaugbouh Kouadio, Boris Tchounga: PACCI, CHU Treichville, Abidjan, Ivory Coast
Central Africa IeDEA 1.0
Dr. Nelly Kamgaing, Centre Hospitalier et Universitaire, Yaoundé, Cameroon; Dr. Pierre Kariyo, Dr. Helene Bukuru, Dr. Martin Nduwimana and Dr. Théodore Niyongabo, Centre Hospitalo-Universitaire de Kamenge, Bujumbura, Burundi; Dr. Marcel Mbaya and Dr. Henri Mukumbi AMO CONGO, Democratic Republic of Congo; Dr. Wilfred Akam, Limbé Provincial Hospital, Limbé, Cameroon; Dr. Modeste Kiumbu and Mr. Joseph Atibu, Ecole de Santé Publique, Kinshasa, Democratic Republic of Congo; Mr. Innocent Azinyue, Vindata Solutions, Yaoundé, Cameroon; Ms. Jennifer Hemingway-Foday, Ms. Jeniffer Iriondo-Perez and Ms. Kristin Stolka, RTI International, Research Triangle Park
Central Africa IeDEA 2.0
Nimbona Pélagie, ANSS, Burundi; Patrick Gateretse, Jeanine Munezero, Valentin Nitereka, Théodore Niyongabo, Christelle Twizere, Centre National de Reference en Matiere de VIH/SIDA, Burundi; Hélène Bukuru, Thierry Nahimana, CHUK, Burundi; Jérémie Biziragusenyuka, Risase Scholastique Manyundo, HPRC, Burundi; Rogers Ajeh, Mark Benwi, Anastase Dzudie, Akindeh Mbuh, CRENC & Douala General Hospital, Cameroon; Kien Atsu, Tabeyang Mbuh, Bamenda Hospital, Cameroon; Djenabou Amadou, Eric Ngassam, Eric Walter Pefura Yone, Jamot Hospital, Cameroon; Alice Ndelle Ewanoge, Norbert Fuhngwa, Chris Moki, Limbe Regional Hospital, Cameroon; Catherine Akele, Faustin Kitetele, Patricia Lelo, Martine Tabala, Kalembelembe Pediatric Hospital, Democratic Republic of Congo; Emile Wemakoy Okitolonda, Landry Wenzi, Kinshasa School of Public Health, Democratic Republic of Congo; Merlin Diafouka, Martin Herbas Ekat, Dominique Mahambou Nsonde, CTA Brazzaville, Republic of Congo; Adolphe Mafou, CTA Pointe-Noire, Republic of Congo; Jean Claude Dusingize, Gallican Kubwimana, Pacifique Mugenzi, Benjamin Muhoza, Athanase Munyaneza, Emmanuel Ndahiro, Diane Nyiransabimana, Jean d'Amour Sinayobye, Vincent Sugira, Rwanda Military Hospital, Rwanda; Fidele Ntarambirwa, Bethsaida Hospital, Rwanda; Yvonne Tuyishimire, Busanza Health Center, Rwanda; Theogene Hakizimana, Gahanga Health Center, Rwanda; Josephine Ayinkamiye, Gikondo Health Center, Rwanda; Sandrine Mukantwali, Kabuga Health Center, Rwanda; Henriette Kayitesi, Olive Uwamahoro, Kicukuro Health Center, Rwanda; Viateur Habumuremyi, Nyarugunga Health Center, Rwanda; Joyce Mukamana, Masaka Health Center, Rwanda; Chantal Benekigeri, Gilbert Mbaraga, WE-ACTx Health Center, Rwanda.
Southern Africa IeDEA
Gary Maartens, Aid for AIDS, South Africa; Michael Vinikoor, Centre for Infectious Disease Research in Zambia (CIDRZ), Zambia; Monique von Lettow, Dignitas, Malawi; Robin Wood, Gugulethu ART Programme, South Africa; Shobna Sawry, Harriet Shezi Children’s Clinic, South Africa; Frank Tanser, Africa Centre for Health & Population Studies (Hlabisa), South Africa; Jonathan Euvrard, Khayelitsha ART Programme, South Africa; Geoffrey Fatti, Kheth’Impilo, South Africa; Sam Phiri, Lighthouse Clinic, Malawi; Cleophas Chimbetete, Newlands Clinic, Zimbabwe; Karl Technau, Rahima Moosa Mother and Child Hospital, South Africa; Brian Eley, Red Cross Children's Hospital, South Africa; Josephine Muhairwe, SolidarMed Lesotho; Anna Jores, SolidarMed Mozambique; Kamelia Kamenova, SolidarMed Zimbabwe, Matthew P Fox, Themba Lethu Clinic, South Africa; Hans Prozesky, Tygerberg Academic Hospital, South Africa.
Footnotes
Presentations: These data were presented at the 6th International Workshop on HIV Pediatrics; Melbourne Australia, July 20-15, 2014.
Contributor Information
Kara Wools-Kaloustian, Indiana University School of Medicine, Indianapolis, IN, USA.
Irene Marete, School of Medicine, Moi University, Eldoret, Kenya.
Samuel Ayaya, School of Medicine, Moi University, Eldoret, Kenya.
Annette H. Sohn, TREAT Asia/amfAR – The Foundation for AIDS Research, Bangkok, Thailand.
Lam Van Nguyen, Department of Infectious Diseases, National Hospital of Pediatrics Hanoi, Vietnam.
Shanshan Li, Fairbanks School of Public Health, Indianapolis, IN, USA.
Valériane Leroy, Inserm U1027, Toulouse 3 University, Toulouse, France.
Beverly S. Musick, Indiana University School of Medicine, Indianapolis, IN, USA.
Jamie E. Newman, RTI International, Public Health Informatics Program, Research Triangle Park, NC, USA.
Andrew Edmonds, Department of Epidemiology, Gillings School of Global Public Health, The University of North Carolina at Chapel Hill.
Mary-Ann Davies, Centre for Infectious Disease Epidemiology and Research, University of Cape Town, Cape Town, South Africa.
François Tanoh Eboua, Department of Pediatrics, Centre Hospitalier Universitaire de Yopougon, Abidjan, Côte d’Ivoire.
Marie-Thérèse Obama, Centre Hospitalier et Universitaire, Yaoundé, Cameroon.
Marcel Yotebieng, Division of Epidemiology, College of Public Health, Ohio State University.
Shobna Sawry, Wits Reproductive Health and HIV Institute, University of the Witwatersrand, School of Clinical Medicine, Johannesburg, South Africa.
Lynne M. Mofenson, Elizabeth Glaser Pediatric AIDS Foundation, Washington DC, USA.
Constantin T. Yiannoutsos, Fairbanks School of Public Health, Indianapolis, IN, USA.
References
- 1.UNICEF. [Accessed Jan 18, 2017];Pediatric Treatment and Care. http://data.unicef.org/hiv-aids/paediatric.html.
- 2.UNAIDS. [Accessed 10 June 2016];FACTS SHEET 2016: Global Statistics. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
- 3.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(12):1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judd A EuroCoord European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) study group. Early antiretroviral therapy in HIV-1-infected infants, 1996–2008: treatment response and duration of first-line regimens. AIDS. 2011;25(18):2279–2287. doi: 10.1097/QAD.0b013e32834d614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry O, Powell J, Renner L, et al. Effectiveness of first-line antiretroviral therapy and correlates of longitudinal changes in CD4 and viral load among HIV-infected children in Ghana. BMC Infect Dis. 2013;13:476. doi: 10.1186/1471-2334-13-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babiker A, Castro nee Green H, Compagnucci A, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11(4):273–283. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. The New England journal of medicine. 2012;366(25):2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A biregional survey and review of first-line treatment failure and second-line paediatric antiretroviral access and use in Asia and southern Africa. J Int AIDS Soc. 2011;14:7. doi: 10.1186/1758-2652-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 10.Kekitiinwa A, Cook A, Nathoo K, et al. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet. 2013;381(9875):1391–1403. doi: 10.1016/S0140-6736(12)62198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. [Accessed 10 June 2016];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2013 http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- 12.Desmonde S, Eboua FT, Malateste K, et al. Determinants of durability of first-line antiretroviral therapy regimen and time from first-line failure to second-line antiretroviral therapy initiation. AIDS. 2015;29(12):1527–1536. doi: 10.1097/QAD.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. [Accessed 10 June 2016];Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed]
- 14.Collins I, Cairns J, Le Coeur S, et al. Five-year trends in antiretroviral usage and drug costs in HIV-infected children in Thailand. J Acquir Immune Defic Syndr. 2013;64(1):95–102. doi: 10.1097/QAI.0b013e318298a309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed TJ, Teeraananchai S, Kerr S, et al. Impact of Viral Load Use on Treatment Switch in Perinatally HIV-Infected Children in Asia. AIDS Res Hum Retroviruses. 2016 doi: 10.1089/aid.2016.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56(3):270–278. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutherford GW, Anglemyer A, Easterbrook PJ, et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS. 2014;28(Suppl 2):S161–169. doi: 10.1097/QAD.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 18.IeDEA Pediatric Working Group. A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa--the International epidemiologic Databases to Evaluate AIDS (IeDEA) J Int AIDS Soc. 2013;16:17998. doi: 10.7448/IAS.16.1.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. 2015;2(3):e107–116. doi: 10.1016/S2352-3018(15)00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed January, 2017];CDC. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 21.Harrison L, Melvin A, Fiscus S, et al. HIV-1 Drug Resistance and Second-Line Treatment in Children Randomized to Switch at Low Versus Higher RNA Thresholds. J Acquir Immune Defic Syndr. 2015;70(1):42–53. doi: 10.1097/QAI.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. The Pediatric Infectious DiseaseJournal. 2011;30(11):974–979. doi: 10.1097/INF.0b013e31822539f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braitstein P, Songok J, Vreeman RC, et al. "Wamepotea" (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr. 2011;57(3):e40–46. doi: 10.1097/QAI.0b013e3182167f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]