Abstract
Adjuvant treatments including Betadine, Dakin’s solution (sodium hypochlorite), or hydrogen peroxide (H2O2) have been attempted to eradicate prosthetic joint infection caused by biofilm or intracellular bacteria. The purpose of this study was to evaluate the in vitro abilities of chemical adjuvants to decrease Staphylococcus aureus (S. aureus) biofilm presence on orthopaedic implant grade materials, including titanium, stainless steel, and cobalt chrome. S. aureus biofilms were grown for 48 h and evaluated for baseline colony forming units/centimeter squared (CFU/cm2) and compared to treatments with Betadine, Dakin’s solution, H2O2, or 1% chlorine dioxide (ClO2). Control discs (n = 18) across all metals had an average of 4.2 × 107 CFU/cm2. All treatments had statistically significant reductions in CFU/cm2 when compared to respective control discs (p <0.05). For all metals combined, the most efficacious treatments were Betadine and H2O2, with an average 98% and 97% CFU/cm2 reduction, respectively. There were no significant differences between reductions seen with Betadine and H2O2, but both groups had statistically greater reductions than Dakin’s solution and ClO2. There was no change in antibiotic resistance patterns after treatment. Analysis of S. aureus biofilms demonstrated a statistically significant reduction in biofilm after a five-minute treatment with the modalities, with an average two log reduction in CFU/cm2. Statement of clinical significance: While statistically significant reductions in CFU/cm2 were accomplished with chemical adjuvant treatments, the overall concentration of bacteria never fell below 105 CFU/cm2, leading to questionable clinical significance. Further techniques to eradicate biofilm should be investigated.
Keywords: prosthetic joint infection, Staphylococcus aureus biofilm, betadine, Dakin’s solution, hydrogen peroxide
Every year approximately one million primary joint arthroplasties are performed in the United States. By 2030, it has been estimated that primary total hip and knee arthroplasties will see a 137% and 601% increase in demand, respectively.1 Of these procedures, up to 1.7% of primary total hip arthroplasties (THA) and 2% of primary total knee arthroplasties (TKA) will result in periprosthetic joint infection (PJI).2–4 The increasing infection burden is a concern both medically and financially as PJIs have a 5-year mortality of 33% and an increased cost of care greater than $100,000.5,6
When addressing biofilms, some of the major obstacles to treatment are their heterogeneity, slow metabolism, exopolysaccharide matrix (glycocalyx), inherent resistance to antibiotics, and strong defensive mechanisms against stress.7 The inherent fastidiousness of biofilm infections are particularly difficult for antibiotic applications and surgical revision techniques, such as one-stage exchange, two-stage exchange, and debridement and irrigation and retention of components (DAIR). The current gold standard, the two-stage exchange, is not only expensive, but it also has a lengthy course of treatment with a high morbidity. More recent studies have demonstrated that two-stage exchange has a failure rate close to 30%, with many patients never undergoing the second stage surgery.8–10 The low cost and lower morbidity associated with DAIR make it an appealing surgical option to both the surgeon and the patient, especially in the setting of an acute infection.11 DAIR has been shown to decrease biofilm mass; however, substantial amounts of residual bacteria and biofilm still remain, which may be a contributing factor in the unacceptably high failure rates with this treatment modality.12,13
In an effort to improve biofilm and infection control strategies, some surgeons employ adjuvant treatments to improve their debridement which has come in the form of a chemical adjuvant with the off-label use of 10% povidone-iodine (Betadine), Dakin’s solution (sodium hypochlorite), and 3% hydrogen peroxide (H2O2).14 These adjuvants employ similar mechanisms in an effort to disrupt cell walls and molecular metabolism.15 Povidone-iodine solutions disrupt cell walls while Dakin’s solution also destroys cell walls and negatively affects the metabolism and enzyme activation of biofilms. Dakin’s solution has been found in previous studies to have effects on Methicillin-resistant Staphylococcus aureus (MRSA).16 Hydrogen peroxide overrides the catalase protection mechanism, subsequently oxidizing and destroying cell walls.17 One additional topical application of interest is 1% chlorine dioxide (Camelbak cleaning tabs, Camelbak, Petaluma, CA), which is not commonly used intra-operatively. However, chlorine dioxide has been shown to be efficacious against biofilm-related problems in water treatments.18 Chlorine dioxide directly kills the bacteria by disrupting transport across cell walls and effectively penetrates the glycocalyx. Chlorine dioxide was chosen as a treatment for this study instead of chlorhexidine, because unlike chlorine dioxide, chlorhexidine has been shown to be efficacious against PJI.19 The purpose of this investigation was to evaluate the efficacy of topical applications against Methicillin-sensitive S. aureus biofilms grown in vitro on metals commonly used in hip and knee arthroplasties, including cobalt chrome (CoCr), titanium alloy (Ti), and stainless steel (SS).
METHODS
Inoculum and Bioreactor Preparation
A single clinical isolate of Methicillin-sensitive S. aureus (MSSA) was used for all biofilm production. Following propagation on 5% sheep’s blood agar plates (Remel, Inc., Lenexa, KS), standard inocula for each experiment was created by suspending MSSA in 50 ml brain heart infusion broth (BHI) (Becton, Dickinson and Company, Sparks, MD) with subsequent incubation for 18 h at 37°C on a rocker platform. The optical density of the bacterial solution was measured and adjusted with BHI broth to standardize the inoculum to a McFarland standard of 2 (~3 × 108 CFU/ml). A fresh inoculum was created for each trial.
Tested biomaterials consisted of steam sterilized, surgical grade CoCr (1 cm diameter 0.1 cm thickness), Ti (0.8 cm diameter and 0.1 cm thickness), and SS discs (0.8 cm diameter and 0.1 cm thickness((Small Parts, Inc., Miami Lakes, FL, wet jet cut by Wilson Works, Morgantown, WV). The discs were aseptically transferred to 12-well flat bottom cell culture plates (Corning Inc., Corning, NY) with one disc per well. A 1:1 ratio of 1 ml S. aureus inoculum and 1 ml fresh BHI was subsequently aliquoted into each well. The 12-well plates were allowed to incubate for 48 h at 37°C on a rocker platform with 2 ml fresh media exchanged every 24 h.
Quantifying Colony Forming Units (CFU/cm2)
Mature biofilm was established on the discs after 48 h of incubation. After 48 h, control discs (no treatment, n =18) were washed to remove non-adherent planktonic bacteria and BHI media present. These discs were then sonicated (Model 575HTA, CREST Ultrasonics, Road Trenton, NJ) for 10 min at 50 Hz (120 V, 4 A) and vortexed for 2 min, then serially diluted and plated via a drop-plate method on 5% blood sheep agar plates (Remel, Inc.) in order to obtain colony forming units (CFU). Plates were incubated at 37°C for 24 h. After the 24 h growth period, colonies were counted and converted to CFU/cm2.
Treatment of S. Aureus Biofilm
Discs not utilized as controls underwent treatment with one of four chemical adjuvants following 48 h incubation (Time-point T0): Dakin’s Solution, 10% Betadine, 3% hydrogen peroxide, and 1% Camelbak™ chlorine dioxide cleaning tablets. BHI broth was removed and each disc was saturated in 1 ml of a treatment solution for 5 min. Following the 5-min period, all discs were washed three times with 1 ml of phosphate buffered saline to remove the treatment and non-adherent planktonic bacteria. Adherent bacteria were then quantified on (6/12) discs via sonication to determine colony forming units (CFU/cm2). The other six discs were aseptically transferred to a new 12-well plate, immersed in 2 ml BHI, and allowed to incubate for another 24 h (T24) at 37°C on a rocker platform in order to determine the longer-term efficacy of the treatments. After the additional 24 h, the same CFU biofilm quantification methods were employed.
Scanning Electron Microscopy
Samples were fixed with 10% formalin for 2 h. Samples were gold-paladium sputtered (Denton Desk V Sputter and Carbon Coater) and imaged with Hitachi S-4700 Scanning Electron Microscope (SEM) (Hitachi, Schaumburg, IL).
Antibiotic Resistance
After adjuvant treatments, all unique colony morphotypes were identified to genus or species level by matrix-assisted laser desorption ionization time of flight mass spectrometry (Vitek MS, Biomériuex, Durham, NC) using manufacturer-recommended protocols. Antimicrobial susceptibility testing (AST) was performed by a routine automated method (Vitek2 GN81 and GP67 cards, Biomériuex) and/or by manual Kirby–Bauer disk diffusion on Mueller Hinton media with breakpoints and interpretive criteria derived from Clinical Laboratory Standards Institute (CLSI) recommendations (M-100 S26). Standard quality control testing for all identification and AST procedures were verified as acceptable.
Statistical Analysis
Statistical analysis consisted of two-way analysis of variance (ANOVA) and Tukey honest significant difference (HSD) analysis to compare CFU count reduction between metals, treatments, treatment, and control, and time points. Statistical significance was set at p <0.05.
RESULTS
Statistical Reductions for All Treatments
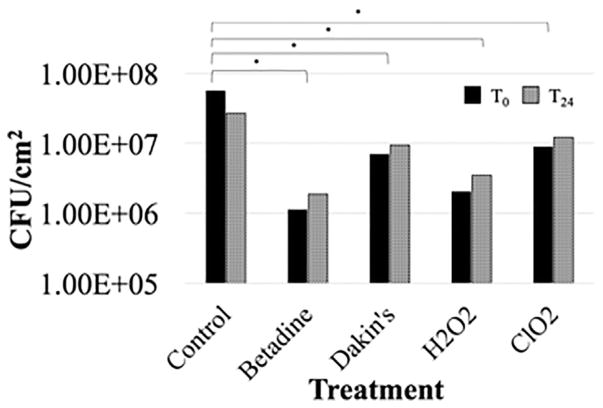
At both T0 and T24, a statistically significant reduction (p <0.05) in CFUs was observed for all treatments of all metals as compared to their respective controls (Fig. 1). All data points for each treatment were averaged to give a comprehensive representation of the efficacies of the treatments. For all metals combined, at each time point, the most efficacious treatments were 10% Betadine and H2O2. At T0, the average log reduction for 10% Betadine and H2O2 was 1.7 (98%) and 1.4 (97%), respectively. At T24, the average log reduction for 10% Betadine and H2O2 was 1.2 (89%) and 0.9 (84%), respectively. However, under the same two-way ANOVA and Tukey HSD analysis, there was no significant difference between 10% Betadine and H2O2, but both were significantly different compared to Dakin’s solution and ClO2 (Fig. 1). Further, internal controls designed to detect residual biofilm following sterilization indicated no viable biofilm growth across all metal surfaces (data not shown).
Figure 1.
A comparison of the average CFU/cm2 at the time of each treatment (T0) and 24 h post-treatment (T24), averaged across all metals (titanium alloy, stainless steel, and cobalt chromium).
Treatment Comparison by Metal
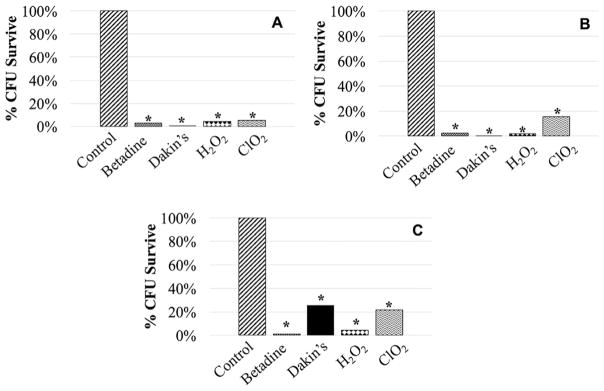
The CoCr discs demonstrated a statistically higher bacterial adherence at all time points when compared to both Ti and SS. Thus, further analysis was performed to examine relative treatment effects in reference to the control group for each metal; analysis was organized by metal and treatment. When comparing the control to treatments for Ti, the largest decrease in CFUs at T0 was a 2.4 log (99.6%) reduction observed with Dakin’s solution and a 1.2 log (94.4%) reduction at T24 with H2O2 (Table 1, Fig. 2A). The remaining adherent bacteria was 1.53 × 105 CFU/cm2 and 1.63 × 106 CFU/cm2, respectively. When comparing the control to treatments for CoCr, the largest reduction was observed with 10% Betadine, in which a 1.9 log (98.7%) reduction was seen at T0 and a 1.4 log (95.8%) reduction was seen at T24 (Table 1, Fig. 2B). The remaining adherent bacteria was 1.07 × 106 CFU/cm2 and 1.74 × 106 CFU/cm2, respectively. For SS, the largest reduction in CFUs for treatments as compared to the control was a 2.8 log (99.8%) reduction at T0 observed with Dakin’s solution and a 0.7 log (80.9%) reduction at T24 with Dakin’s solution (Table 1, Fig. 2C). The remaining adherent bacteria was 7.96 × 104 CFU/cm2 and 1.99 × 106 CFU/cm2, respectively.
Table 1.
Percent Reduction of CFUs With Initial Treatment Application and Growth Recovery at 24 h
| Treatment and Metal | % Reduction from Control at T0 | % Reduction from Control at T24 | Growth Recovery at T24 After Treatment (log10) |
|---|---|---|---|
| Betadine | 98% | 89% | 0.22 |
|
| |||
| CoCr | 98.7% | 95.8% | 0.21% |
| SS | 97.6% | 78.1% | 0.30 |
| Ti | 97.2% | 94.3% | 0.14 |
|
| |||
| Dakin’s | 91% | 71% | 0.97 |
|
| |||
| CoCr | 74.3 | 48.2 | 0.01 |
| SS | 99.8 | 80.9 | 1.40 |
| Ti | 99.6 | 83.0 | 1.51 |
|
| |||
| H2O2 | 97% | 84% | 0.24 |
|
| |||
| CoCr | 95.7% | 85.2% | 0.24 |
| SS | 98.3% | 73.9% | 0.54 |
| Ti | 95.7% | 94.4% | 0.05b |
|
| |||
| ClO2 | 86% | 30% | 0.23 |
|
| |||
| CoCr | 78.5% | 64.0% | 0.07b |
| SS | 84.4% | 54.0%a | 0.34 |
| Ti | 94.9% | 80.5% | 0.42 |
Denotes an increase in CFUs compared to control.
Denotes a negative growth recovery.
Figure 2.
Percent CFU reduction at initial application of treatment (T0) for biofilms cultured on Titanium alloy (A), cobalt chrome (B), and stainless steel (C) as compared to saline control (*p <0.05).
Persistent Effect
There was a trend toward growth recovery with an increase in CFUs when comparing T0 to T24. No statistically significant persistent effect was found when comparing T0 to T24 across all metals for all topical applications. However, there was a statistically significant increase seen between T0 and T24 for Dakin’s solution with titanium and stainless steel.
Antibiotic Resistance Patterns
Across both time points, the antibiotic resistance profiles for S. aureus before and after each treatment for each metal remained the same and showed no changes in resistance profiles.
DISCUSSION
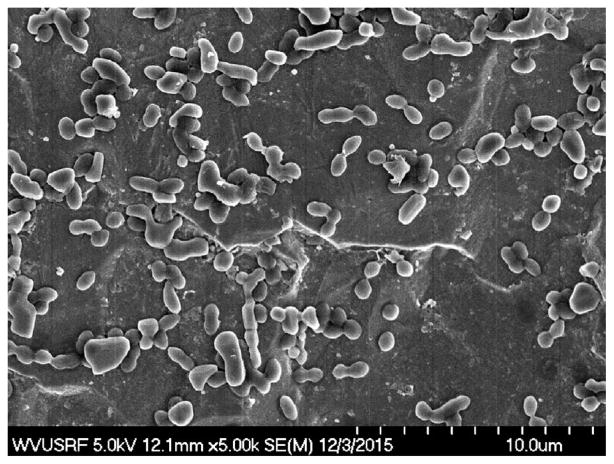
In this in vitro model comparing adjuvant treatment modalities in the elimination of S. aureus biofilm from orthopaedic metals, statistically significant reductions were found with the various topical applications. Immediately following treatment, large numbers of colony forming units remained on the biomaterials, with the largest reduction being a 2.8 log reduction from 107 to 104 with Dakin’s solution treated SS. Following an additional 24 h of incubation after treatment, there were still quantifiable CFUs (Figs. 1 and 2A–C). The results from the 24 h additional incubation further support that these treatments did not demonstrate a sustained effect as there was growth recovery in all cases with the exception of ClO2 treated SS. The threshold of biofilm for a clinically meaningful reduction is unknown, but prior evidence has shown that CFU levels must be reduced below the minimum infectious dose of S. aureus (104 CFU/gram of tissue) for complex extremity wounds.20 However, it is known that the threshold is substantially lower with hardware present and, as demonstrated, there was an average of 5.79 × 106 CFU/cm2 left after treatment. These data are qualitatively supported by S. aureus clusters visible on SEM at T0 (Fig. 3). Further, antibiotic resistance profiles did not change before or after treatment. This factor is important as we focus on antibiotic stewardship in efforts to prevent antibiotic resistance patterns. Though the results may not be clinically significant, it is yet to be determined if the demonstrated reductions could have an additive effect with other treatments. Given these results, other topical applications should be carefully reconsidered in the future, especially with the rising commercialization of new antimicrobial surfactants and clinical products developed for biofilm reduction.
Figure 3.
SEM of Titanium alloy disc treated with H2O2 at T0.
The limited ability to eradicate biofilms may lead to the high failure rates demonstrated in the literature when utilizing the DAIR method. Literature has shown failure rates ranging from 54 to 84%.21–24 A study of 30 patients with S. aureus infected prosthetic joints treated with DAIR showed a 54% (16/30) failure rate within one year and a 69% (21/30) failure rate within 2 years.23 Hartman et al.24 retrospectively reviewed 33 infected total knee arthroplasties treated by irrigation and debridement (I&D) in which there was a 61% (20/33) reinfection rate. Bradbury et al.22 retrospectively reviewed 19 cases of MRSA-infected knee arthroplasties treated with I&D in which 84% (16/19) failed within a two-year follow-up period. Azzam et al.21 retrospectively reviewed hip and knee arthroplasty infections treated with I&D in which 56% (58/104) failed within an average follow-up of 5.7 years. Fehring et al.12 showed that reinfection could occur within 10 days and also reported a 63% (54/86) reinfection rate within 90 days postoperatively. The inability to completely eliminate infection will lead to continued physical, financial, and emotional burdens to patients.25
Limitations of this study consist of the clinical translatability of the results, including biofilm maturation and heterogeneity, biofilm quantification methods, the size, and shape of the coupons as compared to prosthetics, the duration of treatment exposure, and the cytotoxicity of these topical adjuvants to healthy cells. Although it has been shown that mature biofilm can be established in vitro following 48 h incubation, clinical presentations of biofilm-related joint infection generally consist of long-established biofilm.26–30 However, the clinical application of adjuvant treatments as described here would be considered in the setting of acute infections. Further, this study involved infection with clinical isolates of MSSA, but many biofilms are heterogeneous in nature, a characteristic that poses one of the many challenges in treating biofilm-related PJI.31 Based on a previously conducted pilot study, adjuvant treatments administered for 1, 3, and 5 min all showed nearly equal efficacy. Further, biofilm quantification using CFU/cm2 may be an underrepresentation of residual contamination following treatment due to persisters in mature biofilm that are difficult to detect. Despite this, CFU/cm2 is a currently accepted measure of viable bacteria within biofilm, but likely represents an underestimation of the biofouling present.32 Additionally, the treatment exposure time was set at 5 min as this time point was thought to be a clinically feasible amount of time in the operating room. The treatments may not have had enough time for full efficacy; therefore, other time points (i.e., 15 or 20 min) may be worth investigating in the future.33,34 It will also be beneficial to evaluate the additive effects of such modalities or additional treatment strategies to aid in the removal of bacterial biofilm. Lastly, while these topical adjuvants reduce bacterial loads, they also have cytotoxic effects to healthy tissue. Brown et al.35 describe the use of dilute betadine when used as a prophylactic measure in the primary arthroplasty setting. This technique balanced the cytotoxic effects of the beta-dine with the beneficial effects of the wash. In infected tissue, the potential cytotoxic effects on local tissue have to be weighed against the benefits from the full strength delivery of these adjuvant washes. Despite these detrimental effects to healthy tissue, in current clinical practice, these undiluted adjuvants are used intraoperatively as a more aggressive treatment approach is necessary to eradicate infection and prevent further sepsis. Multiple studies support the use of these topical applications, despite their cytotoxicity.33,35,36
In conclusion, the use of adjuvant chemicals led to an average two log reduction in CFU/cm2 from orthopaedic implant materials without affecting the resistance patterns of the organism. However, the treatments left quantifiable amounts of biofilm adherent to orthopaedic metals and are likely not sufficient to treat PJI with planned retention of components in isolation.
Acknowledgments
Grant sponsor: National Institute of General Medical Sciences; Grant number: 1U54GM104942-01.
Support provided by the National Institute Of General Medical Sciences, U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We acknowledge Suzanne Danley for critical review of this manuscript. We acknowledge the use of the WVU Shared Research Facilities.
Footnotes
AUTHORS’ CONTRIBUTIONS
EPE, BAK, ASM, and MJD were all involved in the research design and data acquisition/analysis and interpretation. PRL assisted in data acquisition; in addition, all five authors were involved in the drafting and subsequent revisions of the manuscript. All authors have reviewed and approve of the final submission.
References
- 1.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the united states from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 3.Sculco TP. The economic impact of infected total joint arthroplasty. Instr Course Lect. 1993;42:349–351. [PubMed] [Google Scholar]
- 4.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med. 2014;276:111–119. doi: 10.1111/joim.12233. [DOI] [PubMed] [Google Scholar]
- 5.Choi HR, Beecher B, Bedair H. Mortality after septic versus aseptic revision total hip arthroplasty: a matched-cohort study. J Arthroplasty. 2013;28:56–58. doi: 10.1016/j.arth.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Kapadia BH, Johnson AJ, Issa K, et al. Economic evaluation of chlorhexidine cloths on healthcare costs due to surgical site infections following total knee arthroplasty. J Arthroplasty. 2013;28:1061–1065. doi: 10.1016/j.arth.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 8.Berend KR, Lombardi AV, Jr, Morris MJ, et al. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471:510–518. doi: 10.1007/s11999-012-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortazavi SM, Vegari D, Ho A, et al. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res. 2011;469:3049–3054. doi: 10.1007/s11999-011-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherrell JC, Fehring TK, Odum S, et al. The chitranjan ranawat award: fate of two-stage reimplantation after failed irrigation and debridement for periprosthetic knee infection. Clin Orthop Relat Res. 2011;469:18–25. doi: 10.1007/s11999-010-1434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehring TK, Odum SM, Berend KR, et al. Failure of irrigation and debridement for early postoperative periprosthetic infection. Clin Orthop Relat Res. 2013;471:250–257. doi: 10.1007/s11999-012-2373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urish KL, DeMuth PW, Craft DW, et al. Pulse lavage is inadequate at removal of biofilm from the surface of total knee arthroplasty materials. J Arthroplasty. 2014;29:1128–1132. doi: 10.1016/j.arth.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Urish KL, DeMuth PW, Kwan BW, et al. Antibiotic-tolerant staphylococcus aureus biofilm persists on arthroplasty materials. Clin Orthop Relat Res. 2016;474:1649–1656. doi: 10.1007/s11999-016-4720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen E, Parvizi J. Eradicate periprosthetic infection with irrigation and debridement. Orthop Today 2012 [Google Scholar]
- 15.Estrela C, Estrela CR, Barbin EL, et al. Mechanism of action of sodium hypochlorite. Braz Dent J. 2002;13:113–117. doi: 10.1590/s0103-64402002000200007. [DOI] [PubMed] [Google Scholar]
- 16.Agostinho AM, Hartman A, Lipp C, et al. An in vitro model for the growth and analysis of chronic wound MRSA biofilms. J Appl Microbiol. 2011;111:1275–1282. doi: 10.1111/j.1365-2672.2011.05138.x. [DOI] [PubMed] [Google Scholar]
- 17.Rutala WA, Weber DJ. Healthcare Infection Control Practices Advisory Committee. Guideline for disinfection and sterilization in healthcare facilities. 2008:1–161. [Google Scholar]
- 18.Gagnon GA, Rand JL, O’leary KC, et al. Disinfectant efficacy of chlorite and chlorine dioxide in drinking water biofilms. Water Res. 2005;39:1809–1817. doi: 10.1016/j.watres.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Smith DC, Maiman R, Schwechter EM, et al. Optimal irrigation and debridement of infected total joint implants with chlorhexidine gluconate. J Arthroplasty. 2015;30:1820–1822. doi: 10.1016/j.arth.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azzam KA, Seeley M, Ghanem E, et al. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25:1022–1027. doi: 10.1016/j.arth.2010.01.104. [DOI] [PubMed] [Google Scholar]
- 22.Bradbury T, Fehring TK, Taunton M, et al. The fate of acute methicillin-resistant staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty. 2009;24:101–104. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Brandt CM, Sistrunk WW, Duffy MC, et al. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clinical Infectious Diseases. 1997;24:914–919. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 24.Hartman MB, Fehring TK, Jordan L, et al. Periprosthetic knee sepsis: the role of irrigation and debridement. Clin Orthop Relat Res. 1991:113–118. [PubMed] [Google Scholar]
- 25.Montanaro L, Speziale P, Campoccia D, et al. Scenery of staphylococcus implant infections in orthopedics. Future Microbiol. 2011;6:1329–1349. doi: 10.2217/fmb.11.117. [DOI] [PubMed] [Google Scholar]
- 26.Atshan SS, Shamsudin MN, Sekawi Z, et al. Comparative proteomic analysis of extracellular proteins expressed by various clonal types of staphylococcus aureus and during planktonic growth and biofilm development. Front Microbiol. 2015;6:524. doi: 10.3389/fmicb.2015.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015;86:147–158. doi: 10.3109/17453674.2014.966290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MF, Edwards T, Hobbs G, et al. Acoustic vibration can enhance bacterial biofilm formation. J Biosci Bioeng. 2016;122:765–770. doi: 10.1016/j.jbiosc.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Watters CM, Burton T, Kirui DK, et al. Enzymatic degradation of in vitro staphylococcus aureus biofilms supplemented with human plasma. Infect Drug Resist. 2016;9:71–78. doi: 10.2147/IDR.S103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care. 2010;19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 31.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 32.Welch K, Cai Y, Strømme M. A method for quantitative determination of biofilm viability. J Funct Biomater. 2012;3:418–431. doi: 10.3390/jfb3020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lineaweaver W, Howard R, Soucy D, et al. Topical antimicrobial toxicity. Arch Surg. 1985;120:267–270. doi: 10.1001/archsurg.1985.01390270007001. [DOI] [PubMed] [Google Scholar]
- 34.Rabenberg VS, Ingersoll CD, Sandrey MA, et al. The bactericidal and cytotoxic effects of antimicrobial wound cleansers. J Athl Train. 2002;37:51–54. [PMC free article] [PubMed] [Google Scholar]
- 35.Brown NM, Cipriano CA, Moric M, et al. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27:27–30. doi: 10.1016/j.arth.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 36.Wilson JR, Mills JG, Prather ID, et al. A toxicity index of skin and wound cleansers used on In vitro fibroblasts and keratinocytes. Adv Skin Wound Care. 2005;18:373–378. doi: 10.1097/00129334-200509000-00011. [DOI] [PubMed] [Google Scholar]