Abstract
Objective
Cardiac arrest (CA) etiology may be an important source of between-patient heterogeneity, but the impact of etiology on organ injury is unknown. We tested the hypothesis that asphyxial cardiac arrest (A-CA) results in greater neurologic injury than cardiac etiology CA (VF-CA) whereas VF-CA results in greater cardiovascular dysfunction after return of spontaneous circulation (ROSC).
Design
Prospective observational human and randomized animal study.
Setting
University laboratory and intensive care units (ICU)
Patients
543 CA patients admitted to ICU
Subjects
Seventy-five male Sprague-Dawley rats
Interventions
We examined neurologic and cardiovascular injury in Isoflurane-anesthetized rat CA models matched by ischemic time. Hemodynamic and neurologic outcomes were assessed after 5 min no flow A-CA or VF-CA. Comparison was made to injury patterns observed after human A-CA or VF-CA.
Measurements and Main Results
In rats, cardiac output (20±10 vs. 45±9 ml/min) and pH were lower and lactate higher (9.5±1.0 vs. 6.4±1.3 mmol/L) after ROSC from VF-CA vs. A-CA (all p<0.01). A-CA resulted in greater early neurologic deficits, 7-day neuronal loss and reduced freezing time (memory) after conditioned fear (all p<0.05). Brain antioxidant reserves were more depleted following A-CA. In adjusted analyses, human VF-CA was associated with greater cardiovascular injury based on peak troponin (7.8 [0.8 – 57] vs. 0.3 [0.0 – 1.5] ng/ml) and ejection fraction by echocardiography (20% vs 55%, all p<0.0001) whereas A-CA was associated with worse early neurologic injury and poor functional outcome at hospital discharge (n=46 [18%] vs.102 [44%], p<0.0001). Most VF-CA deaths (54%) were the result of cardiovascular instability whereas most A-CA deaths (75%) resulted from neurologic injury (p<0.0001).
Conclusions
Transcending rat and human studies we find a consistent phenotype of heart and brain injury after CA based on etiology: VF-CA produces worse cardiovascular dysfunction whereas A-CA produces worsened neurologic injury associated with greater oxidative stress.
Keywords: heart arrest, cardiopulmonary resuscitation, animals, laboratory, ventricular fibrillation, asphyxia
Introduction
Almost 500,000 adult patients experience cardiac arrest (CA) in the United States annually.(1) Post-resuscitation care guidelines are independent of etiology.(2, 3) Differences in clinical outcomes based on the presenting rhythm have been reported with: shockable rhythms (ventricular fibrillation [VF] and pulseless ventricular tachycardia [VT]) having the best outcomes.(4–8) But rhythm only loosely corresponds to etiology. The proportion of CA due to asphyxia (A-CA) has steadily increased associated with poor clinical outcomes(9–11) perhaps due to peri-arrest differences in physiology (12) which may result in more severe neurological injury driving worsened outcomes (7, 13).
Animal models have yielded inconsistent results regarding heart and brain injury severity based on etiology.(14, 15) We previously demonstrated more severe histologic brain injury and cerebral blood flow (CBF) alterations induced by A-CA compared to VF of matched duration.(16, 17) Since CBF and systemic acidosis are determined by hemodynamics, heart and brain outcomes are inextricably linked. Our objective was a simultaneous characterization of both organ injury effects within an animal model controlling for factors such as ischemic time and cardio-pulmonary resuscitation (CPR) quality supplemented by clinical observational findings. The goal was to better define the impact of CA etiology on heart and brain injury which drive clinical outcome.(18, 19) We hypothesized that A-CA results in greater neurologic impairment while VF-CA results in greater hemodynamic impairment after return of spontaneous circulation (ROSC). Mechanistically we hypothesized these differences resulted from organ-specific mitochondrial injury and/or oxidant stress.
Materials and Methods
All animal experiments were approved by the Institutional Animal Care and Use Committee and human research were approved by the Institutional Review Board of the University of Pittsburgh. Additional methods in Supplemental Digital Content.
Rat Cardiac Arrest Models
Adult male Sprague Dawley rats were anesthetized with isoflurane, intubated and mechanically ventilated during surgical procedures. Electrocardiogram, respiratory rate, arterial and venous pressure via fluid filled catheters were continuously monitored. Rectal temperature was clamped at 37.0±0.5°C. VF and A-CA models are summarized in Supplemental Figure 1. VF was electrically induced over a period of 120 sec and left untreated for 3 additional min (5 min no flow). Asphyxia was induced by neuromuscular blockade and ventilator disconnection resulting in a pulseless electrical activity (PEA) for 5 min. After 5 min untreated VF/PEA, CPR was initiated by giving intravenous (IV) epinephrine and bicarbonate, resuming mechanical ventilation and initiating manual chest compressions. VF animals received defibrillation 60 sec after CPR start with a second epinephrine dose given at 90 sec before a second shock. Shocks were then repeated every 30 sec if necessary up to 5 min after CPR start. After ROSC, all care was standardized. Sham rats underwent surgery but no CA.
Heart and Brain Injury Endpoints
Cardiac output (CO) was measured for 30 min using an ultrasonic flow probe. Neurologic deficit score (NDS) was obtained on post-arrest days 1, 2, 3 and 8 as previously described: higher NDS indicates greater injury.(20) On days 7 and 8, a 2-day fear conditioning protocol(21) was employed to quantify cue mediated freezing with greater freezing indicative of retained memory. Brain injury based on histology using haematoxylin and eosin stain (H&E) and Fluro-Jade B (FJB) staining was quantified by a blinded neuropathologist. Proportion of surviving hippocampal CA1 neurons (H&E) and neuropathological scoring of other brain regions (FJB) are reported from 0 (no degeneration) to 5 (marked).
Mechanistic endpoints
Brain mitochondria were isolated using a Percoll gradient(22) and heart mitochondria isolated by differential centrifugation.(23) State 2 and 3 mitochondrial respiration rates based on oxygen consumption were quantified using a Clark electrode and normalized to protein content. Respiratory control ratio was calculated as state 3:2 ratio. Ascorbate(22, 24) and antioxidant reserve(25) were assessed as previously described using whole brain homogenate.
Human Cardiac Arrest Population
We validated our experimental findings using the University of Pittsburgh Post-Cardiac Arrest Service (PCAS) registry(19, 26, 27) including in- and out of hospital CA patients. Our survival to hospital discharge exceeds 40% and >50% of survivors have functionally favorable outcome defined as discharge to home or acute inpatient rehabilitation.(28) CA etiology was assigned by structured chart review in 1,007 subjects (2/15/2012-2/15/2016). Etiology was defined as “cardiac” (VF-CA) or “respiratory” (A-CA) as described in Supplemental Appendix 1. A random subset of 15% of etiologies were independently assigned by two reviewers to verify high inter-rater reliability.
Human Cardiac Arrest Injury and Outcome Definitions
We prospectively categorize post-CA injury severity using the Pittsburgh Cardiac Arrest Category (PCAC), a validated 4 point scale based on best neurologic and cardiopulmonary function within 6h of ROSC.(19, 29) PCAC I patients follow commands (best outcome) whereas PCAC IV patients are comatose with some brainstem reflex loss (worst outcome). PCAC is predictive of discharge(19, 27) and long-term CA outcome.(30) PCAC III have greater cardiopulmonary failure than PCAC II patients.(29, 31) Cardiac injury was quantified based on left ventricular ejection fraction (LVEF) on echocardiogram and peak troponin I within 3 days post-arrest. In logistic models these were dichotomized: LVEF, < 35% vs ≥ 35% (32), troponin, ≥4.66 ng/ml vs <4.66 ng/ml.(33) Our primary marker of favorable neurologic/functional outcome was discharge to home or inpatient rehabilitation (28) which predicts long-term survival.(34) Clinical covariates of age, gender, initial arrest rhythm and arrest location (in-hospital vs out-of-hospital) were obtained for model adjustment. We compared cause of death classified as “neurologic” or “cardiovascular” by the PCAS physician, with other causes excluded (Supplemental Digital Content).
Statistics
We report data as mean ± standard deviation (SD) or median and interquartile range. Statistical analysis used Stata v.14.2 (College Station, TX, USA). We compared categorical variables across arrest etiologies using Chi2 or Fisher’s Exact tests, and compared continuous variables using t-tests or Rank Sum tests as appropriate. We built logistic regression models to test the association of CA etiology with binary outcomes (LVEF and peak troponin) adjusting for clinical covariates and used ordered logistic regression to test the association between etiology and neurologic injury (PCAC I vs II/III vs IV). Cause of death based on etiology was compared using Fisher’s exact test.
Results
Organ injury after experimental CA
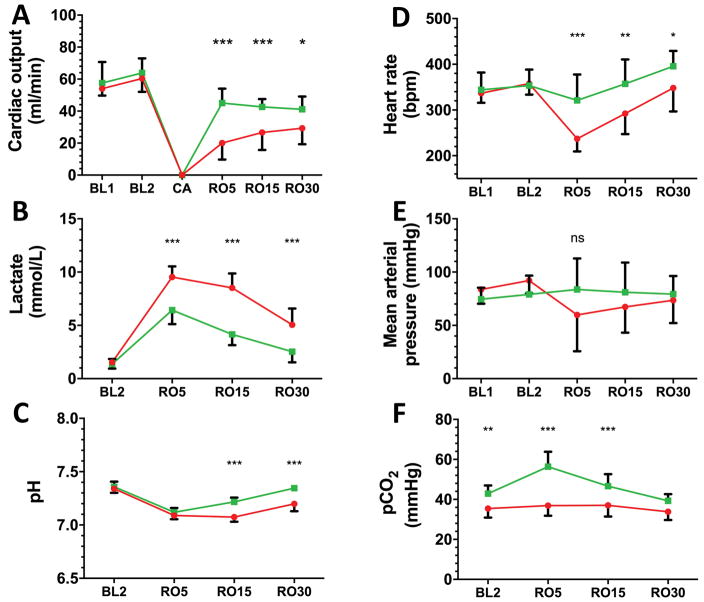
Rats (n=25) weighed 293±18 g and were similar at baseline in terms of cardiac output, pH, gas exchange and hemodynamics (Supplemental Table 1). PEA occurred 154±34 sec after tracheal tube disconnection. CPR time to ROSC was longer for rats undergoing VF-CA [107±24 sec] compared to A-CA [50±11 sec; p=<0.001]. All 20 CA animals survived 30 min after ROSC. CO was significantly lower following VF-CA vs. A-CA at 5 minutes [20±10 vs. 45±9 ml/min] and during the entire 30 min period of observation (Figure 1A; p=0.01). We observed greater metabolic acidosis after VF-CA vs. A-CA [pH 7.09±0.04 vs. 7.12±0.04], all p<0.001; Figure 1B, C). Differences between VF-CA and A-CA were maximal 5 min after ROSC (Supplemental Table 1; RO5) and included lower heart rate [237±28 vs. 321±57 bpm] and mean arterial pressure [54±21 vs. 84±29 mmHg] in VF-CA vs. A-CA (Figure 1D, E).
Figure 1. VF-CA produces greater early post-resuscitation cardiogenic shock compared to A-CA.
Sequential measurements of physiologic and biochemical variables reflecting hemodynamics and perfusion where made at baseline (BL) and after defined times (in minutes) after return of spontaneous circulation (RO; e.g. RO5 = 5 min after RO). VF-CA (red circles; n=10) resulted in significantly lower cardiac output (A), greater lactate (B) and lower pH (C) compared to A-CA (green squares; n=10). Heart rate (D) was significantly more depressed after VF-CA than A-CA and an early reduction in mean arterial pressure (E) was noted at RO5. A-CA resulted in greater hypercarbia than VF-CA (F). Symbols: * p<0.05, ** p<0.01, *** p<0.001
Separately, 25 rats were randomized to VF-CA (n=10), A-CA (n=10) or sham (n=5) and survived 8 d post-injury to assess neurologic injury. Two rats in the VF-CA group died because ROSC was not sustained. Neurologic disability quantified by NDS was worse after A-CA vs. VF-CA on post-CA days 1 [37±18 vs. 14±7 % deficit] and 2 [19±12 vs. 5±3 %], (Figure 2A; p<0.05). Rats undergoing A-CA retained less day 8 cue-mediated freezing compared to VF-CA (Figure 2B; p=0.03). Brain histology revealed greater neuronal loss within several brain regions (CA1, CA2 and cerebellar cortex) after A-CA vs. VF-CA (Table 1). Day 8 hippocampal degeneration was noted in 75 ±14 % of CA1 neurons after A-CA vs. 35 ± 24% after VF-CA (Supplemental Figure 2). CA1 neuronal survival correlated with increased cue-mediated freezing (r=0.54; p=0.05; Supplemental Figure 3).
Figure 2. A-CA produces greater short and long-term neurologic injury than VF-CA.
A standardized neurologic deficit score (A) was assigned blindly on days 1, 2, 3 and 8 after CA revealing significantly greater neurologic injury on days 1 and 2 resulting from A-CA (green; n=10) vs. VF-CA (red; n=8) both of which far exceeded sham animals (blue; n=5). Fear conditioning (B) 8 days after CA shows significantly (p=0.03) diminished cue mediated freezing (memory of the fear conditioning) following A-CA vs. VF-CA (n=7) with similar freezing noted between VF-CA and sham animals (blue, n=5). Symbols: * p<0.05, ** p<0.01, *** p<0.001
Table 1.
Comparison of histopathology 8 days after cardiac arrest using hematoxylin and eosin staining
| Pathology, region | VF-CA (n=8) | ACA (n=10) | p-value |
|---|---|---|---|
| Neuron degeneration, Hippocampus, CA1 | |||
| no degeneration minimal | ● ● ● | ||
| mild | ● ● ● | ● | |
| moderate | ● ● | ● ● ● | |
| marked | ● ● ● ● ● ● | 0.01 | |
| Neuron degeneration, Hippocampus, CA2 | |||
| no degeneration | ● ● | ||
| minimal | ● ● ● | ||
| mild | ● ● | ● ● ● ● ● ● | |
| moderate | ● | ● ● ● | 0.03 |
| marked | ● | ||
| Neuron degeneration, Hippocampus, CA3 | |||
| no degeneration | ● ● ● ● ● ● | ● ● ● ● ● ● ● ● ● | |
| minimal | ● ● | ● | |
| mild | |||
| moderate | ns | ||
| marked | |||
| Neuron degeneration, Cerebellar Cortex | |||
| no degeneration | ● ● ● ● ● ● ● | ||
| minimal | ● | ● | |
| mild | ● ● ● ● ● ● ● ● ● | ||
| moderate | 0.003 | ||
| marked | |||
| Neuron degeneration, Temporal Cortex | |||
| no degeneration | ● ● ● ● ● ● ● | ● ● ● ● ● ● ● ● ● | |
| minimal | ● | ||
| mild | ● | ||
| moderate | ns | ||
| marked |
Abbreviations: VF-CA: Ventricular Fibrillation; A-CA: asphyxial cardiac arrest, FJ-C: Fluorojade C staining;
Mechanism of increased brain injury after A-CA
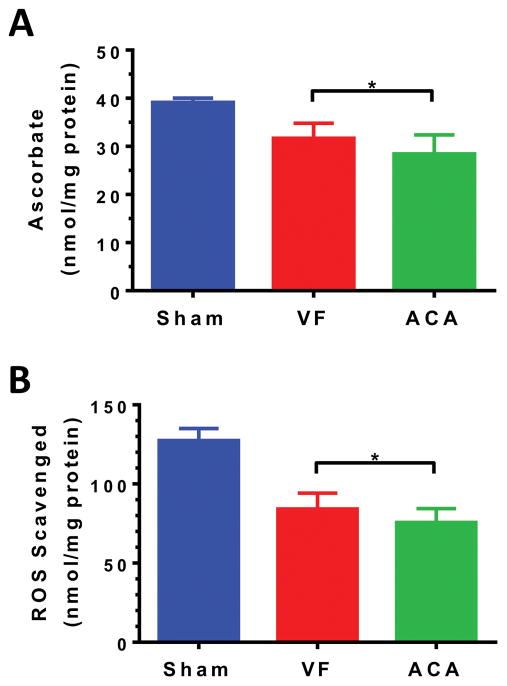
We found similar mitochondrial dysfunction in heart and brain (p=0.19) 15 min post-ROSC independent of CA etiology (Supplemental Figure 4). State 3 oxygen consumption was likewise similar between groups (data not shown). Brain ascorbate (Fig 3A) and total antioxidant reserve (Fig 3B) were significantly depleted compared to sham with greater reductions after A-CA vs. VF-CA.
Figure 3. A-CA results in greater loss of brain antioxidants than VF-CA.
(A) Non-arrested shams had higher brain ascorbate levels (mean=39.1; n=3) than VF (31.7; n=11) or A-CA (28.4; n=10) rats 15 min after ROSC. ACA brain ascorbate was significantly reduced compared to VF brain ascorbate indicating greater ROS burden. (B) Assay of the total antioxidant reserve of the same brain samples were congruous: Sham (127.4 nmol ROS scavenged/mg protein), VF (84.2), and ACA (75.7). Symbols: * p<0.05 after Holm-Šídák multiple comparison adjustment.
Differences in brain vs heart injury after human CA
We examined whether our animal findings were recapitulated in the clinical scenario. Of 1,007 patients with etiology assigned, 254 (25%) had cardiac etiology (VF-CA) while 286 (28%) had asphyxial etiology (A-CA). VF-CA consisted of acute coronary syndrome (n=134), primary arrhythmia (n=5), secondary arrhythmia (n=60), structural heart disease (n=7), left (n=12) and right (n=36) ventricular failure. A-CA consisted of drug-induced asphyxia (n=85), airway obstruction (n=53) and respiratory decompensation (n=148). Patients with VF-CA were older and more likely to present with a shockable rhythm (Supplemental Table 2). A-CA patients were more likely to be hypercapneic and to exhibit severe hypoxemia.
Patients with VF-CA had less neurologic injury within 6 h of ROSC (PCAC) and better survival to hospital discharge (58% vs 42%, P<0.001; Table 2). VF-CA was associated with better functional survival based on discharge disposition (44% vs 18%, P<0.001). VF-CA was associated with increased myocardial dysfunction vs. A-CA based on a lower median LVEF (20%, n=206 vs 55%, n=157) and higher median peak troponin I (7.8 ng/ml vs 0.3 ng/ml). After adjustment, VF-CA was associated with less severe initial brain injury (PCAC IV; OR 0.47 [95% CI: 0.30–0.75]), increased functionally favorable discharge survival (OR 2.15 [95% CI: 1.21–3.80]), and greater frequency of troponin increase (OR 5.46 [95% CI: 3.18–9.36] and reduced LVEF (OR 4.12 [95% CI: 2.27–7.46]).
Table 2.
Unadjusted outcomes stratified by arrest etiology
| Outcome | VF-CA (n=254) | A-CA (n=286) | p-value |
|---|---|---|---|
| Good Neurologic Outcome | 102 (44) | 46 (18) | <0.0001 |
| Hospital Survival | 146 (58) | 100 (35) | <0.0001 |
| Post-ROSC Neurologic Injury | <0.0001 | ||
| PCAC I | 94 (37) | 41 (14) | |
| PCAC II/III | 68 (27) | 57 (20) | |
| PCAC IV | 62 (24) | 138 (48) | |
| Not typable | 30 (12) | 50 (17) | |
| Post-ROSC Cardiac Injury | |||
| LVEF evaluated | 206 (81) | 157 (55) | <0.0001 |
| LVEF (median [IQR]), % | 20 [35 – 55] | 55 [45 – 60] | <0.0001 |
| Troponin I evaluated | 245 (96) | 269 (94) | ns |
| Peak troponin I (ng/ml; median/IQR) | 7.8 [0.8 – 57] | 0.3 [0.0 – 1.5] | <0.0001 |
Abbreviations: VF-CA, cardiac etiology cardiac arrest; A-CA, asphyxial etiology cardiac arrest; ROSC, return of spontaneous circulation; PCAC, Pittsburgh Cardiac Arrest Category; LVEF, left ventricular ejection fraction
In total 294/540 patients with VF-CA or A-CA died in-hospital. We excluded 68 cases where cause of death was multisystem, due to pre-existing wishes or comorbidities. Cardiovascular causes accounted for 70/226 (31%) of the remaining deaths while neurologic injury accounted for 156/226 (69%). Cause of death differed based on CA etiology (p<0.0001) with neurologic death more common after A-CA (117/156; 75%) and cardiovascular death more common after VF-CA (38/70; 54%).
Discussion
This is the first report employing animal modeling of CA coupled with clinical data to elucidate the relationship between CA etiology, end-organ injury and cause of death. Using translational methods we observe a consistent picture wherein cardiac etiology CA (VF-CA) results in greater myocardial injury whereas A-CA results in greater neurologic injury. In rat models with identical no flow times, VF-CA produced worsened myocardial dysfunction (lower CO) and greater cardiogenic shock (lactic acidosis) than A-CA. Despite worsened shock, VF-CA resulted in better neurologic outcomes than A-CA (NDS, fear conditioning, histology). A-CA is associated with greater consumption of brain antioxidant defenses implying an oxidative injury mechanism. Human data recapitulate these findings: VF-CA is associated with increased cardiac injury (LVEF, troponin) vs. A-CA which is associated with worse neurologic injury (PCAC, disposition). In-hospital death is known to result from brain, followed by heart, injury(35) and our study demonstrates that etiology predicts injury which predict mode of death. Thus, etiology is of great clinical significance and understanding the mechanistic and physiological differences that result from etiology is needed to target therapies and improve outcomes. This is particularly poignant now as the rising heroin epidemic drives the incidence of asphyxia etiology CA(11).
Vaagenes and colleagues showed increases in histologic brain injury after 7 min A-CA (13 min total asphyxia) compared to 10 min VF-CA in dogs.(17) Despite shorter no flow time, A-CA increased brain injury. Kamohara and colleagues(14) found greater impairment of myocardial function during 240 min following ROSC after rat VF-CA vs. A-CA of longer no flow time. Vaagenes and Kamohara reported longer CPR times to achieve ROSC after VF-CA vs. A-CA. Our findings are consistent with these studies and demonstrate that etiology determines organ injury. Gazmuri’s data from isolated rat hearts suggests that VF leads to ischemic contracture with increasing coronary vascular resistance(36) which may explain its increased impact on CO. Contradictory results(15) within a pig model show greater myocardial dysfunction, cellular injury and reduced ROSC after A-CA. These disparate findings may reflect species or methodologic differences underscoring the need for clinical correlation.
Appreciating heart vs. brain injury based on etiology may have clinical implications. Prior to an ACA, arterial oxygenation decreases with increasing hypercapnia, whereas in VF-CA oxygenation is usually normal at CA onset. Furthermore, pre-existing comorbidity will also show an impact on outcome (for example COPD patients vs. young patients with drug overdose as a cause of ACA). Pre-hospital cooling with IV fluids resulted in a higher incidence of pulmonary edema and recurrent CA.(37) The hypothesis that VF increased cardiac injury explaining these adverse effects is intriguing though speculative. Myocardial dysfunction after CA has been attributed to calcium overload,(38) which parallels classic findings in myocardial infarction.(39) This mechanism may explain why Vaagenes found that lidoflazine therapy, a calcium antagonist, improved outcomes after VF-CA but not A-CA and free radical scavengers improved outcomes after A-CA not VF-CA.(17) Defining CA injury phenotypes based on etiology may be an important step in targeting therapy to optimize outcomes.
Despite greater cardiovascular dysfunction, neurologic outcome in rats and humans are better after VF-CA. This is consistent with early neurologic injury differences, measured by NDS, in VF-CA vs. A-CA studies on pigs(40) though not corroborated in the setting of a longer no flow time in rats.(14) Brief global ischemia may increase brain>heart injury consistent with Kloner’s findings that cardiomyocyte death does not occur until >15 min ischemia(41) accounting for minimal myocardial apoptosis after rat CA.(42) Most (>90%) patients with good neurologic outcome achieve ROSC within 16 min(43), but ROSC rates remain high (> 10%) even after 20 min of CPR(44) suggesting the brain is more susceptible to prolonged ischemia than the heart.
Our primary mechanistic hypothesis was that mitochondrial dysfunction would parallel organ injury, but this was not confirmed. Prior studies have reported greater cardiac(15, 45) mitochondrial dysfunction after A-CA compared to VF but neither achieved ROSC thus limiting reperfusion injury. We next measured antioxidant reserve in brain, as injury from reactive oxygen species (ROS) represents an early pathway to brain injury.(46–48) We found greater reductions in antioxidant reserve after A-CA implying this etiology increases neurologic injury. This mechanistic hypothesis requires further confirmation.
A strength of our study is our attempt to clinically correlate our findings. Using a high-resolution CA database where etiology and cause of death are expertly assigned, we examined whether our rat results translated to humans. We corroborated that VF-CA causes more severe myocardial dysfunction (troponin and LVEF) which is clinically significant as it results in death due to hemodynamic instability. Since VF-CA had reduced neurologic injury (PCAC), in-hospital mortality and long-term functional impairment was less than A-CA where brain injury is more severe.
Our study naturally has limitations but we have attempted to use animal and clinical data in a complementary way to buttress these. Pre-hospital estimates of no/low flow times have poor reliability within our database and were not adjusted for(49) but our rat models permit precise ischemic time matching. Our rats were young, healthy males hence our clinical findings are important to show these animal results are more generalizable and free of gender specificity. VF rats required more epinephrine to achieve ROSC which we infer is the result of greater myocardial dysfunction but we cannot exclude negative effects of epinephrine on the heart. The corroborating human data are reassuring. The differences noted in ROS injury which presumably originate from mitochondria(46) may have resulted in delayed mitochondrial dysfunction yet we only examined an early time. We matched no flow CA time rather than total insult time (asphyxia), however others recapitulated our findings using different matching strategies.(14, 17) Only 28% of the A-CA patients received a recorded echocardiogram within 48 h of ROSC. These patients were presumably a biased subset with hemodynamic problems, yet their LVEF remained better than VF-CA. Rat VF was induced electrically which could cause direct myocardial injury though Kamohara saw no such effect.(14) The recapitulation of our rat findings in clinical analysis is reassuring that myocardial dysfunction is prevalent after human VF-CA.
Conclusions
Within rat and human CA we find that cardiac etiology CA (vs. A-CA) worsens cardiac injury with increased cardiogenic shock and death due to cardiovascular instability; whereas A-CA worsens neurologic injury resulting in death due to brain injury. These distinct injury phenotypes may have important repercussions in targeted management.
Supplementary Material
Acknowledgments
Financial support: TU was supported by the Laerdal Foundation for Acute Medicine and by the Max Kade Foundation. CD was supported by the Laerdal Foundation for Acute Medicine and by K08SNS069817.
The University of Pittsburgh Post-Cardiac Arrest Service consisted of (alphabetically):
Clifton W. Callaway, MD, PhD
Cameron Dezfulian, MD
Ankur A. Doshi, MD
Jonathan Elmer, MD
Adam Frisch, MD
Francis X. Guyette, MD, MS
Josh C. Reynolds, MD
Jon C. Rittenberger, MD, MS
Footnotes
Copyright form disclosure: Drs. Lamade and Elmer received support for article research from the National Institutes of Health (NIH). Dr. Elmer’s institution received funding from the NIH. Dr. Kochanek’s institution received funding from the NIH, the US Department of Defense, and the Society of Critical Care Medicine (stipend as Editor-in-Chief of Pediatric Critical Care Medicine). He serves as an expert witness, and has several patents/provisional patents (none of which are relevant to this manuscript). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 suppl 2):S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 3.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post–Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 suppl 2):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan PS, McNally B, Tang F, et al. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation. 2014;130(21):1876–1882. doi: 10.1161/CIRCULATIONAHA.114.009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daya MR, Schmicker RH, Zive DM, et al. Out-of-hospital cardiac arrest survival improving over time: Results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. Jama. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Jeung KW, Lee BK, et al. Impact of case volume on outcome and performance of targeted temperature management in out-of-hospital cardiac arrest survivors. The American journal of emergency medicine. 2015;33(1):31–36. doi: 10.1016/j.ajem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted Temperature Management at 33°C versus 36°C after Cardiac Arrest. New England Journal of Medicine. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 9.Kuisma M, Repo J, Alaspaa A. The incidence of out-of-hospital ventricular fibrillation in Helsinki, Finland, from 1994 to 1999. Lancet. 2001;358(9280):473–474. doi: 10.1016/S0140-6736(01)05634-3. [DOI] [PubMed] [Google Scholar]
- 10.Cobb LA, Fahrenbruch CE, Olsufka M, et al. Changing Incidence of Out-of-Hospital Ventricular Fibrillation, 1980–2000. Jama. 2002;288(23):3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 11.Salcido DD, Torres C, Koller AC, et al. Regional incidence and outcome of out-of-hospital cardiac arrest associated with overdose. Resuscitation. 2016;99:13–19. doi: 10.1016/j.resuscitation.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lah K, Krizmaric M, Grmec S. The dynamic pattern of end-tidal carbon dioxide during cardiopulmonary resuscitation: difference between asphyxial cardiac arrest and ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest. Crit Care. 2011;15(1):R13. doi: 10.1186/cc9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uray T, Mayr FB, Fitzgibbon J, et al. Socioeconomic factors associated with outcome after cardiac arrest in patients under the age of 65. Resuscitation. 2015;93(0):14–19. doi: 10.1016/j.resuscitation.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamohara T, Weil MH, Tang W, et al. A Comparison of Myocardial Function after Primary Cardiac and Primary Asphyxial Cardiac Arrest. American journal of respiratory and critical care medicine. 2001;164(7):1221–1224. doi: 10.1164/ajrccm.164.7.2007083. [DOI] [PubMed] [Google Scholar]
- 15.Wu C-J, Li C-S, Zhang Y, et al. Differences of postresuscitation myocardial dysfunction in ventricular fibrillation versus asphyxiation. The American journal of emergency medicine. 2013;31(12):1690–1696. doi: 10.1016/j.ajem.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Drabek T, Foley LM, Janata A, et al. Global and regional differences in cerebral blood flow after asphyxial versus ventricular fibrillation cardiac arrest in rats using ASL-MRI. Resuscitation. 2014;85(7):964–971. doi: 10.1016/j.resuscitation.2014.03.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaagenes P, Safar P, Moossy J, et al. Asphyxiation versus ventricular fibrillation cardiac arrest in dogs: Differences in cerebral resuscitation effects—a preliminary study. Resuscitation. 1997;35(1):41–52. doi: 10.1016/s0300-9572(97)01108-8. [DOI] [PubMed] [Google Scholar]
- 18.Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive care medicine. 2004;30(11):2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 19.Coppler PJ, Elmer J, Calderon L, et al. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015;89:86–92. doi: 10.1016/j.resuscitation.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz L, Ebmeyer U, Safar P, et al. Outcome Model of Asphyxial Cardiac Arrest in Rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1995;15(6):1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 21.Fidan E, Lewis J, Kline AE, et al. Repetitive Mild Traumatic Brain Injury in the Developing Brain: Effects on Long-Term Functional Outcome and Neuropathology. Journal of neurotrauma. 2016;33(7):641–651. doi: 10.1089/neu.2015.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dezfulian C, Kenny E, Lamade A, et al. Mechanistic characterization of nitrite-mediated neuroprotection after experimental cardiac arrest. Journal of neurochemistry. 2016;139(3):419–431. doi: 10.1111/jnc.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dezfulian C, Shiva S, Alekseyenko A, et al. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120(10):897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belikova NA, Glumac AL, Kapralova V, et al. A high-throughput screening assay of ascorbate in brain samples. J Neurosci Methods. 2011;201(1):185–190. doi: 10.1016/j.jneumeth.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayir H, Kagan VE, Tyurina YY, et al. Assessment of Antioxidant Reserves and Oxidative Stress in Cerebrospinal Fluid after Severe Traumatic Brain Injury in Infants and Children. Pediatr Res. 2002;51(5):571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Sabedra AR, Kristan J, Raina K, et al. Neurocognitive outcomes following successful resuscitation from cardiac arrest. Resuscitation. 2015;90:67–72. doi: 10.1016/j.resuscitation.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youn CS, Callaway CW, Rittenberger JC. Combination of initial neurologic examination and continuous EEG to predict survival after cardiac arrest. Resuscitation. 2015;94:73–79. doi: 10.1016/j.resuscitation.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Rittenberger JC, Raina K, Holm MB, et al. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82(8):1036–1040. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rittenberger JC, Tisherman SA, Holm MB, et al. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82(11):1399–1404. doi: 10.1016/j.resuscitation.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elmer J, Rittenberger JC, Coppler PJ, et al. Long-term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation. 2016;108:48–53. doi: 10.1016/j.resuscitation.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent J-L, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Critical care medicine. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Maltais S, Ladouceur M, Cartier R. The influence of a low ejection fraction on long-term survival in systematic off-pump coronary artery bypass surgery. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2011;39(5):e122–127. doi: 10.1016/j.ejcts.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Dumas F, Manzo-Silberman S, Fichet J, et al. Can early cardiac troponin I measurement help to predict recent coronary occlusion in out-of-hospital cardiac arrest survivors? Critical care medicine. 2012;40(6):1777–1784. doi: 10.1097/CCM.0b013e3182474d5e. [DOI] [PubMed] [Google Scholar]
- 34.Elmer J, Rittenberger JC, Coppler PJ, et al. Long-term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation. 2016;108:48–53. doi: 10.1016/j.resuscitation.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts BW, Kilgannon JH, Chansky ME, et al. Multiple Organ Dysfunction After Return of Spontaneous Circulation in Postcardiac Arrest Syndrome. Critical care medicine. 2013;41(6):1492–1501. doi: 10.1097/CCM.0b013e31828a39e9. doi:1410.1097/CCM.1490b1013e31828a31839e31829. [DOI] [PubMed] [Google Scholar]
- 36.Gazmuri RJ, Berkowitz M, Cajigas H. Myocardial effects of ventricular fibrillation in the isolated rat heart. Critical care medicine. 1999;27(8):1542–1550. doi: 10.1097/00003246-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: A randomized clinical trial. Jama. 2014;311(1):45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 38.Woods CE, Shang C, Taghavi F, et al. In Vivo Post-Cardiac Arrest Myocardial Dysfunction Is Supported by Ca2+/Calmodulin-Dependent Protein Kinase II-Mediated Calcium Long-Term Potentiation and Mitigated by Alda-1, an Agonist of Aldehyde Dehydrogenase Type 2. Circulation. 2016;134(13):961–977. doi: 10.1161/CIRCULATIONAHA.116.021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79(2):609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Li CS, Wu CJ, et al. Comparison of Cerebral Metabolism between Pig Ventricular Fibrillation and Asphyxial Cardiac Arrest Models. Chin Med J (Engl) 2015;128(12):1643–1648. doi: 10.4103/0366-6999.158340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104(24):2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 42.Song F, Shan Y, Cappello F, et al. Apoptosis is not involved in the mechanism of myocardial dysfunction after resuscitation in a rat model of cardiac arrest and cardiopulmonary resuscitation. Critical care medicine. 2010;38(5):1329–1334. doi: 10.1097/CCM.0b013e3181d9da8d. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds JC, Frisch A, Rittenberger JC, et al. Duration of Resuscitation Efforts and Functional Outcome After Out-of-Hospital Cardiac Arrest: When Should We Change to Novel Therapies? Circulation. 2013;128(23):2488–2494. doi: 10.1161/CIRCULATIONAHA.113.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagao K, Nonogi H, Yonemoto N, et al. Duration of Prehospital Resuscitation Efforts After Out-of-Hospital Cardiac Arrest. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.115.018788. [DOI] [PubMed] [Google Scholar]
- 45.Tsai M-S, Huang C-H, Tsai S-H, et al. The difference in myocardial injuries and mitochondrial damages between asphyxial and ventricular fibrillation cardiac arrests. The American journal of emergency medicine. 2012;30(8):1540–1548. doi: 10.1016/j.ajem.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu S, Liu X, Nozari A, et al. Evidence for Time-dependent Maximum Increase of Free Radical Damage and Eicosanoid Formation in the Brain as Related to Duration of Cardiac Arrest and Cardio-pulmonary Resuscitation. Free Radical Research. 2003;37(3):251–256. doi: 10.1080/1071576021000043058. [DOI] [PubMed] [Google Scholar]
- 48.Nelson CW, Wei EP, Povlishock JT, et al. Oxygen radicals in cerebral ischemia. American Journal of Physiology - Heart and Circulatory Physiology. 1992;263(5):H1356–H1362. doi: 10.1152/ajpheart.1992.263.5.H1356. [DOI] [PubMed] [Google Scholar]
- 49.Rittenberger JC, Martin JR, Kelly LJ, et al. Inter-rater reliability for witnessed collapse and presence of bystander CPR. Resuscitation. 2006;70(3):410–415. doi: 10.1016/j.resuscitation.2005.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.