Abstract
Exposure to stressors can enhance neuroinflammatory responses, and both stress and neuroinflammation are predisposing factors in the development of psychiatric disorders. Females suffer disproportionately more from several psychiatric disorders, yet stress-induced changes in neuroinflammation have primarily been studied in males. Here we tested whether exposure to inescapable tail shock sensitizes or ‘primes’ neuroinflammatory responses in male and female rats. At 24 h post-stress, male and female rats exposed to a peripheral immune challenge enhanced neuroinflammatory responses and exacerbated anxiety- and depressive-like behaviors. These changes are likely glucocorticoid dependent, as administering exogenous CORT, caused a similar primed inflammatory response in the hippocampus of male and female rats. Further, stress disinhibited anti-inflammatory signaling mechanisms (such as CD200R) in the hippocampus of male and female rats. In males, microglia are considered the likely cellular source mediating neuroinflammatory priming; stress increased cytokine expression in ex vivo male microglia. Conversely, microglia isolated from stressed or CORT treated females did not exhibit elevated cytokine responses. Microglia isolated from both stressed male and female rats reduced phagocytic activity; however, suggesting that microglia from both sexes experience stress-induced functional impairments. Finally, an immune challenge following either stress or CORT in females, but not males, increased peripheral inflammation (serum IL-1β). These novel data suggest that although males and females both enhance stress-induced neuroinflammatory and behavioral responses to an immune challenge, this priming may occur through distinct, sex-specific mechanisms.
Keywords: glucocorticoids, microglia, neuroimmune, sex differences, sickness behavior, stressors
1. Introduction
Current antidepressant therapies have minimal efficacy for ~50% of patients (Kirsch, 2014). One contributing factor to this high failure rate may be the lack of consideration of sex as a biological factor influencing depression. Indeed, most neuroscience research is conducted in males (Klein et al., 2015), yet females suffer disproportionately more from several neuropsychiatric disorders including mood disorders (Seney and Sibille, 2014). Exaggerated innate immune responses in the brain are associated with psychiatric disorders including depression, anxiety, post-traumatic stress disorders, autism, and schizophrenia (Raison et al., 2006). One predisposing factor for both psychiatric disorders and neuroinflammation is stress. Acute and chronic stressors amplify neuroinflammatory responses to a subsequent immune challenge. Indeed, we and others have demonstrated that stress sensitizes or ‘primes’ neuroinflammatory and behavioral responses to subsequent peripheral immune activation (e.g., infection with E. coli) (de Pablos et al., 2014; Espinosa-Oliva et al., 2011; Fonken et al., 2016; Frank et al., 2007; Frank et al., 2012; Johnson et al., 2002; Johnson et al., 2003; Munhoz et al., 2006; Weber et al., 2015; Wohleb et al., 2012; Wohleb et al., 2011).
Well-established sex differences in both the stress response and the immune system suggest that stress-elicited neuroinflammatory priming may vary between the sexes (Bekhbat and Neigh, 2017). Sex differences in the stress response exist throughout the lifespan and relate to both the organizational and activational effects of gonadal hormones (Bourke et al., 2012). In adulthood, females exhibit more robust and prolonged behavioral and physiological responses to stressors. For example, the glucocorticoid (corticosterone; CORT) response to various stressors is enhanced in females (Bourke et al., 2012). Importantly, CORT is the proximal signal through which acute and chronic stress primes neuroinflammatory responses in male rats (Frank et al., 2014; Frank et al., 2010; Frank et al., 2012). This suggests the heightened CORT response to stress may render females more susceptible to neuroinflammatory priming.
As the predominant innate immune cells of the brain, microglia are considered the likely source of neuroinflammatory priming (Frank et al., 2007). Indeed, microglia isolated from male rodents that have experienced prior stress exhibit an enhanced response to an ex vivo challenge with lipopolysaccharide (LPS) (Frank et al., 2007). Endogenously released molecules in the central nervous system (CNS) following stress cause microglia to enter this primed state (Weber et al., 2015). In the adult CNS, there are sex differences in both microglia colonization and morphology (Schwarz et al., 2012). Female rats and mice express greater numbers of microglia than males in brain regions involved in regulating emotional behaviors including the hippocampus and amygdala (Schwarz et al., 2012). Female microglia also have a more activated morphology, including thicker and more branched processes (Nelson et al., 2017; Schwarz et al., 2012). Interestingly, female brains exhibit enhanced immune activation despite the presence of the neuroprotective steroids estrogen and progesterone (Schwarz and Bilbo, 2012).
Overall, the prevalence of several neuropsychiatric disorders differs between the sexes, yet little is known about the contribution of neuroimmune dysregulation to mood disorder etiology in females (Bekhbat and Neigh, 2017). Here, we tested the hypothesis that stress-induced neuroinflammatory priming occurs in female rats, and explored whether priming is exaggerated by enhanced CORT signaling and/or microglial reactivity. Our results indicate that while females demonstrate comparable behavioral and in vivo neuroinflammatory changes following stress exposure, the mechanisms mediating neuroinflammatory priming in males and females are likely distinct. Most notably, whereas microglia from post-stress male rats consistently demonstrated elevated reactivity, microglia from female rats were unaffected (or slightly blunted) by stress exposure.
2. Methods
2.1. General Methods
2.1.1. Animals
Adult (3 mos.) male and female Sprague Dawley rats (Envigo) were pair-housed (unless otherwise specified) with a same-sex conspecifics in separate rooms; food and water were available ad libitum. Rats were maintained on a 12:12 light cycle with lights on at 0700h MST (zeitgeber time 0; ZT0) at an ambient temperature of 22 ± 2°C. All rats were acclimated to the facility for 2 weeks and to handling for 3 days prior to experimental manipulations. Because of the large number of groups represented throughout these experiments and the potential confound of exposing male and female rats to each other’s odors, male and female rats were run on sequential days (i.e. males and females were housed in the facility at the same time but males would undergo stress 24 h prior to females). A total of 258 rats were used in these experiments. All procedures were conducted in accordance with ARRIVE guidelines and the National Institute of Health’s Guide for the Care and Use of Laboratory Animals. Experiments were approved by the University of Colorado Institutional Animal Care and Use Committee.
2.1.2. Inescapable tail shock
This stressor protocol has been extensively used in this laboratory and reliably potentiates hippocampal pro-inflammatory cytokine responses to a peripheral immune challenge in male rats (12). All stress occurred between ZT3 and ZT5 ± 1 h (the stress session is approximately 108 min). Male and female received a single session of inescapable tail shock stress that consisted of rats being placed in Plexiglas tubes and exposed to 100 1.6 mA, 5s tail shocks with a variable inter-shock interval ranging from 30-90s. Following stressor exposure, rats were immediately returned to their home cages. During the stress session, home cage (control) rats remained undisturbed.
2.1.3. Tissue collection
Rats received a lethal IP injection of sodium pentobarbital (Fatal-plus; 150 mg/kg), a cardiac blood sample was collected, and then rats were transcardially perfused with 4°C 0.1 M PBS to remove peripheral immune cells from CNS vasculature. Brains and hippocampal tissue were rapidly extracted on ice. For in vivo experiments, hippocampus was flash frozen in liquid nitrogen and stored at −80°C. For ex vivo experiments, microglia were immediately isolated.
In experiments where tissue was not collected, rats were humanely euthanized with CO2 exposure and donated to the Birds of Prey Foundation (only non-LPS treated rats).
2.1.4. Microglia isolations
Hippocampal microglia were isolated as described in (Frank et al., 2006). Following PBS perfusion, hippocampal tissue was homogenized in 3 mL of 0.2% glucose in 1X DPBS (Gibco, Life Technologies, Waltham, MA) in a sterilized glass homogenizer. The homogenate was strained through a 40 μm filter (Falcon, Sigma) that was rinsed with an additional 2 mL of glucose. The homogenate was transferred to a sterile 5 mL tube and pelleted at 1000 g for 10 min at 22°C. Supernatant was poured off and a Percoll gradient was created by resuspending the pellet in 2 mL of 70% isotonic Percoll (GE Healthcare, Lafayette, CO; isotonic Percoll is 10:1 Percoll with 10X PBS; 100% isotonic Percoll is then diluted with 1X DPBS), followed by a layer of 2 mL 40% Percoll, and topped with 1 mL DPBS. The gradient was spun at 1200 g for 30 min at 22 °C with no acceleration or break. Myelin debris was removed and then microglia were extracted from the 40/70% interface. Microglia were washed in DPBS and then resuspended in filtered media [sterile high glucose DMEM (Gibco, 11960-044) with 10% FBS (Atlanta biological, Flowery Branch, GA, S11050)] and microglia concentration was determined by trypan blue exclusion. Microglia concentration was adjusted to a density of 10,000 cells/100 uL and cells were plated in a 96-well v-bottom plate. Microglia were challenged ex vivo with LPS (LPS; E. coli serotype 0111:B4; Sigma) at a concentration of 1, 10, or 100 ng/mL or media alone (Frank et al., 2007) for 4 h at 37 °C, 5% CO2. Following LPS exposure, cells were washed with DPBS and RNA was immediately extracted using SuperScript III CellsDirect cDNA Synthesis System (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol.
2.1.5. Protein analyses
Blood was allowed to clot, clots were removed, and then samples were centrifuged (4,000 g for 10 min at 4°C) and serum was collected. Hippocampal and liver samples were sonicated on ice using a tissue extraction reagent (Invitrogen) supplemented with protease inhibitor cocktail (Sigma). Homogenates were centrifuged (14,000 g for 10 min at 4°C) and supernatant collected and stored at −20°C.
Enzyme immunoassays for corticosterone (Assay Designs, Inc., Ann Arbor, MI) were run in duplicate according to the manufacturer’s instructions. Serum samples were treated with a steroid displacement reagent and then diluted either 1:20 or 1:40 with assay buffer. The high and low limits of detectability were 80 μg/dL to 0.064 μg/dL (taking into account the dilution factors). All samples fell within the range of detectability.
Rat IL1β ELISAs (R&D systems) were run in duplicate according to the manufacturer’s instructions. All samples fell within the range of detectability (2000 pg/ml and 7.8 pg/ml respectively). The concentration of IL-1β protein is expressed relative to total protein concentrations established in a Bradford assay for the hippocampal and liver homogenates (pg of IL1β/100μg of total protein).
Glucocorticoid receptor (GR) protein concentrations were evaluated in the hippocampus using Western blot. Samples were heated to 75°C for 10 min and loaded into a standard polyacrylamide Bis-Tris gel (Invitrogen). SDS-PAGE was performed in 3-(N-morpholino)-propanesulfonic acid running buffer (Invitrogen) at 175 V for ~75 min. Protein was transferred onto a nitrocellulose membrane using an iBlot dry transfer system (Invitrogen). The membrane was blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h and incubated overnight at 4°C in block buffer with primary antibodies: rabbit anti-rat GR (1:5000; Santa Cruz Biotechnology, SC-1004) and mouse anti-rat β-actin (1:500,000; Sigma). The membrane was washed in 1× PBS + 0.1% Tween and then incubated in blocking buffer containing either goat anti-rabbit or goat anti-mouse (LI-COR) IRDye 800CW secondary antibody at a concentration of 1:10,000 (LI-COR) for 1 h at room temperature. Protein expression was quantified using an Odyssey Infrared Imager (LI-COR) and expressed relative to the housekeeping protein β-actin.
2.1.6. Quantitative real-time PCR (qPCR)
Primers were previously designed using Genbank at the National Center for Biotechnology Information (NCBI), the Operon Oligo Analysis Tool, and the Basic Local Alignment Search Tool at NCBI and obtained from Invitrogen. Primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA. Primer specificity was verified by melt curve analysis. Primer sequences are included in Table 1. RNA was extracted from hippocampal and liver homogenates using TRIZOL reagent and 2 μg of RNA was reversed transcribed to cDNA using Superscript II (Life Technologies) according to the manufacturer’s instructions. RNA was isolated from microglia and reversed transcribed to cDNA using SuperScript III CellsDirect cDNA Synthesis System (Life Technologies). PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA) with a MyiQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA). Gene expression was determined in duplicate and expressed relative to β-actin. There were no group differences in β-actin. All qPCR results were analyzed using the 2−ΔΔCt method and were normalized such that the female control group was set to a value of 1.
Table 1.
Primer sequences.
| Gene | Primer Sequence 5′ → 3′ | Function |
|---|---|---|
| β-Actin | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein (Housekeeping gene) |
| CD200 | F: CTCTCTATGTACAGCCCATAG R: GGGAGTGACTCTCAGTACTAT |
Neuronal antigen that binds CD200R to inhibit microglia |
| CD200R | F: TAGAGGGGGTGACCAATTAT R: TACATTTTCTGCAGCCACTG |
Cognate receptor for CD200 |
| CX3CL1 | F: ATCATCCTGGAGACGAGACAGC R: CCACACGCTTCTCAAACTTGCC |
Neuronal antigen that binds CX3CR1 to inhibit microglia |
| CX3CR1 | F: TCAGGACCTCACCATGCCTA R: CGAACGTGAAGACAAGGGAG |
Cognate receptor for CX3CL1 |
| IL-1β | F: CCTTGTGCAAGTGTCTGAAG R: GGGCTTGGAAGCAATCCTTA |
Pro-inflammatory cytokine |
| IL-6 | F: AGAAAAGAGTTGTGCAATGGCA R: GGCAAATTTCCTGGTTATATCC |
Pro-inflammatory cytokine |
| TNFα | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
Pro-inflammatory cytokine |
2.1.7. Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM). Data were analyzed with StatView and Prism 7 (GraphPad Software, La Jolla, CA). Data were analyzed using analysis of variance (ANOVA) with sex, stress, and immune challenge as the between subjects factors (2 × 2 × 2). A 4-way ANOVA (repeated measures with sex, stress, and immune challenge as between subjects factors) was used to analyze juvenile social exploration results. F values are reported for each ANOVA and serve as the criteria for post hoc analysis (Tukey’s HSD). Threshold for statistical significance was set at p < 0.05. Sample sizes and more details on the exact statistical comparisons are provided in figure captions and degrees of freedom (also indicating sample size) are reported throughout the results section.
2.2. Experimental design
2.2.1. Experiment 1: Are LPS-induced sickness behaviors exaggerated by prior stress in male and female rats?
Male and female rats were assessed for putative anxiety-like responses and behavioral anhedonia in a juvenile social exploration task and a sucrose preference test as previously described (Fonken et al., 2015) (experimental timeline in Fig 1A). Rats were singly housed for the behavioral experiment.
Figure 1. Sickness behavior is increased by prior stress in male and female rats.
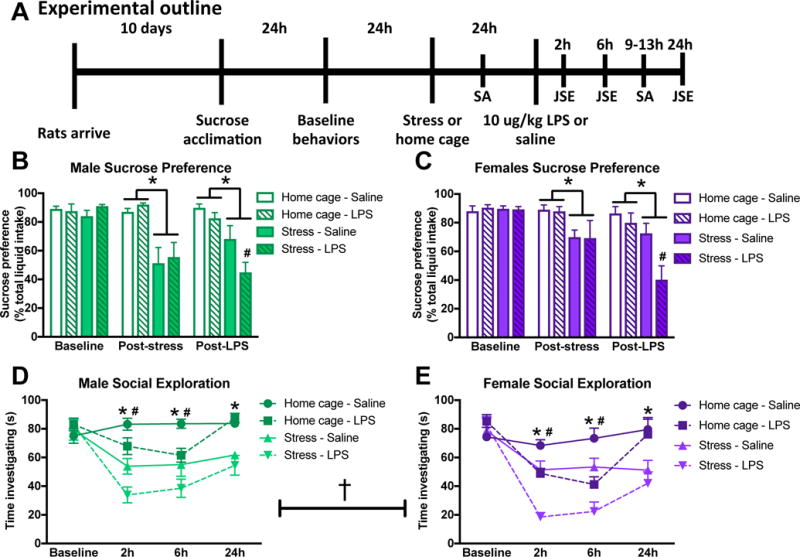
(A) Male and female rats underwent inescapable stress (100 trials of tailshock) or remained in the home cage. 24 h later rats received a single IP injection of 10 ug/kg LPS or saline (vehicle-control) and then underwent behavioral testing (see methods for additional details on timing). Male and female rats are graphed separately to simplify interpretation but sex was analyzed as a variable in both the sucrose preference and social exploration tests. (B) Male and (C) female rats demonstrated reduced sucrose preference following stress. Furthermore, prior stress exaggerated the LPS induced reduction in sucrose preference in both male and female rats. Juvenile social exploration was reduced by both stress and LPS in (D) male and (E) female rats. Furthermore, female rats had lower levels of social investigation as compared to male rats. Sucrose preference results were analyzed for each day using a 2 × 2 × 2 ANOVA with sex, stress, and immune challenge as the between subjects factors (n = 6 per group with a total of 48 rats). Results from the juvenile social exploration test were analyzed using 2 × 2 × 2 repeated measures ANOVAs with sex, stress, and immune challenge as the between subject factors and time as the within subjects factor. Data are expressed as mean ± SEM. †main effect of sex, *main effect of stress, #main effect of LPS, p < 0.05 in all cases. Abbreviations: JSE (juvenile social investigation), SA (sucrose anhedonia), LPS (lipopolysaccharide).
Rats were first acclimated to behavioral tests and baseline values were established. After baseline testing, rats underwent either tail shock stress (described above in 2.1.2.) or remained undisturbed in their home cage (control). 24h following stress exposure, rats received an intraperitoneal (IP) injection of LPS (10 ug/kg; Escherichia coli serotype 0111:B4; Sigma) or vehicle (sterile saline) between ZT3 and ZT5. This LPS dose was selected to maintain consistency with past studies investigating neuroinflammatory priming in this laboratory and because it reliably causes a low-grade hippocampal pro-inflammatory response (Johnson et al., 2002). Low-grade pro-inflammatory response was preferred to avoid robust LPS induced sickness behaviors that may mask stress-induced priming in males and females.
To assess behavioral anhedonia (Willner et al., 1987), rats were provided with two bottles, one containing water and one containing water supplemented with 2% sucrose (side of sucrose bottle was counterbalanced within groups). Rats were acclimated to the two-choice test for 4 h on the night (ZT12 – ZT16) directly prior to baseline assessment and all rats showed a strong preference for the sucrose solution (approximately 90% sucrose intake). Sucrose intake was then measured for 4 h each night (ZT12 – ZT16) at baseline and following the experimental manipulations (stress and LPS) and a percentage of relative sucrose intake was calculated: [sucrose intake/(sucrose intake + water intake)] × 100.
To assess motivation to engage in social exploratory behavior, a novel same-sex juvenile rat (28 ± 4 days old) was introduced into the home cage of the experimental rat for 3 min. A condition-blind observer scored the session live for two mutually-exclusive conditions (engaging with the juvenile versus not engaging with the juvenile) using a program designed with LATEX. Engaging with the juvenile was defined as social exploratory behaviors (sniffing, pinning, allogrooming, and following) initiated by the experimental rat. Juvenile rats were used a maximum of 5 times per session with a > 30 min break between uses and experimental rats always encountered a novel juvenile rat. Juvenile rats were also counterbalanced between the groups and there were no significant order effects or differences in investigation time based on the juvenile. An endpoint criterion for the juvenile social exploration task is the occurrence of aggressive behavior; no aggressive encounters occurred in this experiment. Baseline social behavior was established 24 h prior to stress exposure (ZT3 – ZT5). Rats then underwent stress and 24 h later received LPS. Social behavior was then repeatedly assessed at 2 h (ZT5 – ZT7), 6 h (ZT9 – ZT11), and 24 h (ZT3 – ZT5) following an LPS injection (between ZT3 and ZT5).
24 h after the conclusion of behavioral testing, rats were humanely euthanized (tissue was not collected because euthanasia occurred > 48 h post-LPS and the stress of behavioral testing would confound results).
2.2.2. Experiment 2: Does prior stress potentiate LPS-induced inflammatory responses in the periphery and hippocampus of male and female rats?
Male and female rats underwent either tail shock stress or remained undisturbed in their home cage. 24h following stress exposure, rats received an IP injection of LPS (10 ug/kg) or vehicle between ZT3 and ZT5. Three hours following the LPS or vehicle injection, tissues and serum were collected in order to evaluate the inflammatory response.
2.2.3. Experiments 3: Are microglia isolated from the hippocampus of adult male and female rats primed by prior stress?
Male and female rats underwent either tail shock stress or remained undisturbed in their home cage. 24 h following stress exposure, hippocampal microglia were isolated and challenged ex vivo with lipopolysaccharide (described above in 2.1.4.).
2.2.4. Experiment 4: Does prior stress shift the phagocytic capacity of microglia isolated from male and female rats?
Male and female rats underwent either tail shock stress or remained undisturbed in their home cage. 24 h following stress exposure, microglia were isolated (described above in 2.1.4.) and their phagocytic activity was assessed. For the phagocytosis assay, microglia were plated at a density of 50,000 cells per well in 8-well chambered culture slides (Nunc Lab-Tek II; ThermoFisher 154534PK). The microglia were incubated at 37⁰C, 5% CO2 for 2 h and then latex beads-rabbit IgG-FITC complex (at 1:100; Cayman Chemicals, 400291) were added to the wells. After a 30 min incubation, the beads were washed off with PBS and the cells were fixed with cold 4% paraformaldehyde for 30 min. The slides were stored in PBS. For immunocytochemistry, slides were incubated in 10% normal donkey serum for 30 min, then with primary antibody (rabbit anti-Iba1, 1:1000; Wako 019-19741) overnight. Cells were incubated with secondary antibody (Alexa 546 donkey anti-rabbit, 1:500; ThermoFisher A10040) and DAPI for 2 h. Slides were coverslipped and imaged using an inverted Nikon Ti-E microscope. For image capture and analysis, 20 images per well were taken (random areas within the well). The percent of cells containing green phagocytic beads was assessed. Multiple groups (i.e., stress-home cage; male-female) were included, so female and male rats were run on different days; thus, the data are expressed as percent home cage control.
2.2.5. Experiment 5: Is the serum corticosterone response differentially affected by stress in male and female rats?
After acclimating the rats to handling, we collected a baseline blood sample from the tail vein. Male and female rats then underwent stress or home cage treatment and repeated tail vein blood samples were collected at 0 h (immediately upon cessation of the stress protocol), 24 h, 72 h, 1 week and 4 weeks following the stress paradigm. Rats were humanely euthanized at the conclusion of this experiment and donated to the Birds of Prey Foundation (Broomfield, CO).
2.2.6. Experiment 6: Are microglia isolated from the hippocampus of female rats primed one week following stress when CORT concentrations have normalized?
Male and female rats underwent either tail shock stress or remained undisturbed in their home cage. One week following stress exposure, when serum CORT concentrations had returned to baseline in female rats, hippocampal microglia were isolated and challenged ex vivo with LPS.
2.2.7. Experiment 7: Is CORT sufficient to prime neuroinflammatory responses in male and female rats?
Male and female rats received a single subcutaneous injection of CORT (2.5 mg/kg; Sigma, St. Louis, MO) or vehicle (100% propylene glycol) at ~ZT4 (corresponding to the time of stress). We previously demonstrated that this CORT dose recapitulates inescapable shock-elicited CORT (Fleshner et al., 1995) and induces neuroinflammatory priming in male rats (Frank et al., 2012). 24 h following the CORT or vehicle injection, rats received a single IP injection of LPS (10 ug/kg) or vehicle between ZT3 and ZT5. Three hours following the LPS or vehicle injection, tissues and serum were collected in order to evaluate the inflammatory response.
2.2.8. Experiment 8: Are glucocorticoid sufficient to prime microglia responses in male and female rats?
Male and female rats received a single subcutaneous injection of CORT (2.5 mg/kg) or vehicle at ~ZT4. 24 h later, microglia were isolated and stimulated ex vivo with LPS. Graphs from this experiment are represented in Figure 2, 3, 4.
Figure 2. Male and female rats exhibit comparable primed hippocampal cytokine responses post-stress.
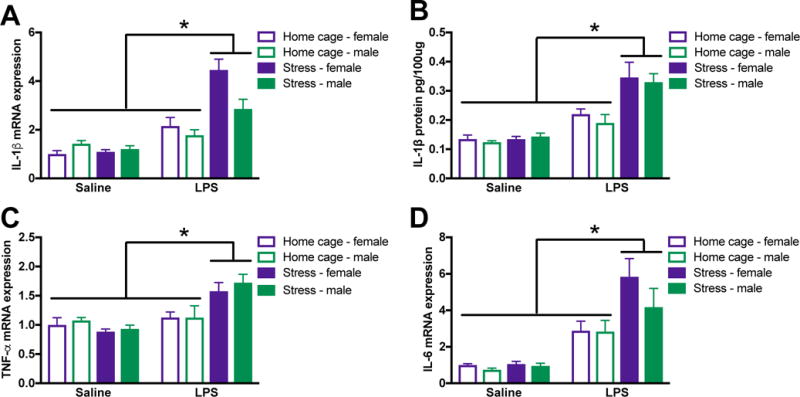
Male and female rats underwent inescapable stress or remained in the home cage. 24 h later rats received a single IP injection of 10 ug/kg LPS or saline and tissue was collected 3 h later. (A) Stress potentiated LPS-induced IL-1β mRNA elevations in the hippocampus of male and female rats. (B) IL-1β protein was similarly regulated in the hippocampus of male and female rats. Additional pro-inflammatory cytokine mRNA including (C) TNFα and (D) IL-6 were elevated by stress followed by LPS. Results were analyzed using 2 × 2 × 2 ANOVAs with sex, stress, and immune challenge as the between subjects factors (n = 6 – 8 per group with a total of 56 rats). Data are expressed as mean ± SEM. *simple effect of stress, p < 0.05 in all cases.
Figure 3. Male and female rats exhibited comparable down-regulation of anti-inflammatory pathway genes following stress.
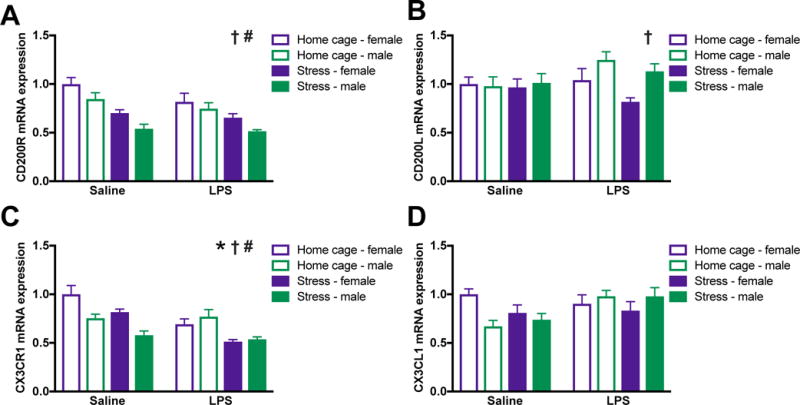
Male and female rats underwent inescapable stress or remained in the home cage. 24 h later rats received a single IP injection of 10 ug/kg LPS or saline and tissue was collected 3 h later. (A) CD200R mRNA was lower in the hippocampus of male as compared to female rats and was suppressed by stress in both sexes. (B) CD200L was increased in the hippocampus of male as compared to female rats and unaffected by and LPS stress. (C) CX3CR1 was reduced in the hippocampus of male as compared to female rats and suppressed by stress and LPS. (D) In contrast, mRNA expression of the CX3CL1 was unaffected by sex, stress, or LPS. Results were analyzed using 2 × 2 × 2 ANOVAs with sex, stress, and immune challenge as the between subjects factors (n = 6 – 8 per group with a total of 56 rats). Data are expressed as mean ± SEM. †main effect of sex, *main effect of LPS, #main effect of stress, p < 0.05 in all cases.
Figure 4. Peripheral cytokine responses are potentiated in female but not male rats in response to stress and LPS.
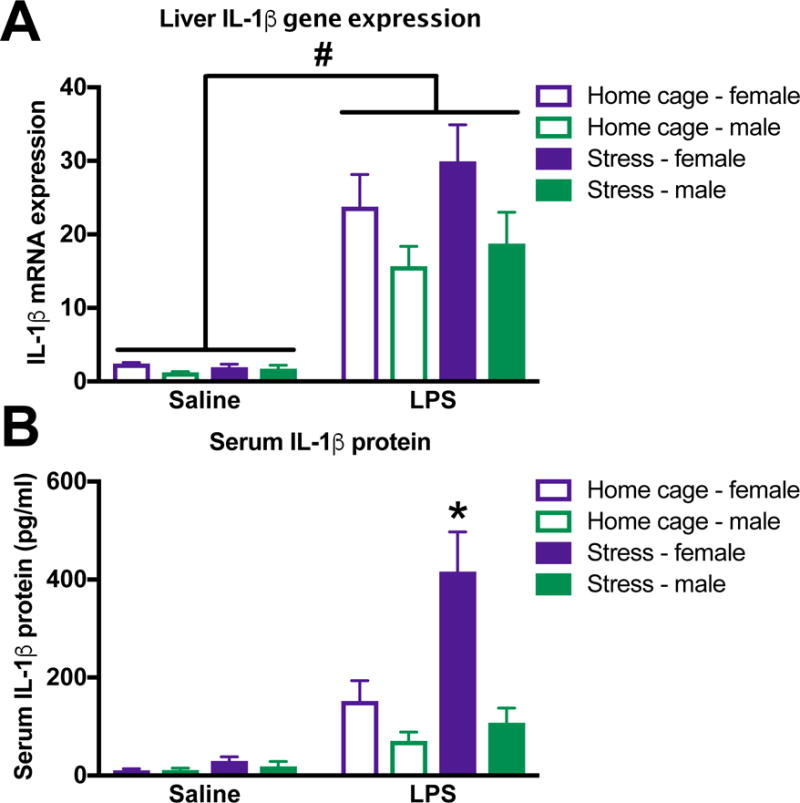
Female and male rats underwent inescapable stress or remained in the home cage. 24 h later rats received a single IP injection of 10 ug/kg LPS or saline and blood and tissue were collected 3 h later. (A) IL-1β mRNA expression in liver was increased by LPS but not stress in both female and male rats. (B) Serum IL-1β concentrations were increased by exposure to stress and LPS in female rats. In contrast, serum IL-1β was not affected by stress exposure in male rats. Results were analyzed using 2 × 2 × 2 ANOVAs with sex, stress, and immune challenge as the between subjects factors (n = 6 – 8 per group). Data are expressed as mean ± SEM. #main effect of LPS, *simple effect of stress, p < 0.05 in all cases.
3. Results
3.1 Stress exacerbates LPS-induced behavioral changes in male and female rats
Male rats that experience stress prior to an immune challenge exhibit an exaggerated behavioral sickness response (Johnson et al., 2003). Here, we tested whether females exhibit similar stress-enhanced behavioral responses to immune challenge (Fig 1A). Male and female rats underwent 100 sessions of inescapable tail shock stress. 24 h later, rats were given either an IP vehicle or LPS (10 ug/kg) injection and sickness behaviors were evaluated (n = 6/group with a total of 48 rats). One female rat was euthanized prior to the conclusion of behavioral testing due to an injury sustained in the stress paradigm and is not included in statistical analyses. Male and female rats are graphed separately for ease of visualization. Sex was analyzed as a variable in all statistical comparisons and all male/female differences are described below and noted in the figures.
There were no baseline differences in sucrose preference between the groups and there was no effect of sex (p > 0.05). On the evening following stress, sucrose preference was reduced in male and female rats that received stress as compared to home cage control treatment (main effect of stress, p < 0.05; Fig 1B&C). 24 h post-stress rats were administered LPS, which additively reduced sucrose intake in male and female stress rats (main effect of LPS and stress, p < 0.05; Fig 1B&C).
There were no baseline differences in juvenile social exploration between groups (p > 0.05) and juvenile social interaction varied over time (time X stress and LPS: F3,117 = 8.2 and 14.6 respectively, p < 0.05; Fig 1D&E). There was a main effect of sex on social exploration (F1,39 = 11.8, p < 0.005); such that females showed an overall reduction in social exploratory behavior compared to males. However, there was no interaction of sex with time, stress, or immune challenge; thus, male and female rats had a comparable response to the experimental manipulations. Stress and LPS reduced juvenile social exploration in an additive manner in male and female rats at 2 h and 6 h post-LPS (post hoc, p <0.05). By 24 h post-LPS, rats had recovered from the effects of the LPS (p > 0.05) but not prior stress (post hoc, p < 0.05).
3.2. Post-stress male and female rats exhibit primed hippocampal cytokine responses
To determine whether female rats demonstrate similarly primed neuroinflammatory responses to male rats following stress, rats received an LPS (10 ug/kg) injection 24 post-stress and hippocampal tissue was collected 3 h later. In agreement with previous findings, stressor exposure 24 h prior to an LPS challenge caused an exaggerated hippocampal cytokine response in male rats (Johnson et al., 2002). Furthermore, there was no effect of sex on hippocampal cytokine expression (p > 0.05): female rats had similarly elevated hippocampal cytokine expression in response to LPS post-stress. Male and female rats showed potentiated IL-1β mRNA (stress X LPS: F1,46 = 26.3, p < 0.0001; Fig 2A) and IL-1β protein (stress X LPS: F1,46 = 17.2; p < 0.0001; Fig 2B) responses in the hippocampus 3 h following LPS. Furthermore, male and female rats had enhanced hippocampal TNFα mRNA (stress X LPS: F1,45 = 17.8, p < 0.0001; Fig 2C) and IL-6 mRNA (stress X LPS: F1,48 = 7.9; p < 0.01; Fig 2D) expression following LPS post-stress.
3.3. In the hippocampus of male and female rats, stress down-regulates anti-inflammatory signaling pathways
The CD200:CD200R and CX3CL1:CX3CR1 (fractalkine) dyads are considered neuroinflammatory “off” signals for microglia (Biber et al., 2007; Paolicelli et al., 2014). Down-regulation or ablation of CX3CR1 or CD200 causes heightened neuroinflammatory responses (Cardona et al., 2006; Denieffe et al., 2013). Thus, here we evaluated whether male and female rats exhibit changes in the CD200 and fractalkine family of genes following stress and LPS exposure. Females had elevated CD200 receptor and reduced CD200 ligand expression in the hippocampus as compared to male rats (sex: F1,48 = 10.6 and 4.8 respectively, p < 0.05; Fig 3A&B). Stress elicited reductions in the CD200 receptor (stress: F1,48 = 38.2; p < 0.0001; Fig 3A) but did not affect expression of the ligand (p > 0.05; Fig 3B) in the hippocampus of male and female rats.
Fractalkine receptor (CX3CR1) expression was significantly affected by sex, stress, and LPS. CX3CR1 was elevated in the hippocampus of female as compared to male rats (sex: F1,46 = 5.9, p < 0.05) and was down-regulated by exposure to stress (stress: F1,46 = 24.2, p < 0.0001; Fig 3C). LPS also reduced CX3CR1 expression (LPS: F1,46 = 16.5). In contrast, there was no effect of sex, stress, or LPS on fractalkine ligand expression (p > 0.05; Fig 3D).
3.4. Stress primes serum IL-1β responses in female but not male rats
Stress-evoked cytokine increases to LPS were limited to the CNS in male but not female rats. In agreement with previous findings, liver IL-1β mRNA expression was increased by LPS but unaffected by stress in both male and female rats (F1,32 = 60.2, p < 0.0001; Fig 4A). Of note, a subset of liver samples (n = 2/group) were lost which is why the degrees of freedom is lower for this comparison. In contrast, female but not male rats that underwent stress 24 h prior showed potentiated serum IL-1β responses to LPS (sex X stress X LPS: F1,46 = 7.0, p < 0.05; Fig 4B).
3.5. Microglia isolated from male, but not female rats are primed following stress
Although microglia appear to contribute to neuroinflammatory priming in males, it is important to establish whether or not microglia similarly drive priming in females. Indeed, other groups have demonstrated that immune cells mediating neuroinflammatory responses can vary between the sexes (Sorge et al., 2015). Male and female rats received stress and 24 h later hippocampal microglia were isolated. Microglia were challenged ex vivo with LPS (0, 1, 10, 100 ng/ml) for 4 h (Fig 5A). There were sex differences in microglia responses to stress followed by an ex vivo challenge with LPS (sex X stress X LPS for IL-1β and IL-6: F3,48 = 3.4 and 2.9 respectively, p < 0.05). As expected, LPS dose dependently increased IL-1β and IL-6 mRNA expression in microglia isolated from both male and female rats (Fig 5B&C). However, while prior-stress potentiated the IL-1β (post hoc, p < 0.05; Fig 5B) and IL-6 (post hoc, p < 0.05; Fig 5C) response in microglia isolated from male rats, microglia isolated from females had comparable IL-1β and IL-6 mRNA expression following stress or control treatment (p > 0.05). The lack of priming in microglia isolated from female rats was confirmed in separate cohort of animals (supplemental Fig 1). This suggests that prior stress primed male, but not female microglia.
Figure 5. Microglia are primed by exposure to stress in male but not female rats.
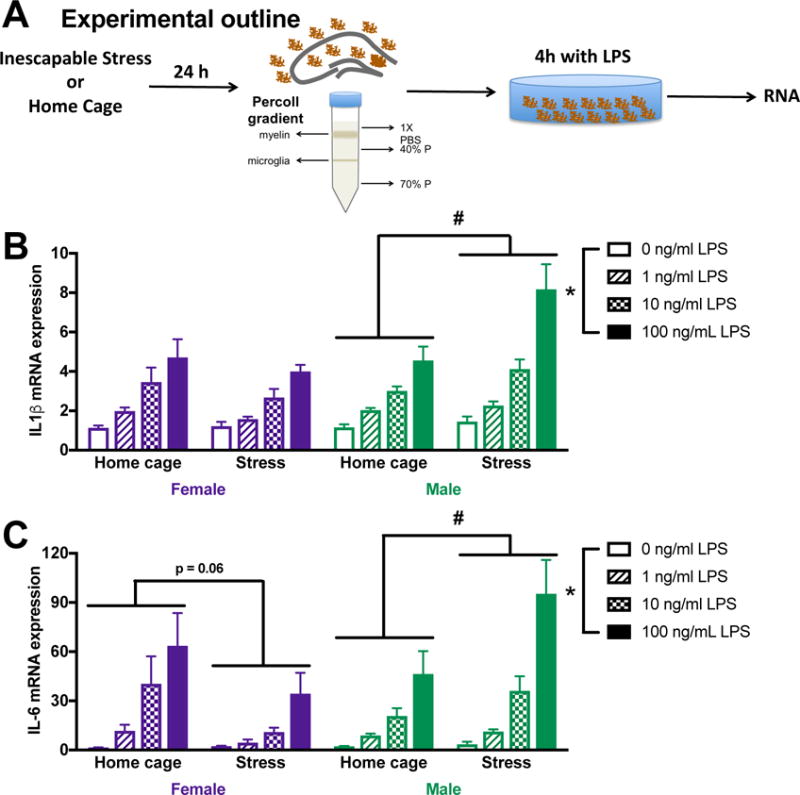
(A) Male and female rats underwent inescapable stress or remained in the home cage. 24 h later microglia were isolated from the hippocampus using a percoll density gradient. Cells were plated (10,000 cell per well) with LPS for 4 h prior to isolating mRNA. (B) Prior stress exposure potentiated IL-1β mRNA responses to LPS in microglia isolated from male rats. In contrast, IL-1β mRNA expression was unaffected by prior stress in female microglia. (C) Similarly, IL-6 mRNA expression was potentiated by stress exposure in males, which trends toward an overall suppression in microglia isolated from the female hippocampus. Results were analyzed using 2 × 2 × 4 ANOVAs with sex, stress, and LPS dose as the independent variables (n = 4 per group and experiment replicated in an additional cohort of 32 female rats [supplemental Fig 1]). Data are expressed as mean ± SEM. *main effect of LPS, #stress X LPS interaction, p < 0.05 in all cases.
3.6. Microglia from stressed male and female rats show reduced phagocytic capacity
Microglia isolated from female rats following stress did not show the enhanced inflammatory profile to LPS typically found in microglia isolated from stressed male rats. This led us to next test whether microglia were functionally shifted toward an alternative activation phenotype. One functional feature of alternative activation is increased phagocytic ability. We evaluated phagocytic capacity of hippocampal microglia isolated from stressed male and female rats 24 h after stress, using fluorescent latex beads. Fewer microglia from stressed, compared to home cage, rats phagocytosed the latex beads (stress: F1,22 = 6.454, p < 0.05; Fig 6). While there was no significant interaction of sex and stress on phagocytosis, microglia from stressed females had particularly robust reductions in phagocytosis with 41% fewer stressed female microglia containing beads compared to home cage female microglia.
Figure 6. Stress reduces phagocytic capacity in microglia isolated from male and female rats.
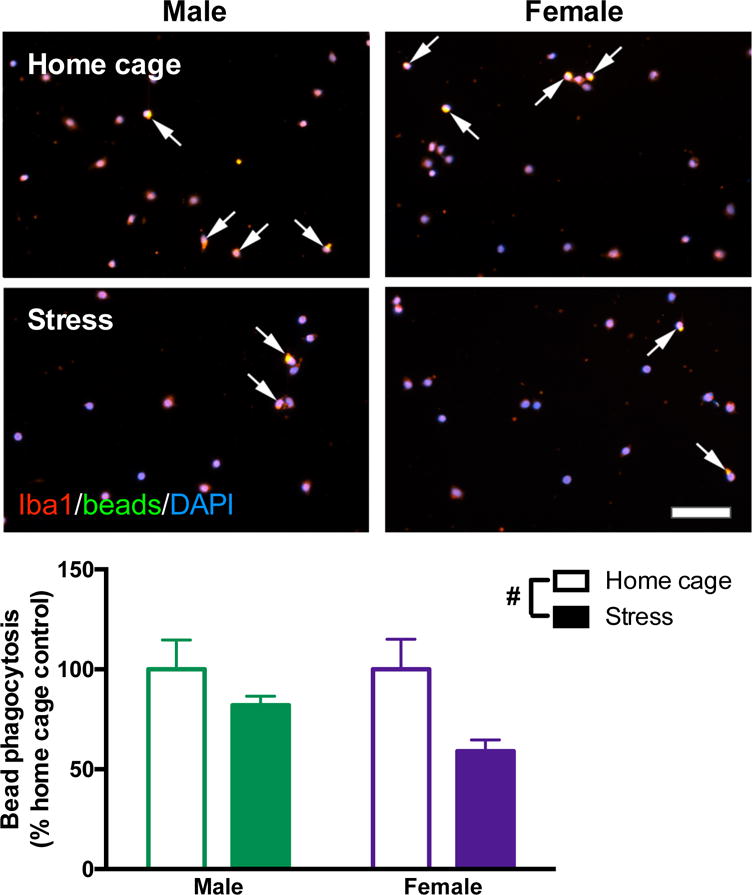
Male and female rats underwent inescapable stress or remained in the home cage. 24 h later microglia were isolated from the hippocampus using a percoll density gradient. Microglia were plated (5 × 104 cells per well) with a latex bead solution (1:100 concentration). Microglia were identified with Iba1 (red), beads were conjugated to a green fluorophore, and nuclei were identified using DAPI (blue). Arrows highlight microglia that phagocytosed latex beads (yellow represents colocalization of red microglia and green beads). Stress caused fewer microglia to phagocytose the beads. Results were analyzed using a 2 × 2 ANOVA with sex and stress as the independent variables (n = 6 per group with a total of 24 rats; 20 images were analyzed per rat and averaged). Data are expressed as mean ± SEM. #main effect of stress, p < 0.05. Scale bar: 50 mm.
3.7. Glucocorticoid responses vary between male and female rats following stress
Previous work from our laboratory demonstrates that in vivo and ex vivo priming in males is driven by CORT (Frank et al., 2010; Frank et al., 2012). Thus, we next evaluated CORT responses in male and female rats that underwent stress followed by an injection of LPS 24 h later. Tissue was collected 3 h post-LPS. Hippocampal CORT was potentiated by stress followed by LPS in male and female rats (stress X LPS: F1,48 = 18.0, p < 0.0001; Fig 7A). There was an interaction of sex and stress on hippocampal GR expression (sex X stress: F1,41 = 7.6, p < 0.01; Fig 7B): such that, there was no effect of stress on GR expression in the hippocampus of male rats (post hoc, p > 0.05), but female rats exhibited an overall down-regulation in GR following stress (post hoc, p < 0.05).
Figure 7. Some aspects of the glucocorticoid response are differentially modulated in male and female rats, but normalizing CORT concentrations does not result in priming in female microglia.
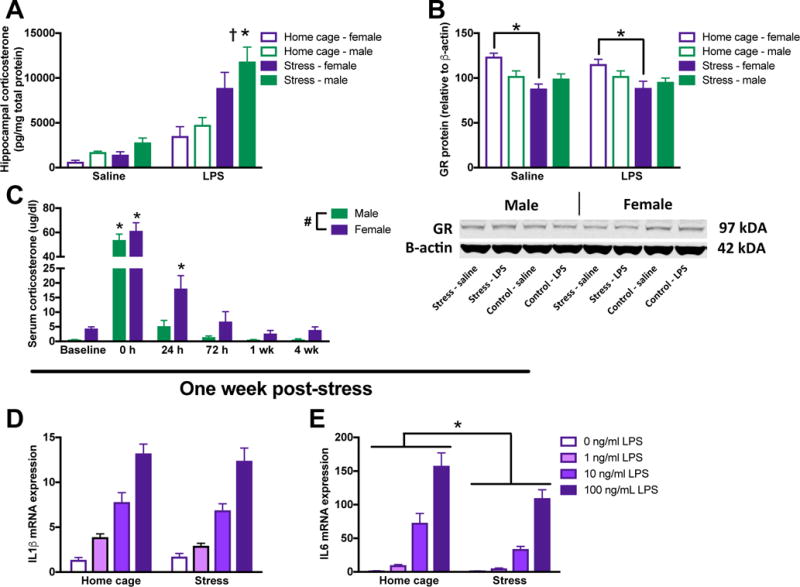
(A) CORT responses are elevated by both prior stress and LPS in the hippocampus of male and female rats. (B) However, while glucocorticoid receptor concentrations are unaffected by stress in the hippocampus of male rats, females show downregulated glucocorticoid receptor expression in the hippocampus following stress. A representative blot is included below the graph (note: groups are represented in a different order in the blot than the figure). (C) The peripheral serum CORT response is elevated in females compared to males and CORT is increased for a longer duration of time following stress in females. Microglia isolated from female rats one week following stress when CORT concentrations have returned to baseline do not exhibit primed (D) IL-1β or (E) IL-6 mRNA expression. Panels A&B were analyzed using 2 × 2 × 2 ANOVAs with sex, stress, and immune challenge as the independent variables (n = 6 – 8 per group). Panel C was analyzed using a 2-way repeated measures ANOVA with sex as the between subjects factor and time as the within subject factor (n = 6 per group). Panels D&E were analyzed using 2 × 4 ANOVAs with stress and dose of LPS as the independent variables (n = 4 per group). Data are expressed as mean ± SEM. *main effect of stress, #main effect of sex, †main effect of LPS, p < 0.05 in all cases.
Serum CORT concentrations were elevated in females as compared to males (F1,10 = 11.93, p < 0.05) and were elevated by stress as compared to home cage treatment (F5,50 = 130.20, p < 0.05; Fig 7C). Elevations in serum CORT concentrations persisted longer in females compared to males: by 24 h post-stress males no longer exhibited higher CORT concentrations than baseline (post hoc, p < 0.05). Females demonstrated elevated CORT concentrations at 24 h (post hoc, p < 0.05). While CORT concentrations were no longer significantly elevated in females at 3 days post–stress, CORT levels were highly variable (CORT from some animals was near baseline levels, while CORT from other female rats remained elevated).
In order to determine whether prolonged elevations in CORT may be preventing priming in microglia at 24 h in females, we next isolated microglia from female rats at 7 days post-stress when serum CORT concentrations were back to baseline. Male microglia exhibit priming for up to one-month post-stress (Frank et al, unpublished observations). Microglia isolated from female rats 7 days post-stress exhibited typical increases in IL-1β and IL-6 mRNA expression in response to LPS (LPS on IL-1β: F3,24=77.59, LPS on IL-6: F3,24=82.59, p < 0.05; Fig 7D&E). However, female microglia still did not appear primed by stress: IL-1β mRNA expression was unaffected by prior stress (p > 0.05) and IL-6 mRNA expression was suppressed by prior stress (stress: F1,24=11.70, p < 0.05).
3.8. CORT induces priming in vivo but not ex vivo in female rats
CORT is a proximate signal through which stress primes neuroinflammatory and microglial responses in male rats (Frank et al., 2014; Frank et al., 2010; Frank et al., 2012). Indeed, a CORT injection that mimics the rise in CORT induced by stress causes neuroinflammatory priming in male rats (Frank et al., 2010). Thus, we next evaluated whether CORT similarly induces priming in female rats. To test this, male and female rats were injected with a single subcutaneous dose of CORT or vehicle (propylene glycol) (Frank et al., 2010). 24 h later, rats received 10 ug/kg IP LPS and then hippocampal tissue was collected after 3 h to evaluate cytokine expression. Prior CORT potentiated LPS induced IL-1β mRNA expression (CORT X LPS: F1,21= 15.0, p < 0.001; Fig 8A) and IL-6 mRNA expression (CORT X LPS: F1,21 = 6.3, p < 0.05; Fig 8B) in the hippocampus of male and female rats. Importantly, prior CORT also potentiated the serum IL-1β response to LPS in female but not male rats (sex X CORT X LPS: F1,23 = 4.3 and post hoc, p < 0.05; Fig 8C).
Figure 8. Prior CORT treatment induces neuroinflammatory priming in vivo in male and female rats but does not prime female microglia.
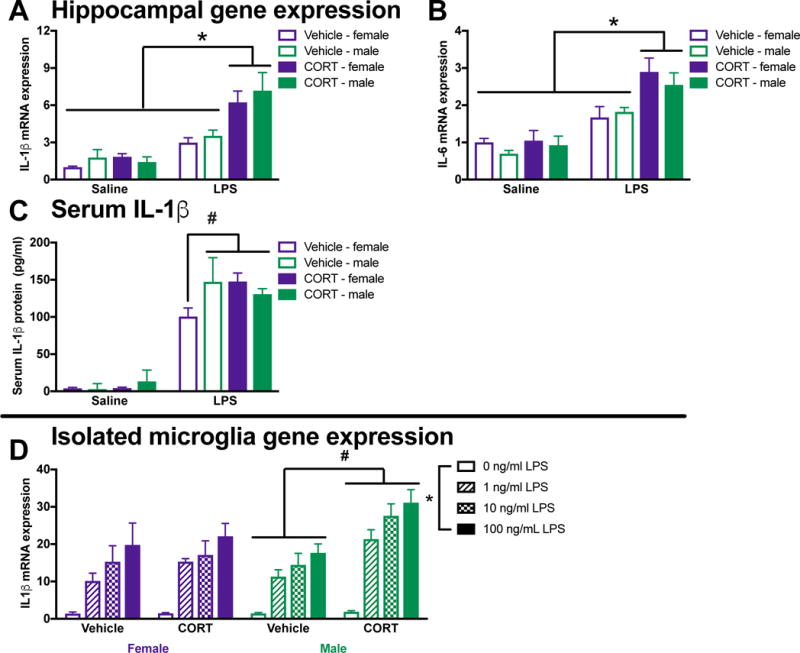
Male and female rats were injected with a single subcutaneous dose of CORT or vehicle (propylene glycol). 24 h later rats received 10 ug/kg IP LPS. Serum and hippocampi were collected after 3 h to evaluate cytokine expression. Prior CORT treatment potentiated LPS induced hippocampal IL-1β mRNA expression in (A) male and female rats. (B) Hippocampal IL-6 expression was similarly potentiated by prior CORT treatment in male and female rats. (C) Serum IL-1β was induced by an LPS injection in male rats but only potentiated by prior CORT treatment in females. In a separate cohort of rats, microglia were isolated 24 h following CORT or vehicle treatment. (D) Ex vivo treatment with LPS produced exaggerated IL-1β mRNA responses in male but not female rats that had received prior CORT treatment. Panels A – C were analyzed using 2 × 2 × 2 ANOVAs with sex, CORT, and LPS as the independent variables (n = 4 per group with a total of 32 rats). Panel D was analyzed using 2 × 2 × 4 ANOVAs with sex, CORT, and LPS dose as the independent variables (n = 4 per group with a total of 16 rats). Data are expressed as mean ± SEM. *main effect of LPS, #interaction of CORT and LPS (G), p < 0.05 in all cases.
In a separate cohort of rats, microglia were isolated 24 h following CORT or vehicle treatment. Microglia were isolated at 24 h post-CORT because this is when the in vivo immune challenge would occur. Microglia priming was differentially affected by CORT treatment in male and female rats (sex X CORT: F1,48 = 7.7, p < 0.05). In agreement with previous findings, in vivo CORT potentiated ex vivo LPS-elicited IL-1β mRNA responses in microglia isolated from male rats (post hoc, p < 0.05; Fig 8D). In contrast, prior CORT treatment did not affect female microglia IL-1β induction in response to ex vivo LPS stimulation (p > 0.05).
Discussion
Exposure to stressors and peripheral immune challenges induce pro-inflammatory cytokines in the CNS. Furthermore, cross-sensitization between these factors can occur; for example, stress potentiates the CNS cytokine response to a subsequent immune challenge (Frank et al., 2016). This phenomenon, termed “neuroinflammatory priming”, has primarily been studied in males. Sex differences in both the stress response (Bourke et al., 2012) and the immune system (Klein and Flanagan, 2016) led us to test whether behavioral and neuroinflammatory changes in response to stress differ in male and female rats. Here we show that male and female rats exhibit comparable behavioral changes in response to stress followed by a peripheral injection of LPS. Exposure to stress increased behavioral anhedonia, as assessed by a sucrose preference test in male and female rats. Furthermore, stress and LPS additively affected sucrose preference with animals that experienced both challenges exhibiting the greatest reduction in sucrose intake. Similarly, male and female rats that underwent stress followed by LPS showed additive reductions in social exploratory behavior in a juvenile social exploration task. Consistent with the greater behavioral changes, neuroinflammatory responses to LPS were enhanced in male and female rats that underwent prior stress. Indeed, the induction of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) in the hippocampus was elevated in male and female rats that received a peripheral LPS injection 24 h post-stress. Importantly, behavioral changes and CNS cytokine induction were similarly altered in male and female rats, suggesting that stress-elicited neuroinflammatory priming is equivalent in vivo.
The anti-inflammatory signature of the hippocampus was comparable in male and female rats post-stress. Stress caused a down-regulation of anti-inflammatory pathway genes, including CD200R and CX3CR1, in the hippocampus of male and female rats. Both the CD200:CD200R and CX3CL1:CX3CR1 dyads are neuroinflammatory “off” signals for microglia (Biber et al., 2007; Paolicelli et al., 2014). CD200R and CX3CR1 are expressed exclusively on microglia and other myeloid cells in the CNS (Cardona et al., 2006; Koning et al., 2009). Interactions between the neuronally expressed CX3CL1 and CD200 ligands and corresponding receptors on microglia inhibit inflammatory responses (Cardona et al., 2006). Our findings support previous work implicating these pathways in neuroinflammatory priming in the context of stress and aging (Fonken et al., 2016; Wynne et al., 2010). Since stress reduces CD200R and CX3CR1, stress may induce neuroinflammatory priming by attenuating neuronal inhibition of microglia. Dampening the pathways through which neurons inhibit microglia could produce a CNS microenvironment permissive to pro-inflammatory activation (Frank et al., 2007).
Thus, next we tested whether microglia contribute to stress-induced potentiation of CNS pro-inflammatory responses. Microglia isolated from male and female home cage control rats exhibit comparable reactivity to ex vivo LPS treatment indicating male and female microglia have equivalent baseline pro-inflammatory capacity. Following stress, male rats exhibit increases in microglia activation in vivo and potentiated ex vivo microglia responses to an LPS challenge (Frank et al., 2007), suggesting that microglia are a neuroimmune substrate leading to stress-elicited neuroinflammatory priming in males. In contrast, while female rats exhibit comparable pro-inflammatory cytokine potentiation in the CNS following stress and LPS, microglia isolated from female rats did not exhibit a primed phenotype. Paradoxically, there was an overall trend towards stress suppressing microglia activation in females (see Fig 5 and supplemental Fig 1). This indicates that neuroinflammatory priming in females may not depend on, or be initiated by, heightened microglia reactivity. The lack of microglia priming in females is somewhat surprising as female rodents express more microglia than males in limbic structures (Schwarz et al., 2012) and female microglia have a more activated morphology (Nelson et al., 2017; Schwarz et al., 2012).
It is possible that microglia from female rats undergo alternative activation in response to stress or display a different time course of neuroinflammatory priming. To test this first possibility, the phagocytic capacity of microglia isolated from rats that had underwent a stressor 24 h prior was assessed. Pro-inflammatory microglia phagocytose less efficiently (Koenigsknecht-Talboo and Landreth, 2005), whereas microglia treated with anti-inflammatory factors (e.g., IL-4) increase phagocytic capacity (Shimizu et al., 2008) – partly via CD200:CD200R signaling (Varnum et al., 2015). Thus, stress was expected to reduce phagocytic ability of microglia isolated from male rats. Interestingly, both male and female rats showed reduced phagocytic capacity 24 h following stress. This reduction in microglial phagocytic activity from both sexes suggests that while microglia from stressed females are less reactive to an in vitro inflammatory challenge than male microglia, they are not more anti-inflammatory.
Second, we evaluated whether the time course of priming was altered in microglia isolated from female rats. Importantly, while male and female rats had comparable peak CORT concentrations directly following stress, the CORT elevation was protracted in female rats. The sustained increase of stress-elicited CORT in females 24 h post-stress could blunt the microglial response to LPS ex vivo at this same time post-stress. Previous work in our laboratory suggests that microglia priming following exposure to stress can last up to a month in male rats (Frank et al, unpublished observations). Thus, we tested whether microglia isolated from female rats 7 days post-stress, when serum CORT concentrations had returned to baseline in female rats, would exhibit an enhanced inflammatory response to an ex vivo challenge. Microglia isolated from female rats one week following stress did not exhibit an enhanced immune response to an ex vivo immune challenge. Moreover, treating rats with exogenous CORT and isolating microglia 24 h later only produced a primed phenotype in microglia isolated from male rats. Taken together, these results suggest that microglia from female rats do not undergo the ex vivo priming in response to in vivo stress that has been described in males. This may indicate that in female, but not male rats continued signaling from other CNS cells is required for priming to occur.
Glucocorticoids are a proximate signal through which stress primes inflammatory responses in male rats (Frank et al., 2013). In male rats, CORT is necessary and sufficient for stress-induced neuroinflammatory and microglia priming (Frank et al., 2013). Thus, we next evaluated whether CORT similarly induces hippocampal neuroinflammatory priming in female rats. In agreement with previous findings, the administration of CORT 24 h prior to LPS potentiated cytokine responses in the hippocampus of male rats. Moreover, female rats also exhibited increases in hippocampal IL-1β and IL-6 mRNA in response to an LPS injection 24 h post-CORT administration. As noted above, microglia responses to CORT injection paralleled findings in microglia isolated post-stress: whereas microglia isolated from males rats 24 h following CORT showed enhanced inflammatory potential to ex vivo LPS, prior CORT treatment did not potentiate cytokine responses in microglia isolated from female rats. One limitation to administering exogenous CORT in females is that it may not parallel the curve produced by stress exposure. Indeed, females typically show more rapid metabolism and clearance of glucocorticoids (reviewed in (Goel et al., 2014)). However, female rats displayed prolonged elevations in serum CORT as compared to males following tail shock stress. Furthermore, it is unclear if the time course of CORT in the hippocampus differs in male and female rats: previous work in our lab suggests that hippocampal and serum CORT responses can differ (Barrientos et al., 2015).
Finally, enhanced inflammatory responses appear limited to the CNS in male rats in these experiments. However, female rats that received stress or CORT prior to LPS had potentiated serum IL-1β responses. Therefore, an enhanced immune-to-brain signal in females may contribute to neuroinflammatory priming. Interestingly, in other models of stress-induced priming, peripheral immune signaling may be critical for neuroinflammatory potentiation (McKim et al., 2017), although see (Lehmann et al., 2016). Alternatively, it is possible that sex differences in serum IL-1β can be attributed to differential kinetics of LPS and CORT responses in males and females since only one time point was assessed.
Overall, these results suggest that the mechanisms mediating stress induced neuroinflammatory potentiation is likely distinct between the sexes. Sex differences in the mechanisms mediating neuroinflammatory changes have been characterized in other contexts. For example, the sexually dimorphic effects of morphine were recently attributed to basal sex differences in microglia reactivity (Doyle et al., 2017). Moreover, chronic pain hypersensitivity is mediated by completely different mechanisms in male and female mice; microglia enable mechanical pain hypersensitivity in males, whereas adaptive immune cells induce pain sensitivity in female mice (Sorge et al., 2015).
Stage of estrous can affect both the stress response and neuroimmune activation (Bekhbat and Neigh, 2017). For example, stress-induced IL-1β responses vary across the estrous cycle (Arakawa et al., 2014). However, stage of estrous does not affect microglia gene expression profiles (Hanamsagar et al., 2017) or behavioral responses to stress in this model (Baratta et al, unpublished observation). Because of the large-scale nature of these experiments, here we focused on sex differences (not estrous cycle-dependent differences) in stress-induced neuroinflammatory priming. One additional variable not addressed in this experiment is whether the stressor (inescapable tail shock) was differentially perceived between the sexes. Indeed, in humans, there are sex difference in both stress perception and coping strategies in response to a variety of stressors (for example see (Brougham et al., 2009)). The perception of stress could possibly account for some of the sex differences in stress response; however, stress perception is difficult to evaluate in rats.
Taken together, our data suggest that stress exaggerates neuroinflammatory responses in male and female rats by distinct mechanisms. Although here we used a single method of stress (tail shock), other stressors including social defeat (Wohleb et al., 2012) and chronic variable stress (de Pablos et al., 2014) also alter neuroinflammatory responses suggesting these results may generalize to other types of stress. Importantly, psychological and physiological stressors that are neuroinflammatory may potentiate the CNS pro-inflammatory and behavioral response in any disease condition characterized by glial activation and/or neuroinflammation. Lifetime risk of experiencing a number of neuroinflammatory disorders, including multiple sclerosis (Ramagopalan et al., 2010), Alzheimer’s disease (Mielke et al., 2014), and stroke (Seshadri et al., 2006), is higher in females. This underscores the importance of studying etiologies underlying or exacerbating diseases/disorders in both sexes. More broadly, our findings suggest that distinct sex-specific cellular and physiologic mechanisms could underlie similar psychological disorders.
Supplementary Material
Research Highlights.
-
*
Male and female rats exhibit similar neuroinflammatory responses to stress
-
*
Stress-induced neuroimmune changes may occur through distinct sex-specific mechanisms
-
*
Microglia are a likely cellular source of priming in male but not female rats
-
*
Prior stress enhances peripheral immune signals in female rats only
-
*
It is critical to investigate mechanisms underlying biological phenomena in both sexes
Acknowledgments
This research was supported by NIH grant R01MH108523 to M.G.F., L.R.W., and S.F.M., NIH grant F32AG048672 to L.K.F., and a NARSAD Young Investigator Grant sponsored by the NY Women’s Committee to L.K.F. Additional support was provided by Craig H. Neilsen Foundation (SFM: 382497) and the Wings for Life Foundation (LRW/ADG; Project number 139). The authors thank University of Colorado animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
References
- Arakawa K, Arakawa H, Hueston CM, Deak T. Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology. 2014;100:162–177. doi: 10.1159/000368606. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, V, Thompson M, Kitt MM, Amat J, Hale MW, Frank MG, Crysdale NY, Stamper CE, Hennessey PA, Watkins LR, Spencer RL, Lowry CA, Maier SF. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol Aging. 2015;36:1483–1495. doi: 10.1016/j.neurobiolaging.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN. Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brougham RR, Zail CM, Mendoza CM, Miller JR. Stress, Sex Differences, and Coping Strategies Among College Students. Curr Psychol. 2009;28:85–97. [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nature neuroscience. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Munoz MF, Machado A, Venero JL. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation. 2014;11:34. doi: 10.1186/1742-2094-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denieffe S, Kelly RJ, McDonald C, Lyons A, Lynch MA. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun. 2013;34:86–97. doi: 10.1016/j.bbi.2013.07.174. [DOI] [PubMed] [Google Scholar]
- Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ. Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. J Neurosci. 2017;37:3202–3214. doi: 10.1523/JNEUROSCI.2906-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, Cano J. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging. 2011;32:85–102. doi: 10.1016/j.neurobiolaging.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology. 1995;136:5336–5342. doi: 10.1210/endo.136.12.7588279. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171–179. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Weber MD, Daut RA, Kitt MM, Frank MG, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology. 2016;66:82–90. doi: 10.1016/j.psyneuen.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol Stress. 2016;4:62–70. doi: 10.1016/j.ynstr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. Journal of neuroscience methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Compr Physiol. 2014;4:1121–1155. doi: 10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia. 2017;65:1504–1520. doi: 10.1002/glia.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kirsch I. The emperor’s new drugs: medication and placebo in the treatment of depression. Handb Exp Pharmacol. 2014;225:291–303. doi: 10.1007/978-3-662-44519-8_16. [DOI] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, Zucker I. Opinion: Sex inclusion in basic research drives discovery. Proc Natl Acad Sci U S A. 2015;112:5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol. 2009;68:159–167. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Cooper HA, Maric D, Herkenham M. Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation. 2016;13:224. doi: 10.1186/s12974-016-0672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, Sobol CG, Quan N, Sheridan JF, Godbout JP. Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LH, Warden S, Lenz KM. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–739. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. Sex, glia, and development: interactions in health and disease. Horm Behav. 2012;62:243–253. doi: 10.1016/j.yhbeh.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Sibille E. Sex differences in mood disorders: perspectives from humans and rodent models. Biol Sex Differ. 2014;5:17. doi: 10.1186/s13293-014-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Kawahara K, Kajizono M, Sawada M, Nakayama H. IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia. J Immunol. 2008;181:6503–6513. doi: 10.4049/jimmunol.181.9.6503. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature neuroscience. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum MM, Kiyota T, Ingraham KL, Ikezu S, Ikezu T. The anti-inflammatory glycoprotein, CD200, restores neurogenesis and enhances amyloid phagocytosis in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2015;36:2995–3007. doi: 10.1016/j.neurobiolaging.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci. 2015;35:316–324. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


