Abstract
Experiences of psychosocial neglect affect the developing brain and may place individuals at increased risk for anxiety. The majority of research in this area has focused on children who have experienced severe psychosocial deprivation; it is not clear whether typical variation in neglect experienced in community samples would have the same neurobiological consequences as those documented in extreme samples. The present study examined the associations among self-reported childhood neglect, amygdala volume, and anxiety symptoms in a community sample of 138 adolescents ages 9 to 15 years (43% male). Linear mixed modeling yielded a three-way interaction of neglect, sex, and brain hemisphere, reflecting a significant positive association between neglect and right amygdala volume in boys. Additional analyses indicated that right amygdala volume significantly mediated the association between neglect and anxiety symptoms in boys. These findings are consistent with previous reports of larger amygdala volumes in previously institutionalized children, and with documented associations between caregiving deprivation and anxiety symptoms. The results suggest that the effects of childhood neglect on limbic structures are sex-specific and lateralized, and provide support for a neural mechanism relating childhood neglect to later difficulties in emotional functioning.
Keywords: neglect, amygdala, anxiety, sex, laterality
Introduction
Child neglect is common; indeed, in the United States neglect accounts for more than 75% of the over 700,000 children with validated child welfare cases each year (U.S. Department of Health & Human Services, Administration for Children and Families, Adminstration on Children, Youth, & Children's Bureau, 2014). Worldwide, it is likely that experiences of neglect are proportionally even more prevalent, with war, famine, natural disasters, and epidemics leaving many children without caregivers. Indeed, a United Nations report indicated that 8 million children worldwide are living in institutions (United Nations, 2006), under conditions known to be associated with severe psychosocial neglect (Zeanah, Smyke, & Settles, 2006). Not surprisingly, a large body of work has documented the association between experiences of neglect and adverse cognitive and socioemotional outcomes (Calem, Bromis, McGuire, Morgan, & Kempton, 2017; De Bellis, 2005; Glaser, 2000; Gould et al., 2012; Humphreys, Fox, Nelson, & Zeanah, 2017). Given the consistency of these findings, there is now growing interest in identifying the mechanisms by which neglect leads to poorer functioning.
In this context, researchers have documented that the lack of a sensitive and reliable caregiver early in life has lasting neuroanatomical consequences (Humphreys, King, & Gotlib, 2018; Humphreys & Zeanah, 2015; McLaughlin, Sheridan, & Nelson, 2017; Sheridan & McLaughlin, 2014; Tottenham & Sheridan, 2009). Experiences of child neglect have been found to be associated with anomalous limbic system structure and connectivity, particularly involving the amygdala, which coordinates behavioral and physiological responses to threat (Ledoux, 2003). Many neural pathways connect sensory brain regions to the amygdala, enabling its central role in fear learning (Davis, 2006); indeed, increased amygdala reactivity has been linked consistently with anxiety disorders (Shin & Liberzon, 2010). Despite evidence from non-human work that maternal deprivation is associated with alterations in the amygdala (Kikusui & Mori, 2009; Ladd, Thrivikraman, Huot, & Plotsky, 2005; Ono et al., 2008; Teicher et al., 2004), data from human research are mixed. For example, two studies found that previously institutionalized children showed increased amygdala volumes relative to never-institutionalized controls (Mehta et al., 2009; Tottenham et al., 2010) and, further, that time spent in institutional care was linearly and positively associated with amygdala volume (Tottenham et al., 2010). In contrast, two other studies found that children who spent time in institutions had smaller left amygdala volumes than did children who had not experienced neglect (Hanson et al., 2015; Hodel et al., 2015); Hodel et al. (2015) found only a trend-level effect for right amygdala volume. Further, in the Bucharest Early Intervention Project, children with and without a history of institutional care did not differ from each other in amygdala volume (Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012).
Studies of adults using retrospective dimensional self-report measures to examine the effects of neglect occurring outside the context of institutional care on neural outcomes have also yielded inconclusive findings (Calem et al., 2017; Hanson et al., 2015; Pechtel, Lyons-Ruth, Anderson, & Teicher, 2014). Two studies found no association between childhood abuse and neglect, considered together, and amygdala volume in adults (Calem et al., 2017; Dannlowski et al., 2012). In two studies that assessed neglect separately from abuse, investigators either found no association with amygdala volume (Pechtel et al., 2014), or found that early neglect was associated with smaller amygdala volume (Hanson et al., 2015).
These inconsistencies may be due to possible sex differences or hemispheric interactions that were not examined in these studies. While some studies did explicitly examine sex differences (e.g., Mehta et al., 2009; Tottenham et al., 2010), others researchers controlled for sex without describing potential differences between boys and girls (Calem et al., 2017; Dannlowski et al., 2012; Hanson et al., 2015; Hodel et al., 2015; Lyons-Ruth, Pechtel, Yoon, Anderson, & Teicher, 2016; Sheridan et al., 2012). Importantly, it is possible that boys are less resilient to experiences of abuse and neglect than are girls (Humphreys, Miron, et al., 2017; Rutter, 1987). For example, in a large sample of adults who as children had experienced abuse and neglect, maltreated males were less likely than were maltreated females to meet threshold criteria for resilience measured across eight domains of functioning, an effect that remained significant even when investigators controlled for domains that may disproportionately affect males (e.g., criminality) (McGloin & Widom, 2001). Further, it is noteworthy that non-human animal studies have found sex differences in amygdala development following maternal separation: male mice, but not female mice, showed premature amygdala myelination (Kikusui & Mori, 2009), which may contribute to increases in amygdala volume in males (see Uematsu et al., 2012). These results suggest that in non-human animals, the amygdala may be more sensitive to early experiences of deprivation in males than in females. Given these findings, it is possible that sex moderates the observed neuroanatomical consequences of neglect, such that the association is stronger in boys than in girls.
Findings are similarly equivocal with respect to the association between neglect and laterality of its effect on amygdala volume. While several investigators did not report examining hemisphere differences (e.g., Sheridan et al., 2012), studies in which hemisphere-specific effects of neglect on amygdala volume were found have yielded inconsistent findings. For example, whereas in one study early deprivation was associated with larger right than left amygdala volumes (Mehta et al., 2009), in two other studies early neglect was associated with reductions in left but not in right amygdala volume (Hanson et al., 2015; Hodel et al., 2015), and a third study reported no significant association between neglect and either right or left amygdala volume (Pechtel et al., 2014). Inconsistencies in the findings of these studies may be due to measurement differences in the assessment and classification of neglect. For example, Pechtel, Lyons-Ruth, Anderson, and Teicher (2014) used the MACE Scale to assess childhood maltreatment and found a significant positive association between severity of maltreatment and right amygdala volume but no significant association between the neglect subscale of the MACE Scale and amygdala volume. It is possible that this approach to measuring neglect may have limited range, given that only physical and emotion neglect are assessed. Hanson et al., Hodel et al. and Mehta et al. all assessed neglect in a binary manner (i.e., previously institutionalized compared to never institutionalized) rather than using a continuous measure of neglect. Given that children likely experience different forms and degrees of neglectful behavior within the same institutional environments (Zeanah et al., 2006), using a dimensional measure of neglect may yield more consistent results with respect to a possible dose-response relation between childhood neglect and amygdala volume.
The present study was designed to examine the association between neglect and amygdala volume and to address gaps in the literature concerning participant sex and amygdala laterality. Rather than using an extreme-groups approach (i.e., comparing those with and without a history of institutional care), we used a dimensional measure of neglect (i.e., self-reported neglect) that includes a range of neglect experiences (i.e., physical, emotional, and supervisory) in a community sample of adolescents. Although extant findings concerning the relation between neglect and amygdala volume are mixed, most studies that did report a directional effect found a positive association between these two variables (Mehta et al., 2009; Tottenham et al., 2010). Therefore, we hypothesized that higher levels of neglect would be associated with larger amygdala volume in our sample. Given possible sex- and hemisphere-related differences in amygdala volume following neglect, we examined both sex and hemisphere as potential moderators of the association between neglect and amygdala volume.
Based on findings that boys are less resilient to psychosocial deprivation than are girls (Humphreys, Miron, et al., 2017; McGloin & Widom, 2001; Rutter, 1987), and that maternal separation is associated with growth-related changes in male, but not in female, amygdalae (Kikusui & Mori, 2009; Ono et al., 2008), we hypothesized that effect of neglect on amygdala volume would be larger in boys than in girls. Based on the results of previous studies that examined hemispheric interactions (Mehta et al., 2009; Pechtel et al., 2014) and on recent evidence that stress has lateralized effects on the brain that often implicate the right side (Ocklenburg, Korte, Peterburs, Wolf, & Güntürkün, 2016), we hypothesized that neglect would be associated more strongly with right than with left amygdala volume, and again, to a greater extent in boys than in girls. Finally, given the link between neglect and anxiety (Humphreys, Gabard-Durnam, et al., 2017; Zeanah et al., 2009) and given that amygdala plays an important role in anxiety in individuals with (Gee et al., 2013; Tottenham et al., 2010, 2011) and without (Barrós-Loscertales et al., 2006; De Bellis et al., 2000) histories of neglect, we examined whether amygdala volume would mediate the association between self-reported neglect and symptoms of anxiety.
Methods
Participants
Participants were 138 children (60 boys, 78 girls) ages 9.11 to 15.58 years (M age=12.14 years, SD=1.55) who were recruited from the San Francisco Bay Area community with the goal of obtaining a sample of children who had experienced a range of severity of early life stress. Pubertal stage was assessed via child report; Tanner Stage ranged from 1-5 (M=2.53, SD=1.18), with the goal of recruiting boys and girls that, as groups, did not differ significantly in pubertal stage (see Table 1). Children self-reported race/ethnicity: 38% White/Caucasian, 14% Asian, 9% African American, 8% Hispanic, 2% Native American, 1% Pacific Islander, 26% Other (primarily children who identify as multiracial), and 1% not provided. Parents reported on annual household income; 7% reported <$25,000, 12% reported $25,001-$75,000, 39% reported $75,001-$150,000, 34% reported >$150,000, and 8% not provided. Given the high cost of living in the area in which these participants live, we also calculated the income-to-needs ratio for participants who provided income information (n=127, M=1.33, SD=0.54), and found that 27% of the sample would be categorized as “low income” (i.e., income-to-needs ratio<1.0). These and additional details about the demographic characteristics of the sample are presented in Table 1. The study was approved by the Stanford University Institutional Review Board; participants and their parents gave assent and informed consent, respectively. Participants were screened for initial inclusion/exclusion criteria through a telephone interview; potentially eligible individuals were then invited to the laboratory for in-person interviews and assessments. The measure of neglect was added midway through data collection for Wave 1 of a longitudinal study. Because we included neglect data in this study only from the first time this construct was assessed for each participant, for 80 participants their neglect data were obtained at Wave 1 and for 58 participants their neglect data were obtained at Wave 2, which occurred approximately two years following the baseline assessment. Thus, all participants provided one measure of neglect and amygdala volume data that were obtained at the same time. At baseline, inclusion criteria were that children be between the ages of 9 and 13 years and be proficient in English. Exclusion criteria were factors that would preclude MRI scan (e.g., metal implants, braces), a history of major neurological or medical illness, severe learning disabilities that would make it difficult for participants to comprehend the study procedures. Females who reported having started menses were excluded, and boys were matched to girls on pubertal stage (Marshall & Tanner, 1968). Participants who provided usable structural MRI scans in which either or both amygdala volumes were obtained were included in the present study.
Table 1. Demographic and Clinical Variables by Participant Sex (N=138).
| Girls | Boys | t or χ2 | |
|---|---|---|---|
| Age | 11.93 (1.70) | 12.41 (1.30) | -1.88 |
| Pubertal Stage | 2.64 (1.17) | 2.38 (1.16) | 1.33 |
| Race/Ethnicity | 7.26 | ||
| White/Caucasian | 37% | 40% | |
| African American | 13% | 3% | |
| Hispanic | 6% | 10% | |
| Asian | 10% | 18% | |
| Native American | 1% | 3% | |
| Pacific Islander | 1% | 2% | |
| Other | 30% | 22% | |
| No response given | 1% | 2% | |
| Mother Education | 2.84 | ||
| <High School | 1% | 0% | |
| High School | 1% | 0% | |
| Some College | 24% | 29% | |
| College Degree | 48% | 40% | |
| Graduate Degree | 25% | 31% | |
| Father Education | 6.24 | ||
| <High School | 2% | 0% | |
| High School | 4% | 2% | |
| Some College | 18% | 30% | |
| College Degree | 49% | 28% | |
| Graduate Degree | 27% | 40% | |
| Family Income | 6.68 | ||
| Less than $25,000 | 10% | 3% | |
| $25,001-$75,000 | 12% | 12% | |
| $75,001-$150,000 | 33% | 47% | |
| More than $150,000 | 33% | 35% | |
| No response given | 12% | 3% | |
| Income-to-needs ratio | 1.28 (0.59) | 1.38 (0.48) | -1.03 |
| Neglect Score | 2.9 (3.2) | 2.0 (2.7) | 1.65 |
| Anxiety Symptoms (MASC Score) | 21.0 (12.3) | 19.1 (11.2) | 0.91 |
| Left Amygdala Volume | 1451.2 (196.2) | 1606.6 (225.9) | -4.30*** |
| Right Amygdala Volume | 1552.6 (225.4) | 1653.5 (256.6) | -2.42* |
Note. M (SD) or %.
p<.05.
p<.001.
Procedure
Participants visited the laboratory and completed self-report measures assessing experiences of neglect, as well as other measures and interviews not included here. Participants also returned to the MRI center within two weeks of their laboratory visit, where structural scans were obtained. Participants were compensated for their participation.
Measures
Multidimensional Neglectful Behavior Scale (MNBS; Straus, Kinard, & Williams, 1995)
To assess neglect, participants completed the MNBS (Adult Recall Short Form), an 8-item self-report measure of neglectful behavior designed to be completed by adolescents and adults regarding neglectful behavior by their caretakers during childhood. The MNBS yields data on the four dimensions of neglect: cognitive, emotional, physical, and supervisory; responses are scored on a 4-point scale, from 0 (strongly disagree) to 3 (strongly agree) (see Appendix for subscale descriptive statistics). The total score can range from 0 to 24, with higher scores indicating greater neglect. In our sample, the MNBS demonstrated good internal consistency (α=.89). Scores greater than 2 SD from the mean were winsorized and replaced with the next closest value.
Multidimensional Anxiety Scale for Children (MASC; March, Parker, Sullivan, Stallings, & Connings, 1997)
To assess anxiety, participants completed the MASC, 2nd edition, a self-report measure that assesses a broad range of symptoms of anxiety in children ages 8 to 19 years (Fraccaro, Stelnicki, & Nordstokke, 2013; March et al., 1997). Items are scored on a 4-point scale, from 0 (never true about me) to 3 (often true about me), with higher scores indicating greater anxiety symptoms. The MASC has high internal consistency (α=92; Fraccaro, Stelnicki, & Nordstokke, 2013) and has strong psychometric properties (Baldwin & Dadds, 2007; Wood, Piacentini, Bergman, McCracken, & Barrios, 2002). Because the MASC measures symptoms and yields scale scores that correspond to DSM-IV criteria and categorization of anxiety disorders in children, it has been found to have widespread utility in clinical and research contexts (Wood et al., 2002). The MASC yields data on four scales of anxiety; in order to reduce participant burden in this study, only the Social Anxiety and Physical Symptoms scales were administered. The scores from these two scales were summed to obtain a total anxiety score.
MRI data acquisition
MRI scans were acquired at the Center for Cognitive and Neurobiological Imaging at Stanford University using a 3T Discovery MR750 (GE Medical Systems, Milwaukee, WI, USA) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA). Whole-brain T1-weighted images (T1w) were collected using the following spoiled gradient echo (SPGR) pulse sequence: 186 sagittal slices; TR (repetition time)/TE (echo time)/TI (inversion time)=6.24/2.34/450ms; flip angle=12°; voxel size = 0.9 mm×0.9 mm×0.9 mm; scan duration=315s.
Amygdala Segmentation
To obtain volumes from T1w images, we used the FreeSurfer software suite (v5.3; available at: http://surfer.nmr.mgh.harvard.edu/) for automated segmentation of subcortical volumes (Fischl et al., 2002). This segmentation approach is widely used, is robust against anatomic variability, has comparable accuracy to manual labeling techniques (Fischl et al., 2002; Fischl & Dale, 2000) and has acceptable scan-rescan reliability (Jovicich et al., 2009). Using the FreeView image viewer, amygdala segmentations were visually inspected for processing and segmentation errors. Only individuals with usable left (n=137) or right (n=134) amygdala volumes were included.
Data Analysis
To examine the association of self-reported neglect and amygdala volume, we used linear mixed modeling (LMM) given that individuals provided both left and right volume measurements and LMM accounts for the non-independence of nested measures within individuals. We used restricted maximum likelihood estimation in Mixed Models in SPSS (version 23, IBM Corporation), specifying fixed and random intercepts. Neglect, sex, and hemisphere were included as main effects and in two- and three-way interactions. Age, pubertal stage, income-to-needs ratio, and race (dummy-coded as White/Caucasian vs. else) and intracranial volume (ICV) were included as covariates. Degrees of freedom were calculated using the Satterthwaite method (Satterthwaite, 1946), which can yield fractional values.
To test whether amygdala volume mediated the association between self-reported neglect and anxiety symptoms, we conducted a single-step mediation analysis using the PROCESS macro (Hayes, 2013) in SPSS. To assess the significance of the indirect effect, we implemented a non-parametric bootstrap procedure using sampling with replacement (n=1000) and 95% bias correction, and calculated accelerated confidence intervals (CIs) for coefficients. If the CI does not include zero, the indirect effect is considered statistically significant. Age, pubertal stage, and ICV were included as covariates.
Results
Demographic and variables of interest assessed in this study are presented in Table 1 as a function of sex. Boys and girls did not differ on demographic variables or neglect. As expected, boys had greater left and right amygdala volumes than did girls.
Neglect and Amygdala Volume
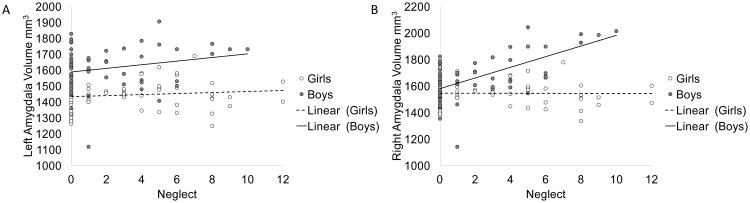
We conducted a single LMM to test the association between neglect and amygdala volume, with sex and hemisphere included as potential moderators of interest, and covarying for age, pubertal stage, income-to-needs ratio, race, and ICV. As predicted, this analysis yielded a significant three-way interaction of neglect, sex, and hemisphere (see Table 2). As shown in Figure 1, the three-way interaction was driven by differences in right and left amygdala volume in boys as a function of neglect, such that higher levels of self-reported neglect were associated with significantly larger right (compared to left) amygdala volume in boys. Post-hoc analyses using linear regression within the sample of boys indicated that neglect explained 7 percent of variance in right amygdala volume (p=009) over and above the effects of age, pubertal stage, income-to-needs ratio, race, and ICV.
Table 2. Linear Mixed Model Results.
| df | F | p | |
|---|---|---|---|
| Intercept | 1, 119.34 | 2.37 | .126 |
| Race (white = 1) | 1, 114.30 | 4.75 | .031 |
| Income-to-needs ratio | 1, 115.21 | .22 | .640 |
| Age | 1, 114.47 | 3.30 | .072 |
| Pubertal stage | 1, 114.47 | 1.46 | .229 |
| Intracranial volume | 1, 124.87 | 32.45 | <.001 |
| Neglect | 1, 117.25 | 1.40 | .239 |
| Sex (male=1) | 1, 116.05 | 0.59 | .446 |
| Hemisphere | 1, 116.67 | 7.35 | .008 |
| Neglect × sex | 1, 116.06 | 1.11 | .294 |
| Neglect × hemisphere | 1, 118.46 | 5.53 | .020 |
| Sex × hemisphere | 1, 116.66 | 9.04 | .003 |
| Neglect × sex × hemisphere | 1, 118.42 | 8.91 | .003 |
Note. Bold indicates statistical significance.
Figure 1.
Amygdala volume as a function of neglect and sex for left (A) and right (B) hemispheres.
Test of Mediation
We tested whether larger right amygdala volume mediated the association between neglect and anxiety in males, given that a hierarchical linear regression analysis supported an association between right amygdala volume and anxiety symptoms after controlling for age, pubertal stage, income-to-needs ratio, race, and ICV (β=.39, t(47)=2.09, p=.042, ΔR2=.08). Using a single-step mediation with age, pubertal stage, and ICV as covariates, there was no significant direct effect of the self-reported neglect on anxiety symptoms (B=0.39, SE=0.65 [95%CI: -0.92, 1.71], p=.548); however, right amygdala volume significantly mediated the association between neglect and anxiety symptoms (indirect effect=0.46, SE=0.31 [95% CI: 001, 1.27]), indicating that the variance between self-reported neglect and anxiety symptoms in boys is partially explained by increased right amygdala volume. When income and race were included as covariates, the sample size was reduced (n=54) and the estimate of the indirect effect was similar (indirect effect=0.43, SE=0.32), but the confidence interval no longer indicated that the effect reached statistical significance (95% CI: -.025, 1.16).
Discussion
In a community sample of 138 adolescents, we found that higher levels of self-reported neglect were significantly associated with larger volume of the right amygdala in boys. These results are consistent with previous reports that early experiences of adversity are related to greater amygdala volume (Mehta et al., 2009; Pechtel et al., 2014; Tottenham et al., 2010). Importantly, the present findings are also consistent with reports of larger amygdala volumes in children who experienced neglect in the form of institutional rearing, a severe form of psychosocial deprivation, suggesting that the association between neglect and increased amygdala volume is evident even in less severe forms of neglect experienced by children in the community (i.e., who are not referred for maltreatment). Thus, our findings support the utility of considering neglect along a continuum (Humphreys, King, & Gotlib, 2018) in examining the association between experiences of deprivation and neurobiological outcomes. Further, we provide support that, in boys, right amygdala volume mediates the association between self-reported neglect and anxiety symptoms.
Notably, the current findings were sex-specific: only in boys was neglect associated with amygdala volume. Previous reports have found sex-specific neurobiological correlates of adverse childhood experiences (De Bellis & Keshavan, 2003; Teicher et al., 2003, 2004). Further, boys have been found to exhibit greater amygdala functional connectivity and reactivity to emotional material than do girls (Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004; Kilpatrick, Zald, Pardo, & Cahill, 2005; Schneider et al., 2011). Our results indicate that early experiences of deprivation may affect neural structure differentially in males and females.
Sex differences in the behavioral consequences of early adversity have been well documented, arguably starting 40 years ago with findings of greater vulnerability in boys than in girls in response to family discord (Rutter, 1987). Boys faced with an emotional challenge may elicit more negative responses from parents, due to increased oppositional or disruptive behavior (Rutter, 1987). Gender differences in coping strategies may also contribute to sexually dimorphic responses to psychosocial stress. In general, males are less likely than are females to engage in adaptive coping strategies (see Tamres, Janicki, & Helgeson, 2002 for review). In particular, boys are less likely to seek social support or use problem-solving strategies in response to stressful situations, and are more likely to use avoidant coping mechanisms (Eschenbeck, Kohlmann, & Lohaus, 2007). Thus, it is possible that sex and gender-related differences in family dynamics and coping styles have downstream effects on the amygdala, given that boys showed a more pronounced effect of neglect on amygdala volume than was observed in girls. While previous research has tended to focus on females' vulnerability to early adversity based on immunological or hormonal pathways (e.g. Cordero, Ansermet, & Sandi, 2013; Machado et al., 2013; Palanza, 2001; Ruttle, Shirtcliff, Armstrong, Klein, & Essex, 2015), our findings suggest that males may be more vulnerable to the negative effects of neglect than are females, and are consistent with other work examining sex differences in outcomes in children with histories of neglect (Humphreys, Miron, et al., 2017; McGloin & Widom, 2001).
Further, because the limbic system undergoes considerable development over adolescence, it is possible that sex-specific responses to early experiences of neglect are mediated by sex hormones such as estrogens and androgens (Rose et al., 2004; Tottenham & Sheridan, 2009; Uematsu et al., 2012). Indeed, amygdala volume has been found to be positively associated with higher levels of circulating testosterone in adolescent boys (M age = 11.70 years) (Neufang et al., 2009); moreover, the amygdala has a high proportion of androgen receptors relative to other brain regions (Martini & Melcangi, 1991). Given the host of changes in neural structure and circuitry that occur during adolescence and the critical role of sex hormones in myelination (Arain et al., 2013), it is possible that early experiences of deprivation convey sexually dimorphic effects through the neuroendocrine system. Further research is needed to explore the mechanisms by which testosterone may potentiate sex differences in amygdala volume in typical and atypical development.
The present results indicate that the effects of neglect on amygdala volume in boys are moderated by hemisphere: higher levels of neglect were associated with larger right, but not left, amygdala volume in boys. This finding is consistent with earlier reports of sex-specific hemispheric lateralization of amygdala connectivity and reactivity (Cahill et al., 2004; Kilpatrick et al., 2005); more specifically, compared to girls, boys have been found to exhibit greater functional connectivity of the right amygdala and greater activation of the right amygdala in response to emotionally arousing stimuli. Laterality differences in amygdala volume have been found to be associated with a wide range of stressors (e.g., Barry et al., 2017; Hill et al., 2001; Lyons-Ruth, Pechtel, Yoon, Anderson, & Teicher, 2016; Woon & Hedges, 2008, 2009). In particular, larger right amygdala volume has been associated with trauma exposure and PTSD in adults (F. L. Woon & Hedges, 2009), suggesting that trauma contributes to asymmetrically lateralized amygdala volumes. Our finding that neglect only explained variation in right amygdala volume are consistent with these findings, but not with other studies that found different patterns of lateralization in other groups at heightened risk for psychological difficulties. For example, Lyons-Ruth et al. (2016) found that disorganized attachment was associated with increased left amygdala volume in adults. The functional significance of these asymmetries in response to stress, however, is far from clear, and is an important question to be addressed in future research.
Our results underscore the importance of examining possible neurobiological consequences of neglect. Previous research has typically assessed structural differences in relation to maltreatment, irrespective of type of maltreatment. For example, in a meta-analysis of brain structure in adults with and without early experiences of adversity, Calem and colleagues (2017) found no association between level of adversity and amygdala volume. Importantly, this report did not consider the type of adversity, but instead examined global effects of child maltreatment on structural outcomes. Given that early neglect appears to be sufficiently potent to confer neural alterations independent of experiences of other types of adversity, these divergent results may be explained by the fact that Calem et al. did not distinguish neglect from other forms of maltreatment. Researchers and theorists examining child maltreatment have emphasized the importance of differentiating environments that lack adequate input (e.g., neglect) from environments that are characterized by the presence of harmful input (e.g., abuse) (Humphreys & Zeanah, 2015; McLaughlin, Sheridan, & Lambert, 2014; Sheridan & McLaughlin, 2014; Zeanah & Sonuga-Barke, 2016). For instance, although abuse and neglect often co-occur (Dong, Anda, & Felitti, 2004), amygdala volume may be differentially affected by different types of stressors. Indeed, caregiving deprivation appears to affect the amygdala in particular. Lupien et al. (2011) found that children who were exposed to maternal depressive symptomatology, which is associated with reduced caregiving sensitivity, had larger bilateral amygdala volumes compared with children who had not been exposed to maternal depressive symptoms. Further, greater maternal symptom severity was associated with larger amygdala volume in offspring. No equivalent effect was found for the hippocampus, indicating that the amygdala may be uniquely sensitive to inadequate care.
Increased amygdala volume may have functional significance; we found preliminary evidence that amygdala volume mediates the association between self-reported neglect and anxiety symptoms in boys, although the finding was attenuated in a smaller sample size that included additional covariates. This finding is consistent with an earlier report that documented associations between previous institutionalization and amygdala volumes, and between amygdala volumes and internalizing behavior, but did not explore a possible mediation because of limited sample size (Tottenham et al., 2010). Previous research has demonstrated a positive association between amygdala volumes and anxiety in both pediatric and adult samples (Barrós-Loscertales et al., 2006; De Bellis et al., 2000), and a growing literature suggests that the amygdala mediates the association between adversity and difficulties in self-regulation. Children rely on caregivers as external sources of emotion regulation until they develop their own internal mechanisms of regulation (Atzil & Gendron, 2017); further, the amygdala is instrumental in fear learning and threat processing (Duvarci & Pare, 2014; Tovote, Fadok, & Lüthi, 2015). Taken together, previous evidence suggests that inadequate input from caregivers may result in altered development of amygdala circuitry (McLaughlin et al., 2014; Tottenham & Sheridan, 2009), amplifying neural activation in response to threatening and fearful stimuli (Maheu et al., 2010) and ultimately increasing the likelihood of psychopathology (Humphreys & Zeanah, 2015).
We should note several limitations of this study. First, the children in our sample reported mild to moderate levels of neglect, indicating that our sample represents a restricted range in terms of neglect experience. The majority of our sample was relatively high income, which likely limited the range of neglect we observed. Given the association between income and early adversity, more research is needed to determine whether income level and neglect experience contribute independently to neuroanatomical changes. Importantly, however, finding an association between neglect severity and amygdala volume even in this restricted sample indicates that even more minor forms of neglect may have measureable neuroanatomical consequences. Second, while our community sample was reasonably diverse with respect to race and ethnicity (see Table 1), the relatively small sample size within each identified racial group limits our ability to assess differences in self-reported neglect or amygdala volume in terms of race. Race is an important factor that may be associated with the likelihood of experiencing neglect given inequitable social conditions for minorities; the most recent report on child maltreatment cases in the U.S. finds that American Indian and African American children are at the greatest risk for maltreatment (U.S. Department of Health & Human Services, Administration for Children and Families, Adminstration on Children, & Children's Bureau, 2018). Third, we relied on adolescent self-report to assess neglect and anxiety. Self-report has some advantages over parent report for this construct, given concerns regarding bias due to potential social desirability. Nevertheless, adolescent report is not without potential for bias given difficulty in accurately reporting on experiences in very early life (i.e., infantile amnesia) and the potential for psychopathology to result in inflated perceptions of neglect (Chae, Goodman, Eisen, & Qin, 2011). Fourth, the MNBS does not provide information regarding the timing of exposure. This is an important area for future research given previous reports documenting the importance of timing and chronicity of early adversity on brain development (Pechtel, Lyons-Ruth, Anderson, & Teicher, 2014; Tottenham & Sheridan, 2009). Finally, while we found differences in amygdala volume associated with variations in neglect, the correlational nature of these associations makes it difficult to draw causal conclusions. Given evidence from non-human animal studies and other studies in humans (e.g., children with institutional care histories), we believe that it is likely that neglect affects brain development. Nevertheless, future research is needed to replicate and extend these findings in order to increase confidence in our obtained pattern of results.
In conclusion, we document a positive association between self-reported neglect in adolescent boys reared outside of institutional settings and right amygdala volume and, in addition, provide evidence that right amygdala volume mediates the association between neglect and symptoms of anxiety in boys. Early caregiver deprivation has been found to increase children's risk for psychological difficulties later in life; we suggest that the amygdala plays a key role in mediating this association given its importance in emotional functioning, especially in threat responding and fear conditioning. Because neglect occurs on a continuum even among typical community families, and increased exposure to neglect confers risk for negative outcomes, policy efforts to reduce exposure to neglect should be a priority.
Supplementary Material
Acknowledgments
Funding for this study was provided by the National Institutes of Health (R01 MH101495 to IHG and F32 MH107129 to KLH); the Brain and Behavior Research Foundation (Young Investigator Award 23819 to KLH); the National Science Foundation Graduate Research Fellowship (LSK); and the Klingenstein Third Generation Foundation (KLH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, et al. Sharma S. Maturation of the adolescent brain. Neuropsychiatric Disease and Treatment. 2013;9:449–61. doi: 10.2147/NDT.S39776. https://doi.org/10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Gendron M. Bio-behavioral synchrony promotes the development of conceptualized emotions. Current Opinion in Psychology. 2017;17:162–169. doi: 10.1016/j.copsyc.2017.07.009. https://doi.org/10.1016/j.copsyc.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JS, Dadds MR. Reliability and validity of parent and child versions of the multidimensional anxiety scale for children in community samples. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(2):252–260. doi: 10.1097/01.chi.0000246065.93200.a1. https://doi.org/10.1097/01.chi.0000246065.93200.a1. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Meseguer V, Sanjuán A, Belloch V, Parcet MA, Torrubia R, Ávila C. Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. NeuroImage. 2006;33(3):1011–1015. doi: 10.1016/j.neuroimage.2006.07.025. https://doi.org/10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Barry TJ, Murray L, Fearon P, Moutsiana C, Johnstone T, Halligan SL. Amygdala volume and hypothalamic-pituitary-adrenal axis reactivity to social stress. Psychoneuroendocrinology. 2017;85:96–99. doi: 10.1016/j.psyneuen.2017.07.487. https://doi.org/10.1016/j.psyneuen.2017.07.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an fMRI investigation. Learning & Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. https://doi.org/10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calem M, Bromis K, McGuire P, Morgan C, Kempton MJ. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage Clinical. 2017;14:471–479. doi: 10.1016/j.nicl.2017.02.016. https://doi.org/10.1016/j.nicl.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae Y, Goodman GS, Eisen ML, Qin J. Event memory and suggestibility in abused and neglected children: Trauma-related psychopathology and cognitive functioning. Journal of Experimental Child Psychology. 2011;110(4):520–538. doi: 10.1016/j.jecp.2011.05.006. https://doi.org/10.1016/j.jecp.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Ansermet F, Sandi C. Long-term programming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology. 2013;38(11):2758–2769. doi: 10.1016/j.psyneuen.2013.07.005. https://doi.org/10.1016/j.psyneuen.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Kugel H. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. https://doi.org/10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. https://doi.org/10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. The psychobiology of neglect. Child Maltreatment. 2005;10(2):150–172. doi: 10.1177/1077559505275116. https://doi.org/10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, et al. Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry. 2000;48(1):51–57. doi: 10.1016/s0006-3223(00)00835-0. https://doi.org/10.1016/S0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews. 2003;27:103–117. doi: 10.1016/s0149-7634(03)00013-7. https://doi.org/10.1016/S0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Dong M, Anda R, Felitti V. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect. 2004;28:771–784. doi: 10.1016/j.chiabu.2004.01.008. https://doi.org/10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82(5):966–980. doi: 10.1016/j.neuron.2014.04.042. https://doi.org/10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbeck H, Kohlmann CW, Lohaus A. Gender differences in coping strategies in children and adolescents. Journal of Individual Differences. 2007;28(1):18–26. https://doi.org/10.1027/1614-0001.28.1.18. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. https://doi.org/10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. https://doi.org/10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fraccaro RL, Stelnicki AM, Nordstokke DW. Test review: Multidimensional Anxiety Scale for Children by J. S. March. Canadian Journal of School Psychology. 2013;30(1) https://doi.org/10.1177/0829573514542924. [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(39):15638–43. doi: 10.1073/pnas.1307893110. https://doi.org/10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain-A review. Journal of Child Psychology and Psychiatry. 2000;41(1):97–116. https://doi.org/10.1111/1469-7610.00551. [PubMed] [Google Scholar]
- Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. Journal of Psychiatric Research. 2012;46:500–506. doi: 10.1016/j.jpsychires.2012.01.005. https://doi.org/10.1016/j.jpsychires.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. Davidson RJ. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. https://doi.org/10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. New York: Guilford Press; 2013. Introduction to mediation, moderation, and conditional process analysis New York, NY: Guilford. https://doi.org/978-1-60918-230-4. [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. https://doi.org/10.1016/S0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, Thomas KM. Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. https://doi.org/10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Fox NA, Nelson CA, Zeanah CH. Child Maltreatment in Long-Term Residential Centers: History, Research, and Current Implications. New York: Springer; 2017. Psychopathology following severe deprivation: History, research, and implications of the Bucharest Early Intervention Project; pp. 129–148. [Google Scholar]
- Humphreys KL, Gabard-Durnam L, Goff B, Telzer EH, Flannery J, Gee DG, et al. Tottenham N. Social processes and friendship following early parental deprivation: Potential roles of anxiety and ADHD symptoms 2017 [Google Scholar]
- Humphreys KL, King LS, Gotlib IH. Neglect. In: Zeanah CH, editor. Handbook of Infant Mental Health. 4th. New York: Guilford Press; n.d. [Google Scholar]
- Humphreys KL, Miron D, McLaughlin KA, Sheridan MA, Nelson CA, Fox NA, Zeanah CH. Foster care promotes adaptive functioning in early adolescence among children who experienced severe, early deprivation. 2017 doi: 10.1111/jcpp.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Zeanah CH. Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology. 2015;40(1):154–170. doi: 10.1038/npp.2014.165. https://doi.org/10.1038/npp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46(1):177–192. doi: 10.1016/j.neuroimage.2009.02.010. https://doi.org/10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. Journal of Neuroendocrinology. 2009;21(4):427–431. doi: 10.1111/j.1365-2826.2009.01837.x. https://doi.org/10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2005;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. https://doi.org/10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30(6):520–533. doi: 10.1016/j.psyneuen.2004.12.004. https://doi.org/10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The Emotional Brain, Fear, and the Amygdala. 2003;23(October):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Séguin JR. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14324–9. doi: 10.1073/pnas.1105371108. https://doi.org/10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K, Pechtel P, Yoon SA, Anderson CM, Teicher MH. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behavioural Brain Research. 2016;308:83–93. doi: 10.1016/j.bbr.2016.03.050. https://doi.org/10.1016/j.bbr.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado TD, Dalle Molle R, Laureano DP, Portella AK, Werlang ICR, Benetti CdaS, et al. Silveira PP. Early life stress is associated with anxiety, increased stress responsivity and preference for “comfort foods” in adult female rats. Stress. 2013;16(5):549–556. doi: 10.3109/10253890.2013.816841. https://doi.org/10.3109/10253890.2013.816841. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, et al. Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective & Behavioral Neuroscience. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. https://doi.org/10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JDA, Sullivan K, Stallings P, Connings CK. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. https://doi.org/10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual Review of Medicine. 1968;19(1):283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC. Androgen metabolism in the brain. Journal of Steroid Biochemistry and Molecular Biology. 1991;39(5 PART 2):819–828. doi: 10.1016/0960-0760(91)90031-y. https://doi.org/10.1016/0960-0760(91)90031-Y. [DOI] [PubMed] [Google Scholar]
- McGloin JM, Widom CS. Resilience among abused and neglected children grown up. Development and Psychopathology. 2001;13(4) doi: 10.1017/s095457940100414x. S095457940100414X https://doi.org/10.1017/S095457940100414X. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews. 2014;47(11):578–591. doi: 10.1016/j.neubiorev.2014.10.012. https://doi.org/10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Nelson CA. Neglect as a violation of species-expectant experience: neurodevelopmental consequences. Biological Psychiatry. 2017;82(7):462–471. doi: 10.1016/j.biopsych.2017.02.1096. https://doi.org/10.1016/j.biopsych.2017.02.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, et al. Sonuga- Barke EJS. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. https://doi.org/10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. https://doi.org/10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S, Korte SM, Peterburs J, Wolf OT, Güntürkün O. Stress and laterality – The comparative perspective. 2016 doi: 10.1016/j.physbeh.2016.06.020. https://doi.org/10.1016/j.physbeh.2016.06.020. [DOI] [PubMed]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156(4):1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. https://doi.org/10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: How are females different? Neuroscience and Biobehavioral Reviews. 2001;25(3):219–233. doi: 10.1016/s0149-7634(01)00010-0. https://doi.org/10.1016/S0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: The role of maltreatment in preadolescence. NeuroImage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. https://doi.org/10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB, Merke DP, Clasen LS, Rosenthal MA, Wallace GL, Vaituzis AC, et al. Giedd JN. Effects of hormones and sex chromosomes on stress-influenced regions of the developing pediatric brain. Annals of the New York Academy of Sciences. 2004;1032(1):231–233. doi: 10.1196/annals.1314.027. https://doi.org/10.1196/annals.1314.027. [DOI] [PubMed] [Google Scholar]
- Rutter M. Psychosocial resilience and protective mechanisms. American Journal of Orthopsychiatry. 1987 doi: 10.1111/j.1939-0025.1987.tb03541.x. https://doi.org/10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology. 2015;57(6):688–704. doi: 10.1002/dev.21138. https://doi.org/10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, et al. Büchel C. Boys do it the right way: Sex-dependent amygdala lateralization during face processing in adolescents. NeuroImage. 2011;56(3):1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. https://doi.org/10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(32):12927–32. doi: 10.1073/pnas.1200041109. https://doi.org/10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends in Cognitive Sciences. 2014;18(11):580–585. doi: 10.1016/j.tics.2014.09.001. https://doi.org/10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. https://doi.org/10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA, Kinard EM, Williams LM. Durham, HN: University of New Hampshire: Family Research Laboratory; 1995. The multidimensional neglectful behavior scale, Form A: Adolescent and adult-recall version. Retrieved from https://www.researchgate.net/profile/Murray_Straus/publication/228920879_The_multidimensional_neglectful_behavior_scale_Form_A_Adolescent_and_adult-recall_version/links/0912f50ef3906d4048000000.pdf. [Google Scholar]
- Tamres LK, Janicki D, Helgeson VS. Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personality and Social Psychology Review. 2002;6(1):2–30. https://doi.org/10.1207/S15327957PSPR0601_1. [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. https://doi.org/10.1016/S0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry. 2004;56(2):80–85. doi: 10.1016/j.biopsych.2004.03.016. https://doi.org/10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. https://doi.org/10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. https://doi.org/10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:1–18. doi: 10.3389/neuro.09.068.2009. https://doi.org/10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16(6):317–331. doi: 10.1038/nrn3945. https://doi.org/10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services, Administration for Children and Families, Adminstration on Children, Youth, and F., & Children's Bureau. Child maltreatment 2014. 2014 Retrieved from https://www.acf.hhs.gov/cb/research-data-technology/statistics-research/child-maltreatment.
- U.S. Department of Health & Human Services, Administration for Children and Families, Adminstration on Children, Youth and Families, & Children's Bureau. Child maltreatment 2016. 2018 Retrieved from https://www.acf.hhs.gov/cb/research-data-technology/statistics-research/child-maltreatment.
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE. 2012;7:10. doi: 10.1371/journal.pone.0046970. https://doi.org/10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. Violence Against Children. United Nations General Assembly. 2006 Retrieved from http://eric.ed.gov/?id=ED459272.
- Wood JJ, Piacentini JC, Bergman RL, McCracken J, Barrios V. Concurrent validity of the anxiety disorders section of the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. Journal of Clinical Child and Adolescent Psychology. 2002;31(3):335–342. doi: 10.1207/S15374424JCCP3103_05. https://doi.org/10.1207/S15374424JCCP3103_05. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus. 2008;18(8):729–736. doi: 10.1002/hipo.20437. https://doi.org/10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Amygdala volume in adults with posttraumatic stress disorder: A meta-analysis. Journal of Neuropsychiatry. 2009;21(1):5–12. doi: 10.1176/jnp.2009.21.1.5. https://doi.org/10.1176/appi.neuropsych.21.1.5. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. The American Journal of Psychiatry. 2009;166(7):777–85. doi: 10.1176/appi.ajp.2009.08091438. https://doi.org/10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Settles L. Children in orphanages. In: McCartney K, Phillips D, editors. Blackwell Handbook of Early Childhood Development. Malden, MA: Blackwell Publishing; 2006. pp. 224–254. [Google Scholar]
- Zeanah CH, Sonuga-Barke EJS. Editorial: The effects of early trauma and deprivation on human development - from measuring cumulative risk to characterizing specific mechanisms. Journal of Child Psychology and Psychiatry. 2016;57(10):1099–1102. doi: 10.1111/jcpp.12642. https://doi.org/10.1111/jcpp.12642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.