Abstract
The second-most common neurodegenerative disease, Parkinson’s Disease (PD) has three hallmarks: dysfunctional dopamine transmission due, at least in part, to dopamine neuron degeneration; intracellular inclusions of α-synuclein aggregates; and neuroinflammation. The origin and interplay of these features remains a puzzle, as does the underlying mechanism of PD pathogenesis and progression. When viewed in the context of neuroimmunology, dopamine also plays a role in regulating peripheral immune cells. Intriguingly, plasma dopamine levels are altered in PD, suggesting collateral dysregulation of peripheral dopamine transmission. The dopamine transporter (DAT), the main regulator of dopaminergic tone in the CNS, is known to exist in lymphocytes and monocytes/macrophages, but little is known about peripheral DAT biology or how DAT regulates the dopaminergic tone, much less how peripheral DAT alters immune function. Our review is guided by the hypothesis that dysfunctional peripheral dopamine signaling might be linked to the dysfunctional immune responses in PD and thereby suggests a potential bidirectional communication between central and peripheral dopamine systems. This review seeks to foster new perspectives concerning PD pathogenesis and progression.
Keywords: Dopamine transporter, Parkinson’s Disease, Macrophages, Dopamine, Peripheral immunity
1. Introduction
Dopaminergic regulation of peripheral immune function has gained much attention within the field of neuroimmunology. Similarly, the role of the immune system in Parkinson’s Disease (PD) has recently attracted increasing attention. However, there is little research on these two sub-fields in the context of one another. This review seeks to summarize the existing literature on dopaminergic regulation of peripheral immunity and the role of the immune system in PD. Additionally, we review the function and regulation of the dopamine transporter (DAT), which exists both centrally and peripherally and regulates dopaminergic tone in general. We therefore explore the possibility that peripheral DAT modulates peripheral dopaminergic tone. By extension, peripheral DAT dysregulation could also induce immune dysfunction, a hallmark of PD. DAT dysfunction could also disrupt or be influenced by alterations in peripheral and/or central dopamine homeostasis, the latter of which is another hallmark of PD, suggesting both peripheral and central dopamine systems may be dysregulated in PD. Thus, we review the central hypothesis that there is a bidirectional communication between peripheral and central dopamine systems, and that this communication may play a role in some characteristics of PD pathology.
1.1. The immune system
The peripheral immune system is comprised of a vast and complex network of both circulating and resident cells, proteins, and peptides that protect the body against pathogens and regulate other physiological functions. This system is further divided into two subsystems, the innate and adaptive immune systems, which work to protect the body against pathogens and regulate a variety of homeostatic functions. Neutrophils, eosinophils, basophils, natural killer cells (NK cells), dendritic cells (DCs), monocytes and macrophages, and the complement system all fall under innate categorization. T-cells, B-cells, and immunoglobulins are categorized under the adaptive branch. All of these cell types can communicate through secretion and binding of functionally diverse cytokines and chemokines.
In the central nervous system (CNS), immune cells are represented by microglia, astrocytes, and several distinct populations of macrophages (Prinz et al., 2017) all of which belong to the innate branch. Under normal, non-pathological conditions these cells carry out surveillance functions and maintain homeostasis in the CNS (Ousman and Kubes, 2012). T-cells also have a presence in the CNS, albeit in lower numbers, and mostly survey specific sub-compartments outside the parenchyma (Wekerle et al., 1986; Hickey et al., 1991; Ousman and Kubes, 2012; Prinz and Priller, 2017).
1.2. Dopamine
Dopamine, one of the five biogenic amines, is a neuromodulator active in several CNS pathways implicated in sensorimotor function, cognition, and reward (Iversen and Iversen, 2007). Dopamine also acts peripherally regulating important physiological functions such as blood pressure, glucose metabolism and plasma glucose levels, endocrine function, immune and neuro-immune regulation (Basu et al., 1993; Basu and Dasgupta, 2000; Jose et al., 2003; Mignini et al., 2003; Rubi and Maechler, 2010). Many of dopamine’s functions are initiated by dopamine receptor activation. There are 5 subtypes of dopamine receptors, which are divided into two subgroups, D1-like dopamine receptors and D2-like dopamine receptors. The D1-like dopamine receptors, D1R and D5R, increase cAMP levels by activating adenylyl cyclase through Gαs. The D2-like receptors, D2R, D3R, and D4R, block the production of cAMP by inhibiting adenylyl cyclase through the activation of GαI (Missale et al., 1998; Beaulieu and Gainetdinov, 2011). Peripheral dopamine may be involved in the cross talk within the immune system, between the immune and nervous systems, and thus in disease states involving neuro-immune dysfunction.
In the CNS, dopaminergic transmission is tightly controlled and regulated via complex mechanisms, which can either act independently or in concert with other dopamine-related cellular mechanisms. Dopamine transporter (DAT) is one of the monoamine transporters and falls under the highly homologous SLC6 sym-porter family meaning it moves dopamine across the plasma membrane along with sodium and chloride ions (Rudnick and Clark, 1993; He et al., 2009; Manepalli et al., 2012) thereby potently regulating dopaminergic tone. DAT dysfunction and dysregulation is thought to play a role in diseases associated with dysregulated dopamine transmission such as Parkinson’s Disease, ADHD, and drug addiction (Vaughan and Foster, 2013).
Here, we outline the evidence for three topics—dopamine regulation of the immune system, DAT regulation of dopamine signaling, and how these two phenomena may intersect in PD which is characterized by dysfunctional dopamine signaling and neuroinflammation.
2. Dopamine regulation of the peripheral immune system
Peripheral dopamine has several different sources. The human gastrointestinal tract is reported to be a significant source of peripheral dopamine (Eisenhofer et al., 1997) providing the potential for dopamine signaling on tissue-resident immune cells of the gut. Dopamine stores, dopaminergic terminals and key dopaminergic proteins such as VMAT2 have also been detected in rat spleen and thymus (Mignini et al., 2009), both of which contain developing or resident immune cells. This suggests that the sympathetic nervous system (SNS) releases dopamine into immune-resident tissue thereby creating the potential for SNS dopamine-mediated regulation of immune cells as they develop. While norepinephrine is the main neurotransmitter of the SNS, these data indicating presence of dopaminergic stores, vesicles, and endogenous receptors support the idea that peripheral dopamine system might be involved in the regulation of biological responses and potentially regulation of peripheral immune system.
2.1. Dopamine and the innate immune system
2.1.1. Granulocytes
Granulocytes, such as neutrophils, basophils, eosinophils, and mast cells, act as effectors in the body’s first line of defense. They are involved in various first-response reactions, but more recent evidence also suggests they play a role in adaptive immune activation and regulation (Rothenberg and Hogan, 2006; Mantovani et al., 2011; Erjefalt, 2014). Mast cells, eosinophils, and neutrophils express either some or most of the dopamine receptors (Table 1). Mast cells specifically contain mRNA for tyrosine hydroxylase (TH) as well as dopamine receptors (Sookhai et al., 1999; McKenna et al., 2002; Ronnberg et al., 2012). Consistent with these reports, it has been shown that dopamine can induce nitric oxide (NO) release from mast cells (Seol et al., 2004), and recent reports have shown that D1R/D5R activation induces mast cell degranulation and enhances the Th2-mediated cutaneous immune reaction using real time PCR analysis in a murine model (Seol et al., 2004; Mori et al., 2013).
Table 1.
Dopaminergic proteins expressed in subsets of human leukocytes.
| Cell Type | Receptor Expression | Other Dopaminergic | Proteins References |
|---|---|---|---|
| Monocytes/Macrophages | D1/D5, D2, D3, D4 | DAT, TH, VMAT2 | McKenna et al. (2002), Gaskill et al., (2009, 2012), Coley et al. (2015) |
| Dendritic Cells | D1, D2 | TH | Nakano et al. (2008, 2009) |
| Granulocytes | D1/D5, D2, D3, D4 | TH | McKenna et al. (2002), Ronnberg et al. (2012) |
| Microglia | D1, D2, D3, D4, (D5?) | Mastroeni et al. (2009) | |
| T-cells | D1/D5, D2, D3, D4 | DAT, TH, VMAT | McKenna et al. (2002), Besser et al. (2005), Cosentino et al. (2007) |
Dopamine’s effects on neutrophils are extensively characterized. Dopamine induces neutrophil apoptosis and, in high concentrations, alters neutrophil morphological changes in response to stimuli (Sookhai et al., 1999; Trabold et al., 2007). Dopamine treatment of activated neutrophils attenuates chemotaxis and cytokine production, reduces Th17-mediated inflammatory airway response, and decreases phagocytic activity and reactive oxygen species production in a dose-dependent manner (Wenisch et al., 1996; Sookhai et al., 2000; Nakagome et al., 2011). Dopamine regulation of peripheral immune system is further supported by a recent finding by Cordano and colleagues showing a case of L-DOPA-induced neutropenia in a human PD patient where the neutrophil mRNA levels for DR5 and TH were increased and the mRNAs for dopamine receptors 2, 3, and 4 were decreased compared to healthy controls (Cordano et al., 2015), and by a study in a rat model where peripheral blood eosinophil levels were altered even when L-DOPA was administered directly into the CNS (Podolec et al., 1979). These reports collectively support the postulate of a potential communication between the central and peripheral dopamine systems.
2.1.2. Dendritic cells
The functions of dendritic cells (DC) are sensitive to dopaminergic regulation (Levite, 2012; De Kleer et al., 2014; Prado et al., 2012; Chen et al., 2012). Functions of antigen-presenting DC change with differentiation and maturation. Immature DCs show mostly phagocytic activity while mature DCs have a prolific cytokine profile and are thought to be important in peripheral immune responses (Mellman and Steinman, 2001). Dopamine receptor expression has been detected in DCs, and they are proposed to be part of the maturation process. Dendritic cells can also be induced to express TH (Nakano et al., 2008; Nakano et al., 2009; Prado et al., 2012). These findings are consistent with the proposed role of DCs as mediators of the innate immune system both within and between the innate and adaptive immune systems. In both cases, DC functions have been shown to be sensitive to dopaminergic regulation (Levite, 2012; De Kleer et al., 2014). Specifically, D5R activation, but not D2R inhibition, modulates DC stimulation and increases production of IL-12 and IL-23 (Prado et al., 2012; Chen et al., 2012). However, D2R inhibition reduces IL-10, IL-12, and TNF-α secretion, although this may be partially due to serotonin receptor antagonism (Chen et al., 2012).
In addition, dopamine alters DC-mediated induction of a polarized Th2 or Th17 response, as evidenced in response to a short peptide sequence associated with experimental autoimmune encephalomyelitis (Bailey et al., 2007; Segura et al., 2013). DCs are potent regulators of naïve CD4+ T cell differentiation, inducing a Th17 polarized response which can then be potentiated by DR5 activation of DCs (Prado et al. 2012). CD4+ T cell interaction with DC stimulate dopamine release from DCs (Nakano et al., 2009), which then induces polarized Th2 differentiation mediated by D1R (Nakano et al., 2009). Although the exact amounts of dopamine synthesized by peripheral immune cells and the conditions for its release remain unknown, these data along with several other studies suggest that immune cells synthesize neurotransmitters such as dopamine (Cosentino et al., 2002; Franco et al., 2007). The existence of dopaminergic proteins in multiple antigen-presenting cells (Table 1) suggests that these cells may also synthesize dopamine and release it in their interactions with other immune cells. Future studies using fast-scan cyclic voltammetry, amperometry after drug stimulation, high-performance liquid chromatography, or mass spectroscopy of cell lysate can address the existing knowledge gap regarding the source and dynamic of dopamine release in the periphery.
Overall, these data show that multiple arms of the innate immune system are regulated by dopamine supporting the hypothesis that altered peripheral dopamine homeostasis may regulate innate immune function and modulate the communication between the innate and adaptive systems. Next, we review the literature on dopamine and mononuclear phagocytes, a functionally diverse subset of the innate immune system present in the periphery and the CNS.
2.2. Mononuclear phagocytes—microglia, monocytes, and macrophages
2.2.1. Dopaminergic protein expression in human microglia, monocytes, and macrophages
A number of studies have examined the dopaminergic system in monocytes, macrophages and microglia. Similar to its function in neurons, the actions of dopamine in monocytes and macrophages seem to be mediated primarily by dopamine receptors. In neurons and HEK cells, dopamine receptors have also been shown to induce alternative signaling pathways mediated by beta-arrestin or calcium release (Beaulieu and Gainetdinov, 2011; Gaskill et al., 2014), but the specific pathways involved in dopamine receptor responses in macrophages remain unclear.
Studies examining human cells of the myeloid lineage have shown that these cells express all of the proteins involved in the dopaminergic system (Table 1 for reference). Gaskill and colleagues have shown expression of dopamine receptor mRNA, protein expression of all five dopamine receptors, and expression of D1R, D2R, D3R and D4R proteins on the plasma membrane of human monocyte-derived macrophages. These studies have also demonstrated that the dopamine receptors are functional and modulate intracellular signaling and cytokine production (Gaskill et al., 2009, 2012). Expression of all five dopamine receptor subtypes was also found on monocytes using mRNA and flow cytometry (Coley et al., 2015; Gaiazzi et al., 2016; Calderon et al., 2017). Robust expression of protein and mRNA for D1R, D2R, D3R and D4R was also shown in elderly human microglia, while expression of D5R is less clear (Mastroeni et al., 2009). Other studies have also confirmed the expression of dopamine receptors on human monocytes, macrophages and monocyte-derived osteoclasts (McKenna et al., 2002; Liang et al., 2008; Hanami et al., 2013)
In addition to dopamine receptor expression, human macrophages also express DAT, VMAT2, and the enzymes mediating the production of dopamine, TH and amino acid decarboxylase (AADC) (Gaskill et al., 2012). Examination of mRNA in human primary monocyte-derived macrophages (MDM) from 13 donors suggests that the expression levels of DAT and TH are similar among different donors, suggesting the expression of these proteins may be relatively homogeneous across the population (Fig. 1). Further, using immunofluorescent labeling we found that monocytes cultured from the blood of 5 healthy donors express DAT and TH proteins (Fig. 2). Both the macrophages and the monocytes were isolated from human blood using Ficoll density gradient separation. The macrophages were cultured for 6 days in macrophage media (as described in Gaskill et. al., 2012); the mRNA was extracted and analyzed for DAT and TH expression. The monocytes were plated in monocyte adhesion media for 1 day, then fixed and stained for IBA1 (Wako LKF6437, Richmond VA), DAT, and TH (Millipore Mab369 and T1299, respectively, Burlington MA) and imaged using a Nikon A1 laser-scanning confocal microscope and a 60x 1.49NA Nikon Plan-Apo objective (Nikon Instruments, Melville NY). Studies in human cells lines corroborate these findings, showing enzymatically active AADC in human promyelocytic U937 cells (Kokkinou et al., 2009), and that LPS-treatment of these cells induces TH and VMAT2 expressions (Capellino et al., 2010). Cross species examinations have further substantiated the potential generalization of dopaminergic proteins’ expression in rodent macrophages and microglia (Brown et al., 2003; Farber et al., 2005a; Flierl et al., 2007; Nakashioya et al., 2011; Yao et al., 2016).
Fig. 1.
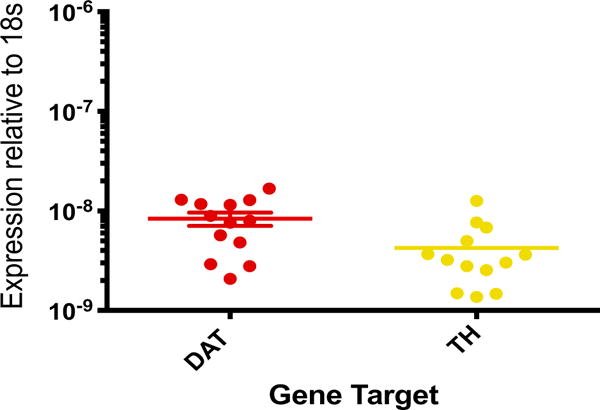
Primary human macrophages express low but variable levels of both the dopamine transporter and tyrosine hydroxylase. Peripheral blood mononuclear cells (PBMC) were isolated from human blood by Ficoll gradient centrifugation. Monocytes were isolated from the PBMC by adherence, and matured into monocyte-derived macrophages (MDM) through 6 days of culture in the presence of M-CSF (10 ng/mL). MDM RNA was isolated, converted into cDNA and analyzed for expression of DAT and TH by quantitative reverse transcriptase PCR (qRT-PCR). We utilized Taqman primers for the dopamine transporter (DAT) and tyrosine hydroxylase (TH) designed by Applied Biosystems. Both DAT and TH transcripts were found in all human samples probed (n = 15). All samples were normalized to an 18S control primer. Data were transformed using the equation 2−ΔCt and are expressed relative to the expression of 18S in each donor.
Fig. 2.
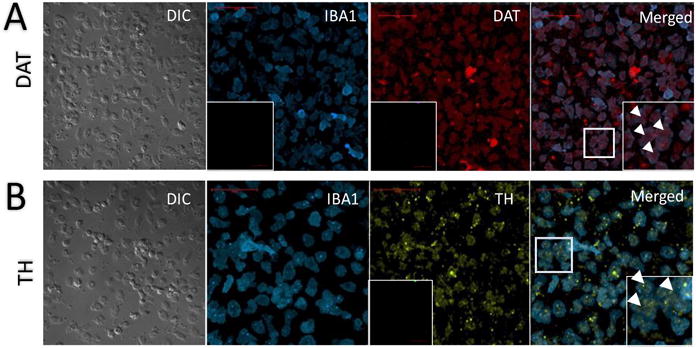
Monocytes constitutively express DAT and TH proteins. Confocal microscopy of immuno-stained monocytes isolated and cultured from human blood. A) DIC imaging (far left) of macrophages isolated and cultured from human blood are immuno-positive for IBA1 (second from left, with secondary Ab only labelled negative control inset), a macrophage-specific marker, and for DAT (third from left, with secondary Ab only labelled negative control inset). The same cells co-express both proteins (far right, inset); examples indicated by arrowheads in inset. B) DIC imaging of macrophages isolated and cultured from human blood are immuno-positive for IBA1 (second from left), a macrophage-specific marker, and TH (third from left, with secondary Ab only labelled negative control inset). Cells co-express both proteins, with zoomed-in examples indicated by arrowheads in far right inset. Scale bar is 50 μM; images are all representative of experiments repeated at least 5 times (n = 5).
Although more direct evidence is needed, the mere presence of dopamine receptor proteins, VMAT2, and DAT in the macrophages suggests the capacity of vesicular release or other alternative mechanism(s) of dopamine release from these cells (Marino et al., 1999). Supported by the above-described literature, Fig. 3 depicts a proposed model for dopamine transmission and dopamine signaling in the macrophages. This surmounts to the idea that macrophages contain dopaminergic protein machinery similar to dopaminergic neurons although the regulation of the dopaminergic system in macrophages and its effects on macrophage function are not well understood. Future studies are needed to determine whether or not, in the macrophages, these proteins have a similar capacity and sensitivity for dopamine signaling as well as commensurately sensitive regulatory mechanisms of dopamine transmission that are described for other dopaminergic cell types in the body.
Fig. 3.
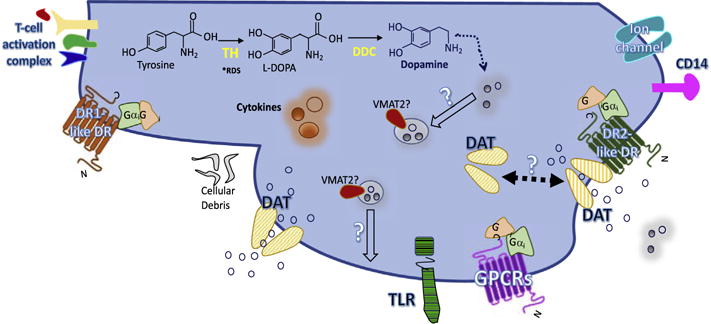
Postulated Dopamine System in Macrophages. Macrophages function as phagocytic and antigen presenting cells of the innate immune system and as such express receptors such as Toll-like receptor, T-cell activating proteins (MHC molecules with peptides, adhesion molecules, and costimulatory proteins), and markers such as CD14. They are also capable of engulfing cellular debris or pathogens and can be induced to secrete cytokines. Furthermore, studies have shown macrophages contain mRNA for all DRs, DAT, and TH. The latter may indicate DA is endogenously synthesized in macrophages from tyrosine via TH—the rate-limiting enzyme—to L-dihydroxyphenylalanine then to DA via AADC. There is conflicting evidence regarding the expression of these enzymes at protein level and regarding expression of VMAT2 at any level. This raises the question of vesicular storage and release of DA. Similarly, evidence regarding the level of DAT expression is unclear, and whether or not it functions as it does in neurons, with complex uptake, efflux, and trafficking mechanisms, is also inconsistent. Functional studies have shown that DA treatment and DR activation may modulate macrophage functions such as cytokine production and secretion.
2.2.2. Dopamine regulation of macrophage function
Recent research has shed more light on the effects of dopamine on macrophage function. Functional studies have revealed that treatment with dopamine or DR-specific agonists/antagonists can modulate proliferation, cytokine profiles, and phagocytosis. Activation of D1R on CD14+/CD16+ monocytes has been shown to increase chemokinesis, adhesion, and transmigration across an in-vitro blood–brain barrier model (Coley et al., 2015; Calderon et al., 2017). Treatment of rodent macrophages with dopamine antagonists was shown to modulate oxidative burst (Carvalho-Freitas et al., 2008; Carvalho-Freitas et al., 2011) through an effect mediated by prolactin. Dopamine treatment and D1R activation has also been shown to inhibit NLRP-3 inflammasome-dependent inflammation which includes LPS-induced and neurotoxin-induced systemic inflammation (Yan et al., 2015). D1R activation has also been shown to inhibit macrophage proliferation induced by oxidized low-density lipoprotein in a MAPK and Akt-dependent manner (Yao et al., 2016). In unstimulated human monocyte-derived macrophages dopamine treatment increased IL-6 and CCL2 production (Gaskill et al., 2012). In LPS-stimulated human monocyte-derived macrophages, dopamine has a broader range of effects on the cytokine profile resulting in increased secretions of IL-10, IL-6, CCL2, and CXCL8, and decreased production of TNF-α (Gaskill et al., 2012). In a separate study, dopamine also decreased production of IL-12p40, although this may be mediated by a beta-adrenergic receptor mechanism (Hasko et al., 2002), since dopamine is a partial agonist of beta-2 adrenergic receptors (Katritch et al., 2009). This demonstrates the potential complexity of multiple interacting neurotransmitter systems in macrophages and implies that dopamine may act on various receptors to carry out diverse functions.
In non-human model system, murine RAW 264.7, LPS stimulation increased dopamine synthesis, and, at high enough concentrations, dopamine decreased proliferation and increased apoptosis in these cells (Brown et al., 2003). In an in vivo mouse model, the D2-like dopamine receptor antagonist chlorpromazine increased IL-10 production in a D1R-dependent manner (Tarazona et al., 1995), and the D2-like dopamine receptor antagonist metoclopramide enhanced mRNA levels of IL-1β, IL-6, and TNF-α in both resting and LPS-stimulated macrophages subjected to sepsis (Zhu et al., 1997). Along the same lines, methamphetamine treatment of stimulated murine macrophages decreases CD14 expression and reduces production of NO, TNF-α, IL-1, and IL-6 (In et al., 2004). In macrophages isolated from chickens and wall lizards dopamine had paradoxical effects on phagocytosis depending on length of exposure and concentration of dopamine with longer exposure or higher concentrations reversing initial increase in phagocytic activity (Ali et al., 1994). Furthermore, in rat models, long-term inhibition of D2-like dopamine receptors with haloperidol increased phagocytic activity (Lourenço et al., 2005), whereas 14 day treatment with methamphetamine, known to increase extracellular dopamine in the CNS, decreased phagocytic activity (In et al., 2004). Overall these studies suggest dopamine mediates an inhibitory effect on macrophage phagocytosis. Collectively, these data suggest the idea that dopamine treatment of macrophages may have pro- or anti-inflammatory effects, implying that peripheral dopamine tone must be tightly regulated to coordinate a proper immune response. In addition, the particularly conflicting literature on the roles of CCL2 and the delicate balance of TNF-α required for immunological health (Gaskill et al., 2013) underscore the complexity underlying the meaning of these dopamine-induced effects, with particular regard to how the overall effect can depend on receptor specificity. Therefore, a lot of unanswered questions remain regarding the effects of dopamine on macrophage function, necessitating further study in this area.
2.2.3. Microglia
Microglia are CNS-resident mononuclear phagocytes derived from prenatal hematopoietic cells. During steady-state conditions, microglia are the only myeloid cells in the CNS parenchyma (Prinz et al., 2017). One of their main functions is the production of neurotrophic factors, which are critical for sustaining neuronal function and viability (Frade and Barde, 1998; Marin-Teva et al., 2004; Wakselman et al., 2008; Ueno et al., 2013; Ji et al. 2013). Microglia are major mediators in the immune response to CNS infection or tissue damage releasing a variety of cytokines upon activation (Mack et al., 2003; Davalos et al., 2005; Saijo, Crotti and Glass, 2013; Nayak et al., 2014). Their dysregulation has been implicated with many neurodegenerative diseases (Pinoli et al., 2017).
To date, a limited number of studies have examined dopamine signaling in microglia. Mastroeni and colleagues showed that dopamine treatment of human microglia enhanced their immune responsiveness and cytotoxicity (Mastroeni et al., 2009). In a non-human primate model of SIV, SIV-infected rhesus macaques treated with L-DOPA showed an increase in TNF-α production (Czub et al., 2004). Isolated rat microglia showed that activation of D1R and D2R modulates K+ currents and LPS-induced NO release (Farber and Pannasch, 2005b). High concentrations of dopamine are also thought to inhibit microglial nitric oxide production (Chen et al., 2012). Collectively these data suggest dopamine modulates microglial responses to stimuli and their subsequent effects on neurons.
However, microglia are not the only immune cells in the CNS. In healthy states, non-parenchymal macrophages and T-cells may exist in the CNS while in disease states various other immune cell populations can migrate across the BBB. (Sallusto et al., 2012, Korn and Kallies, 2017, Prinz and Priller, 2017). The evidence above also suggests that many of the immune cells in the periphery and the CNS are subjected to dopaminergic regulation or can produce dopamine themselves. Furthermore, the CNS-resident macrophages are thought to be sensitive to signaling molecules in the blood due to their localization (Goldmann et al., 2016) thereby providing a link between peripheral and central immune signaling. These data highlight the possibility that myeloid cells mediate a potential communication between peripheral (immune) dopamine and central (nervous system) dopamine. Extrapolation of this postulate implies that dysregulated dopamine transmission in the peripheral innate immune system could alter microglial function in the CNS and vice versa. To our knowledge there have been no studies investigating this connection in healthy and disease states delineating the causal relationship between dysregulated peripheral dopamine signaling and CNS dysfunction, providing an important direction for future studies.
Overall, the evidence shows that many distinct functions of monocytes and macrophages are sensitive to dopamine regulation. The role of different dopamine receptor subtypes is key, and a closer analysis of the different populations and subpopulations of macrophages, which may have varying expression of receptors and congruently varying responses to dopamine treatment, is an important future direction. This research could shed significantly more light onto how dopamine may play a role in macrophage function, dysfunction, and how faulty signaling may contribute to pathological states.
2.3. Dopamine and the adaptive immune system
There is a large body of research focusing on dopaminergic regulation of the adaptive immune system, specifically T-lymphocytes. These cells have been shown to express the proteins necessary for dopaminergic transmission and signaling including TH, VMAT2, all five dopamine receptor subtypes, and DAT (Table 1, Amenta et al., 2001; McKenna et al., 2002; Cosentino et al., 2007; Watanabe et al., 2006; Sarkar et al., 2006). Additionally, studies have also shown dopamine-mediated autocrine regulation of cytokine production in T-cells (Cosentino et al., 2007) indicating that dopamine is synthesized and acts an active signaling molecule in these cells.
Multiple investigations show that dopamine receptor activation can alter T cell functions. Lymphocyte proliferation, IFN-γ production, and differentiation decreased in the presence of dopamine (Bergquist et al., 1994). Incubation of CD4+ and CD8+ T-cells with reserpine, which induces dopamine release by inhibiting VMAT2 function, resulted in decreased CD4+ T cell-suppression of CD8+ T cell activity via reduction of IL-10 and TGF-β. This effect is mediated by DR1-like dopamine receptors (Cosentino et al. 2007). Treatment of resting T-cells with dopamine can stimulate them and induce secretion of TNF-α within 48 h, but more prolonged treatment (72 h) induces secretion of IL-10. Treating resting T-cells with dopamine did not affect the secretion of IFN-γ or IL-4 (Besser, Ganor and Levite 2005); whereas dopamine treatment of activated CD4+ and CD8+ T-cells can increase the number of cells producing IFN-γ, TNF-α, or IL-4 (Torres et al. 2005). On the other hand, it has been shown that dopamine treatment of activated T-cells can induce their quiescence in a DR4-dependent manner (Sarkar et al. 2006), reducing their secretion of IL-4, IL-2, and IFN-γ (Ghosh et al. 2003). These and other studies demonstrate the dynamic nature of dopamine as a versatile regulator of adaptive immunity, suggesting that dysregulated dopamine signaling in the periphery could alter adaptive immune system function.
The current literature supports the idea that T-cells contain dopaminergic protein machinery. They are sensitive to and may regulate dopaminergic tone in the periphery, and disruptions in dopaminergic signaling may contribute to pathologies characterized by adaptive immune dysfunction. Furthermore, the evidence suggests that dopamine may mediate interactions between the innate and adaptive immune systems. Thus, dysregulation of dopamine systems in either innate or adaptive immune cells may have consequences on the immune system in general, further suggesting the importance of proper regulation of peripheral dopamine tone.
3. DAT regulation of dopamine metabolism and signaling
The complexity of dopamine’s effects on immune cells suggests that the peripheral dopaminergic tone is strictly regulated in order to coordinate a proper immune response. In the CNS, DAT is the principle regulator of dopaminergic tone. Human lymphocytes have been found to express both DAT and VMAT2 with the former localizing largely at the plasma membrane and the latter in various cellular locations (Amenta et al., 2001). Intriguingly, similar to DAT function in the CNS, DAT in peripheral lymphocytes displays a Michaelis-Menten-like uptake kinetic profile in a sodium-chloride and temperature-dependent manner (Faraj et al., 1995). Additionally, cocaine, which is a DAT blocker and binds to a site that overlaps with the dopamine binding site on the DAT molecule, was shown to inhibit the DAT-dependent dopamine uptake into lymphocytes in a dose-dependent manner (Faraj et al., 1995; Beuming et al., 2008). This body of evidence suggests that DAT expressed in peripheral lymphocytes is functionally similar to DAT studied in heterologous system and dopamine neurons, implying that DAT on immune cells plays a role in modulating peripheral dopaminergic tone and may also abide by established regulatory mechanisms. It should be noted that over the last two decades, the DAT biology has been primarily studied in the HEK, CHO or PC12 cells expressing the transporter. Therefore, it is likely that some of the regulatory mechanisms described for DAT are shared amongst most DAT-expressing cells, including peripheral immune cells. We therefore review the literature on DAT regulation as established in both heterologous system and neurons.
3.1. DAT structure and function
The dopamine transporter (DAT) belongs to a group of homologous proteins known as the neurotransmitter transporters (NTTs), which have high structural alignment with and belong to the larger family of sodium chloride symporter proteins, the SLC6 family (Beuming et al., 2006; Saier et al., 2009). Analogous to other members of the SLC6 family, DAT transports two sodium cations and a chloride anion along with dopamine across the membrane (Gu et al., 1994), although evidence indicates the chloride currents are non-stoichiometric (De Felice, 2016). Indeed, x-ray crystallographic structures have shown two substrate binding sites, an allosteric binding site, and the presence of ions in the substrate pocket (Shan et al., 2011; Penmatsa et al., 2013). The transporters follow Michaelis-Menten kinetic profiles with respect to both substrate concentration and ion concentration, with each transporter having distinct kinetic parameters (Gu et al., 1994; Kristensen et al., 2011).
DAT functions to terminate synaptic transmission by uptake of the neurotransmitter back into the pre-synaptic terminal (Iversen, 1971) and thus is crucial in the regulation of neurotransmitter signaling and metabolism, both centrally and peripherally (Rudnick and Clark, 1993; Chen et al., 2004b; Bala et al., 2013). The transporter can also work in reverse mode. The reverse transport of dopamine, termed dopamine efflux, through DAT occurs when the transporter is in either an inward-facing or channel-like conformation and can be induced by drugs such as amphetamine and its derivatives (Fischer and Cho, 1976; Eshleman et al., 1994; Wall et al., 1995) resulting in an increase of extracellular dopamine. Structurally, several N-terminal residues along with trans-membrane domains 2, 7, and 8 have all been shown to play important roles in stabilizing transporter conformations (Loland et al., 1999, 2002; Norregaard et al., 2003; Chen et al., 2004a; Sen et al., 2005; Kanner and Zomot, 2008). The protein–protein interactions and other factors that mediate these conformational and functional changes are discussed below.
DAT regulates intracellular and extracellular levels of dopamine; therefore, dysregulated DAT function leads to altered dopamine signaling in the target regions. Dysregulated DAT activity is associated with various neurological disorders and neuropathologies (Kurian, 2009; Bala et al., 2013), and thus DAT is one of the main targets of psychostimulants, antidepressants, and drugs used for attention-deficit hyperactivity disorder therapies (Rudnick and Clark, 1993; Kristensen et al., 2011; Penmatsa et al., 2013). Here we review evidence regarding regulation of DAT function and, by extension, dopamine metabolism and homeostasis.
3.2. DAT trafficking
Although DAT function is commonly studied when the protein is localized to the plasma membrane, it is subject to an intricate network of trafficking regulatory mechanisms. DAT is constitutively trafficked to and from the surface membrane, but it can also be induced to internalize or be recruited to the membrane by exogenous and endogenous agents. Psychostimulants have proven to be useful agents and are frequently employed to study membrane expression of DAT. Specifically, amphetamines have been shown to induce transporter internalization as evidenced by decreased DAT function. However, in the striatal region, DAT membrane expression is upregulated for a short period of time following administration of amphetamine (Melikian and Buckley, 1999; Gorentla and Vaughan, 2005; Johnson et al., 2005a; Richards, 2009). Cocaine, conversely, has been shown to upregulate DAT surface expression in HEK cells following acute exposure (Daws et al., 2002) while Mash and colleagues showed upregulated DAT surface expression in chronic cocaine users (Mash et al., 2002). There is also evidence that links D2R and D3R activation to alterations in DAT surface levels (Zapata et al., 2007; Chen et al., 2013) which implies that endogenous agonists, i.e. dopamine, can putatively regulate DAT trafficking. Estrogens have also been shown to regulate internalization and sequestration of DAT into intracellular compartments (Watson et al., 2006; Chavez et al., 2010).
Kinases, directly or indirectly, regulate DAT trafficking. Protein Kinase C (PKC), specifically the beta isoform, has been shown to mediate several endocytic mechanisms such as those induced by amphetamines, D2R, and phorbol esters (Holton et al., 2005; Cervinski et al., 2005; Chen et al., 2013). Amphetamines induce DAT endocytosis through both PKC-dependent and PKC-independent mechanisms (Boudanova et al., 2008a). Additional evidence indicates that PKC-induced DAT internalization, as well as basal DAT trafficking, is mediated by clathrin (Sorkina et al., 2005). PKC-dependent endocytosis can occur via different pathways including phosphorylation or ubiquitylation, mediated by E3 ubiquitin ligase, at N-terminal residues (Miranda et al., 2007) with ubiquitylation leading to higher rates of degradation (Vaughan et al., 1997; Miranda et al., 2005; Block et al., 2007). Several residues along the C-terminus are also necessary for PKC-induced and constitutive endocytosis (Holton et al., 2005; Boudanova et al., 2008b).
Other kinases shown to interact directly or indirectly with DAT include mitogen activated protein kinase (MAPK), whose inhibition results in down-regulation of cell surface DAT in HEK cells, and phosphatidylinositol 3-kinase (PI-3 K), whose inhibition also results in DAT internalization (Carvelli et al., 2002; Moron et al., 2003). D2R-linked regulation of DAT trafficking is also sensitive to MAPK inhibition (Bolan et al., 2007). While it has been shown that cholesterol and lipid-rich membrane rafts regulate cell-surface redistribution of DAT, the existing reports regarding the role of Flortillin-1, a lipid raft-associating protein, on DAT trafficking are conflicting (Foster et al., 2008; Cremona et al., 2011; Sorkina et al., 2013). DAT is targeted approximately equally to lipid raft and non-raft domains of the membrane (Foster et al., 2008), and cholesterol’s importance in DAT kinetic regulation (discussed below), implies that there may be mechanisms that intentionally target DAT to or away from cholesterol-rich membrane domains. Importantly, membrane potential, which is an intrinsically dynamic cellular property to which DAT inherently contributes, can regulate DAT trafficking (Sonders et al., 1997; Ingram et al., 2002). Taken together, these studies show how extracellular signaling may putatively regulate levels of DAT surface expression, and, therefore, dopaminergic tone. Furthermore, innate cellular properties, such as cholesterol metabolism and membrane potential, may also regulate DAT trafficking and thus dopaminergic tone. Further study of these interactions and properties in peripheral immune cells will reveal how DAT is trafficked in this system.
DAT trafficking is a potent regulator of dopamine homeostasis in the CNS, and its regulation is intricately coordinated by multiple mechanisms. Abnormal DAT trafficking has been implicated in disorders such as attention-deficit hyperactivity disorder (Wu et al., 2015). This and the above studies suggest that disruptions in peripheral DAT trafficking may play a role in disease states via alterations in peripheral dopamine signaling and immune functions. However, although a significant number of the studies cited above have extrapolated the data in the heterologous expression systems to the biology of DAT in the CNS, the nature and mechanism of DAT activity in the immune cells before and after immuno-stimulation is less understood. In Figs. 1 and 2, we have shown that monocytes constitutively express DAT, but to our knowledge, there have been no studies investigating the mechanism of DAT trafficking in these cells. As described in previous sections, peripheral dopamine system is implicated in the regulation of biological responses and potentially regulation of peripheral immune system. Future studies needed to determine what influence DAT trafficking may have on peripheral immune system.
3.3. Kinetic and conformational regulation of DAT
DAT localized to the plasma membrane is subject to kinetic and conformational regulation by many extrinsic and intrinsic mechanisms, which can be signaled by psychostimulants, therapeutic drugs, and endogenous molecules, whose actions are all mediated by protein–protein interactions and changes in membrane potential or electrochemical gradients. Highly addictive drugs of abuse including amphetamine and its derivatives, MDMA, and cocaine, have been popular focal points for DAT research due to their ability to compete with dopamine for recognition by, and binding to DAT. In the case of amphetamine and its derivatives, there is also interest in its ability to induce a conformational change to either a reverse or channel-like conformer (Kilty et al., 1991; Norregaard et al., 2003; Kahlig et al., 2005; Beuming et al., 2008; Hagino et al., 2011; Penmatsa et al., 2013; Wang et al., 2015).
Most work on non-trafficking regulation of DAT examines DAT in an efflux conformation, which inhibits dopamine uptake, but releases dopamine via the reverse transport mechanism. Exogenous agents such as amphetamine and its derivatives enhance dopamine release via disruption of vesicular storage of dopamine and an efflux mechanism, while cocaine and other inhibitors such as nomifensine and GBR-12935 block both the uptake and DAT-mediated dopamine efflux (Eshleman et al., 1994; Goodwin, 2009). Research on endogenous aspects of DAT regulation exist to examine and characterize the interactions between DAT and other intracellular proteins, which may serve to mediate the regulation attributed to psychostimulants and possibly other endogenous agents (Butler et al., 2015; Sambo et al., 2017).
The intracellular domains of DAT are subject to interactions with a number of intracellular proteins. Amphetamines induce DAT internalization through a PKC-dependent mechanism, but also reduce DAT uptake in a trafficking-independent manner (German et al., 2012). Amphetamine-induced reverse transport through either a reverse conformation or a channel-like conformation is mediated by PKC (Johnson et al., 2005b; Kahlig et al., 2005), and thus blocks uptake and increases extracellular dopamine without changes in DAT surface expression. In addition to PKC, αCaMKII (Calmodulin-dependent Protein Kinase IIα) also plays a role in regulating amphetamine-induced dopamine efflux as its inhibition attenuates DAT-mediated dopamine efflux (Steinkellner et al., 2012). Because DAT transport is a sodium dependent transporter, sodium concentrations and membrane potential have also been shown to regulate uptake and efflux with more positive membrane potential—affected by sodium and calcium gradients—reducing uptake and favoring efflux (Khoshbouei et al., 2003; Khoshbouei et al., 2004; Gnegy et al., 2004; Richardson et al., 2016). Along the same lines, site-directed mutagenesis and functional studies have shown that N-terminal T62 is crucial for determining the favored DAT conformation, and that N-terminal phosphorylation is critical for amphetamine-induced efflux (Khoshbouei et al., 2004; Guptaroy et al., 2009; Guptaroy et al., 2011; Fraser et al., 2014), This indicates that these are sites of modification necessary for changing and stabilizing DAT conformation.
DAT can be kinetically regulated through allosteric modulators, sodium, or cholesterol. Specific quinzolinamines are able to allosterically modulate amphetamine induced DAT-mediated dopamine efflux, with some acting as agonists and others taking an antagonist role (Rothman et al., 2009). Intracellular sodium concentrations also serve to regulate amphetamine-induced efflux as seen by increases in increased amperometric currents at several membrane potentials (Khoshbouei et al., 2003). Membrane cholesterol has been shown to be important in stabilizing an outward-facing conformation, and, importantly, different levels of cholesterol can alter DAT transport and efflux without altering surface expression (Adkins et al., 2007; Hong and Amara, 2010; Jones et al., 2012).
The kinetic and conformational states of DAT function are equally important in maintaining dopamine homeostasis, and the mechanisms controlling each are tightly regulated. Aberrant changes in the thermodynamic equilibrium that determines the conformational state of the transporter can dramatically alter dopamine levels as reported following administration of amphetamines. In fact, anomalous efflux is seen in ADHD patients with specific mutation on DAT molecules (Mazei-Robison et al., 2008) implicating dysregulated DAT in the molecular pathology. Therefore, it is possible that conformational state of DAT expressed in the peripheral immune cell modulates immune function via changes in peripheral dopamine levels. It will be important to delineate to what extent peripheral DAT conformation affects peripheral immune function in healthy and disease states.
3.4. DAT and immunity
Knowledge of the role of peripheral DAT in the context of the immune system remains incomplete. More specifically, how the milieu of cytokines and signaling cascades affects DAT function, and how peripheral DAT function alters immune function remains unknown. A recent study by Kavelaars and colleagues has shown that deletion of DAT gene (DAT−/−) impaired cellular immune responses and has substantial consequences for both the acquired and the innate immune response (Kavelaars et al., 2005). Although this study does not reveal if the immune effects are due to changes in DA levels and/or DAT on immune cells or in the peripheral/central nervous systems. Nevertheless, these data suggest there is a dynamic interaction between peripheral DAT function and immune cells, underscoring the need for future investigation into the molecular interactions between peripheral DAT and the immune system. Specifically, immune cell modification and re-implantation by knockdown/knockout of DAT or employment of a BBB-impermeable DAT inhibitor is needed to directly confirm this potential interaction as well as its directionality.
3.5. DAT and Parkinson’s Disease
The current role of DAT in Parkinson’s Disease is two-fold. First, the availability of radiolabeled DAT-specific ligands has enabled the quantification of dopaminergic degeneration following clinical diagnoses of Parkinson’s Disease. Initial studies revealed that PD patients exhibited 40–50% loss of striatal uptake capacity by the time of diagnosis as well as a yearly loss that is greater than 10-fold that of normal age-matched healthy individuals (Marek et al., 2001). Further, it was recently revealed that decreased DAT-mediated dopamine uptake was correlated to earlier onsets of L-DOPA induced dyskinesia when compared to patients that did not show the aversive reaction (Yoo et al., 2017). As the current standard in visualizing the degree of cell death in PD, quantifying DAT uptake capacity represents a valuable longitudinal readout in order to better understand the links between symptom severity, L-DOPA efficacy, and overall progression of the disease. Although, peripheral dopamine level is decreased in PD patients (Kustrimovic et al., 2016), whether peripheral DAT uptake capacity and/or its level are changed in PD are unknown. Addressing this important knowledge gap may provide a potential connection, or lack thereof, between changes in the peripheral dopamine and DAT levels and the central nervous system. Future studies employing DAT knockdown/knockout via immune cell modification or a BBB-impermeable DAT inhibitor and subsequent measures of CNS dopamine via fast-scan cyclic voltammetry, micro-dialysis, or HPLC measurements could shed more light on these outstanding questions. A potential relationship between peripheral and central DAT levels can potentially assess PD progression and the effectiveness of pharmacotherapies in PD.
Early studies show DAT knockout mice develop Parkinsonian-like symptoms, which correlated with the gradual and severe loss of DA in striatal tissue from DAT-KO mice corroborating the role of DAT-mediated DA recycling and DA homeostasis throughout life (Giros et al., 1996). As this suggests, DAT dysfunction has been directly linked to PD: mutations in the gene encoding the transporter (SLC6A3) result in severe motor deficiencies in infancy with PD-like symptoms appearing before 3 years of age (Kurian et al., 2011). Additional mutations were recently identified in patients presenting PD-like symptoms at a later stage (Ng et al., 2014). And while familial cases of PD remain a minority of total cases, the presence of multiple unique mutations unequivocally establish a role of DAT dysfunction in PD.
In addition to the direct genetic role in familial PD, alterations of DAT function have also been linked to more general elements of PD progression that suggest a role in idiopathic PD. Most prominently, DAT has been shown to directly interact with α-synuclein, the main constituent of Lewy Bodies (Butler et al., 2015). Increases in α-synuclein lowers DAT uptake and overall trafficking to the membrane, while simultaneously reorganizing the surface topography such that DAT becomes more heavily associated with cholesterol-rich membrane microdomains (Swant et al., 2011; Butler et al., 2015; Oaks et al., 2013). Detailed examinations of how distinct pathological insults associated with PD (Lewy Body presence, mitochondrial dysfunction, neuroinflammation) impact DAT function remain to be conducted, but the transporter remains a potentially unifying factor throughout PD’s etiology.
Although there are no studies examining peripheral DAT in the context of PD, these data suggest the possibility that alterations in peripheral DAT function may be linked to PD in a similar fashion to central DAT dysfunction. Moreover, this would further support the idea that dysregulated peripheral immune function in PD may be associated with fluctuations in peripheral dopamine levels due to change in DAT function. Future investigations could further elucidate the dynamics of this potential relationship as well as the potential causal or chronological mechanism.
In summary, an intricate network exists to regulate DAT surface expression, conformation, and kinetics, which, in turn, all serve to regulate overall dopaminergic tone. Characterization of DAT regulation and function has been done both in heterologous systems and in neurons. Innate properties and protein activity of all cells such as membrane potential and kinase-dependent modifications contribute to DAT regulation in these models, and it is therefore reasonable to postulate that other non-neuronal cells expressing DAT can be similarly subjected to the same regulatory mechanisms as depicted in Fig. 4. However, the biology of DAT in the immune system is currently unknown. Specifically, whether or not DAT function and regulation are similar in the immune system as compared to other studied systems remains unclear. Therefore, it is necessary to examine DAT activity before and after immune stimulation before investigating any possible connections to disease pathology. However, assuming that peripheral DAT is tightly regulated, dopaminergic tone in the periphery may be just as sensitive to these mechanisms as dopaminergic tone in the CNS, implying that dysfunction of these mechanisms may manifest itself in the periphery and central nervous system.
Fig. 4.
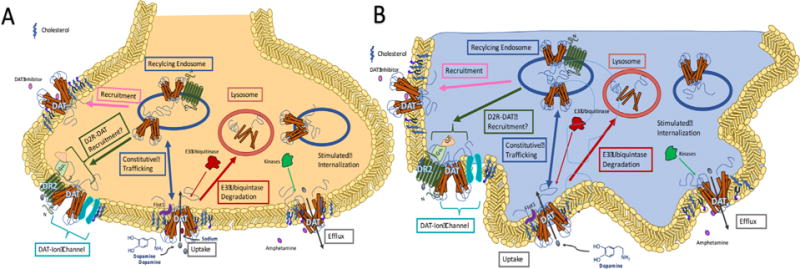
Regulation and Trafficking of DAT. In dopaminergic neurons, DAT is under several regulatory mechanisms modulating its surface expression and its kinetics. Regarding trafficking to and from the membrane, psychostimulants, DAT inhibitors, and dopamine receptor ligands can all regulate membrane expression. This regulation is mediated by intracellular proteins such as kinases and ubiquitinases and occurs in addition to the constitutive trafficking of DAT. DAT activity also induces an inward current, and thus membrane potential. Reciprocally, membrane potential also regulates DAT trafficking. These mechanisms differentially recycle or degrade DAT. DAT conformation and kinetics of transport can also be regulated by endogenous signals and other drugs, such as amphetamine, whose actions are mediated by kinases. Conformation and kinetics of DAT are also subject to regulation by cholesterol and membrane potential. All of these regulatory mechanisms have been established in HEK cells and DA neurons (A). However, it remains unknown whether or not macrophages, which we have shown express DAT, utilize the same regulatory mechanisms, thus we depict our proposed model in the macrophages based on existing data in heterologous system and neurons (B).
4. Parkinson’s Disease and the immune system
Parkinson’s Disease (PD) is the second most common neurodegenerative disorder, after Alzheimer’s, which affects 10 million people globally and is characterized by rigidity, bradykinesia, tremors, and gait instability (O’sullivan et al., 2007). The pathological hallmarks of PD include reduced dopamine due to loss of dopaminergic neurons in the basal ganglia and the substantia nigra (SN) (Hirsch et al., 1988; Fearnley and Lees, 1991), as well as accumulation of α-synuclein, ubiquitin, and neurofilaments, also called Lewy bodies (LB).
Pharmacological dopaminergic substitution therapy remains a central treatment for symptom management. Immune-related side effects have been reported in patients taking antiparkinson’s medications such as L-DOPA, dopamine agonists, and catabolic inhibitors (Table 2) suggesting alterations in peripheral dopamine levels may lead to dysfunctional immune responses. Indeed, plasma dopamine is elevated in patients on dopamine substitution therapy compared to healthy controls (Kustrimovic et al., 2016) and thus this may lead to increased dopamine signaling on immune cells. On a more clinically relevant scale, L-DOPA treatment in a 6-hydroxydopamine rat model of Parkinson’s increases immunogenic response after xenogeneic ventral mesencephalon graft (Breger et al., 2017), implicating over-activation of microglia. Additionally, PD patients on dopaminergic therapies showed increased expression of D1R on CD4+ T-cell when compared to drug-naïve patients (Kustrimovic et al., 2016). This evidence, along with the report by Cordano and colleagues, shows that while dopaminergic therapies are aimed at rescuing CNS dopamine levels, they also have effects on peripheral dopamine tone and, consequently, immunological effects. This then further implies that the dysregulation of dopamine in Parkinson’s Disease may alter peripheral immunity, suggesting a connection between central dopamine, peripheral dopamine, and immune function.
Table 2.
Immune-related effects of anti-Parkinson drugs.
| Drug Name | Immune Effects | Reference |
|---|---|---|
| L-DOPA | Promotes immune response in xenogenic graft (rat) | Breger et al. (2017) |
| Possible therapy for fibromyalgia and chronic fatigue syndrome | Hitzig (1997) | |
| Neutropenia | Cordano et al. (2015) | |
| Alters T-cell proteome | Alberio et al. (2012) | |
| Dopamine Agonists | Prevents experimental autoimmune encephalomyelitis in mice | Lieberknecht et al. (2017) |
| Alters T-cell proteome | Alberio et al. (2012) | |
| Possible therapy for fibromyalgia and chronic fatigue syndrome | Hitzig (1997) | |
| COMT Inhibitors | Increased pain sensitivity (may have inflammatory component) | Nackley et al. (2007) |
| MAO Inhibitors | Remission of rheumatoid arthritis | Lieb (1983) |
Braak and colleagues developed a widely-accepted hypothesis suggesting PD starts in the gastrointestinal tract, spreading to the dorsal motor nucleus of the vagus nerve, and then throughout the CNS (Braak et al. 2003a, 2003b). Although the exact etiology is still unknown, environmental, genetic, and immune system interactions are reported as the most common contributing factors in the disease onset and progression (Olson and Gendelman, 2016). Therefore, we review the literature concerning the for the role of the immune system in PD, including involvement of neuroinflammation and peripheral immunity. Consistent with the Braak hypothesis of a peripheral origin for PD, we specifically explore the possibility of dopamine-related peripheral immune cell dysfunction association to PD pathogenesis and/or progression.
4.1. Parkinson’s Disease and innate immunity
Neuroinflammation is thought to be one of the main pathological features for PD. Indeed it has been shown that a positive correlation exists between levels of CD68 expression and PD duration (Croisier et al., 2005), implicating microglia and macrophage activation in PD progression. In conjunction with this body of evidence, Castano et al showed that bacterial endotoxin LPS injections into the SN reduce dopamine and TH+ neurons in SN via activation of microglial TLR4 receptor and neuroinflammation (Castano et al., 1998). The above reports suggest that there is a direct relationship between immune system activation and PD progression.
Furthermore, postmortem studies of patients with degenerative disorders such as PD show increased levels of cytokines, including IL-1β, TGF-β, IFN-γ, and IL-6, in nigrostriatal regions as well as CSF (Boka et al., 1994; Mogi et al., 1994a, 1994b). These studies also suggest HLA-DR, HLA-DQ and MHCII positive microglia as the most common associated neuropathology in PD (McGeer et al., 1988). Animal models of PD also show increased pro-inflammatory cytokines, such as IFN-γ, IL-2, IL-17, and IL-22 but equal concentrations immunomodulatory cytokines such as IL4, IL10, and TGF-β, in serum and peripheral lymphoid tissues of PD-models versus healthy controls (Huang and Halliday, 2012). This body of evidence provides additional support that activation of the immune system is involved in PD pathology. Since dopamine may modulate peripheral immune cell cytokine secretion, these data may be reflective of dysregulated peripheral dopamine, suggesting a link between the central and peripheral dopamine system in PD. Whether these data represent a cause or effect remains a topic of intense debate, however the correlation between central and peripheral immune markers in PD still holds.
The studies mentioned previously demonstrate that dopamine levels can dynamically modulate cytokine profiles of both innate and adaptive immune cells. Being that PD is a disease of altered dopamine homeostasis, there is a chance that the differential cytokines profiles between PD and healthy patients is associated with PD-related dopaminergic dysregulation. Additionally, this further emphasizes the potential for peripheral dopamine system communication with the central dopamine system analogous to, and possibly mediated by, the interactions between the central and peripheral immune systems. Pending direct experimental evidence as mentioned above, this presents the exciting possibility that PD may be monitored, treated, or studied via the peripheral dopamine system on innate immune cells.
4.2. Parkinson’s Disease and adaptive immunity
Activated microglia and aggregated misfolded α-synuclein in the CNS can activate antigen-presenting cells in the periphery and recruit neurotoxic T-cells to the CNS via the damaged BBB (Mosley et al., 2012). Importantly, IFN-γ, which is shown to be elevated in PD patients, promotes migration of CD4+ T-cells across the BBB (Sonar et al., 2017). Furthermore, a study by González et al. (2013), in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD showed that D3R expression on CD4+ T-cells may contribute to dopaminergic neuron degeneration in the SN. D3R stimulation is known to decrease CD4+ T-cells synthesis of IL-4 and IL-10 and increase IFN-γ production. The results suggest D3R-deficient mice are protected against microglial activation and loss of dopaminergic neurons (González et al., 2013).
However, conflicting literature exists about the role of CD4+ T-cells in PD. In a study by Stevens et al. (2012), reduced number of TCRαβ+, CD4+ (T helpers) and CD19+ (B) cells but equal populations of CD14+ (monocytes) and CD56+ (natural killer cells) were reported in PD patients compared to controls (Stevens et al., 2012). This study is consistent with the previous studies reporting neuroprotective effects of CD4+ CD25+ regulatory T-cells in animal models of PD (Baba et al., 2005; Reynolds et al., 2007). Therefore, CD4+ T-cells are paradoxically associated with both neuroprotection and neurodegeneration. However, the neurodegenerative effects of CD4+ T-cells are associated with D3R expression, thereby implicating the dysregulation of the peripheral dopamine system in dopaminergic neuron degeneration in PD.
Furthermore, not only are T-cell levels different between healthy and PD samples, but studies have also shown that dopamine receptor expression varies between the two populations, and treatment with α-synuclein resulted in different effects on dopamine receptor expression in healthy vs PD patient T-cells (Kustrimovic et al., 2016). Since dopamine signaling has been shown to regulate T-cell function and DR activation is potentially associated with overall pro-inflammatory immune activation, it is reasonable to postulate that dopamine signaling on peripheral T cells may cause a pro-inflammatory response that may migrate to the CNS and lead to neuroinflammation. This would corroborate the aforementioned hypothesis that the role CD4+ T-cells assume in PD is dependent on central and peripheral dopaminergic systems.
4.3. Parkinson’s Disease and autoimmunity
Autoimmunity emerged as a popular theory of PD after elevated levels of inflammatory cytokines and γ/δ+ T cells were found in the peripheral blood and CSF of patients with PD (Fiszer et al. 1994). Accumulated α-synuclein inside dopaminergic neurons acts as a foreign antigen that triggers a T-cell regulated response and subsequent autophagy of dopaminergic neurons (Papachroni et al. 2007), which provides a possible explanation for induction of an autoimmune response. Moreover, recently it was reported that T cells from PD patients and healthy controls age-matched differentially recognize and react to certain epitopes of α-synuclein, the main protein component in Lewy Bodies (Sulzer et al., 2017). These studies indicate a role for adaptive autoimmunity in PD.
Autoantibodies against one or multiple forms of α-synuclein are among the most common in PD patients (Papachroni et al., 2007; Benner et al., 2008; Spillantini et al., 1998; Gruden et al., 2011). These reports suggest that α-synuclein may play a role in mounting the auto-immune response in PD, bridging the innate immune response to the specific adaptive immune response. In fact, α-synuclein is expressed in macrophages, which are antigen presenting cells, and its expression increases in response to LPS and IL-1 (Tanji et al., 2002). Macrophages may therefore link innate immunity, α-synuclein, and adaptive immunity. In conjunction with the data showing dopamine-induction and stabilization of α-synuclein pre-fibrillary adducts (Conway et al., 2001; Mor et al., 2017) along with the functioning dopamine system in macrophages, there is an intriguing possibility that α-synuclein may mediate the proposed connection between peripheral dopamine dysregulation and PD pathology.
4.4. Parkinson’s Disease: Genetics and the immune system
Genetic studies have also highlighted the inflammatory nature of PD. Leucine repeat rich kinase 2 (LRRK2, PARK8), PTEN-induced kinase 1 (PINK1, PARK6) and DJ-1 (PARK8) are linked to familial PD, and their mutations have been described with aberrant expressions of immune responses (Hardy et al., 2006) SNCA encodes α-synuclein and was the first identified mutated gene in PD. α-synuclein protein is expressed in a wide variety of immune cells including T cells, B cells, NK cells, microglia, and monocytes. Although the comprehensive function of α-synuclein remains unknown, it plays a homeostatic role in microglia. Microglia from mice lacking SNCA has a more activated phenotype in terms of morphology and cytokine secretion in addition to decreased phagocytic ability (Zappia et al., 2002).
Lymphocyte-activation gene-3 (LAG-3), present on multiple immune cells and a ligand for human leukocyte antigen class II molecules, specifically binds to pre-fibrillary forms of α-synuclein (PFF α-synuclein), and knockout of this gene attenuates PFF α-synuclein-induced neurodegeneration (Mao et al., 2016) Based on the premises that exogenous pre-fibrillary α-synuclein can induce dopaminergic neurodegeneration (Volpicelli-Daley et al., 2011); and that PD T-cells in the CNS respond differentially to α-synuclein peptides presented by HLA (Sulzer et al., 2017), there is sufficient evidence to postulate that α-synuclein may be a link between immune dysfunction and neurodegeneration in PD. This aligns with our hypothesis that there is a potential bidirectional communication between the peripheral (immune) and central dopaminergic systems. Whether or not T-cell migration, recognition of, and reaction to α-synuclein precede clinical onset of PD symptoms or further contribute to later disease progression is unclear. Future studies may examine the potential for T-cells that cannot bind HLA-α-synuclein complexes as a method for resolving this matter.
5. Conclusion
Here we have reviewed three bodies of interconnected evidence regarding dopamine regulation of the immune system, DAT regulation of dopamine signaling, and the role of the immune system in PD, a pathology of the dopamine system. Considering the evidence collectively, we return to our central hypothesis that the peripheral dopamine system on immune cells may be connected to the central dopamine system. This communication could be mediated by immune cells of both the periphery and the CNS and regulated by DAT. By extension, failure of DAT regulation in the periphery and thus dysregulated dopamine signaling, particularly in the immune cells, could transpire into central dopamine dysfunction and aberrant immune function, both of which are hallmarks of PD. These hypotheses surmount to the idea that peripheral DAT function may play a key and unexplored role in PD pathogenesis or progression. Direct and thorough investigation of each of the central postulates put forth is needed in order to evaluate the potential for a novel way to research PD.
Abbreviations
- CNS
Central Nervous System
- DA
Dopamine
- DAT
Dopamine Transporter
- DR
Dopamine Receptor
- LPS
Lipopolysaccharide
- MDM
Monocyte-Derived Macrophages
- PD
Parkinson’s Disease
- PKC
Protein Kinase C
- TH
Tyrosine Hydroxylase
- VMAT2
Vesicular Monoamine Transporter 2
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry. 2007;46:10484–10497. doi: 10.1021/bi700429z. [DOI] [PubMed] [Google Scholar]
- Alberio T, Pippione AC, Comi C, Olgiati S, Cecconi D, Zibetti M, Lopiano L, Fasano M. Dopaminergic therapies modulate the T-CELL proteome of patients with Parkinson’s disease. IUBMB Life. 2012;64:846–852. doi: 10.1002/iub.1073. [DOI] [PubMed] [Google Scholar]
- Ali RA, Qureshi MA, McCorkle FM. Profile of Chicken Macrophage Functions After Exposure to Catecholamines In Vitro. Immunopharmacol Immunotoxicol. 1994;16:611–625. doi: 10.3109/08923979409019742. [DOI] [PubMed] [Google Scholar]
- Amenta F, Bronzetti E, Cantalamessa F, El-Assouad D, Felici L, Ricci A, Tayebati SK. Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J Neuroimmunol. 2001;117 doi: 10.1016/s0165-5728(01)00317-4. [DOI] [PubMed] [Google Scholar]
- Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson’s disease phenotype. J Neurol. 2005;252:1201–1205. doi: 10.1007/s00415-005-0835-7. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ’preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Bala PA, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 2000;102:113–124. doi: 10.1016/s0165-5728(99)00176-9. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS, Lahiri T, Chowdhury JR. Uptake and biodistribution of dopamine in bone marrow, spleen and lymph nodes of normal and tumor bearing mice. Life Sci. 1993;53:415–424. doi: 10.1016/0024-3205(93)90645-j. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci USA. 1994;91:12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFα or both. J Neuroimmunol. 2005;169:161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8 doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Melikian HE. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology. 2008a;54:605–612. doi: 10.1016/j.neuropharm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, Stevens Z, Melikian HE. Dopamine transporter endocytic determinants: carboxy terminal residues critical for basal and PKC-stimulated internalization. Mol Cell Neurosci. 2008b;39:211–217. doi: 10.1016/j.mcn.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003a;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Trans (Vienna) 2003b;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Breger LS, Kienle K, Smith GA, Dunnett SB, Lane EL. Influence of chronic L-DOPA treatment on immune response following allogeneic and xenogeneic graft in a rat model of Parkinson’s disease. Brain Behav Immun. 2017:155–164. doi: 10.1016/j.bbi.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, Ryan JJ, Stewart JK, Fischer-Stenger K. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135 doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- Butler B, Saha K, Rana T, Becker JP, Sambo D, Davari P, Goodwin JS, Khoshbouei H. Dopamine transporter activity is modulated by alpha-synuclein. J Biol Chem. 2015;290:29542–29554. doi: 10.1074/jbc.M115.691592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon TM, Williams DW, Lopez L, Eugenin EA, Cheney L, Gaskill PJ, Veenstra M, Anastos K, Morgello S, Berman JW. Dopamine increases CD14+CD16+ monocyte transmigration across the blood brain barrier: implications for substance abuse and HIV neuropathogenesis. J Neuroimmune Pharmacol. 2017;12:353–370. doi: 10.1007/s11481-017-9726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellino S, Cosentino M, Wolff C, Schmidt M, Grifka J, Straub RH. Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Ann Rheum Dis. 2010;69:1853–1860. doi: 10.1136/ard.2009.119701. [DOI] [PubMed] [Google Scholar]
- Carvalho-Freitas MI, Rodrigues-Costa EC, Nasello AG, Palermo-Neto J, Felicio LF. In vitro macrophage activity: biphasic effect of prolactin and indirect evidence of dopaminergic modulation. Neuroimmunomodulation. 2008;15:131–139. doi: 10.1159/000148196. [DOI] [PubMed] [Google Scholar]
- Carvalho-Freitas MIR, Anselmo-Franci JA, Maiorka PC, Palermo-Neto J, Felicio LF. Prolactin differentially modulates the macrophage activity of lactating rats: possible role of reproductive experience. J Reprod Immunol. 2011;89:38–45. doi: 10.1016/j.jri.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera A, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280:40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: An autoradiography study. Brain Res. 2010;1321:51–59. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Chen ML, Tsai TC, Wang LK, Lin YY, Tsai YM, Lee MC, Tsai FM. Risperidone modulates the cytokine and chemokine release of dendritic cells and induces TNF-alpha-directed cell apoptosis in neutrophils. Int Immunopharmacol. 2012;12:197–204. doi: 10.1016/j.intimp.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Chen N, Rickey J, Berfield JL, Reith ME. Aspartate 345 of the dopamine transporter is critical for conformational changes in substrate translocation and cocaine binding. J Biol Chem. 2004a;279:5508–5519. doi: 10.1074/jbc.M306294200. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004b;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, Gnegy ME. Protein kinase Cβ is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem. 2013;125:663–672. doi: 10.1111/jnc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, Berman JW. Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis. PLoS One. 2015;10:e0117450. doi: 10.1371/journal.pone.0117450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- Cordano C, Pardini M, Cellerino M, Schenone A, Marino F, Cosentino M. Levodopa-induced neutropenia. Parkinsonism Relat Disord. 2015;21:423–425. doi: 10.1016/j.parkreldis.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S. Human CD4+ CD25+ regulatory T cells selectively express tyrosinehydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109 doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- Cosentino M, Marino F, Bombelli R, Ferrari M, Rasini E, Lecchini S, Frigo G. Stimulation with phytohaemagglutinin induces the synthesis of catecholamines in human peripheral blood mononuclear cells: role of protein kinase C and contribution of intracellular calcium. J Neuroimmunol. 2002;125:125–133. doi: 10.1016/s0165-5728(02)00019-x. [DOI] [PubMed] [Google Scholar]
- Cremona ML, Matthies HJG, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RKB, Graeber MB. J Neuroinflammation. London: 2005. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition; p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Muller JG, Stahl-Hennig C, Riederer P, Ter Meulen V, Gosztonyi G. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol. 2004;107:216–226. doi: 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- De Felice LJ. Chloride Requirement for Monoamine Transporters. Pflugers Arch. 2016;468:503–511. doi: 10.1007/s00424-015-1783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kleer I, Willems F, Lambrecht B, Goriely S. Ontogeny of myeloid cells. Front Immunol. 2014;5 doi: 10.3389/fimmu.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Åneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady Bl, Mezey E. Substantial Production of Dopamine in the Human Gastrointestinal Tract. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- Erjefalt JS. Mast cells in human airways: the culprit? Eur Respir Rev. 2014;23:299–307. doi: 10.1183/09059180.00005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Henningsen RA, Neve KA, Janowsky A. Release of dopamine via the human transporter. Mol Pharmacol. 1994;45:312–316. [PubMed] [Google Scholar]
- Faraj BA, Olkowski ZL, Jackson RT. A cocaine-sensitive active dopamine transport in human lymphocytes. Biochem Pharmacol. 1995;50:1007–1014. doi: 10.1016/0006-2952(95)00230-w. [DOI] [PubMed] [Google Scholar]
- Farber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005a;29:128–138. doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Farber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005b;29 doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114 (Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Properties of dopamine efflux from rat striatal tissue caused by amphetamine and p-hydroxyamphetamine. Proc West Pharmacol Soc. 1976;19:179–182. [PubMed] [Google Scholar]
- Fiszer U, Mix E, Fredrikson S, Kostulas V, Olsson T, Link H. gamma delta + T cells are increased in patients with Parkinson’s disease. J Neurol Sci. 1994;121:39–45. doi: 10.1016/0022-510x(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Franco R, Pacheco R, Lluis C, Ahern GP, O’Connell PJ. The emergence of neurotransmitters as immune modulators. Trends Immunol. 2007;28:400–407. doi: 10.1016/j.it.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fraser R, Chen Y, Guptaroy B, Luderman KD, Stokes SL, Beg A, DeFelice LJ, Gnegy ME. An N-terminal threonine mutation produces an efflux-favorable, sodium-primed conformation of the human dopamine transporter. Mol Pharmacol. 2014;86:76–85. doi: 10.1124/mol.114.091926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiazzi M, Rasini E, Marino F, Zaffaroni M, Cosentino M. Dopaminergic receptors on human monocytes and peripheral blood dendritic cells. J Neuroimmune Pharmacol. 2016;11:214–226. [Google Scholar]
- Gaskill PJ, Calderon TM, Coley JS, Berman JW. Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. J Neuroimmune Pharmacol. 2013;8:621–642. doi: 10.1007/s11481-013-9443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human Immunodeficiency Virus (HIV) Infection of human macrophages is increased by Dopamine : a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175:1148–1159. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Carvallo L, Eugenin EA, Berman JW. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. J Neuroinflammation. 2012;9:203. doi: 10.1186/1742-2094-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW. Dopamine Receptor Activation Increases HIV Entry into Primary Human Macrophages. PLoS One. 2014;9:e108232. doi: 10.1371/journal.pone.0108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Hanson GR, Fleckenstein AE. Amphetamine and methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. J Neurochem. 2012;123:288–297. doi: 10.1111/j.1471-4159.2012.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, Dasgupta PS. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol. 2003;3 doi: 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. 1996;379:606. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gnegy ME, Khoshbouei H, Berg KA, Javitch JA, Clarke WP, Zhang M, Galli A. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol. 2004;66:137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González H, Contreras F, Prado C, Elgueta D, Franz D, Bernales S, Pacheco R. Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J Immunol. 2013;190:5048–5056. doi: 10.4049/jimmunol.1203121. [DOI] [PubMed] [Google Scholar]
- Goodwin JS. Amphetamine and Methamphetamine Differentially Affect Dopamine. 2009:2978–89. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorentla BK, Vaughan RA. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology. 2005;49:759–768. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gruden MA, Sewell RD, Yanamandra K, Davidova TV, Kucheryanu VG, Bocharov EV, Bocharova OA, Polyschuk VV, Sherstnev VV, Morozova-Roche LA. Immunoprotection against toxic biomarkers is retained during Parkinson’s disease progression. J Neuroimmunol. 2011;233:221–227. doi: 10.1016/j.jneuroim.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269:7124–7130. [PubMed] [Google Scholar]
- Guptaroy B, Fraser R, Desai A, Zhang M, Gnegy ME. Site-directed mutations near transmembrane domain 1 alter conformation and function of norepinephrine and dopamine transporters. Mol Pharmacol. 2011:520–532. doi: 10.1124/mol.110.069039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptaroy B, Zhang M, Bowton E, Binda F, Shi L, Weinstein H, Galli A, Javitch JA, Neubig RR, Gnegy ME. A juxtamembrane mutation in the N terminus of the dopamine transporter. Mol Pharmacol. 2009:514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagino Y, Takamatsu Y, Yamamoto H, Iwamura T, Murphy DL, Uhl GR, Sora I, Ikeda K. Effects of MDMA on extracellular dopamine and serotonin levels in mice lacking dopamine and/or serotonin transporters. Curr Neuropharmacol. 2011:91–95. doi: 10.2174/157015911795017254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanami K, Nakano K, Saito K, Okada Y, Yamaoka K, Kubo S, Kondo M, Tanaka Y. Dopamine D2-like receptor signaling suppresses human osteoclastogenesis. Bone. 2013;56:1–8. doi: 10.1016/j.bone.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A. Genetics of Parkinson’s disease and parkinsonism. Ann Neurol. 2006;60:389–398. doi: 10.1002/ana.21022. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Deitch EA. Dopamine suppresses IL-12 p40 production bylipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediatedmechanism. J Neuroimmunol. 2002;122 doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- He L, Vasiliou K, Nebert DW. Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics. 2009:195–205. doi: 10.1186/1479-7364-3-2-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hitzig P. Combined use of dopamine and serotonin agonists in the treatment of immune disorders. Google Patents 1997 [Google Scholar]
- Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WC, Amara SG. J Biol Chem. 9650 Rockville Pike, Bethesda, MD 20814, U.S.A: 2010. Membrane Cholesterol Modulates the Outward Facing Conformation of the Dopamine Transporter and Alters Cocaine Binding; pp. 32616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Halliday GM. Aspects of innate immunity and Parkinson’s disease. Front Pharmacol. 2012;3 doi: 10.3389/fphar.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In SW, Son EW, Rhee DK, Pyo S. Modulation of murine macrophage function by methamphetamine. J Toxicol Environ Health A. 2004;67:1923–1937. doi: 10.1080/15287390490514589. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Iversen LL. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005a;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Johnson LAA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of Amphetamine-stimulated Dopamine Efflux by Protein Kinase C β. J Biol Chem. 2005b;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Jones KT, Zhen J, Reith ME. Importance of cholesterol in dopamine transporter function. J Neurochem. 2012;123:700–715. doi: 10.1111/jnc.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose PA, Eisner GM, Felder RA. Regulation of blood pressure by dopamine receptors. Nephron Physiol. 2003;95:19–27. doi: 10.1159/000073676. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. PNAS. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI, Zomot E. Sodium-coupled neurotransmitter transporters. Chem Rev. 2008;108:1654–1668. doi: 10.1021/cr078246a. [DOI] [PubMed] [Google Scholar]
- Katritch V, Reynolds KA, Cherezov V, Hanson MA, Roth CB, Yeager M, Abagyan R. Analysis of full and partial agonists binding to β(2)-adrenergic receptor suggests a role of transmembrane Helix V in agonist-specific conformational changes. J Mol Recognit. 2009;22:307–318. doi: 10.1002/jmr.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavelaars A, Cobelens PM, Teunis MA, Heijnen CJ. Changes in innate and acquired immune responses in mice with targeted deletion of the dopamine transporter gene. J Neuroimmunol. 2005;161:162–168. doi: 10.1016/j.jneuroim.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced Dopamine Efflux: a voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem. 2003;278:12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- Kilty J, Lorang D, Amara S. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991;254:578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Kokkinou I, Nikolouzou E, Hatzimanolis A, Fragoulis EG, Vassilacopoulou D. Expression of enzymatically active L-DOPA decarboxylase in human peripheral leukocytes. Blood Cells Mol Dis. 2009;42:92–98. doi: 10.1016/j.bcmd.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Korn T, Kallies A. T cell responses in the central nervous system. 2017;17:179–194. doi: 10.1038/nri.2016.144. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jørgensen TN, Sørensen L, Eriksen J, Loland CJ, Strømgaard K, Gether U. SLC6 Neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kurian MA. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. 2009:1595–603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, Hoffmann GF, Jardine P, von Moers A, Mordekar SR, O’Callaghan F, Wassmer E, Wraige E, Dietrich C, Lewis T, Hyland K, Heales S, Jr, Sanger T, Gissen P, Assmann BE, Reith ME, Maher ER. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol. 2011;10:54–62. doi: 10.1016/S1474-4422(10)70269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustrimovic N, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, Comi C, Mauri M, Minafra B, Riboldazzi G, Sanchez-Guajardo V, Marino F, Cosentino M. Dopaminergic Receptors on CD4+ T Naive and Memory Lymphocytes Correlate with Motor Impairment in Patients with . Parkinson’s Disease. 2016;6:33738. doi: 10.1038/srep33738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite M. Dopamine in the immune system: dopamine receptors in immune cells, potent effects, endogenous production and involvement in immune and neuropsychiatric diseases. In: Levite M, editor. Nerve-Driven Immunity: Neurotransmitters and Neuropeptides in the Immune System. Springer; Vienna, Vienna: 2012. pp. 1–45. [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb J. Remission of rheumatoid arthritis and other disorders of immunity in patients taking monoamine oxidase inhibitors. Int J Immunopharmacol. 1983;5:353–357. doi: 10.1016/0192-0561(83)90039-5. [DOI] [PubMed] [Google Scholar]
- Lieberknecht V, Junqueira SC, Cunha MP, Barbosa TA, de Souza LF, Coelho IS, Santos ARS, Rodrigues ALS, Dafré AL, Dutra RC. Pramipexole, a Dopamine D2/D3 receptor-preferring agonist, prevents experimental autoimmune encephalomyelitis development in mice. Mol Neurobiol. 2017;54:1033–1045. doi: 10.1007/s12035-016-9717-5. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Gether U. Defining proximity relationships in the tertiary structure of the dopamine transporter identification of a conserved glutamic acid as a third coordinate in the endogenous Zn2+-binding site. J Biol Chem. 1999;274:36928–36934. doi: 10.1074/jbc.274.52.36928. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn2+ switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci. 2002;99:1683–1688. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço GA, Dorce VAC, Palermo-Neto J. Haloperidol treatments increased macrophage activity in male and female rats: influence of corticosterone and prolactin serum levels. Eur Neuropsychopharmacol. 2005;15:271–277. doi: 10.1016/j.euroneuro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Mack CL, Vanderlugt-Castaneda CL, Neville KL, Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler’s virus model of multiple sclerosis. J Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Manepalli S, Surratt CK, Madura JD, Nolan TL. Monoamine transporter structure, function, dynamics, and drug discovery: a computational perspective. Aaps J. 2012;14:820–831. doi: 10.1208/s12248-012-9391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Goncalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DA, Dawson, Ko HS, Dawson TM. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353 doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, Oakes D, Seibyl J. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology. 2001;57:2089–2094. doi: 10.1212/wnl.57.11.2089. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Marino F, Cosentino M, Bombelli R, Ferrari M, Lecchini S, Frigo G. Endogenous catecholamine synthesis, metabolism storage, and uptake in human peripheral blood mononuclear cells. Exp Hematol. 1999;27:489–495. doi: 10.1016/s0301-472x(98)00057-5. [DOI] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Mastroeni D, Grover A, Leonard B, Joyce JN, Coleman PD, Kozik B, Bellinger DL, Rogers J. Microglial responses to dopamine in a cell culture model of Parkinson’s disease. Neurobiol Aging. 2009;30:1805–1817. doi: 10.1016/j.neurobiolaging.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 2002;132 doi: 10.1016/s0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Mignini F, Tomassoni D, Traini E, Amenta F. Dopamine, vesicular transporters and dopamine receptor expression and localization in rat thymus and spleen. J Neuroimmunol. 2009;206:5–13. doi: 10.1016/j.jneuroim.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Miranda M, Dionne KR, Sorkina T, Sorkin A. Three Ubiquitin Conjugation Sites in the Amino Terminus of the Dopamine Transporter Mediate Protein Kinase C–dependent Endocytosis of the Transporter. In: Lemmon S, editor. Mol Biol Cell. 2007. pp. 313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by Protein Kinase C. J Biol Chem. 2005;280:35617–35624. doi: 10.1074/jbc.M506618200. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78 doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994a;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994b;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mor DE, Tsika E, Mazzulli JR, Gould NS, Kim H, Daniels MJ, Doshi S, Gupta P, Grossman JL, Tan VX, Kalb RG, Caldwell KA, Caldwell GA, Wolfe JH, Ischiropoulos H. Dopamine induces soluble alpha-synuclein oligomers and nigrostriatal degeneration. Nat Neurosci. 2017;20:1560–1568. doi: 10.1038/nn.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kabashima K, Fukamachi S, Kuroda E, Sakabe JI, Kobayashi M, Nakajima S, Nakano K, Tanaka Y, Matsushita S, Nakamura M, Tokura Y. D1-like dopamine receptors antagonist inhibits cutaneous immune reactions mediated by Th2 and mast cells. J Dermatol Sci. 2013;71:37–44. doi: 10.1016/j.jdermsci.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harbor Perspect Med. 2012;2:a009381. doi: 10.1101/cshperspect.a009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both β2- and β3-adrenergic receptors. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagome K, Imamura M, Okada H, Kawahata K, Inoue T, Hashimoto K, Harada H, Higashi T, Takagi R, Nakano K, Hagiwara K, Kanazawa M, Dohi M, Nagata M, Matsushita S. Dopamine D1-like receptor antagonist attenuates Th17-mediated immune response and ovalbumin antigen-induced neutrophilic airway inflammation. J Immunol. 2011;186:5975–5982. doi: 10.4049/jimmunol.1001274. [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: Preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun. 2008;373:286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Takagi R, Hashimoto K, Tanaka Y, Matsushita S. Dopamine released by dendritic cells polarizes Th2 differentiation. Int Immunol. 2009;21:645–654. doi: 10.1093/intimm/dxp033. [DOI] [PubMed] [Google Scholar]
- Nakashioya H, Nakano K, Watanabe N, Miyasaka N, Matsushita S, Kohsaka H. Therapeutic effect of D1-like dopamine receptor antagonist on collagen-induced arthritis of mice. Mod Rheumatol. 2011;21:260–266. doi: 10.1007/s10165-010-0387-2. [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakar N, Ahmad J, Thiele H, Kubisch C, Rider NL, Morton DH, Strauss KA, Puffenberger EG, D’Agnano D, Anikster Y, Carducci C, Hyland K, Rotstein M, Leuzzi V, Borck G, Reith ME, Kurian MA. Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain. 2014;137:1107–1119. doi: 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norregaard L, Loland CJ, Gether U. Evidence for distinct sodium-, dopamine-, and cocaine-dependent conformational changes in transmembrane segments 7 and 8 of the dopamine transporter. J Biol Chem. 2003;278:30587–30596. doi: 10.1074/jbc.M303854200. [DOI] [PubMed] [Google Scholar]
- Oaks AW, Marsh-Armstrong N, Jones JM, Credle JJ, Sidhu A. Synucleins antagonize endoplasmic reticulum function to modulate dopamine transporter trafficking. PLoS One. 2013;8:e70872. doi: 10.1371/journal.pone.0070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KE, Gendelman HE. Immunomodulation as a neuroprotective and therapeutic strategy for Parkinson’s disease. Curr Opin Pharmacol. 2016;26:87–95. doi: 10.1016/j.coph.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’sullivan SS, Evans AH, Lees AJ. Punding in Parkinson’s disease. Pract Neurol. 2007;7:397–399. doi: 10.1136/jnnp.2007.129015. [DOI] [PubMed] [Google Scholar]
- Papachroni KK, Ninkina N, Papapanagiotou A, Hadjigeorgiou GM, Xiromerisiou G, Papadimitriou A, Kalofoutis A, Buchman VL. Autoantibodies to alpha-synuclein in inherited Parkinson’s disease. J Neurochem. 2007;101:749–756. doi: 10.1111/j.1471-4159.2006.04365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinoli M, Marino F, Cosentino M. Dopaminergic regulation of innate immunity: a review. J Neuroimmune Pharmacol. 2017 doi: 10.1007/s11481-017-9749-2. [DOI] [PubMed] [Google Scholar]
- Podolec Z, Vetulani J, Bednarczyk B, Szczeklik A. Central Dopamine Receptors Regulate Blood Eosinophilia in the Rat. 1979;34:103–110. doi: 10.1111/j.1398-9995.1979.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Prado C, Contreras F, Gonzalez H, Diaz P, Elgueta D, Barrientos M, Herrada AA, Lladser A, Bernales S, Pacheco R. Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J Immunol. 2012;188:3062–3070. doi: 10.4049/jimmunol.1103096. [DOI] [PubMed] [Google Scholar]
- Prinz M, Erny D, Hagemeyer N. Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol. 2017;18:385–392. doi: 10.1038/ni.3703. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+ CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukocyte Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Richards TL. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. 2009;108:1575–1584. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Saha K, Krout D, Cabrera E, Felts B, Henry LK, Swant J, Zou MF, Newman AH, Khoshbouei H. Membrane potential shapes regulation of dopamine transporter trafficking at the plasma membrane. Nat Commun. 2016;7:10423. doi: 10.1038/ncomms10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnberg E, Calounova G, Pejler G. Mast cells express tyrosine hydroxylase and store dopamine in a serglycin-dependent manner. Biol Chem. 2012;393:107–112. doi: 10.1515/BC-2011-220. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Dersch CM, Ananthan S, Partilla JS. Studies of the biogenic amine transporters. 13. Identif. J Pharmacol Exp Ther. 2009:718–728. doi: 10.1124/jpet.108.149088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubi B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance. Endocrinology. 2010;151:5570–5581. doi: 10.1210/en.2010-0745. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Saier MH, Jr, Yen MR, Noto K, Tamang DG, Elkan C. The Transporter Classification Database: recent advances. Nucl Acids Res. 2009;37:D274–D278. doi: 10.1093/nar/gkn862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Crotti A, Glass CK. Regulation of microglia activation and deactivation by nuclear receptors. 2013;61:104–111. doi: 10.1002/glia.22423. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Impellizzieri D, Basso C, Laroni A, Uccelli A, Lanzavecchia A, Engelhardt B. T-cell trafficking in the central nervous system. Immunol Rev. 2012;248:216–227. doi: 10.1111/j.1600-065X.2012.01140.x. [DOI] [PubMed] [Google Scholar]
- Sambo DO, Lin M, Owens A, Lebowitz JJ, Richardson B, Jagnarine DA, Shetty M, Rodriquez M, Alonge T, Ali M, Katz J, Yan L, Febo M, Henry LK, Bruijnzeel AW, Daws L, Khoshbouei H. The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission. Nat Commun. 2017;8:2228. doi: 10.1038/s41467-017-02087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar C, Das S, Chakroborty D, Chowdhury UR, Basu B, Dasgupta PS, Basu S. Cutting Edge: Stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J Immunol. 2006;177:7525–7529. doi: 10.4049/jimmunol.177.11.7525. [DOI] [PubMed] [Google Scholar]
- Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, Dalod M, Soumelis V, Amigorena S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Sen N, Shi L, Beuming T, Weinstein H, Javitch JA. A pincer-like configuration of TM2 in the human dopamine transporter is responsible for indirect effects on cocaine binding. Neuropharmacology. 2005;49:780–790. doi: 10.1016/j.neuropharm.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Seol IW, Kuo NY, Kim KM. Effects of dopaminergic drugs on the mast cell degranulation and nitric oxide generation in RAW 264.7 cells. Arch Pharm Res. 2004;27:94–98. doi: 10.1007/BF02980053. [DOI] [PubMed] [Google Scholar]
- Shan J, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. In: Uversky V, editor. PLoS One. San Francisco, USA: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonar SA, Shaikh S, Joshi N, Atre AN, Lal G. IFN-gamma promotes transendothelial migration of CD4+ T cells across the blood-brain barrier. Immunol Cell Biol. 2017 doi: 10.1038/icb.2017.56. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. The Journal of Neuroscience. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookhai S, Wang JH, McCourt M, O’Connell D, Redmond HP. Dopamine induces neutrophilapoptosis through a dopamine D-1 receptor-independent mechanism. Surgery. 1999;126 [PubMed] [Google Scholar]
- Sookhai S, Wang JH, Winter D, Power C, Kirwan W, Redmond HP. Dopamine attenuates thechemoattractant effect of interleukin-8: a novel role in the systemic inflammatoryresponse syndrome. Shock. 2000;14 doi: 10.1097/00024382-200014030-00009. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Caltagarone J, Sorkin A. Flotillins regulate membrane mobility of the dopamine transporter but are not required for its Protein Kinase C dependent endocytosis. Traffic. 2013;14:709–724. doi: 10.1111/tra.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and Protein Kinase C-Induced Internalization of the Dopamine Transporter is Mediated by a Clathrin-Dependent Mechanism. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Yang JW, Montgomery TR, Chen WQ, Winkler MT, Sucic S, Lubec G, Freissmuth M, Elgersma Y, Sitte HH, Kudlacek O. Ca2+/Calmodulin-dependent Protein Kinase IIα (αCaMKII) controls the activity of the dopamine transporter: implications for angelman syndrome. J Biol Chem. 2012;287:29627–29635. doi: 10.1074/jbc.M112.367219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, Ward C, Halliday GM. Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol. 2012;252:95–99. doi: 10.1016/j.jneuroim.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546:656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swant J, Goodwin JS, North A, Ali AA, Gamble-George J, Chirwa S, Khoshbouei H. alpha-Synuclein stimulates a dopamine transporter-dependent chloride current and modulates the activity of the transporter. J Biol Chem. 2011;286:43933–43943. doi: 10.1074/jbc.M111.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Mori F, Imaizumi T, Yoshida H, Matsumiya T, Tamo W, Yoshimoto M, Odagiri H, Sasaki M, Takahashi H, Satoh K, Wakabayashi K. Upregulation of alpha-synuclein by lipopolysaccharide and interleukin-1 in human macrophages. Pathol Int. 2002;52:572–577. doi: 10.1046/j.1440-1827.2002.01385.x. [DOI] [PubMed] [Google Scholar]
- Tarazona R, Gonzalez-Garcia A, Zamzami N, Marchetti P, Frechin N, Gonzalo JA, Ruiz-Gayo M, van Rooijen N, Martinez C, Kroemer G. Chlorpromazine amplifies macrophage-dependent IL-10 production in vivo. J Immunol. 1995;154 [PubMed] [Google Scholar]
- Torres KC, Antonelli LR, Souza AL, Teixeira MM, Dutra WO, Gollob KJ. Norepinephrine, dopamine and dexamethasone modulate discrete leukocyte subpopulations andcytokine profiles from human PBMC. J Neuroimmunol. 2005;166 doi: 10.1016/j.jneuroim.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Trabold B, Gruber M, Frohlich D. Functional and phenotypic changes in polymorphonuclear neutrophils induced by catecholamines. Scand Cardiovasc J. 2007;41:59–64. doi: 10.1080/14017430601085948. [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci. 2013;34 doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein Kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley Laura A, Luk Kelvin C, Patel Tapan P, Tanik Selcuk A, Riddle Dawn M, Stieber A, Meaney David F, Trojanowski John Q, Lee Virginia MY. Exogenous α-synuclein fibrils induce lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall SC, Gu H, Rudnick G. Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol Pharmacol. 1995;47:544–550. [PubMed] [Google Scholar]
- Wang KH, Penmatsa A, Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. 2015;521:322–327. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Nakayama T, Nagakubo D, Hieshima K, Jin Z, Katou F, Hashimoto K, Yoshie O. Dopamine selectively induces migration and homing of naive CD8+ T cells via dopaminereceptor D3. J Immunol. 2006;176 doi: 10.4049/jimmunol.176.2.848. [DOI] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Hawkins BE, Thomas ML, Cunningham KA, Jakubas AA. Estradiol effects on the dopamine transporter – protein levels, subcellular location, and function. J Mol Signal. 2006;5 doi: 10.1186/1750-2187-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. 1986;9:271–277. [Google Scholar]
- Wenisch C, Parschalk B, Weiss A, Zedwitz-Liebenstein K, Hahsler B, Wenisch H, Georgopoulos A, Graninger W. High-dose catecholamine treatment decreases polymorphonuclear leukocyte phagocytic capacity and reactive oxygen production. Clin Diagn Lab Immunol. 1996;3:423–428. doi: 10.1128/cdli.3.4.423-428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Bellve KD, Fogarty KE, Melikian HE. Ack1 is a dopamine transporter endocytic brake that rescues a trafficking-dysregulated ADHD coding variant. Proc Natl Acad Sci USA. 2015;112:15480–15485. doi: 10.1073/pnas.1512957112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- Yao Y, Yang D, Han Y, Wang W, Wang N, Yang J, Zeng C. Dopamine D1-Like Receptors Suppress the Proliferation of Macrophages Induced by Ox-LDL. 2016 doi: 10.1159/000438640. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Chung SJ, Moon H, Oh JS, Kim JS, Hong JY, Ye BS, Sohn YH, Lee PH. Presynaptic dopamine depletion determines the timing of levodopa-induced dyskinesia onset in Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2017 doi: 10.1007/s00259-017-3844-8. [DOI] [PubMed] [Google Scholar]
- Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, Jaligam V, Oz M, Jayanthi LD, Samuvel DJ, Ramamoorthy S, Shippenberg TS. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282:35842–35854. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- Zappia M, Crescibene L, Bosco D, Arabia G, Nicoletti G, Bagala A, Bastone L, Napoli I, Caracciolo M, Bonavita S. Anti-GM1 ganglioside antibodies in Parkinson’s disease. Acta Neurologica Scandinavica. 2002;106:54–57. doi: 10.1034/j.1600-0404.2002.01240.x. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zellweger R, Wichmann MW, Ayala A, Chaudry IH. Effects of prolactin and metoclopramide on macrophage cytokine gene expression in late sepsis. Cytokine. 1997;9:437–446. doi: 10.1006/cyto.1996.0186. [DOI] [PubMed] [Google Scholar]


