SUMMARY
Understanding the molecular defects underlying cardiovascular disease is necessary for the development of therapeutics. The most common method to lower circulating lipids, which reduces the incidence of cardiovascular disease, is statins, but other drugs are now entering the clinic, some of which have been approved. Nevertheless, patients cannot tolerate some of these therapeutics, the drugs are costly, or the treatments are approved for only rare forms of disease. Efforts to find alternative treatments have focused on other factors, such as apolipoproteinB (apoB), which transports cholesterol in the blood stream. The levels of apoB are regulated by endoplasmic reticulum (ER) associated degradation as well as by a post ER degradation pathway in model systems, and we suggest that these events provide novel therapeutic targets. We discuss first how cardiovascular disease arises and how cholesterol is regulated, and then summarize the mechanisms of action of existing treatments for cardiovascular disease. We then review the apoB biosynthetic pathway, focusing on steps that might be amenable to therapeutic interventions.
Keywords: Cardiovascular Disease, Cholesterol, ApolipoproteinB, Very Low Density Lipoprotein (VLDL), Endoplasmic Reticulum Associated Degradation (ERAD), Autophagy
1.0 INTRODUCTION
Cardiovascular disease is one of the leading causes of mortality worldwide, accounting for ~31% of all deaths [1]. In the United States, this disease is responsible for more deaths than the next two conditions, cancer and chronic lower respiratory disease, combined. The most recent report from the American Heart Association estimates that approximately 1 in 3 people will be affected by some form of cardiovascular disease in their lifetime [2]. It is no surprise that a better understanding of biological pathways to combat this disease is imperative.
Coronary artery disease (CAD) is the most prevalent form of cardiovascular disease, and its incidence has been steadily increasing, representing about 20% of all cases of cardiovascular disease in 2011 to a current level of ~45% of all cases [2]. Stroke is the second most common condition, accounting for 16.5% of all deaths [3, 4]. Both of these maladies are the direct consequences of atherosclerosis, which begins as a response to the retention in the arteries of apolipoprotein B (apoB)-containing lipoproteins [5]. These lipoproteins primarily transport triacylglycerol, along with cholesterol, which is mainly synthesized in the liver but accumulates in macrophages in atherosclerotic plaques. Even though triacylglycerol is the main component of the particles, the level of low density lipoprotein (LDL) cholesterol is the statistically strongest single risk factor for CAD, which has stimulated interest in the pathways that control its metabolism. However, links between CAD and circulating triacylglycerol have also been noted [6]. Of particular relevance to this review is that LDL is not directly produced by the liver, but is a metabolic product of very low density lipoproteins (VLDL), the primary apoB-containing lipoprotein produced by the liver. Thus, to understand LDL production, one must start with the assembly and secretion of VLDL.
2.0 LIPOPROTEIN BIOGENESIS
2.1 Properties of Lipoprotein Particles
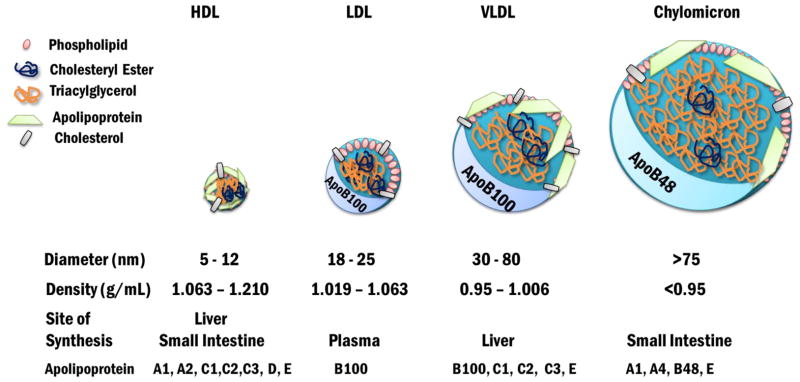
There are four main classes of lipoproteins: high density lipoproteins (HDLs), LDLs, VLDLs, and chylomicrons (Figure 1), each of which is a macromolecular complex containing a neutral lipid core surrounded by an amphipathic phospholipid monolayer as well as apolipoprotein partners. Lipoproteins are classified based on density and size. HDLs are the most dense particles (1.063–1.210 g/mL), whereas chylomicrons are the least dense (<0.95 g/mL) [7–10]. Each class has a range of diameters, ranging in size from 5–1000 nm, with the diameter inversely related to density [10]. The relative percent composition of, for example, an LDL particle is 23% protein, 10% triacylglycerol, 45% cholesterol and cholesterol esters, and 20% phospholipid, and for a VLDL particle it is 10% protein, 50% triacylglycerol, 19% cholesterol and cholesterol esters, and 18% phospholipid.
Figure 1. Lipoprotein Particles of Blood Plasma.
The four main classes of plasma lipoproteins are HDL, LDL, VLDL, and chylomicrons. HDLs are the smallest particles and are the most dense. Chylomicrons are the largest particle and are the least dense. LDL and VLDL contain apoB100 as their major apolipoprotein while chylomicrons in humans contain apoB48. HDL contains apoAI and apoAII as the major apolipoproteins. VLDL is synthesized in the liver whereas chylomicrons are synthesized in the small intestine. LDL is a product of VLDL modifications made in circulation. HDL is made in both tissues.
Apolipoproteins provide structural rigidity by scaffolding the growing lipoprotein, and due to their amphipathic nature they act as detergents to solubilize fatty acids (i.e., mainly triacylglycerol) and cholesterol. Apolipoproteins also bind to select cell surface receptors, regulate the enzymes involved in lipid metabolism in the circulation, and can be transferred between lipoproteins [7, 10]. In this review, we will focus on apoB, but there are at least 10 apolipoproteins found in human blood plasma.
ApoB-containing lipoproteins are associated with delivery of triacylglycerol and cholesterol transported by LDLs, VLDLs, and chylomicrons. As a result of its scaffolding function, apoB provides the backbone for lipoprotein particles during their synthesis, maturation and modification. The full length apoB protein in LDLs and VLDLs also contains a recognition motif for LDL receptor binding and is present in only a single copy in LDLs, VLDLs, and chylomicrons [7, 10]. In contrast to apoB, the exchangeable apolipoproteins include apolipoprotein AI, CI, CII, CIII, and E [7, 10].
The apoB gene is transcribed and translated into a ~550 kDa full length protein, known as apoB100, but can also be processed into an isoform that contains the N-terminal 48% of apoB, termed apoB48 [10], which is discussed further in the next section. In the small intestine of humans, where apoB48 is required for chylomicron formation (also see below), the apoB mRNA editing complex-1 (Apobec-1) is expressed. Apobec-1 is the founding member of a large family of RNA editing enzymes that deaminate nucleotides [11–13], and deamination of the apoB100 mRNA at codon 2153 changes a CAA to a UAA, converting a glutamine into a stop codon in the mRNA [11, 14, 15]. Interestingly, rodent livers also express Apobec-1, producing both apoB100 and apoB48 in the same tissues.
2.2 Very Low Density Lipoprotein Maturation and Secretion
2.2.1 Introduction
Chylomicrons are formed in enterocytes and serve to transport lipids that were absorbed from dietary and biliary sources. Their major lipid content is triacylglycerol, and the protein component includes a number of apolipoproteins, with apoB48 being a major component. Details about the assembly and metabolism of chylomicrons will not be further discussed, but the reader is referred to recent reviews [16, 17]. Similarly, primordial VLDL is initially assembled in the ER in a process in which membrane bound ribosomes synthesize apoB (i.e. apoB100 in human liver, apoB100 and apoB48 in rodent liver) and the protein is then translocated into the ER through the Sec61 translocation channel. Next, apoB is co-translationally lipidated by the MTP complex in the ER [18, 19]. The MTP complex consists of an “M” subunit and a molecule of protein disulfide isomerase (PDI). The M subunit is required for lipid transfer while PDI, which in other cases facilitates disulfide bond formation on nascent ER proteins, acts as a chaperone for the M moiety [20, 21]. PDI is also required for the proper folding of apoB, which contains 8 intramolecular disulfide bonds, but this may result from a separate pool of PDI that is distinct from that complexed with MTP [22]. The net result of the MTP-mediated transfer of lipids, which mainly include triglycerides but also cholesterol ester and phospholipids, is a primordial VLDL particle. Two lipid associating domains in apoB are sites for the co-translational association of lipids by the MTP complex, facilitating apoB’s incorporation into a pre-VLDL (or chylomicron) only when triglycerides and other neutral lipids are sufficiently abundant [23, 24]. In this way, as apoB is synthesized it is continuously available to assemble with lipids as they are derived from endogenous or dietary sources.
The resulting particle exits the ER in COPII coated vesicles, but there is some diversity of opinion whether these vesicles are conventional or require specialized factors [25–27]. It was recently proposed that TANGO, a receptor for pre-collagen (another large secretory protein), and its partner TALI recruit lipids to ER exit sites to facilitate pre-VLDL secretion [28]. However, another factor that clearly impacts VLDL secretion from the ER is KLHL12, which co-localizes with apoB and facilitates the construction of transport vesicles from the ER that are also able to accommodate large cargo (e.g., collagen) [27, 29]. In addition, a number of lipid droplet-related factors have been implicated as regulators of how much triacylglycerol is loaded onto nascent VLDL particles in the ER. These include TM6SF2, Cideb, and Ces1. The first two appear to function in the transport of neutral lipids (particularly triacylglycerol) from lipid droplets to the ER. The third is an enzyme that resides in the ER, coordinating both triacylglycerol loading of VLDL and the hydrolysis of triacylglycerol in lipid droplets [30–32].
In the Golgi, the pre-VLDL particles are further lipidated and apoB is posttranslationally modified and conformationally altered, which results in the mature VLDL particle [26, 33–37]. After exiting the Golgi, the particles enter the blood stream and migrate to peripheral tissues. Upon reaching endothelial cells lining the blood vessels in peripheral tissues, the apoCII component in VLDL activates the plasma membrane-associated lipoprotein lipase in endothelial cells. Through its hydrolytic activity, lipoprotein lipase releases free fatty acids from the triacylglycerols, which are taken up into the endothelial cells and transferred to the underlying cells, such as heart muscle, skeletal muscle, and adipose tissue. These molecules are in turn oxidized for ATP production or stored for future energy generation [7].
Due to the reduced amount of hydrolyzed triacylglycerol, the VLDL particle is relatively enriched in cholesterol and is referred to as a VLDL remnant or intermediate density lipoprotein (IDL), which can also deliver cholesterol to cells. Promoting the clearance of VLDL and IDL is the conformational change of another apolipoprotein, apoE. This conformational change follows triacylglycerol hydrolysis, so that the LDL receptor binding domain becomes accessible [38]. The remaining triglycerides are subsequently hydrolyzed in the circulatory system, and the particles are now considered to be a bona fide LDL. LDL then delivers cholesterol to peripheral tissues and the liver because a specific sequence in apoB/LDL binds the LDL receptor (see below), which allows for endocytosis. The resulting LDL-containing endocytic vesicles ultimately transfer LDL to the lysosome.
2.2.2 ApoB structure and isoforms
ApoB is essential for synthesis of chylomicrons and VLDL, and as alluded to above, apo48 and apoB100 are the main structural components of chylomicrons, VLDL, and LDL, and interact with cholesterol, cholesterol esters, phospholipids, and triacylglycerols through extended hydrophobic β-sheets to form a lipoprotein particle. However, apoB must also be able to function in the aqueous environment of blood plasma. Although a structure is currently unavailable, apoB is believed to contain multiple α-helical domains which give rise to its amphipathic nature (Figure 2) [39]. Based on computer predictions, apoB is >25% α-helical, which is common to the exchangeable apolipoproteins. During apoB translocation into the ER, “pause transfer” sequences are also present throughout the protein to aid lipid loading by slowing translocation and allowing for apoB modifications in the ER [40, 41], although the β-sheet domain may contribute substantially to this process as well [42]. In addition, the N-terminal 1000 amino acids in apoB interact with the MTP complex as this region in the protein forms a lipid binding pocket [43].
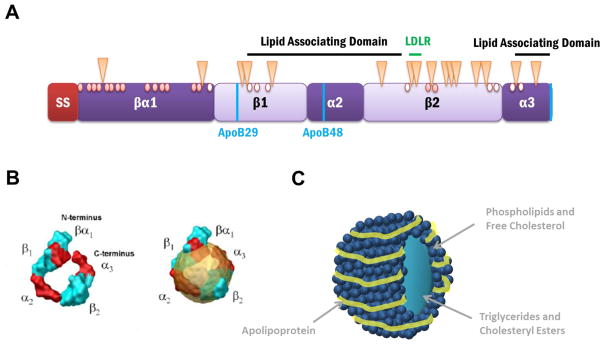
Figure 2. Proposed Structural Domains and Modifications of ApoB.
(A) The predicted apoB domain structure contains a 27 amino acid signal sequence (SS), which targets apoB to the ER membrane and allows for translocation or entry into the ER, and a β-barrel domain, which is directly followed by α-helix, β-sheet, α-helix motifs, and is interspersed by 2 lipid association domains. The naturally occurring isoforms, apoB100 and apoB48, and the hypolipidemic truncation, apoB29, are depicted using vertical blue lines. The LDL receptor binding region is found in the β2 domain and is indicated with a horizontal green line. The 16 glycosylated asparagine residues in apoB are indicated with triangles. Of the 25 cysteines, only 16 participate in disulfide bond formation (red circles) while the remaining 9 are free sulfhydryls (open circles).
(B) Computer simulated model of proposed apoB structure (left) alone and (right) in a lipoprotein. The image is reproduced with permission from [49].
(C) A representation of a lipoprotein particle. The apolipoprotein wraps around the circumference of the lipoprotein which consists of phospholipids, free cholesterol, triglycerides, and cholesteryl esters.
The current view of the overall apoB domain structure is that it is organized in a βα1-β1-α2-β2-α3 fashion [44]. The N-terminus is termed βα1, as it is predicted to form a β-barrel followed by an α-helical region based upon homology to lipovitellin, an egg yolk lipoprotein [44–46]. ApoB has two additional, large β-sheet-rich domains from amino acids 827-2001 and 2571-4032. There are also two smaller α-helical domains containing amino acids 2045-2587 and 4017-4515 [43, 44, 46]. In addition, apoB contains 2 lipid associating domains that span amino acids 1701-3101 and 4101-4536 [44, 47]. The LDL receptor binding region resides near the C-terminus between amino acids 3345-3381, and allows for receptor mediated endocytosis [47, 48]. It is also thought that the N- and C-termini interact with one another once apoB is completely synthesized to provide structural rigidity to the spherical lipoprotein particle (Figure 2B and C) [49].
ApoB also contains 25 cysteine resides but only 16 of these residues are used to form 8 disulfide bonds (Figure 2A) [34, 50]. The formation of these bonds helps assemble the final structure of the protein and hence the lipoprotein particle as each domain can fold co-translationally as disulfide bonds are formed. Furthermore, as with many secretory proteins, apoB is glycosylated by the oligosaccharyltransferase in the ER. ApoB has 19 potential sites for glycosylation, which are designated by an asparagine-X-serine/threonine motif (where X can be any amino acid except proline), but only 16 of these sites are used. In total, these secondary modifications likely position and stabilize apoB in the lipoprotein particle during synthesis and while circulating in plasma. Each modification is also carefully orchestrated to ensure proper synthesis, folding, and incorporation into lipoprotein particles. For example, if cysteine 4326 is mutated, then apoB fails to incorporate into a lipoprotein [51]. Furthermore, if the cysteines involved in forming disulfide bonds 2 and 4 (the side chains of amino acids 51 and 70 and 218 and 234) are substituted, then apoB secretion is reduced to 3% of wildtype levels and is unable to adopt its proper conformation [52]. Moreover, if cysteines located in the N-terminus are mutated, then apoB similarly fails to form a lipoprotein and the protein is rapidly degraded by the proteasome [53].
A homozygous knockout of apoB results in embryonic lethality in mice, whereas heterozygous knockout mice developed normally but are protected from hypercholesterolemia [43, 54]. Over 100 coding and non-coding polymorphisms have been identified in the APOB gene and several significantly alter cholesterol homeostasis [7, 55]. For example, a rare arginine to proline substitution mutation at codon 3480 in the α-helical region (see below) results in hypobetalipoproteinemia [55]. Hypobetalipoproteinemia is characterized by very low levels of circulating apoB-containing lipoproteins and the lipids associated with them. This can result in steatosis (from decreased triacylglycerol export from the liver) and fat and vitamin malabsorption (from impaired chylomicron assembly), as well as a failure to thrive. Two other common substitutions are found in the 27 amino acid signal peptide through which apoB is targeted to and translocated into the ER. These mutations consequently affect the ability of apoB to become incorporated into lipoprotein particles [56]. Yet another alteration in the apoB encoding gene generates a premature stop codon due to a C to T nucleotide transition [57, 58]. Even though the resulting protein harbors only the N-terminal 29% of apoB, lipids still associate with the truncated product but lipid-poor VLDL particles are ultimately produced. This apoB29 protein—which has a radius of ~42.9 Å and a density of 1.50 g/ml [59]—traffics through the secretory pathway as efficiently as larger apoB variants, but smaller sized lipoprotein particles are evident [60]. However, this species is undetectable in plasma, possibly due to decreased apoB synthesis or increase lipoprotein clearance [57]. Interestingly, a condition known as familial defective apoB100 results from by a substitution of glutamine or tryptophan for arginine at residue 3500 in apoB100 and is primarily linked to significantly reduced binding between apoB and the LDL receptor [61]. This mutation affects ~1 in 500 Caucasians and leads to high levels of serum LDL cholesterol [62].
2.2.3 The intracellular level of apoB is regulated by Endoplasmic Reticulum Associated Degradation (ERAD) as well as by Post-ER Pre-Secretory Proteolysis (PERPP)
Nearly one third of all newly synthesized proteins enter the secretory pathway. Once these proteins enter the ER, they fold, establish intra-domain interactions, and assemble with additional protein partners or subunits (if necessary). If secreted proteins do not achieve their proper conformations, they risk damaging the cell, which may manifest as human disease [63]. Therefore, the cell must have a way to monitor and remove such proteins. One route to eliminate aberrant proteins in the secretory pathway is through ER associated degradation (ERAD). ERAD is a quality control pathway that normally monitors and selectively degrades misfolded or misassembled secretory proteins. Thus, the discovery that even wild type forms of apoB are targeted for this pathway was unexpected (see below).
ERAD is generally divided into four steps: substrate recognition, ubiquitination, retrotranslocation, and degradation [63, 64]. Nascent proteins, such as apoB, entering the ER are monitored by molecular chaperones, which prevent the formation of off-pathway folding intermediates, and thereby prevent aggregation and help maintain the protein in a productive folding pathway [65, 66]. More generally, misfolded proteins commonly expose stretches of hydrophobic amino acids. Molecular chaperones bind these amino acid stretches and prevent hydrophobic regions from interacting and potentially aggregating in the hydrophilic environment of the ER lumen [65]. After recognition, an ERAD substrate is tagged with a polyubiquitin motif, which is built on lysine-48 of the ubiquitin moiety and signals degradation by the 26S proteasome [67]. To access this cytoplasmic protease, ERAD substrates must also be removed from the ER lumen or membrane and into the cytoplasm. The mechanical force for this event is provided by an ATPase known as Cdc48 in yeast or p97 in mammals. The Cdc48/p97 protein forms a complex with two cofactors, Npl4 and Ufd1, which help bind ubiquitinated proteins and recruit the protein to the ER membrane [68]. Degradation is then completed by the action of the 26S proteasome, which consists of a 20S core proteolytic particle and a 19S regulatory particle, or “cap”, which binds ubiquitinated proteins and drives substrates into the core in an ATP-dependent process [69]. In addition, there are deubiquitinating enzymes associated with the cap to remove the appended polyubiquitin chain. In this manner, the 26S proteasome efficiently degrades only polyubiquitinated proteins.
As noted above, apoB is a unique ERAD substrate as its levels are regulated by the amount of lipids available for lipoprotein assembly, but not necessarily by the presence of misfolded protein domains. Since these early discoveries, other wild type proteins that are metabolically targeted for ERAD have also been identified [70–72]. The decision-making process that selects apoB for ERAD begins co-translationally as the protein is translocated into the ER through a proteinaceous channel composed of the Sec61 complex [73]. This poises apoB for a quick transition from degradation by the ERAD pathway to incorporation into a pre-VLDL, depending upon successful lipid assembly (Figure 3A). As described above, the MTP complex transfers cholesterol, cholesterol esters, triacylglycerols, and phospholipids to apoB via its lipid associating domains, a process that is further facilitated by pause transfer sequences and β-sheets in apoB [40–43]. These features slow translocation, which confers additional time for lipids to associate with apoB (also see above). Once fully lipidated, translated, and translocated into the ER, apoB exits the ER and enters the secretory pathway to become a mature lipoprotein particle. However, when lipid “ligands” (such as triglycerides) are either in short supply or there is insufficient MTP activity, the presence of apoB is not needed for VLDL assembly, and without stabilization of its conformation by lipids, it begins to misfold (Figure 3B). In this case, apoB translocation slows, the ribosome slightly detaches from the ER through an unknown mechanism, and large loops of apoB are exposed to cytoplasmic factors, including molecular chaperones and the ubiquitination machinery [23, 74, 75]. Again, in contrast to most misfolded, soluble ERAD substrates that utilize the Hrd1 complex as a retrotranslocation channel [76], apoB is instead retrotranslocated back to the cytoplasm through the Sec61 translocation channel.
Figure 3. ApoB is Metabolically Regulated by ERAD.
(A) ApoB is required for the assembly of a pre-VLDL. (1) ApoB is co-translationally translocated into the ER where the MTP complex attaches lipids (purple diamonds) to apoB. (2) This results in a pre-VLDL particle that contains apoB. (3) ApoB exits the ER in a (perhaps non-canonical) COPII coated vesicle. (4) The pre-VLDL can undergo further maturation in the Golgi apparatus. (5) If the particle fully matures, it will be secreted and enter the bloodstream to deliver lipids. If the particle does not fully mature, it can also be sent to the lysosome for degradation by post-ER presecretory proteolysis.
(B) If lipids are limiting or MTP function is blunted, then apoB fails to become lipidated. Translocation slows, exposing large cytoplasmic loops. These loops can be acted upon by chaperones (e.g. Hsp70 and Hsp90) and the ubiquitination machinery. Another chaperone, Hsp110, protects apoB from degradation (see text for details). Once the cytoplasmically-disposed peptide loop is ubiquitinated, apoB is retrotranslocated back through the Sec61 complex and targeted to the proteasome for degradation.
By applying biochemical analyses in mammalian cell systems and through studies in yeast-based biochemical and genetic assays, several chaperones that interact with apoB have been identified. These include the ER luminal lectin, Calnexin, PDI family members that do not reside in the MTP complex (namely ERp57 and ERp72), and the cytosolic Hsp70, Hsp90, and Hsp110 chaperones, which either help to promote (Hsp70, Hsp90) or inhibit (Hsp110) apoB degradation [75, 77–80]. Each of these chaperones has also been shown to play a role in the degradation of other ERAD substrates [81]. The role of Calnexin and ERp57 during the ERAD of apoB is consistent with the interaction of these factors as effectors of nascent protein folding [82], and ERp72 associates with ERdj5, a lumenal disulfide reductase/chaperone that plays a critical role during the retrotranslocation of ERAD substrates by breaking disulfide bonds that might impede transit through the Sec61 pore [83]. Similarly, the Hsp70 and Hsp90 chaperones can deliver substrates to the ubiquitination machinery and can even act as a “bridge” between chaperones and E3 ubiquitin ligases [84, 85]. The function of Sse1, the yeast Hsp110, and Hsp110 in mammalian cells as an apoB protector is particularly intriguing: This chaperone can act as a “holdase”, potentially preventing transfer of a substrate to the degradation machinery, although the essential function of the chaperone appears instead to be as a co-chaperone for Hsp70 [86]. Also in accordance with the requirements for other ERAD substrates, apoB is ubiquitinated on the exposed cytoplasmic loop by the E3 ubiquitin ligase Hrd1 (in a yeast model) or gp78 (in human cells) [87, 88]. As outlined above, apoB is also removed from the ER by the p97 ATPase and degraded by the 26S proteasome [89, 90].
Following translation, translocation, and transport from the ER, the apoB residing in a pre-VLDL particle may be degraded by a post-ER and thus proteasome-independent process after it has reached the Golgi. This phenomenon has been termed Post-ER Pre-Secretory Proteolysis (PERPP), which unlike ERAD, destroys apoB in the presence of normal levels of triglycerides after it has assembled into a pre-VLDL [91, 92]. PERPP is a specialized form of autophagy that occurs post Golgi when the final maturation of the VLDL particle is not successfully completed [35, 93, 94]. In the best characterized example, when liver cells are incubated with polyunsaturated fatty acids (such as fish oils), lipid peroxides derived from these fatty acids cause oxidative damage of apoB in the Golgi and the formation of large aggregates containing modified apoB [91–94]. Because the resulting aggregates are likely too large to be targeted for proteasomal degradation, the particles are instead sequestered into autophagic vesicles and delivered to the lysosome for degradation. Consistent with this idea, when the proteasome is inhibited (and the levels of polyubiquitinated and aggregation-prone apoB rise), increased amounts of apoB are found in the lysosome [95].
Recent data from our laboratories have now led to a deeper understanding of how apoB is delivered for PERPP. First, the over-expression of Sortillin, which facilitates the delivery of other trafficking cargo to the lysosome for degradation, increases apoB degradation [96]. Second, Sortillin directly captures apoB and the protein is routed through an amphisome intermediate, which functions at the intersection of the trafficking and autophagy pathways [97]. Third, two particular fatty acids that stimulate PERPP are the fish oil N-3 fatty acids, eicosapentaenoic and docosahexaenoic acid, which oxidatively damage apoB through a pathway involving superoxide [93, 94, 98, 99]. Interestingly, a diet rich in these fish oil-enriched N-3 fatty acids correlates with lower triglyceride level [100]. This may be a result of PERPP-mediated degradation of apoB, which reduces VLDL secretion. In fact, reduced systolic and diastolic blood pressure, plasma triglycerides, and apoB levels – as well as increased artery elasticity – were evident in familial hypercholesterolemia patients taking statins whose diet was supplemented with N-3 fatty acids [101]. Moreover, individuals with a polymorphism near the Sortillin locus on the chromosome were more likely to have decreased levels of LDL-associated cholesterol and were protected from cardiovascular disease. Further analyses of whether Sortillin activity and/or levels can be modulated are warranted.
ApoB degradation by PERPP can also be stimulated by an acute increase in insulin, which decreases VLDL and apoB secretion [102–105]. This process is mediated through multiple factors, including MAP kinase, TNFα, and PI3-kinase [106–108]. In one aspect of the insulin-stimulated process, activated PI3-kinase leads to the production of multiple PIPs, which likely serve to direct secretory vesicles containing VLDL particles to an autophagic degradation compartment [106, 109–111]. In addition, apoB and VLDL secretion rise in insulin resistance and type 2 diabetes mellitus, suggesting that this is in part a consequence of the loss of insulin-stimulated apoB degradation that in turn increases the amount secretion-competent VLDL [112]. Through the use of an insulin-resistant mouse model, another study indicated that the effect on apoB secretion is via triglyceride levels [113].
3.0 EXISTING AND POTENTIAL THERAPEUTIC TARGETS
Not surprisingly, the development of treatments for reducing the risk of cardiovascular diseases is an active area of research. Prescribed to nearly 40% of American adults, the most common treatment is the use of statins. Statins are a class of drugs that inhibit cholesterol synthesis by acting as competitive inhibitors of 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG CoA) reductase, the rate limiting step during cholesterol biosynthesis. Consequently, statins prevent the conversion of HMG CoA to mevalonate, a cholesterol precursor. This results in two major effects in the liver: less cholesterol is synthesized and available for export on VLDL particles, and there is an increase in LDL receptor levels because of increased processing of SREBP, which regulates LDL receptor transcription through its SRE promoter element [114]. These drugs can reduce LDL associated cholesterol levels by up to 60% at the highest doses, and reduced cardiovascular disease by ~5% for every 40 mg/dl decrease in LDL cholesterol [115, 116]. Furthermore, when combined with ezetimibe, a drug which prevents intestinal cholesterol uptake by inhibiting the activity of Niemann-Pick C1-like protein 1 (NPC1L1), statin treatment reduced cardiovascular disease by another 6.4% [117]. NPC1L1 is a multispanning membrane protein localized to the surface of enterocytes that binds lipids through its sterol sensing domain and is rapidly internalized in the presence of dietary cholesterol and fatty acids. Ezetimibe blocks NPC1L1 internalization, thus inhibiting cholesterol uptake [118, 119].
Although statins remain an attractive and well established first-line treatment to reduce the amount of cholesterol synthesized in the body (and have been used for ~30 years), they are not without complications, as hinted at above. First, statins are not universally effective. In a meta-analysis of >38,000 patients, Boekholdt and coworkers discovered that statin effectiveness varied significantly and that >40% of patients undergoing high dose statin therapy did not achieve an optimal level of circulating cholesterol [115]. Moreover, certain patients fail to respond to statin therapy, possibly due to genetic variation and polymorphisms in genes related to statin metabolism, including intestinal P-glycoprotein, organic anion transporter 2, coenzyme Q10, and cytochrome P450 3A4 [120–126]. Second, statins can exhibit antagonistic or unwanted side effects when combined with other drugs [127, 128]; 25–30% of patients discontinue statin use due to side effects [129]. The most common side effects, affecting ~15% of patients, include muscle wasting, leg cramps, and myopathy [127, 130, 131]. Although many of these relatively minor side effects disappear by simply switching to a different statin, other more serious side effects can occur, such as acute liver failure and peripheral neuropathy [128], although these conditions are exceedingly rare. Third, another exceedingly rare condition is statin-associated myopathy with significant elevation of serum creatine kinase, which affects ~1:10,000 to 1:100,000 people who take normal doses of statins [132]. Fourth, a meta-analysis identified a 9% increase in the incidence of diabetes associated with statin use [133]. Due to these deficiencies and observed side effects, it is important that alternative therapies are at least considered as a means to control cholesterol production.
One alternative therapy to control circulating cholesterol levels includes the use of antibodies. The most promising antibody was developed against proprotein convertase subtilisin/kexin type 9 (PCSK9), a serine protease whose levels appear to positively correlate with CAD lesions [134, 135]. In humans, PCSK9 targets the LDL receptor for degradation by the lysosome through a mechanism that has not been fully elucidated [134, 136]. By inhibiting PCSK9, more LDL receptors are present on the cell surface to bind apoB, thereby reducing circulating LDLs by up to 60%. Two antibody therapeutics, Evolocumab and Alirocumab, were recently approved for specific patient groups [137–139]. The initial trial for Evolocumab followed >27,000 patients for 2 years and investigated if an Evolucumab/statin combination had additional value compared to statin alone. Indeed, patients taking both drugs saw a 59% reduction of LDL cholesterol with no increase in side effects [134, 140]. The effect on cardiovascular disease was also significant. The risk of myocardial infarction, stroke, and coronary revascularization was reduced by ~27% [140]. Another Phase 3 clinical trial followed nearly 5000 patients to investigate the effects of Alirocumab compared to either placebo or ezetimibe only. At week 24 of the 104 week trial, >70% of patients taking both Alirocumab and statins had obtained optimal or near optimal levels of cholesterol and apoB compared to the other treatments [141]. Interestingly, Alirocumab decreased both LDL-associated cholesterol and apoB while Evolucumab appeared to only affect LDL levels [142, 143].
While anti-PCSK9 therapy may be a promising strategy for treating CAD, additional investigation into the mechanism of action of the antibodies is critical. Moreover, the antibody therapeutics are approved thus far only for patients with familial hypercholesterolemia, which most often arises from mutations in the LDL receptor, or for patients with severe atherosclerosis who require additional lipid lowering treatments [144, 145]. Most likely, new cholesterol lowering drugs would also initially be used for individuals with these severe diseases as well. Finally, these drugs are cost prohibitive and may increase health care costs by 38% over the next 5 years [135, 146].
The next most promising alternative therapy to treat CAD is by directly reducing apoB levels. One such method to reduce apoB levels is via knock-down strategies, which target the apoB message and decrease protein levels [147, 148]. The first proof-of-principle study underlying the clinical approach of targeting the apoB message with an antisense oligonucleotide was conducted by Ionis (formerly Isis) in a mouse model [149]. The use of this technology was extended to reduce circulating apoB100 in mice engineered to express human apoB100 [150]. In addition, Zimmerman and colleagues [151] used an siRNA to apoB and observed a reduction in apoB levels as well as a reduction in circulating LDL and serum cholesterol levels in cytomogolous monkeys. However, non-apoB containing lipoprotein particles (i.e. HDLs) remained unaffected, supporting the idea that altering apoB levels can specifically regulate cholesterol delivery [151]. This led to the development of Mipomersen, the first apoB-specific anti-sense oligonucleotide treatment approved for use in humans. Prior to its approval in 2013, phase 3 clinical trials indicated a reduction in LDL-associated circulating cholesterol by 25–40% [152, 153]. However, Mipomersen is similarly only approved for those suffering from homozygous familial hypercholesteremia and has not been approved for use in Europe due to concerns with liver toxicity and other side effects [135, 137, 148, 154]. Further investigation is undoubtedly required to understand the long-term effects of this drug in humans.
A drug that specifically prevents lipid loading onto apoB, Lomitapide, was also recently approved for patients with homozygous familial hypercholesterolemia. Lomitapide inhibits the activity of the MTP complex. Lomitapide treatment lowered triglyceride levels by 65% and LDL levels by 50% [155]. However, Lomitapide may cause liver damage and steatosis, and users must still maintain a low lipid diet [135, 156].
As discussed above in section 2.2.3., apoB can be degraded via PERPP, which represents a specialized form of autophagy. Thus, the relationship between cardiovascular disease and autophagy is also an active area of research interest. Autophagy is a pathway in which aggregated proteins, damaged organelles, and bulk cytoplasmic proteins become sequestered into double membranous vesicles in the cytoplasm, which are then engulfed by the lysosome and degraded [157, 158]. The autophagy pathway can be activated by a transcription factor known as TFEB, which stimulates the degradation of lipids and fatty acids in the lysosome [159]. This phenomenon led to the discovery that TFEB overexpression reduced cholesterol levels in macrophages by promoting cholesterol efflux as well as the aggregation of ubiquitinated proteins [160]. Moreover, TFEB overexpression not only increased the degradation of aggregated proteins associated with p62, an autophagy receptor/chaperone, but also reduced inflammatory signaling, resulting in smaller atherosclerotic plaques [161]. Current studies in patients with non-alcoholic fatty liver disease are being undertaken to determine if polymorphisms in autophagy related genes affect lipid storage [162]. For this reason, and because autophagy may clear aggregated proteins that lead to neurodegenerative disorders, the development of small molecules that regulate autophagy is being actively pursued [163–166]. In the future, it will be exciting to see if autophagy inhibitors exhibit a combined beneficial effect on both neurodegenerative disease and atherosclerosis
Although the mechanism of action may be independent of apoB targeting, in another recent clinical trial the impact of tamoxifen on VLDL production was investigated [167]. Tamoxifen is effectively used for specific breast cancer subtypes [168] and reduces IGF-1 levels and increases fatty acid oxidation, consequently preventing the secretion of growth hormones, such as EGF [169]. In the liver, this drug may reduce VLDL levels because growth hormone stimulates VLDL synthesis. Notably, tamoxifen treatment lowered VLDL and apoB levels and secretion by ~30% [167]. Thus, it will be important to investigate whether this drug affects apoB ERAD and PERPP.
4.0 CONCLUSIONS AND PERSPECTIVES
As outlined in this review article, cardiovascular disease is a pressing and growing problem. While the vast majority of patients benefit from statins, the ongoing continued development of new therapeutics for cardiovascular disease remains a critical undertaking, and recent exciting progress in this area has led to the identification of new targets (e.g., PCSK9) and reagents (e.g., RNA interference technologies and antisense oligonucleotides). However, these achievements have been met with tempered enthusiasm due to several considerations, which were outlined above. Nevertheless, as with any disease that can be defined at the molecular level, there are ample targets that—in principle—are available for therapeutic development. What has lagged is the availability of potent small molecules that target these pathways, but here too some progress has now been made.
We propose here that other cellular pathways and protein targets may offer still more opportunities. Research in some of these areas is in its infancy but in other areas clinical trials could soon start. For example, both the ERAD pathway and PERPP that degrade and lower apoB levels are regulated by various factors, including lipids (e.g., phosphatidyl inositols), post-translational modifications (e.g., ubiquitination and ubiquitin-like modifications in the autophagy pathway), molecular chaperones (e.g., Hsp70 and Hsp90), and hormones (e.g., insulin). Since apoB levels directly correlate with circulating LDL, VLDL, serum cholesterol, and triacylglycerol, factors that regulate apoB could also represent therapeutic targets to regulate cholesterol levels. Thus, these varied factors may represent new and alternative approaches to treat CAD.
There is significant work toward the identification of small molecule modulators of the Hsp70 and Hsp90 chaperones, which target apoB to the ERAD pathway [75, 78, 170]. While activators of Hsp90 have not been identified (even though Hsp90 inhibitors are in clinical trials), a new class of Hsp70 enhancers have been identified [171]. We anticipate that these molecules will enhance the turnover of apoB. Similarly, regulators of the ubiquitin-proteasome pathway have been actively sought [172]. Inhibitors of the proteasome have now entered the clinic as a front-line therapy for multiple myeloma, and an activator of proteasome-dependent degradation was also identified [173]. More specifically, activators of both the proteasome and autophagy have shown efficacy in neurodegenerative disease models without apparent side-effects [173, 174]. To our knowledge, none of these compounds have been investigated for their effects on the levels of circulating cholesterol and lipoproteins. Finally, the fact that Sortillin over-expression enhances the PERPP of apoB—and that individuals with polymorphisms that increase Sortillin expression exhibit decreased incidents of cardiovascular disease—suggests that this cargo receptor can and should be therapeutically modulated. Recent studies indicate that the production of Sortillin is triggered by neurotensin, providing a potential route to further explore the action of this cargo receptor in apoB metabolism [175].
In sum, we envision that future studies will give rise to synergistic effects from the use of drug combinations that target more than one pathway/molecule. This advance will allow patients to lower the doses of drugs that currently give rise to adverse side effects, further reducing the levels of circulating LDL cholesterol and decreasing the medical and economic burden associated with heart disease.
Highlights.
ApolipoproteinB levels correlate with serum cholesterol and cardiovascular disease
ApolipoproteinB is regulated by two cellular degradation pathways
Statins are used to reduce serum cholesterol but other therapies should be considered
Alternate therapies may regulate ApolipoproteinB levels directly or via degradation
Acknowledgments
The authors would like to thank the Fisher and Brodsky labs for thoughtful discussions and comments. We appreciate the many labs doing excellent research that contribute to the field but cannot be discussed due to space limitations.
Funding
This work was supported by the National Institutes of Health [grants GM075061 and DK079307 (to JLB) and HL127930 (to JLB and EAF)].
Abbreviations
- CAD
Coronary artery disease
- VLDL
Very low density lipoprotein
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- apoB
apolipoproteinB
- ERAD
endoplasmic reticulum associated degradation
- PERPP
post ER presecretory proteolysis
- PDI
protein disulfide isomerase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, Naghavi M, Mensah GA, Murray CJ. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. doi: 10.1161/STR.0b013e31829734f2. [DOI] [PubMed] [Google Scholar]
- 4.Fang MC, Coca Perraillon M, Ghosh K, Cutler DM, Rosen AB. Trends in stroke rates, risk, and outcomes in the United States, 1988 to 2008. The American journal of medicine. 2014;127:608–615. doi: 10.1016/j.amjmed.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arteriosclerosis, thrombosis and vascular biology. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg-Hansen A, Watts GF. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. European heart journal. 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominiczak MH, Caslake MJ. Apolipoproteins: metabolic role and clinical biochemistry applications. Annals of clinical biochemistry. 2011;48:498–515. doi: 10.1258/acb.2011.011111. [DOI] [PubMed] [Google Scholar]
- 8.Christie WW. Plasma Lipoproteins. In: Harwood JLaRJW., editor. Plasma Lipoproteins. Vol. 2014. AOCS Lipid Library; 2014. [Google Scholar]
- 9.Cox RA, Garcia-Palmieri MR. Cholesterol, Triglycerides, and Associated Lipoproteins. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: 1990. [Google Scholar]
- 10.Wasan KM, Brocks DR, Lee SD, Sachs-Barrable K, Thornton SJ. Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: implications for drug discovery. Nature reviews. Drug discovery. 2008;7:84–99. doi: 10.1038/nrd2353. [DOI] [PubMed] [Google Scholar]
- 11.Blanc V, Davidson NO. APOBEC-1-mediated RNA editing. Wiley interdisciplinary reviews. Systems biology and medicine. 2010;2:594–602. doi: 10.1002/wsbm.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salter JD, Bennett RP, Smith HC. The APOBEC Protein Family: United by Structure, Divergent in Function. Trends in biochemical sciences. 2016;41:578–594. doi: 10.1016/j.tibs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King JJ, Larijani M. A Novel Regulator of Activation-Induced Cytidine Deaminase/APOBECs in Immunity and Cancer: Schrodinger’s CATalytic Pocket. Frontiers in immunology. 2017;8:351. doi: 10.3389/fimmu.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjiagapiou C, Giannoni F, Funahashi T, Skarosi SF, Davidson NO. Molecular cloning of a human small intestinal apolipoprotein B mRNA editing protein. Nucleic acids research. 1994;22:1874–1879. doi: 10.1093/nar/22.10.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannoni F, Bonen DK, Funahashi T, Hadjiagapiou C, Burant CF, Davidson NO. Complementation of apolipoprotein B mRNA editing by human liver accompanied by secretion of apolipoprotein B48. The Journal of biological chemistry. 1994;269:5932–5936. [PubMed] [Google Scholar]
- 16.Julve J, Martin-Campos JM, Escola-Gil JC, Blanco-Vaca F. Chylomicrons: Advances in biology, pathology, laboratory testing, and therapeutics. Clin Chim Acta. 2016;455:134–148. doi: 10.1016/j.cca.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Mansbach CM, 2nd, Siddiqi S. Control of chylomicron export from the intestine. American journal of physiology Gastrointestinal and liver physiology. 2016;310:G659–668. doi: 10.1152/ajpgi.00228.2015. [DOI] [PubMed] [Google Scholar]
- 18.Kolovou G, Vasiliadis I, Gontoras N, Kolovou V, Hatzigeorgiou G. Microsomal transfer protein inhibitors, new approach for treatment of familial hypercholesterolemia, review of the literature, original findings, and clinical significance. Cardiovascular therapeutics. 2015;33:71–78. doi: 10.1111/1755-5922.12105. [DOI] [PubMed] [Google Scholar]
- 19.Hooper AJ, Burnett JR, Watts GF. Contemporary aspects of the biology and therapeutic regulation of the microsomal triglyceride transfer protein. Circulation research. 2015;116:193–205. doi: 10.1161/CIRCRESAHA.116.304637. [DOI] [PubMed] [Google Scholar]
- 20.Wetterau JR, Combs KA, Spinner SN, Joiner BJ. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. The Journal of biological chemistry. 1990;265:9800–9807. [PubMed] [Google Scholar]
- 21.Lamberg A, Jauhiainen M, Metso J, Ehnholm C, Shoulders C, Scott J, Pihlajaniemi T, Kivirikko KI. The role of protein disulphide isomerase in the microsomal triacylglycerol transfer protein does not reside in its isomerase activity. The Biochemical journal. 1996;315(Pt 2):533–536. doi: 10.1042/bj3150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelness GS, Thornburg JT. Role of intramolecular disulfide bond formation in the assembly and secretion of apolipoprotein B-100-containing lipoproteins. Journal of lipid research. 1996;37:408–419. [PubMed] [Google Scholar]
- 23.Pariyarath R, Wang H, Aitchison JD, Ginsberg HN, Welch WJ, Johnson AE, Fisher EA. Co-translational interactions of apoprotein B with the ribosome and translocon during lipoprotein assembly or targeting to the proteasome. The Journal of biological chemistry. 2001;276:541–550. doi: 10.1074/jbc.M007944200. [DOI] [PubMed] [Google Scholar]
- 24.Bakillah A, Hussain MM. Binding of microsomal triglyceride transfer protein to lipids results in increased affinity for apolipoprotein B: evidence for stable microsomal MTP-lipid complexes. The Journal of biological chemistry. 2001;276:31466–31473. doi: 10.1074/jbc.M100390200. [DOI] [PubMed] [Google Scholar]
- 25.Tiwari S, Siddiqi SA. Intracellular trafficking and secretion of VLDL. Arteriosclerosis, thrombosis and vascular biology. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gusarova V, Brodsky JL, Fisher EA. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. The Journal of biological chemistry. 2003;278:48051–48058. doi: 10.1074/jbc.M306898200. [DOI] [PubMed] [Google Scholar]
- 27.Butkinaree C, Guo L, Ramkhelawon B, Wanschel A, Brodsky JL, Moore KJ, Fisher EA. A regulator of secretory vesicle size, Kelch-like protein 12, facilitates the secretion of apolipoprotein B100 and very-low-density lipoproteins--brief report. Arteriosclerosis, thrombosis and vascular biology. 2014;34:251–254. doi: 10.1161/ATVBAHA.113.302728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos AJ, Nogueira C, Ortega-Bellido M, Malhotra V. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. The Journal of cell biology. 2016;213:343–354. doi: 10.1083/jcb.201603072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, Rape M. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. The Journal of biological chemistry. 2016;291:10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahitham W, Watts R, Nelson R, Lian J, Lehner R. Liver-specific expression of carboxylesterase 1g/esterase-x reduces hepatic steatosis, counteracts dyslipidemia and improves insulin signaling. Biochimica et biophysica acta. 2016;1861:482–490. doi: 10.1016/j.bbalip.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Quiroga AD, Lian J, Lehner R. Carboxylesterase1/Esterase-x regulates chylomicron production in mice. PloS one. 2012;7:e49515. doi: 10.1371/journal.pone.0049515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gusarova V, Seo J, Sullivan ML, Watkins SC, Brodsky JL, Fisher EA. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. The Journal of biological chemistry. 2007;282:19453–19462. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- 34.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. The Journal of biological chemistry. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 35.Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. Journal of lipid research. 2009;50(Suppl):S162–166. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olofsson SO, Boren J. Apolipoprotein B secretory regulation by degradation. Arteriosclerosis, thrombosis and vascular biology. 2012;32:1334–1338. doi: 10.1161/ATVBAHA.112.251116. [DOI] [PubMed] [Google Scholar]
- 37.Yao Z, Zhou H, Figeys D, Wang Y, Sundaram M. Microsome-associated lumenal lipid droplets in the regulation of lipoprotein secretion. Current opinion in lipidology. 2013;24:160–170. doi: 10.1097/MOL.0b013e32835aebe7. [DOI] [PubMed] [Google Scholar]
- 38.Beisiegel U. Receptors for triglyceride-rich lipoproteins and their role in lipoprotein metabolism. Current opinion in lipidology. 1995;6:117–122. doi: 10.1097/00041433-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Segrest JP, Jones MK, Dashti N. N-terminal domain of apolipoprotein B has structural homology to lipovitellin and microsomal triglyceride transfer protein: a “lipid pocket” model for self-assembly of apob-containing lipoprotein particles. Journal of lipid research. 1999;40:1401–1416. [PubMed] [Google Scholar]
- 40.Chuck SL, Lingappa VR. Pause transfer: a topogenic sequence in apolipoprotein B mediates stopping and restarting of translocation. Cell. 1992;68:9–21. doi: 10.1016/0092-8674(92)90202-n. [DOI] [PubMed] [Google Scholar]
- 41.Kivlen MH, Dorsey CA, Lingappa VR, Hegde RS. Asymmetric distribution of pause transfer sequences in apolipoprotein B-100. Journal of lipid research. 1997;38:1149–1162. [PubMed] [Google Scholar]
- 42.Yamaguchi J, Conlon DM, Liang JJ, Fisher EA, Ginsberg HN. Translocation efficiency of apolipoprotein B is determined by the presence of beta-sheet domains, not pause transfer sequences. The Journal of biological chemistry. 2006;281:27063–27071. doi: 10.1074/jbc.M606809200. [DOI] [PubMed] [Google Scholar]
- 43.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. Journal of lipid research. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 44.Segrest JP, Jones MK, De Loof H, Dashti N. Structure of apolipoprotein B-100 in low density lipoproteins. Journal of lipid research. 2001;42:1346–1367. [PubMed] [Google Scholar]
- 45.Mann CJ, Anderson TA, Read J, Chester SA, Harrison GB, Kochl S, Ritchie PJ, Bradbury P, Hussain FS, Amey J, Vanloo B, Rosseneu M, Infante R, Hancock JM, Levitt DG, Banaszak LJ, Scott J, Shoulders CC. The structure of vitellogenin provides a molecular model for the assembly and secretion of atherogenic lipoproteins. Journal of molecular biology. 1999;285:391–408. doi: 10.1006/jmbi.1998.2298. [DOI] [PubMed] [Google Scholar]
- 46.Segrest JP, Jones MK, Mishra VK, Anantharamaiah GM, Garber DW. apoB-100 has a pentapartite structure composed of three amphipathic alpha-helical domains alternating with two amphipathic beta-strand domains. Detection by the computer program LOCATE. Arteriosclerosis and thrombosis: a journal of vascular biology/American Heart Association. 1994;14:1674–1685. doi: 10.1161/01.atv.14.10.1674. [DOI] [PubMed] [Google Scholar]
- 47.Yang CY, Gu ZW, Weng SA, Kim TW, Chen SH, Pownall HJ, Sharp PM, Liu SW, Li WH, Gotto AM, Jr, et al. Structure of apolipoprotein B-100 of human low density lipoproteins. Arteriosclerosis. 1989;9:96–108. doi: 10.1161/01.atv.9.1.96. [DOI] [PubMed] [Google Scholar]
- 48.Boren J, Lee I, Zhu W, Arnold K, Taylor S, Innerarity TL. Identification of the low density lipoprotein receptor-binding site in apolipoprotein B100 and the modulation of its binding activity by the carboxyl terminus in familial defective apo-B100. The Journal of clinical investigation. 1998;101:1084–1093. doi: 10.1172/JCI1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johs A, Hammel M, Waldner I, May RP, Laggner P, Prassl R. Modular structure of solubilized human apolipoprotein B-100. Low resolution model revealed by small angle neutron scattering. The Journal of biological chemistry. 2006;281:19732–19739. doi: 10.1074/jbc.M601688200. [DOI] [PubMed] [Google Scholar]
- 50.Harazono A, Kawasaki N, Kawanishi T, Hayakawa T. Site-specific glycosylation analysis of human apolipoprotein B100 using LC/ESI MS/MS. Glycobiology. 2005;15:447–462. doi: 10.1093/glycob/cwi033. [DOI] [PubMed] [Google Scholar]
- 51.Callow MJ, Rubin EM. Site-specific mutagenesis demonstrates that cysteine 4326 of apolipoprotein B is required for covalent linkage with apolipoprotein (a) in vivo. The Journal of biological chemistry. 1995;270:23914–23917. doi: 10.1074/jbc.270.41.23914. [DOI] [PubMed] [Google Scholar]
- 52.Huang XF, Shelness GS. Identification of cysteine pairs within the amino-terminal 5% of apolipoprotein B essential for hepatic lipoprotein assembly and secretion. The Journal of biological chemistry. 1997;272:31872–31876. doi: 10.1074/jbc.272.50.31872. [DOI] [PubMed] [Google Scholar]
- 53.Tran K, Boren J, Macri J, Wang Y, McLeod R, Avramoglu RK, Adeli K, Yao Z. Functional analysis of disulfide linkages clustered within the amino terminus of human apolipoprotein B. The Journal of biological chemistry. 1998;273:7244–7251. doi: 10.1074/jbc.273.13.7244. [DOI] [PubMed] [Google Scholar]
- 54.Farese RV, Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benn M, Nordestgaard BG, Jensen JS, Nilausen K, Meinertz H, Tybjaerg-Hansen A. Mutation in apolipoprotein B associated with hypobetalipoproteinemia despite decreased binding to the low density lipoprotein receptor. The Journal of biological chemistry. 2005;280:21052–21060. doi: 10.1074/jbc.M413877200. [DOI] [PubMed] [Google Scholar]
- 56.Sturley SL, Talmud PJ, Brasseur R, Culbertson MR, Humphries SE, Attie AD. Human apolipoprotein B signal sequence variants confer a secretion-defective phenotype when expressed in yeast. The Journal of biological chemistry. 1994;269:21670–21675. [PubMed] [Google Scholar]
- 57.Collins DR, Knott TJ, Pease RJ, Powell LM, Wallis SC, Robertson S, Pullinger CR, Milne RW, Marcel YL, Humphries SE, et al. Truncated variants of apolipoprotein B cause hypobetalipoproteinaemia. Nucleic acids research. 1988;16:8361–8375. doi: 10.1093/nar/16.17.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang LS, Ripps ME, Korman SH, Deckelbaum RJ, Breslow JL. Hypobetalipoproteinemia due to an apolipoprotein B gene exon 21 deletion derived by Alu-Alu recombination. The Journal of biological chemistry. 1989;264:11394–11400. [PubMed] [Google Scholar]
- 59.Spring DJ, Chen-Liu LW, Chatterton JE, Elovson J, Schumaker VN. Lipoprotein assembly. Apolipoprotein B size determines lipoprotein core circumference. The Journal of biological chemistry. 1992;267:14839–14845. [PubMed] [Google Scholar]
- 60.McLeod RS, Zhao Y, Selby SL, Westerlund J, Yao Z. Carboxyl-terminal truncation impairs lipid recruitment by apolipoprotein B100 but does not affect secretion of the truncated apolipoprotein B-containing lipoproteins. The Journal of biological chemistry. 1994;269:2852–2862. [PubMed] [Google Scholar]
- 61.Vrablik M, Ceska R, Horinek A. Major apolipoprotein B-100 mutations in lipoprotein metabolism and atherosclerosis. Physiol Res. 2001;50:337–343. [PubMed] [Google Scholar]
- 62.Innerarity TL, Mahley RW, Weisgraber KH, Bersot TP, Krauss RM, Vega GL, Grundy SM, Friedl W, Davignon J, McCarthy BJ. Familial defective apolipoprotein B-100: a mutation of apolipoprotein B that causes hypercholesterolemia. Journal of lipid research. 1990;31:1337–1349. [PubMed] [Google Scholar]
- 63.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiological reviews. 2012;92:537–576. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stevenson J, Huang EY, Olzmann JA. Endoplasmic Reticulum-Associated Degradation and Lipid Homeostasis. Annu Rev Nutr. 2016;36:511–542. doi: 10.1146/annurev-nutr-071715-051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 66.Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 67.Komander D, Rape M. The ubiquitin code. Annual review of biochemistry. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 68.Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochimica et biophysica acta. 2012;1823:117–124. doi: 10.1016/j.bbamcr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Budenholzer L, Cheng CL, Li Y, Hochstrasser M. Proteasome Structure and Assembly. Journal of molecular biology. 2017 doi: 10.1016/j.jmb.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardner RG, Shearer AG, Hampton RY. In vivo action of the HRD ubiquitin ligase complex: mechanisms of endoplasmic reticulum quality control and sterol regulation. Molecular and cellular biology. 2001;21:4276–4291. doi: 10.1128/MCB.21.13.4276-4291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foresti O, Rodriguez-Vaello V, Funaya C, Carvalho P. Quality control of inner nuclear membrane proteins by the Asi complex. Science. 2014;346:751–755. doi: 10.1126/science.1255638. [DOI] [PubMed] [Google Scholar]
- 72.Olzmann JA, Richter CM, Kopito RR. Spatial regulation of UBXD8 and p97/VCP controls ATGL-mediated lipid droplet turnover. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1345–1350. doi: 10.1073/pnas.1213738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell DM, Zhou M, Pariyarath R, Wang H, Aitchison JD, Ginsberg HN, Fisher EA. Apoprotein B100 has a prolonged interaction with the translocon during which its lipidation and translocation change from dependence on the microsomal triglyceride transfer protein to independence. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14733–14738. doi: 10.1073/pnas.95.25.14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeung SJ, Chen SH, Chan L. Ubiquitin-proteasome pathway mediates intracellular degradation of apolipoprotein B. Biochemistry. 1996;35:13843–13848. doi: 10.1021/bi9618777. [DOI] [PubMed] [Google Scholar]
- 75.Fisher EA, Zhou M, Mitchell DM, Wu X, Omura S, Wang H, Goldberg AL, Ginsberg HN. The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. The Journal of biological chemistry. 1997;272:20427–20434. doi: 10.1074/jbc.272.33.20427. [DOI] [PubMed] [Google Scholar]
- 76.Schoebel S, Mi W, Stein A, Ovchinnikov S, Pavlovicz R, DiMaio F, Baker D, Chambers MG, Su H, Li D, Rapoport TA, Liao M. Cryo-EM structure of the protein-conducting ERAD channel Hrd1 in complex with Hrd3. Nature. 2017;548:352–355. doi: 10.1038/nature23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao W, Yeung SC, Chan L. Proteasome-mediated degradation of apolipoprotein B targets both nascent peptides cotranslationally before translocation and full-length apolipoprotein B after translocation into the endoplasmic reticulum. The Journal of biological chemistry. 1998;273:27225–27230. doi: 10.1074/jbc.273.42.27225. [DOI] [PubMed] [Google Scholar]
- 78.Gusarova V, Caplan AJ, Brodsky JL, Fisher EA. Apoprotein B degradation is promoted by the molecular chaperones hsp90 and hsp70. The Journal of biological chemistry. 2001;276:24891–24900. doi: 10.1074/jbc.M100633200. [DOI] [PubMed] [Google Scholar]
- 79.Hrizo SL, Gusarova V, Habiel DM, Goeckeler JL, Fisher EA, Brodsky JL. The Hsp110 molecular chaperone stabilizes apolipoprotein B from endoplasmic reticulum-associated degradation (ERAD) The Journal of biological chemistry. 2007;282:32665–32675. doi: 10.1074/jbc.M705216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grubb S, Guo L, Fisher EA, Brodsky JL. Protein disulfide isomerases contribute differentially to the endoplasmic reticulum-associated degradation of apolipoprotein B and other substrates. Molecular biology of the cell. 2012;23:520–532. doi: 10.1091/mbc.E11-08-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nature reviews Molecular cell biology. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maattanen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21:500–511. doi: 10.1016/j.semcdb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Kadokura H, Saito M, Tsuru A, Hosoda A, Iwawaki T, Inaba K, Kohno K. Identification of the redox partners of ERdj5/JPDI, a PDI family member, from an animal tissue. Biochemical and biophysical research communications. 2013;440:245–250. doi: 10.1016/j.bbrc.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 84.Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl FU. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakatsukasa K, Huyer G, Michaelis S, Brodsky JL. Dissecting the ER-associated degradation of a misfolded polytopic membrane protein. Cell. 2008;132:101–112. doi: 10.1016/j.cell.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia VM, Nillegoda NB, Bukau B, Morano KA. Substrate binding by the yeast Hsp110 nucleotide exchange factor and molecular chaperone Sse1 is not obligate for its biological activities. Molecular biology of the cell. 2017;28:2066–2075. doi: 10.1091/mbc.E17-01-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubenstein EM, Kreft SG, Greenblatt W, Swanson R, Hochstrasser M. Aberrant substrate engagement of the ER translocon triggers degradation by the Hrd1 ubiquitin ligase. The Journal of cell biology. 2012;197:761–773. doi: 10.1083/jcb.201203061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang JS, Kim T, Fang S, Yamaguchi J, Weissman AM, Fisher EA, Ginsberg HN. Overexpression of the tumor autocrine motility factor receptor Gp78, a ubiquitin protein ligase, results in increased ubiquitinylation and decreased secretion of apolipoprotein B100 in HepG2 cells. The Journal of biological chemistry. 2003;278:23984–23988. doi: 10.1074/jbc.M302683200. [DOI] [PubMed] [Google Scholar]
- 89.Cardozo C, Wu X, Pan M, Wang H, Fisher EA. The inhibition of microsomal triglyceride transfer protein activity in rat hepatoma cells promotes proteasomal and nonproteasomal degradation of apoprotein b100. Biochemistry. 2002;41:10105–10114. doi: 10.1021/bi025749w. [DOI] [PubMed] [Google Scholar]
- 90.Fisher EA, Lapierre LR, Junkins RD, McLeod RS. The AAA-ATPase p97 facilitates degradation of apolipoprotein B by the ubiquitin-proteasome pathway. Journal of lipid research. 2008;49:2149–2160. doi: 10.1194/jlr.M800108-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Pan M, Maitin V, Parathath S, Andreo U, Lin SX, St Germain C, Yao Z, Maxfield FR, Williams KJ, Fisher EA. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maitin V, Andreo U, Guo L, Fisher EA. Docosahexaenoic acid impairs the maturation of very low density lipoproteins in rat hepatic cells. Journal of lipid research. 2014;55:75–84. doi: 10.1194/jlr.M043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. The Journal of biological chemistry. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 94.Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. The Journal of clinical investigation. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohsaki Y, Cheng J, Fujita A, Tokumoto T, Fujimoto T. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Molecular biology of the cell. 2006;17:2674–2683. doi: 10.1091/mbc.E05-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strong A, Ding Q, Edmondson AC, Millar JS, Sachs KV, Li X, Kumaravel A, Wang MY, Ai D, Guo L, Alexander ET, Nguyen D, Lund-Katz S, Phillips MC, Morales CR, Tall AR, Kathiresan S, Fisher EA, Musunuru K, Rader DJ. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. The Journal of clinical investigation. 2012;122:2807–2816. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amengual J, Guo L, Strong A, Madrigal-Matute J, Wang H, Kaushik S, Brodsky JL, Rader DJ, Cuervo AM, Fisher EA. Autophagy Is Required for Sortilin-Mediated Degradation of Apolipoprotein B100. Circulation research. 2018;122:568–582. doi: 10.1161/CIRCRESAHA.117.311240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H, Chen X, Fisher EA. N-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. The Journal of clinical investigation. 1993;91:1380–1389. doi: 10.1172/JCI116340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andreo U, Elkind J, Blachford C, Cederbaum AI, Fisher EA. Role of superoxide radical anion in the mechanism of apoB100 degradation induced by DHA in hepatic cells. FASEB J. 2011;25:3554–3560. doi: 10.1096/fj.11-182725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Djousse L, Hunt SC, Arnett DK, Province MA, Eckfeldt JH, Ellison RC. Dietary linolenic acid is inversely associated with plasma triacylglycerol: the National Heart, Lung, and Blood Institute Family Heart Study. The American journal of clinical nutrition. 2003;78:1098–1102. doi: 10.1093/ajcn/78.6.1098. [DOI] [PubMed] [Google Scholar]
- 101.Chan DC, Pang J, Barrett PH, Sullivan DR, Mori TA, Burnett JR, van Bockxmeer FM, Watts GF. Effect of omega-3 fatty acid supplementation on arterial elasticity in patients with familial hypercholesterolaemia on statin therapy. Nutrition, metabolism and cardiovascular diseases: NMCD. 2016;26:1140–1145. doi: 10.1016/j.numecd.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 102.Sparks JD, Sparks CE. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. The Journal of biological chemistry. 1990;265:8854–8862. [PubMed] [Google Scholar]
- 103.Chirieac DV, Chirieac LR, Corsetti JP, Cianci J, Sparks CE, Sparks JD. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. American journal of physiology Endocrinology and metabolism. 2000;279:E1003–1011. doi: 10.1152/ajpendo.2000.279.5.E1003. [DOI] [PubMed] [Google Scholar]
- 104.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell metabolism. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. The Journal of biological chemistry. 2000;275:8416–8425. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 106.Phung TL, Roncone A, Jensen KL, Sparks CE, Sparks JD. Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. The Journal of biological chemistry. 1997;272:30693–30702. doi: 10.1074/jbc.272.49.30693. [DOI] [PubMed] [Google Scholar]
- 107.Tsai J, Qiu W, Kohen-Avramoglu R, Adeli K. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arteriosclerosis, thrombosis and vascular biology. 2007;27:211–218. doi: 10.1161/01.ATV.0000249861.80471.96. [DOI] [PubMed] [Google Scholar]
- 108.Qin B, Qiu W, Avramoglu RK, Adeli K. Tumor necrosis factor-alpha induces intestinal insulin resistance and stimulates the overproduction of intestinal apolipoprotein B48-containing lipoproteins. Diabetes. 2007;56:450–461. doi: 10.2337/db06-0518. [DOI] [PubMed] [Google Scholar]
- 109.Au CS, Wagner A, Chong T, Qiu W, Sparks JD, Adeli K. Insulin regulates hepatic apolipoprotein B production independent of the mass or activity of Akt1/PKBalpha. Metabolism: clinical and experimental. 2004;53:228–235. doi: 10.1016/j.metabol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 110.Chirieac DV, Davidson NO, Sparks CE, Sparks JD. PI3-kinase activity modulates apo B available for hepatic VLDL production in apobec-1−/− mice. American journal of physiology Gastrointestinal and liver physiology. 2006;291:G382–388. doi: 10.1152/ajpgi.00472.2005. [DOI] [PubMed] [Google Scholar]
- 111.Andreo U, Guo L, Chirieac DV, Tuyama AC, Montenont E, Brodsky JL, Fisher EA. Insulin-stimulated degradation of apolipoprotein B100: roles of class II phosphatidylinositol-3-kinase and autophagy. PloS one. 2013;8:e57590. doi: 10.1371/journal.pone.0057590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khavandi M, Duarte F, Ginsberg HN, Reyes-Soffer G. Treatment of Dyslipidemias to Prevent Cardiovascular Disease in Patients with Type 2 Diabetes. Current cardiology reports. 2017;19:7. doi: 10.1007/s11886-017-0818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moon BC, Hernandez-Ono A, Stiles B, Wu H, Ginsberg HN. Apolipoprotein B secretion is regulated by hepatic triglyceride, and not insulin, in a model of increased hepatic insulin signaling. Arteriosclerosis, thrombosis and vascular biology. 2012;32:236–246. doi: 10.1161/ATVBAHA.111.241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM, Jr, Ridker PM, Grundy SM, Kastelein JJ. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. Journal of the American College of Cardiology. 2014;64:485–494. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 118.Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell metabolism. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 119.Chang TY, Chang C. Ezetimibe blocks internalization of the NPC1L1/cholesterol complex. Cell metabolism. 2008;7:469–471. doi: 10.1016/j.cmet.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 120.Baker SK, Samjoo IA. A neuromuscular approach to statin-related myotoxicity. Can J Neurol Sci. 2008;35:8–21. doi: 10.1017/s0317167100007514. [DOI] [PubMed] [Google Scholar]
- 121.Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 122.Fiegenbaum M, da Silveira FR, Van der Sand CR, Van der Sand LC, Ferreira ME, Pires RC, Hutz MH. The role of common variants of ABCB1, CYP3A4, and CYP3A5 genes in lipid-lowering efficacy and safety of simvastatin treatment. Clinical pharmacology and therapeutics. 2005;78:551–558. doi: 10.1016/j.clpt.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Frudakis TN, Thomas MJ, Ginjupalli SN, Handelin B, Gabriel R, Gomez HJ. CYP2D6*4 polymorphism is associated with statin-induced muscle effects. Pharmacogenetics and genomics. 2007;17:695–707. doi: 10.1097/FPC.0b013e328012d0a9. [DOI] [PubMed] [Google Scholar]
- 124.Mulder AB, van Lijf HJ, Bon MA, van den Bergh FA, Touw DJ, Neef C, Vermes I. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clinical pharmacology and therapeutics. 2001;70:546–551. doi: 10.1067/mcp.2001.120251. [DOI] [PubMed] [Google Scholar]
- 125.Zuccaro P, Mombelli G, Calabresi L, Baldassarre D, Palmi I, Sirtori CR. Tolerability of statins is not linked to CYP450 polymorphisms, but reduced CYP2D6 metabolism improves cholesteraemic response to simvastatin and fluvastatin. Pharmacol Res. 2007;55:310–317. doi: 10.1016/j.phrs.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 126.Oh J, Ban MR, Miskie BA, Pollex RL, Hegele RA. Genetic determinants of statin intolerance. Lipids in health and disease. 2007;6:7. doi: 10.1186/1476-511X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nature reviews Cardiology. 2014;11:563–575. doi: 10.1038/nrcardio.2014.84. [DOI] [PubMed] [Google Scholar]
- 128.Sikka P, Kapoor S, Bindra VK, Sharma M, Vishwakarma P, Saxena KK. Statin intolerance: now a solved problem. J Postgrad Med. 2011;57:321–328. doi: 10.4103/0022-3859.90085. [DOI] [PubMed] [Google Scholar]
- 129.Rosenson RS. Statin non-adherence: clinical consequences and proposed solutions. F1000Research. 2016;5 doi: 10.12688/f1000research.8215.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fitchett DH, Hegele RA, Verma S. Cardiology patient page. Statin intolerance. Circulation. 2015;131:e389–391. doi: 10.1161/CIRCULATIONAHA.114.013189. [DOI] [PubMed] [Google Scholar]
- 131.Mancini GB, Tashakkor AY, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng DS, Pearson GJ, Pope J. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol. 2013;29:1553–1568. doi: 10.1016/j.cjca.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 132.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgozoglu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, Marz W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. European heart journal. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA: the journal of the American Medical Association. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 134.Narasimhan SD. Beyond Statins: New Therapeutic Frontiers for Cardiovascular Disease. Cell. 2017;169:971–973. doi: 10.1016/j.cell.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 135.Weber C, Badimon L, Mach F, van der Vorst EPC. Therapeutic strategies for atherosclerosis and atherothrombosis: Past, present and future. Thrombosis and haemostasis. 2017;117:1258–1264. doi: 10.1160/TH16-10-0814. [DOI] [PubMed] [Google Scholar]
- 136.Schulz R, Schluter KD. PCSK9 targets important for lipid metabolism. Clinical research in cardiology supplements. 2017;12:2–11. doi: 10.1007/s11789-017-0085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okopien B, Buldak L, Boldys A. Current and future trends in the lipid lowering therapy. Pharmacological reports: PR. 2016;68:737–747. doi: 10.1016/j.pharep.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 138.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 139.Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, Xie J, Kang LN, Xu B. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC medicine. 2015;13:123. doi: 10.1186/s12916-015-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 141.Bays HE, Leiter LA, Colhoun HM, Thompson D, Bessac L, Pordy R, Toth PP. Alirocumab Treatment and Achievement of Non-High-Density Lipoprotein Cholesterol and Apolipoprotein B Goals in Patients With Hypercholesterolemia: Pooled Results From 10 Phase 3 ODYSSEY Trials. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.117.005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reyes-Soffer G, Pavlyha M, Ngai C, Thomas T, Holleran S, Ramakrishnan R, Karmally W, Nandakumar R, Fontanez N, Obunike J, Marcovina SM, Lichtenstein AH, Matthan NR, Matta J, Maroccia M, Becue F, Poitiers F, Swanson B, Cowan L, Sasiela WJ, Surks HK, Ginsberg HN. Effects of PCSK9 Inhibition With Alirocumab on Lipoprotein Metabolism in Healthy Humans. Circulation. 2017;135:352–362. doi: 10.1161/CIRCULATIONAHA.116.025253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Watts GF, Chan DC, Dent R, Somaratne R, Wasserman SM, Scott R, Burrows S, RBPH Factorial Effects of Evolocumab and Atorvastatin on Lipoprotein Metabolism. Circulation. 2017;135:338–351. doi: 10.1161/CIRCULATIONAHA.116.025080. [DOI] [PubMed] [Google Scholar]
- 144.Brown MS, Goldstein JL. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975;6:307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- 145.Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science. 1976;191:150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- 146.Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins-Domingo K. Cost-effectiveness of PCSK9 Inhibitor Therapy in Patients With Heterozygous Familial Hypercholesterolemia or Atherosclerotic Cardiovascular Disease. JAMA: the journal of the American Medical Association. 2016;316:743–753. doi: 10.1001/jama.2016.11004. [DOI] [PubMed] [Google Scholar]
- 147.Ricotta DN, Frishman W. Mipomersen: a safe and effective antisense therapy adjunct to statins in patients with hypercholesterolemia. Cardiol Rev. 2012;20:90–95. doi: 10.1097/CRD.0b013e31823424be. [DOI] [PubMed] [Google Scholar]
- 148.Wong E, Goldberg T. Mipomersen (kynamro): a novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. P & T: a peer-reviewed journal for formulary management. 2014;39:119–122. [PMC free article] [PubMed] [Google Scholar]
- 149.Crooke RM, Graham MJ, Lemonidis KM, Whipple CP, Koo S, Perera RJ. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. Journal of lipid research. 2005;46:872–884. doi: 10.1194/jlr.M400492-JLR200. [DOI] [PubMed] [Google Scholar]
- 150.Merki E, Graham MJ, Mullick AE, Miller ER, Crooke RM, Pitas RE, Witztum JL, Tsimikas S. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118:743–753. doi: 10.1161/CIRCULATIONAHA.108.786822. [DOI] [PubMed] [Google Scholar]
- 151.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 152.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 153.McGowan MP, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni-Berthold I, Wagener G, Chasan-Taber S. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PloS one. 2012;7:e49006. doi: 10.1371/journal.pone.0049006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liscinsky M. FDA News Release. U.S. Food and Drug Administration; Silver Spring: 2013. FDA Approves New Orphan Drug Kynamro to Treat Inherited Cholesterol Disorder. [Google Scholar]
- 155.Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AM, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roeters van Lennep J, Averna M, Alonso R. Treating homozygous familial hypercholesterolemia in a real-world setting: Experiences with lomitapide. Journal of clinical lipidology. 2015;9:607–617. doi: 10.1016/j.jacl.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 157.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yin Z, Pascual C, Klionsky DJ. Autophagy: machinery and regulation. Microb Cell. 2016;3:588–596. doi: 10.15698/mic2016.12.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Emanuel R, Sergin I, Bhattacharya S, Turner JN, Epelman S, Settembre C, Diwan A, Ballabio A, Razani B. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arteriosclerosis, thrombosis and vascular biology. 2014;34:1942–1952. doi: 10.1161/ATVBAHA.114.303342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, Javaheri A, Crowley JR, Ballabio A, Schilling JD, Epelman S, Weihl CC, Diwan A, Fan D, Zayed MA, Razani B. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nature communications. 2017;8:15750. doi: 10.1038/ncomms15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu-Cheng Lin MD., Ph.D Far East Memorial Hospital, The Influence of Autophagy on Fatty Liver. Clinical Trials. 2017;2017 [Google Scholar]
- 163.Ramesh N, Pandey UB. Autophagy Dysregulation in ALS: When Protein Aggregates Get Out of Hand. Frontiers in molecular neuroscience. 2017;10:263. doi: 10.3389/fnmol.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nature reviews Drug discovery. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fulda S. Autophagy in Cancer Therapy. Frontiers in oncology. 2017;7:128. doi: 10.3389/fonc.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hua F, Shang S, Hu ZW. Seeking new anti-cancer agents from autophagy-regulating natural products. J Asian Nat Prod Res. 2017;19:305–313. doi: 10.1080/10286020.2017.1304385. [DOI] [PubMed] [Google Scholar]
- 167.Birzniece V, Barrett PHR, Ho KKY. Tamoxifen reduces hepatic VLDL production and GH secretion in women: a possible mechanism for steatosis development. Eur J Endocrinol. 2017;177:137–143. doi: 10.1530/EJE-17-0151. [DOI] [PubMed] [Google Scholar]
- 168.Jordan VC. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions. Journal of medicinal chemistry. 2003;46:883–908. doi: 10.1021/jm020449y. [DOI] [PubMed] [Google Scholar]
- 169.Birzniece V, Sata A, Sutanto S, Ho KK. Paracrine regulation of growth hormone secretion by estrogen in women. The Journal of clinical endocrinology and metabolism. 2010;95:3771–3776. doi: 10.1210/jc.2010-0476. [DOI] [PubMed] [Google Scholar]
- 170.Zhou M, Wu X, Huang LS, Ginsberg HN. Apoprotein B100, an inefficiently translocated secretory protein, is bound to the cytosolic chaperone, heat shock protein 70. The Journal of biological chemistry. 1995;270:25220–25224. doi: 10.1074/jbc.270.42.25220. [DOI] [PubMed] [Google Scholar]
- 171.Wisen S, Bertelsen EB, Thompson AD, Patury S, Ung P, Chang L, Evans CG, Walter GM, Wipf P, Carlson HA, Brodsky JL, Zuiderweg ER, Gestwicki JE. Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS chemical biology. 2010;5:611–622. doi: 10.1021/cb1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang X, Dixit VM. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res. 2016;26:484–498. doi: 10.1038/cr.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nature reviews Neuroscience. 2011;12:437–452. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 175.Patel AB, Tsilioni I, Leeman SE, Theoharides TC. Neurotensin stimulates sortilin and mTOR in human microglia inhibitable by methoxyluteolin, a potential therapeutic target for autism. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1604992113. [DOI] [PMC free article] [PubMed] [Google Scholar]