Abstract
Background
Both diabetes and antidiabetic drugs (ADDs) increase the risk for cardiovascular (CV) diseases. Due to the increasing concern about CV safety associated with ADDs, the US FDA revised regulatory guidelines in 2008 to include CV safety as an endpoint.
Objective
The objective of the current study was to conduct a systematic review with meta-analysis to compare CV mortality of oral ADDs approved before and after the FDA’s 2008 guidance.
Methods
Three electronic databases (PubMed, Scopus, and the Clinical Trial Registry) were searched to retrieve studies published up to 24 February 2017. Randomized clinical trials were included in this study if they (1) were published in the English language; (2) included adults with type 2 diabetes mellitus with or without CV risk factors, who were taking at least one oral antidiabetic drug; and (3) had at least one study outcome as CV mortality. Meta-analysis was performed using a random-effects model. Small-study effects were accessed using funnel plot symmetry. The primary outcome was CV mortality.
Results
We found that there was no significant increase in CV mortality for drugs approved before and after 2008. The overall odds ratio (OR) and the upper bound of the two-sided 95% confidence interval (CI) for all drugs approved after 2008 (OR 0.74, 95% CI 0.52–1.07) were lower than the overall OR for all drugs approved before 2008 (OR 1.03, 95% CI 0.89–1.19). In addition, the upper bounds of the two-sided 95% CI for both groups of drugs before and after 2008 were below 1.3. Empagliflozin, which was approved after the guidance, was significantly associated with a reduction in CV mortality.
Conclusion
The 2008 FDA guidance appears to have a positive impact on CV risk assessment of recently marketed drugs for the management of diabetes.
1 Introduction
In 2015, there were 30.3 million people with diabetes in the US, which accounted for 9.4% of the population [1]. The estimated cost of treating diabetes in 2012 was $245 billion [1]. Type 2 diabetes mellitus (T2DM) is considered a controllable risk factor for cardiovascular (CV) diseases [2]. Adults with T2DM are two- to fourfold more likely to have a heart attack or stroke [2]. T2DM also increases CV mortality by more than twofold [3].
The management and maintenance of glycemic control is one of the most important components of diabetes treatment [4–6]. The association between glycated hemoglobin (HbA1c) control and reduction in microvascular complications is well established [7, 8]; however, there are mixed results for the association between HbA1c management, especially for the use of antidiabetic drugs (ADDs) and macrovascular complications. A recent study by Currie et al. showed a significant association between HbA1c and CV mortality [9]. According to this study, with every 1% increase in the HbA1c, the risk of CV mortality increased 1.15-fold [10]. On the contrary, several randomized clinical trials (RCTs), including the United Kingdom Prospective Diabetes Study, have failed to show any significant association between glycemic control and reduction in the risk of macrovascular complications [11, 12]. Patients with T2DM have a higher risk of developing CV complications (both micro- and macrovascular) and their risk increases with an increase in HbA1c level; however, it remains unclear whether the use of ADDs to control HbA1c is associated with reduced macrovascular complications. Recent findings called into question the CV safety of ADDs [8, 13–18]. For example, rosiglitazone users were observed to have a higher risk of heart attacks, strokes, and CV mortality [19]. Dipeptidyl peptidase-4 (DPP-4) inhibitors were also shown to be associated with decreased hospitalization due to heart failure in one study [20], while another study reported a higher rate of hospitalization due to heart failure among those using DPP-4 inhibitors [17].
Prior to 2008, the manufacturers of ADDs were only required to provide evidence for drug efficacy in terms of reducing HbA1c levels for gaining market access; however, this criterion led to the inclusion of only low-risk people, who were less likely to have any CV complications, in premarketing clinical trials [21]. Moreover, reduction in fasting blood glucose or improvement in lipids was sometimes used as a surrogate endpoint to predict CV events in clinical trials on ADDs approved before 2008. Unfortunately, some ADDs approved before 2008 were later found to be associated with increased risks of CV events. Given the high uncertainty around CV safety of ADDs, the US FDA issued the ‘Guidance for Industry: Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes’ in 2008 [22, 23]. The 2008 FDA guidance recommends conducting clinical trials to assess CV death, myocardial infarction, and stroke, as well as hospitalization for acute coronary syndrome and urgent revascularization procedures as endpoints. These outcomes are generally referred to as major adverse CV events (MACEs). New ADDs need to prove that the CV risk is within an acceptable range of increase by demonstrating the upper bound of the two-sided 95% confidence interval (CI) of the risk ratio of MACEs < 1.8. If the upper bound of the two-sided 95% CI of the risk ratio is between 1.3 and 1.8, a postmarketing clinical trial will be needed to ensure the upper bound of the two-sided 95% CI of the risk ratio is below 1.3.
Healthcare professionals were enthusiastic about the influence of this guidance and had hoped that safer and effective drugs would come to market [24]. Most of the clinical trials in the past included those patients who were at lower risk for CV complications. However, the 2008 FDA guidance suggested the inclusion of high-risk patients as well, such as elderly and those with advanced disease and renal impairment [24]. This 2008 FDA guidance is intended to bring to the market drugs that do not increase the risk of CV diseases. In fact, after 2008, we have newly approved ADDs showing CV benefits. However, there has not yet been a comprehensive evaluation of intended and unintended impacts of the 2008 FDA guidance on CV-related outcomes in diabetes drug development [25].
The increasing global incidence and prevalence of T2DM coupled with the CV consequences associated with both T2DM and ADDs represent an international public health crisis, demanding higher levels of certainty and evidence on how to safely and effectively treat these patients. We therefore conducted a systematic review with meta-analysis to compare CV mortality of ADDs approved for market before and after the 2008 FDA Guidance for Industry. Our hypothesis was that the ADDs approved after the release of the FDA’s 2008 Guidance for Industry will have lower risks of CV mortality compared with those that were approved before 2008.
2 Methods
2.1 Study Eligibility
Study eligibility was decided a priori. Studies with the following characteristics were included in the meta-analysis: (1) adults with T2DM with or without CV risk factors; (2) participants taking at least one oral ADD; (3) at least one study outcome on CV mortality; (4) RCTs; and (5) studies published in the English language up until 24 February 2017. We included studies that explicitly reported CV mortality. In addition, to reduce the heterogeneity of the study population, we only included oral ADDs. Because insulin and other injectable drugs are generally not used as first-line therapies for T2DM, they are generally prescribed for patients who cannot achieve appropriate glucose levels with other therapies.
The ADDs were classified into two groups: drugs approved before the 2008 FDA Guidance for Industry and those approved after the 2008 FDA guidance. The search was limited to articles published in peer-reviewed journals. The unpublished literature was not searched because of the difficulty in retrieving them and the uncertainty about the quality of their study designs.
2.2 Data Sources
We retrieved the literature from the following sources: (1) PubMed; (2) Scopus; and (3) the Clinical Trial Registry (ClinicalTrials.gov). The exact search strategy varied based on the database searched, however basic search terms included ‘type-2 diabetes’, ‘drug class’, ‘cardiovascular mortality’, and ‘random*’. An image of the search strategy is included in Online Resource eFig. 1. All duplicate studies were discovered and deleted using the ‘Find Duplicates’ tool in Endnote® 7.4, as well as through manual examination by the first author (RG), while all nonduplicate studies were stored in Endnote® version 7.4. All searches were performed by the first author (RG).
2.3 Study Selection
After removing the duplicate studies, study selection was performed by the first (RG) and second (PR) authors independent of each other by reviewing the title and abstract. The full text of the studies was retrieved for all titles and abstracts that appeared to meet the inclusion criteria or where there was any uncertainty. Reasons for exclusion were characterized as one or more of the following: inappropriate population, inappropriate intervention, inappropriate comparison, inappropriate outcome(s), and inappropriate study design. After selection, the first and second authors reviewed their selections and resolved any discrepancies by consensus. If consensus could not be reached, two other authors (JC, XT) were consulted. The overall agreement rate before correcting discrepant items was calculated using Cohen’s kappa statistic (κ). Once discrepancies were resolved, the overall precision of searches was calculated by dividing the number of studies included by the total number of studies screened after removing duplicates. The number needed to read (NNR) was then calculated as the inverse of precision.
2.4 Data Abstraction
Before data abstraction, a detailed codebook was developed in Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA). The general categories for which the included studies were coded comprised: (1) study participant characteristics; (2) study intervention regimens (drug dose, route of administration, and frequency); (3) study control (oral ADDs or placebo), with control also coded for dose, route of administration and frequency; (4) primary outcome (CV mortality); (5) study design; and (6) source of funding. The first two authors (RG, PR) performed data abstraction from selected studies, independent of each other, using the codebook in Microsoft Excel® 2013. Upon completion, both authors reviewed the codebook and resolved discrepancies by consensus. If consensus could not be reached, two other authors (JC, XT) were consulted. Before correcting disagreements, the overall agreement rate was calculated using Cohen’s kappa statistic (κ).
2.5 Outcomes and Prioritization
The primary outcome was CV mortality. As aforementioned, there is no consensus on the relation of HbA1c with CV mortality. Therefore, the secondary outcome was HbA1c levels.
2.6 Risk of Bias Assessment in Individual Studies
The risk of bias was assessed using the Cochrane Risk of Bias instrument [26]. Bias was evaluated for six domains: (1) random sequence generation (clinical trials usually assign study subjects to the treatment and control groups randomly to minimize selection bias and to ensure that the groups are comparable. The first domain of the risk of bias instrument evaluated whether the study subjects were randomly assigned to the treatment groups); (2) allocation concealment (the allocation of treatment is often performed using a computer-generated or otherwise randomized sequence. The second domain was used to evaluate whether the sequence or method used for treatment allocation was concealed from patients, researchers, or any other study personnel); (3) blinding of participants and personnel (a lot of clinical trials have a double-blind study design to conceal the treatment from study subjects and the researcher to minimize selection bias. If the study was blinded, the third domain was used to evaluate whether the study subjects and the personnel involved were blinded); (4) blinding of outcome assessors (the fourth domain was used to evaluate if the person assessing the primary outcomes was blinded or not. All participants who are enrolled in the study often do not complete the study; the extent to which the authors had described the loss of participants to follow-up, along with the reason for the loss to follow-up, was used to determine whether there was any attrition bias or not); (5) incomplete outcome data; and (6) selective reporting (selective reporting bias was used to assess the consideration of the possibility of selective outcome reporting by the review authors). Each study was classified as having a high, low, or unclear risk of bias both overall and for each domain. The overall risk of bias was classified as high if any one of the domains was considered high risk. No study was excluded based on the results of the risk of bias assessment. The first two authors (RG, PR) conducted all risk of bias assessments independent of each other, and then reviewed the results for risk of bias assessment and resolved any discrepancies by consensus. If consensus could not be reached, two other authors (JC, XT) were consulted.
3 Data Synthesis
3.1 Outcome Summary Measures
The primary outcome for this study was CV mortality, calculated as an odds ratio (OR). All analyses were conducted by using the natural log of the OR and then transformed back to ORs for presentation purposes. If the OR was not reported, then it was calculated from data reported in the study. If data were not available to calculate the OR, then we contacted the study authors and requested relevant data. The secondary outcome was reported as standardized mean difference, calculated by dividing the change in mean baseline HbA1c and standard deviation (SD).
3.2 Pooled Estimates for Changes in Outcomes
Effect size changes in both CV mortality and HbA1c were pooled using the random-effects model [27]. Heterogeneity for each pooled outcome was estimated using Cochran’s Q [28, 29]. An alpha level of ≤ 0.10 for Q was considered to represent statistically significant heterogeneity. Inconsistency, which was estimated using the I2 statistic, was categorized as very low (≤ 25%), low (25% to ≤50%), moderate (50% to ≤ 75%) or large (≥ 75%) [29].
Influence analysis was conducted with each study deleted from the model once, to examine the effect of each study on our overall results. In addition, cumulative meta-analysis, ranked by year, was used to study the accumulation of results over time. Subgroup analysis was performed to examine the association between CV mortality and the year in which a drug was approved by the FDA.
3.3 Meta-Biases
Small-study effects (publication bias, etc.) for the primary and secondary outcomes were assessed using a funnel plot [30], which is a scatter plot of the effect estimates from individual studies against precision (inverse of standard error). The effect estimates from smaller studies scatter at the bottom and larger studies are concentrated at the top. The plot resembles a symmetrical inverted funnel in the absence of bias.
4 Results
4.1 Study Characteristics
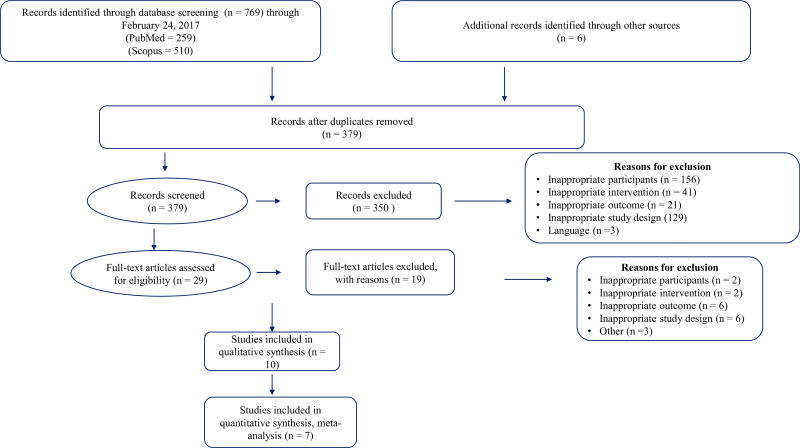
Table 1 presents the general description of the characteristics of each of the included studies. Of the 392 citations reviewed, a total of 54,951 participants (27,670 in the oral ADD groups and 27,281 in the control groups) nested within ten studies were included in the final analysis [20, 31–39]. All ten studies were used in the qualitative analysis, whereas only seven studies were included in the quantitative analysis due to incomplete reporting. The three studies that were not included in the quantitative analysis did not report data to calculate an effect size for CV mortality. The precision of the searches was 0.02 and the NNR was 39. A description of the study selection process, along with the number of studies screened, included, and excluded, and broad categories of reason for exclusion, are presented in Fig. 1. A list of excluded studies along with reasons for exclusion is provided in Online Resource eTable 1. The number of subjects in each class of drug from each study varied from 151 to 8280. Among the studies included in the systematic review, one was conducted in the US, one in the UK, one in China, and seven studies were carried out in multiple countries simultaneously. The average follow-up period was 3.9 years (SD ± 2.6, median 3.05 years, range 0.5–5.7 years). Six studies included in the systematic review had an active comparator (either an oral ADD or insulin) and four studies were placebo controlled. The included studies accessed four different classes of drug that were approved before 2008: biguanides (metformin), thiazolidinediones (pioglitazone and rosiglitazone), sulfonylureas (glipizide, chlorpropamide, glibenclamide, glyburide, and glimepiride), DPP-4 inhibitor (sitagliptin); and two drug classes that were approved after 2008: DPP-4 inhibitor (linagliptin, and saxagliptin) and sodium-glucose co-transporter-2 (SGLT-2) inhibitors (empagliflozin).
Table 1.
General characteristics of the included studies
| Author | Year | Approval year |
Type of RCT | Study location | Intervention type | Participants |
|---|---|---|---|---|---|---|
| Doehner et al. [31] | 2012 | 1999 | Randomized, double-blind | Multiple countries | Placebo-controlled | Pioglitazone (n = 2592, age 61.9 ± 7.6 years, CVD risk = yes, approval year = 1999) |
| Pioglitazone vs. placebo | ||||||
| Placebo (n = 2610, age 61.6 ± 7.7 years, CVD risk = yes) | ||||||
| Giles et al. [32] | 2010 | 1999 | Randomized, double-blind | US | Active control | Pioglitazone (n = 151, age 64 years, CVD risk = yes, HbA1c 8.6 ± 1.5, approval year = 1999) |
| Pioglitazone vs. glyburide | ||||||
| Glyburide (n = 149, age 64 years, CVD risk = yes, HbA1c 8.3 ± 1.4, approval year = 1984) | ||||||
| Giles et al. [33] | 2008 | 1999 | Prospective, double-blind | Multiple countries | Active control | Pioglitazone (n = 262, age 64.2 ± 9.9 years, CVD risk = yes, HbA1c 8.7 ± 1.5, approval year = 1999) |
| Pioglitazone vs. glyburide | ||||||
| Glyburide (n = 256, age 63.4 ± 9.4 years, CVD risk = yes, HbA1c 8.9 ± 1.8, approval year = 1984) | ||||||
| Hong et al. [34] | 2013 | 1997 | Prospective, randomized, double-blind | China | Active control | Glipizide (n = 148, age 63.8 ± 9.4 years, CVD risk = yes, HbA1c 7.6 ± 1.7, approval year = 1997) |
| Glipizide vs. metformin | ||||||
| Metformin (n = 156, age 62.8 ± 8.5 years, CVD risk = yes, HbA1c 7.6 ± 1.7, approval year = 1994) | ||||||
| Scirica et al. [20] | 2013 | 2009 | Randomized, double-blind | Multiple countries | Placebo-controlled | Saxagliptin (n = 8280, age 65.1 ± 8.5 years, CVD risk = yes, HbA1c 8 ± 1.4, approval year = 2009) |
| Saxagliptin vs. placebo | ||||||
| Placebo (n = 8212, age 65 ± 8.6 years, CVD risk = yes, HbA1c 8 ± 1.4) | ||||||
| Zinman et al. [36] | 2015 | 2014 | Randomized, double-blind | Multiple countries | Placebo-controlled placebo | Empagliflozin (n = 2345, age 63 ± 8.6 years, CVD risk = yes, HbA1c 8.1 ± 0.9, approval year = 2014) |
| Empagliflozin vs. | ||||||
| Placebo (n = 2333, age 63.2 ± 8.8 years, CVD risk = yes, HbA1c 8.1 ± 0.9) | ||||||
| Gallwitz et al. [37] | 2012 | 2011 | Randomized, double-blind | Multiple countries | Active control | Linagliptin (n = 764, age 59.8 ± 9.4 years, CVD risk = no, HbA1c 7.7 ± 0.9, approval year = 2011) |
| Linagliptin vs. Glimepiride | ||||||
| Glimepiride (n = 755, age 59.8 ± 9.4 years, CVD risk = no, HbA1c 7.7 ± 0.9, approval year = 1999) | ||||||
| Green et al. [38] | 2015 | 2006 | Randomized, double-blind | 38 countries; multiple sites: 673 | Placebo-controlled | Sitagliptin (n = 7332, age 65.4 ± 7.9 years, CVD risk = yes, HbA1c 7.2 ± 0.5, approval year = 2006) |
| Sitagliptin vs. placebo | ||||||
| Placebo (n = 7339, age 65.5 ± 8 years, CVD risk = yes, HbA1c 7.2 ± 0.5) | ||||||
| Home et al. [39] | 2009 | 1999 | Prospective, randomized, open-label | Europe, Australasia | Active control | Rosiglitazonea (n = 1117, age 57 ± 8 years, CVD risk = no, HbA1c 7.8 ± 0.7, approval year = 1999) |
| Rosiglitazone vs. metformin + SU | ||||||
| Rosiglitazoneb (n = 1103, age 59.8 ± 8.3 years, CVD risk = no, HbA1c 8 ± 0.7) | ||||||
| Metformin (n = 1105, age 57.2 ± 8.1 years, CVD risk = no, HbA1c 7.8 ± 0.7, approval year = 1994) | ||||||
| SU (n = 1122, age 59.7 ± 8.2 years, CVD risk = no, HbA1c 8 ± 0.7) | ||||||
| Turner et al. [35] | 1998 | 1958 | Randomized | UK | Active control | Chlorpropamide (n = 619, age 54 ± 9 years, HbA1c 6.3 ± 1.4, approval year = 1958) |
| Chlorpropamide/glibenclamide vs. insulin | Glibenclamide (n = 615, age 54 ± 8 years, HbA1c 6.3 ± 1.3, approval year = 1998) | |||||
| Insulin (n = 911, age 54 ± 8 years, HbA1c 6.1 ± 1.1) |
RCT randomized clinical trial, CVD cardiovascular disease, HbA1c glycated hemoglobin, SU sulfonylurea
Patients with type 2 diabetes on monotherapy with metformin at baseline who were assigned to rosiglitazone treatment
Patients with type 2 diabetes on monotherapy with sulfonylurea at baseline who were assigned to rosiglitazone treatment
Fig. 1.
Study selection process
4.2 Participant Characteristics
Characteristics of the participants are also presented in Table 1. The mean ages in the intervention and control groups were 61.1 years (SD ± 3.8) and 61.6 years (SD ± 3.2), respectively, and the mean baseline HbA1c level in the intervention group was 7.7% (SD ± 0.7), and 7.8% (SD ± 0.7) in the control group.
4.3 Primary Outcome
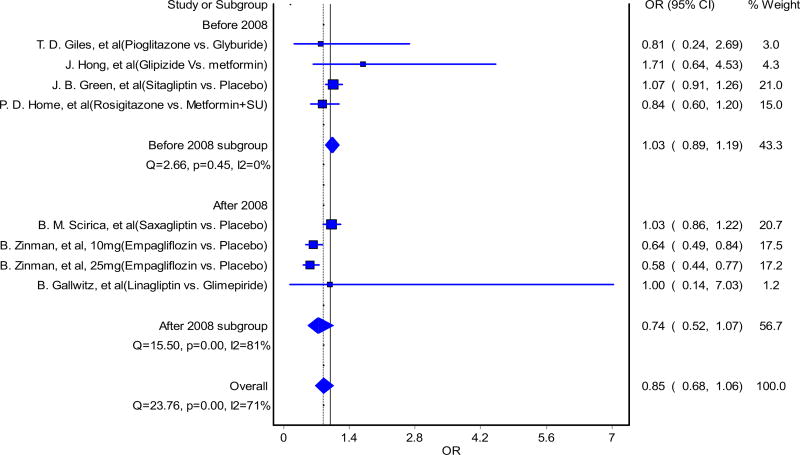
The forest plot of CV mortality of oral ADDs is shown in Fig. 2. When including all oral ADDs (both approved before and after 2008) into the analyses, there was no significant increase in the risk of CV mortality, and the upper bound of the two-sided 95% CI of the OR was < 1.3 (OR 0.85, 95% CI 0.68–1.06). The results from the influence analysis revealed a similar conclusion, with ORs for CV mortality ranging between 0.80 (95% CI 0.62–1.05) and 0.92 (95% CI 0.76–1.12). The pooled effect size, Cochran’s Q statistics, I squared statistics, and tau-squared are presented in Table 2.
Fig. 2.
Impact of oral hypoglycemic agents on cardiovascular mortality, approved before and after 2008. Forest plot from the randomeffects model for association between oral antidiabetic drugs and cardiovascular mortality, comparing those approved before and after the 2008 FDA Guidance for Industry. Seven studies were included in the analysis. The results of Zinman et al. [36] (10 and 25 mg) were from one study where cardiovascular safety of two different strengths of empagliflozin were assessed. OR odds ratio, CI confidence interval
Table 2.
Changes in study outcomes
| Analysis | Studies | Comparisons | Summary statistics | 95% CI | Q (p) | I2 | 95% CI |
|---|---|---|---|---|---|---|---|
| CV mortality for all eligible studies | 7 | 9 | 0.85 | 0.68–1.06 | 23.8 (0.001) | 71 | 39–86 |
| HbA1c | 5 | 6 | −0.08 | − 0.26 to 0.09 | 63.6 (0.0001) | 92 | 85.6–95.7 |
Summary statistics for CV mortality are reported as odds ratio, and for HbA1c are reported as standardized mean difference
CV cardiovascular, CI confidence interval, Q Cochran’s Q, I2 inconsistency, HbA1c glycated hemoglobin
When comparing the risks of CV mortality between the drugs approved before and after 2008, we found there were no significant increases in CV mortality for both groups of drugs approved before and after 2008 (Fig. 2). The OR and the upper bound of the two-sided 95% CI for drugs approved after 2008 (OR 0.74, 95% CI 0.52–1.07) were lower than the OR for drugs approved before 2008 (OR 1.03, 95% CI 0.89–1.19). Furthermore, the upper bounds of the two-sided 95% CI for both drugs before and after 2008 were below 1.3.
The forest plot of CV mortality of oral ADDs by the comparator groups (placebo vs. active control) is presented in Online Resource eFig. 5. We did not find any significant association between the risk of CV or mortality of oral ADDs and the studies in which the control group was an active comparator group (OR 0.91, 95% CI 0.66–1.24) versus those in which the control group was placebo (OR 0.82, 95% CI 0.61–1.06). The upper bounds of the two-sided 95% CI for drugs in both groups were also within the range prespecified by the FDA in their 2008 Guidance to Industry (below 1.3).
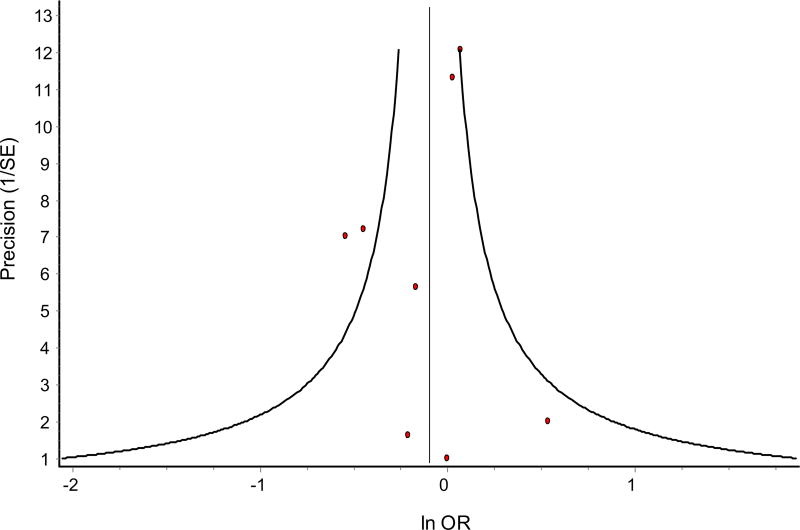
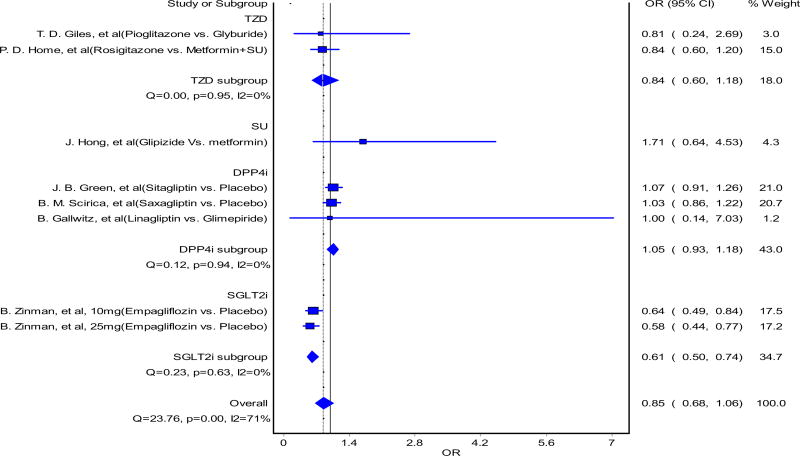
The funnel plot is presented in Fig. 3. Minor asymmetry was observed due to small-study effects. The results from subgroup analysis of examining CV mortality among different drug classes are shown in Fig. 4. Only the SGLT-2 inhibitor drug class was shown to have a significant reduction in CV mortality. Both 10 and 25 mg doses of empagliflozin were associated with a 36 and 42% reduction in CV mortality, respectively, compared with placebo [36]. All other drug classes did not show a significant increase in CV mortality, but sulfonylureas had a higher OR of CV mortality and the upper bound of the two-sided 95% CI of the OR was greater than 1.8, which is outside the limits specified by the FDA [23].
Fig. 3.
Funnel plot for the association between oral antidiabetic drugs and cardiovascular mortality. Small-study effects are present, as observed by little asymmetry in the plot. The figure depicts small-study effects of seven studies included for meta-analysis. There are eight data points in the plot because the results of Zinman et al. [36] (10 and 25 mg) were from one study where cardiovascular safety of two different strengths of empagliflozin were assessed. Egger’s bias = − 0.5960166, p = 0.650
Fig. 4.
Forest plot from the random-effects model for the association between oral antidiabetic drugs and cardiovascular mortality, comparing different pharmacological drug classes. The results of Zinman et al. [36] (10 and 25 mg) were from one study where cardiovascular safety of two different strengths of empagliflozin were accessed. OR odds ratio, CI confidence interval
4.4 Secondary Outcome
The forest plot of the efficacy of oral ADDs (HbA1c) is presented in Online Resource eFig. 2. The comparison of the efficacy of oral ADDs approved before (standard mean difference [SMD] −0.12, 95% CI −0.34 to 0.22) and after 2008 (SMD 0, 95% CI −0.16 to 0.16) did not show significant differences. In addition, taking all included oral ADDs together (both approved before and after 2008), we did not find significant reductions in HbA1c (SMD −0.08, 95% CI −0.26 to 0.09). The funnel plot (Online Resource eFig. 3) shows minor asymmetry due to small-study effects. Influence analysis revealed that the SMD of HbA1c ranged between −0.05 (95% CI −0.22 to 0.16) and −0.16 (95% CI −0.27 to −0.05). The pooled effect size, Cochran’s Q statistics, I-squared statistics, and tau-squared are all presented in Table 2.
Another subgroup analysis was performed by assessing the effects on HbA1c among different ADD classes (Online Resource eFig. 4). We observed a significant decrease in HbA1c in SGLT-2 inhibitors (empagliflozin 10 mg: SMD −0.24, 95% CI −0.40 to −0.08; empagliflozin 25 mg: SMD −0.36, 95% CI −0.51 to −0.20). No other drug classes were found to have a significant influence on HbA1c. A significant reduction in HbA1c was seen in the studies where baseline HbA1c was > 8% compared with those where baseline HbA1c was < 8%.
4.5 Risk of Bias
Risk of bias results are shown in Online Resource eFig. 6, while the results for each item from each study are shown in Online Resource eTable 2. Nine of ten studies [20, 31, 32, 34–39] were considered to be at low risk on random sequence generation, while one study was considered to be at high risk [33]. Most the studies (eight of ten) were at low risk [31–35, 37, 39] with respect to allocation concealment, while two studies were considered to be at high risk [36, 39]. Blinding of participants and personnel was poorly reported; three studies were considered to be at low risk [34, 37, 38], three at high risk [35, 36, 39], and the remaining four studies at unclear risk [20, 31–33]. Blinding of outcome assessment was also poorly reported; two studies were at low risk [34, 38], three studies at high risk [35, 36, 39], and the remaining five studies at unclear risk [20, 31–33, 37]. On incomplete outcome data reporting, four studies were considered to be at low risk [32–34, 37], while six studies were considered to be at high risk [20, 31, 35, 36, 38, 39]. Most of the studies (six of ten) were considered to be at low risk [20, 32–34, 36, 38] on selective reporting, while four studies were considered to be at high risk [31, 35, 37, 39].
5 Discussion
To the best of our knowledge, this was the first study to evaluate whether or not the implementation of the 2008 FDA guidance resulted in the approval of ADDs with a safer CV profile. We did not find significant increases in CV mortality for both groups of drugs approved before and after 2008, and the ORs of both groups were within the acceptable range. Nonetheless, the group of drugs that were approved before 2008 was edging on a higher risk of CV mortality, with a higher OR and upper bound of the two-sided 95% CI, compared with the group approved after 2008. After 2008, we have approved drugs with significant reductions in CV mortality [25].
We did not find a significant increase in CV mortality for drugs approved before 2008, and the upper bound of the two-sided 95% CI of the overall OR in this group was also in the acceptable range; however, this should be interpreted in the following context. First, since manufacturers were only required to prove efficacy before the implementation of the guideline, there are only a few clinical trials evaluating the CV safety of the ADDs that were approved before 2008. Second, clinical trials of drugs that were approved before 2008 included more low-risk patients. For example, the study by Hong et al. included patients with a low or properly managed HbA1c, who had normal body mass index, and were mostly males [34]. Therefore, these were low-risk patients who were less likely to develop any CV complications with or without oral ADDs. Third, the non-significant results from RCTs of the drugs approved before 2008 could be attributed to the relatively short duration of follow-up or the small sample size. The RCTs that had CV mortality or CV safety as an endpoint either had a short follow-up of < 1 year [32, 33] or a small sample size [35], which may be potential barriers to establishing significance.
In addition, the purpose of the FDA guidance of 2008 was not only to have specific CV endpoints to evaluate the CV safety of the ADD but was also to emphasize that HbA1c could not be used as a surrogate of CV safety and is a poor predictor of macrovascular complications and CV mortality [40]. Our study findings were consistent with the guidance recommendations. Three studies for drugs approved after 2008 showed a significant reduction in HbA1c; however, two did not show any significant association with CV mortality [20, 37, 41]. Nonetheless, a trial using empagliflozin, which was approved after 2008, was shown to have a significant reduction in CV mortality [36], yet the ADD drug class differences in CV mortality may confound the influences of the 2008 FDA guidance on CV mortality. Although the overall trend was that drugs approved after 2008 had a safer CV mortality risk profile than those approved before 2008, it is not certain whether this was influenced primarily by the FDA 2008 guidance or the effect of the drug class.
There are opposing opinions on the implementation of the 2008 FDA guidance. On the one hand, researchers believe that the 2008 FDA guidance leads to uncovering of unrecognized CV side effects. In this way, it is thought that the landscape of ADD development has been radically changed by establishing CV safety, recognizing side effects, and including high-risk populations [21]. On the other hand, some question the application of stricter rules to all ADD classes. Thus, a more targeted approach was suggested to assess CV safety in new ADDs [25]. Along with the paradigm shift in the industry to bring drugs with a better CV safety profile to the market after 2008, our results further strengthen the recommendations of 2008 FDA guidance use for the preapproval stage of ADD development.
Given the difference in the CV risk profile of different ADD classes, clinicians should weigh CV safety against the potential benefits in treatment decisions. Our study provides summarized evidence about the CV mortality of oral ADDs. DPP-4 inhibitors and SGLT-2 (empagliflozin) have a fairly low risk for CV mortality. Clinicians might consider prescribing ADDs with relatively lower CV risk implications to diabetes patients who have high risks of developing CVD.
This study has some limitations. Although CV mortality is one of the outcomes suggested by the FDA to be reported in trials evaluating the CV safety of ADDs, most of the studies report them as a composite primary endpoint, along with nonfatal myocardial infarction and stroke; therefore, only a few RCTs report CV mortality individually. Most of the studies included in our meta-analysis did not adequately report the outcomes. Six of ten studies had a high risk of bias on incomplete outcome data. All the meta-analyses inherit the weaknesses and limitations of the studies that are included for analysis, which can impact the findings and conclusions drawn from the meta-analysis. Another limitation of a meta-analysis is that it may be prone to ecological fallacy; since we have used an aggregate data approach in the current study, drawing inferences for an individual based on the study findings might be inappropriate.
Despite these limitations, this study is the first meta-analysis evaluating the impact of the 2008 FDA guidance on CV mortality of oral ADDs, comparing the CV mortality of ADDs approved for market before and after the 2008 FDA guidance. This is a comprehensive systematic review including ten trials with a total of 54,951 adults with T2DM. We also used a rigorous method to conduct our meta-analysis (e.g. use of the Cochrane Risk of Bias tool to assess the risk of bias of the included studies).
6 Conclusions
Overall, there appears to be a positive influence of the 2008 FDA guidance on CV mortality. The overall trend was that the drugs approved after 2008 had a safer CV mortality risk profile than those approved before 2008. Empagliflozin was found to be associated with a significant reduction in CV mortality. After 2008, there has been a paradigm shift in the ADD development industry to bring more drugs with a safer CV profile to the market. With the continued implementation of the 2008 FDA Guidance for Industry, we expect to see more drugs that do not increase, and even reduce, the risk of CV mortality among these patients.
Supplementary Material
Key Points.
This is the first study to evaluate the impact of the US FDA’s 2008 guidance to industry on cardiovascular (CV) mortality of antidiabetes drugs (ADDs).
Our study found that there was no significant increase in CV mortality for drugs approved before and after 2008.
The 2008 FDA guidance to industry has successfully directed more focus to the CV safety of ADDs, along with efficacy, thereby resulting in marketing of ADDs with a safer CV profile.
Acknowledgments
Funding No funding was received to conduct this study.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s40261-018-0639-z) contains supplementary material, which is available to authorized users.
Conflicts of interest Rashmi Goyat, Pragya Rai, Jongwha Chang, Charles D. Ponte, and Xi Tan declare no conflicts of interest.
NOTICE WARNING CONCERNING COPYRIGHT RESTRICTIONS
The copyright law of the United States [Title 17, United States Code] governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the reproduction is not to be used for any purpose other than private study, scholarship, or research. If a user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of "fair use," that use may be liable for copyright infringement. This institution reserves the right to refuse to accept a copying order if, in its judgement, fullfillment of the order would involve violation of copyright law. No further reproduction and distribution of this copy is permitted by transmission or any other means.
References
- 1.National Diabetes Statistics Report. Atlanta: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 2.Lemmens VE, et al. Which comorbid conditions predict complications after surgery for colorectal cancer? World J Surg. 2007;31(1):192–9. doi: 10.1007/s00268-005-0711-8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 4.Standards of medical care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4–5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 5.Ryden L, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 6.Skyler JS, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119(2):351–7. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–9. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 10.Eeg-Olofsson K, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) J Intern Med. 2010;268(5):471–82. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 11.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 12.Manley S. Haemoglobin A1c: a marker for complications of type 2 diabetes: the experience from the UK Prospective Diabetes Study (UKPDS) Clin Chem Lab Med. 2003;41(9):1182–90. doi: 10.1515/CCLM.2003.182. [DOI] [PubMed] [Google Scholar]
- 13.Nichols GA, et al. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–9. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 14.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370(9593):1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 15.Tzoulaki I, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. Br Med J. 2009;339:b4731. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maru S, et al. Antidiabetic drugs and heart failure risk in patients with type 2 diabetes in the U.K. primary care setting. Diabetes Care. 2005;28(1):20–6. doi: 10.2337/diacare.28.1.20. [DOI] [PubMed] [Google Scholar]
- 17.Fu AZ, et al. Association between hospitalization for heart failure and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes: an observational study. Diabetes Care. 2016;39(5):726–34. doi: 10.2337/dc15-0764. [DOI] [PubMed] [Google Scholar]
- 18.Spinar J, Smahelova A. SAVOR TIMI 53 - Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus [in Czech] Vnitr Lek. 2013;59(11):1003–7. [PubMed] [Google Scholar]
- 19.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs: insights from the rosiglitazone experience. N Engl J Med. 2013;369(14):1285–7. doi: 10.1056/NEJMp1309610. [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 21.Menon V, Lincoff AM. Cardiovascular safety evaluation in the development of new drugs for diabetes mellitus. Circulation. 2014;129(25):2705–13. doi: 10.1161/CIRCULATIONAHA.113.008221. [DOI] [PubMed] [Google Scholar]
- 22.Jin T. Why diabetes patients are more prone to the development of colon cancer? Med Hypotheses. 2008;71(2):241–4. doi: 10.1016/j.mehy.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed 12 Mar 2018];Guidance for Industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008 Available at: https://www.fda.gov/downloads/Drugs/…/Guidances/ucm071624.pdf.
- 24.Hirshberg B, Katz A. Cardiovascular outcome studies with novel antidiabetes agents: scientific and operational considerations. Diabetes Care. 2013;36(Suppl 2):S253–8. doi: 10.2337/dcS13-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith RJ, Goldfine AB, Hiatt WR. Evaluating the cardiovascular safety of new medications for type 2 diabetes: time to reassess? Diabetes Care. 2016;39(5):738–42. doi: 10.2337/dc15-2237. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–11. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. [Google Scholar]
- 29.Higgins JP, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 31.Doehner W, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162(1):20–6. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Giles TD, et al. Comparison of pioglitazone vs glyburide in early heart failure: insights from a randomized controlled study of patients with type 2 diabetes and mild cardiac disease. Congest Heart Fail. 2010;16(3):111–7. doi: 10.1111/j.1751-7133.2010.00154.x. [DOI] [PubMed] [Google Scholar]
- 33.Giles TD, et al. Pioglitazone and heart failure: results from a controlled study in patients with type 2 diabetes mellitus and systolic dysfunction. J Card Fail. 2008;14(6):445–52. doi: 10.1016/j.cardfail.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Hong J, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36(5):1304–11. doi: 10.2337/dc12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner R. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 36.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 37.Gallwitz B, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–83. doi: 10.1016/S0140-6736(12)60691-6. [DOI] [PubMed] [Google Scholar]
- 38.Green JB, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 39.Home PD, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 40.McGuire DK. Regulating drugs that regulate glucose: cardiovascular assessment of type 2 diabetes medications. Dallas: University of Texas Southwestern Medical Center; 2014. [Google Scholar]
- 41.Zinman B, et al. Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: a 16-week, randomised, open-label, phase 2 trial. Lancet. 2011;377(9769):924–31. doi: 10.1016/S0140-6736(10)62305-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.