Abstract
The folate receptor (FR) is a valued target that is highly expressed in various cancers, which will expedite the development of ligand-receptor binding based cancer therapeutics. In the present investigation, through tissue microarray analysis, we report higher levels of folate receptor expression in prostate cancer (PCa) tissue derived from patients, which were minimal in normal tissue. For folate-receptor based targeted therapy of PCa, we generated novel planetary ball milled (PBM) nanoparticles (NPs) encapsulated with resveratrol (RES), and in combination with docetaxel (DTX) and conjugated with folic acid (FA) on the surface. The cytotoxic effect of FA-conjugated DTX-nanoparticles was found too effectual that reduced the concentration of free drug (DTX) to 28 times. Flow cytometry analysis showed a significant increase in the number of apoptotic cells by 30.92% and 65.9% in the FA-conjugated RES and in combination with DTX nanoparticle formulation respectively. However, only 8.9% apoptotic cells were found with empty NP. The expressions of NF-kB p65, COX-2, pro (BAX, BAK) and anti-apoptotic (BCL-2, BCL-XL) genes were significantly reduced after treatment with FA-RES+DTX-NP. In addition, the FA-conjugated DTX formulation exhibited additional cytotoxic effects with the down-regulation of survivin and an increased expression of Cleaved Caspase-3 in PCa cells. Further, we observed that treating DTX resistant PCa cells with FA-RES+DTX-NP exhibited a negative effect on the ABC-transporter markers thereby limiting the multidrug resistance phenotype of the cancer cells. Our results strongly suggested that FA coated nanoparticle drugs acted as effective inhibitors of drug efflux that effectually enhances the intracellular concentration of the drug to exhibit their cytotoxic effect.
Keywords: ATP-Transporter genes, Docetaxel, Resveratrol, Nanoparticles, Drug resistance, Folate receptors
1. Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed cancers and is the second-leading cause of cancer-related death in men [1]. Among all newly diagnosed cancers in the United States, 15% of men will be diagnosed with PCa in their lifetime. As PCa cells depend on androgen receptor (AR) signaling for growth and survival, initial treatments for PCa focus on the use of androgen deprivation therapies (ADT) to reduce levels of circulating androgens to inhibit tumor growth. This is achieved either through surgery or via bilateral orchiectomy to inhibit androgen synthesis by the testes or with medical castration using drugs [2].
Historically, PCa was considered moderately resistant to cytotoxic therapy [3]. Docetaxel is the primary drug used to treat cancer [4]. It exerts cytotoxicity initiating mitotic arrest and, ultimately, apoptosis. However, resistance to docetaxel has emerged in PCa cells, and the known mechanisms include that limit of the intracellular drug concentration [3]. Among the various studies that were conducted to uncover the potential mechanisms for the development of resistance in PCa, over-expression of several ABC transporters was found to be responsible for the efflux of chemotherapeutic drugs [5]. Thus, the development of agents able to modulate multidrug resistance (MDR) mediated by efflux pumps remained a major goal for PCa therapy.
In this context, with the mounting drawbacks of conventional chemotherapy, nanotechnology has made emerged as revolutionary in the selective treatment of cancer [6]. Although nanoparticle formulations have proven to have the potential in improving the therapeutic efficacy of anticancer agents [7], a recent survey showed a median nanoparticle delivery efficiency of only 0.7% to the targeted solid tumors [8]. The greater challenges that are faced by passive drug treatments include transporting a high concentration of the drug to the target site and limiting undesirable systematic adverse effects. However, these hurdles could be circumvented through nano-therapeutics that target the cancer cells by permitting the preferential accumulation of the drug within selected tissues, individual cancer cells, or intracellular organelles that are associated with specific molecules in cancer cells termed as “targeted drug delivery” (TDD) [9].
TDD or active targeting is formulated to describe the specific interactions between the drug carrier and the target cells, usually through specific ligand-receptor interactions [10]. The efficiency of the ligand-receptor binding in active targeting depends on various factors, such as a selective expression of the receptor on the surface at the target cells, its availability, and the rate of internalization vs the shedding of the receptor following ligand binding [6, 11, 12]. One such receptor that was highly targeted in nanotherapeutics is the folate receptor, a glycosylphosphatidylinositol-anchored cell-surface protein, which is highly expressed in many cancer cells [13]. Utilizing this concept, folic acid is now being used to coat the nanoparticles because of such advantages as its stability over a wide range of temperatures, pH values, cost-effectiveness, non-immunogenic and its ability to bind the folate receptor after conjugating with the drug or the diagnostic markers [14].
In our recent report, we found that RES and DTX combination blocks the cell cycle arrest by modulation of key regulators and promotes apoptosis via p53 dependent and independent mechanism in PCa [15]. In the present investigation, we present the data showing the expression of folate receptor on clinical samples of prostate cancer. The expression of folate receptor was exploited in designing a nanoparticle using the combination of RES and DTX to treat DTX resistant prostate cancer cells. In addition, we focused on the possible mechanisms by which the RES-DTX nanoparticle formulation inhibits anti-apoptotic markers and promotes cell death by re-sensitizing the DTX resistant PCa cells.
2. Materials and methods
2.1 Cell lines and cell culture treatment
The human prostate cancer cell lines PC3; C4-2B and LNCaP cells were grown in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS), L-glutamine, nonessential amino acid, HEPES, 2mM L-glutamine and penicillin/streptomycin antibiotic solution (Fisher Scientific, Pittsburgh, PA). RWPE-1 cells were grown in keratinocyte serum-free medium (K-SFM), supplemented with 0.05 mg/ml bovine pituitary extracts (BPE), 5 ng/ml human recombinant epidermal growth factor (EGF), and penicillin/streptomycin antibiotic solution (Fisher Scientific, Pittsburgh, PA). All the cell lines were maintained in standard condition incubator at 37°C and 5% CO2.
Docetaxel resistant cells were prepared from the parental PC3 cells. The resistant cells were generated by gradually increasing the docetaxel concentration starting from 4nM to 40nM and were maintained in standard condition incubator for 8 months at 37°C and 5% CO2. The docetaxel resistant cells were labeled as PC3-R and were maintained in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS), L-glutamine, nonessential amino acid, HEPES, 2mM L-glutamine and penicillin/streptomycin antibiotic solution (Fisher Scientific, Pittsburgh, PA).
2.2 Materials and reagents
Resveratrol (RES) was purchased from Fisher Scientific (Pittsburgh, PA) and docetaxel (DTX) from Sigma (St. Louis, MO). Dimethyl Sulphoxide (DMSO) (Fisher Scientific, Pittsburgh, PA) was used to dissolve for both RES and DTX and further diluted with media used for cell growth. Soluble starch, Diethyl Ether, Acetone, 4-(dimethyl amino) pyridine, Dicyclohexylcarbodiimide were purchased from Fisher Scientific (Pittsburgh, PA) and fluorescein sodium salt, folic acid, N-Hydroxysuccinimide ester, Triethyl amine, Polyethylene Glycol, Dioxane, N, N’-disuccinimidyl carbonate (DSC), Polycaprolactone, and Potassium Bromide was purchased from Sigma (St. Louis, MO).
2.3 PBM nanoparticle formulation method
The milling jar holds heat absorbent zirconium oxide planetary milling balls rotate about its own axis as well as in the opposite direction around a common axis of the chamber wheel. This produces the rotation of planetary balls and milling of the particles from the macro-particles containing starch, fluorescein sodium salt and resveratrol or docetaxel alone. The controlled centrifugal force was applied by varying the revolution/sec (Ω), jar velocity, the size as well as a number of the zirconium oxide balls, duration and number of cycle control the size of particles. More detail is available in Issued Patent US 8,231,907[16].
2.4 Synthesis of N-Hydroxysuccinimide ester-activated folate (NHS-FA)
The carboxylate group of folic acid was activated by N-hydroxysuccinimide (NHS) and dicyclohexylcarbodiimide (DCC). Briefly, folic acid (250 μM) and triethylamine dissolved in dimethylsulfoxide (DMSO, 2 mL) were reacted with NHS (500 μM) and DCC (500 μM) at room temperature for 16 h (folic acid: NHS: DCC molar ratio= 1:2:2). The by-product, dicyclohexylurea, was removed by filtration (0.22μm). The dimethyl sulfoxide solution was then concentrated under reduced pressure and heating, and NHS-folate was precipitated in diethyl ether. The product, NHS-folate, was washed several times with anhydrous ether, dried under vacuum, and stored as a powder.
2.5 Preparation of N-Hydroxysuccinimide ester (NHS)-activated folate-conjugated PEG
Briefly, for PEG activation 5g polyethylene glycol (PEG) dissolve in 25 ml of dry dioxane and heating in a water bath to solubilize fully the polymer and react with 6 mmol of N, N’-disuccinimidyl carbonate (DSC) in presence of 6 mmol of 4-(dimethylamino) pyridine in 10 ml of dry acetone with continuous stirring for 6h at room temperature. Precipitate the succinimidyl carbonate (SC)-PEG formed by the addition of diethyl ether until no further precipitation was observed (typically 3-4 vol. of solvent), re-dissolve the precipitated product in acetone and precipitated again using diethyl ether. For PCL activation, 2gm PCL and dry dioxane (6mL) mixed and heated in a water bath and then N, N’-disuccinimidyl carbonate and pyridine were added and placed in a shaker to react for 6 hours. Subsequently filtered the precipitate with diethyl ether and re-dissolve in acetone and placed to become a dry powder. Next, NHS-FA stock solution (148 mM) was added to activate PEG (147 mM) in DMSO (5.0 ml), in the presence of triethylamine (4.0 ml), and the mixture was stirred overnight. The product was purified by a glass filter (Fisher Scientific, Pittsburgh, PA) to remove unconjugated folic acid and lyophilized.
2.6 Characterization of PCa cell-specific PBM nanoparticles
Size and zeta potential of RES-loaded folate-PCL-PEG coated and RES+DTX-loaded folate-PCL-PEG coated or uncoated PBM nanoparticles were measured at pH 6.8 using a Malvern Zetasizer ZS instrument at a concentration of 0.1 mg/mL(5% mass, assuming a density of 1 g/cm3) of nanoparticles.
The morphologies of NPs were examined employing scanning electron microscope (SEM). The prepared samples of SEM, PCL-PEG coated NPs were mounted on the stubby a double-sided adhesive tape followed by coating with platinum–palladium layer and examined by JEOL 820 SEM (Tokyo, Japan).
For surface chemistry, Fourier transform infrared (FTIR) spectroscopy was performed. Initially, the sample was prepared in the form of a transparent pellet (KBr pellet) combining with nanoparticles and potassium bromide (KBr). The FTIR spectra of the RES-free drug and folic acid conjugated NPs (FA-RES-NP) were examined using a Nicolet 6700 spectrometer (Thermo Scientific, USA). Data were analyzed by OMNIC ver.9.2.86 software and collected in absorption mode with 64 background scans and the wave number ranging from 4000 to 500 cm−1.
2.7 Immunohistochemistry
High-density tissue microarrays (TMA) slides were procured from US BIOMAX, Inc. (Derwood, MD). TMA slides containing clinical samples of 80 cases (77 cases of adenocarcinoma, one transitional cell carcinoma, two normal tissue) were diagnosed with n=39 (stage II), n=12 (stage III), n=24 (stage IV). First, paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated through a graded alcohol series (100%, 95%, and 70%, 5 min each), deionized water and phosphate buffer saline (PBS) for 5 min. To enhance immunogenicity and epitope availability, we performed antigen retrieval method by following the protocol for retrieving all antigens unmasking system from BioLegend, (San Diego, CA). Briefly, tissue was incubated for 10 mins in retrieve buffer (BioLegand, San Diego,CA) at 92°C then cooled at room temperature for 5-10 mins and rinsed with PBS. Endogenous peroxidase was blocked by 3% H2O2. Slide was rinsed with PBST (PBS+ 0.05% Tween 20) and tissue sections were blocked with normal donkey serum (NDS, 5%) for 1h at room temperature and then incubated with rabbit anti-human FOLR1 (1:15), (Thermofisher Scientific, Grand Island, NY) antibody at 4°C overnight. After washing (1×PBS, three times, 15 min each), tissue sections were incubated with secondary antibody donkey anti-rabbit (R&D Systems, Minneapolis, MN) for 1hour at room temperature. After washing with PBST, tissue section was incubated with the Streptavidin-horseradish peroxidase (HRP, BioLegend) and developed in DAB coloring reagent. The slide was counterstained with hematoxylin and dehydrated and mounted. Digital images were captured and analyzed using an AperioScanScope scanning system (Aperio Technologies, USA).
2.8 Cell toxicity and viability assay (MTT assay)
Cell proliferation assays were estimated by the MTT (3-(4,5-dimethylthiazol-2yl)2,5-diphenyltetrazolium bromide) (Sigma, St. Louis, MO) as described previously [15]. Briefly, PC3-R growing cells were trypsinised and collected using 0.25% Trypsin-EDTA (Fisher Scientific, Pittsburgh, PA) and seeded in 96-well plates at 10,000 cells/well. After 24 hours, cells were treated with different concentration of docetaxel (5, 10, 50, 100, 150, 200, 300, 500 nM) and resveratrol (10, 15, 20, 25, 50, 75, 100, 150, 200 μM) in each well per concentration. Cells were incubated for 24, 48 and 72 hours at 37°C and 5% CO2 incubator. Cell viability was detected by adding MTT (5 mg/mL) to each well and incubated for 2-3 hr at 37°C. The formazan crystals were dissolved in 100 μL dimethyl sulfoxide (DMSO) (Sigma), and the absorbance was measured at 570 nm using a spectrophotometer (Spectramax M5, Molecular Devices, Sunnyvale,CA). Similar protocol was applied for folate conjugated nanoparticles with different concentration of resveratrol (0.5, 1, 3, 5, 10, 20, 30, 50 μM), docetaxel (0.05, 0.1, 1, 3, 5, 10 nM) and the combination each well per concentration. The IC50 (half maximum inhibitory concentration) value was calculated for the PC3-R cells. Combination index (CI) was calculated for DTX and RES after determining the IC50 values of individual drug using the formula; CI= RES/(RES) × + DTX/(DTX) × + (RES)(DTX)/(RES) × (DTX) ×. CI values were tested for synergy (CI<0.9), additive (0.9<CI<1.1) and antagonism (CI>1.1), respectively, to determine the effect of drug combination [17].
2.9 Apoptosis Assay
The cells (PC3 and PC3-R) were grown and treated with folate conjugated resveratrol (RES-NP) and combination (RES+DTX-NP) nanoparticles for 48h. After treatment, cells were trypsinized with 0.25% trypsin, and counted using a hemocytometer (Countess II FL, Life Technology). Fifty thousand cells were used from each of the cell lines (PC3 and PC3-R) and washed in phosphate-buffered saline (PBS) (Fisher Scientific, Pittsburgh, PA). The apoptosis was detected by APC Annexin V apoptosis detection kit with PI (BioLegend, San Diego, CA) as described previously [12]. Briefly, cells were washed thrice with PBS and resuspended in Annexin V Binding Buffer. The apoptotic cells were analyzed using a flow cytometer using guava easyCyte HT (EMD Millipore, Billerica, MA).
2.10 Immunofluorescence Assay
For the membrane-bound folate receptor (FR) expression, PCa cells (RWPE-1, PC3, and PC3-R) were seeded in 48 well plate overnight at 37°C and 5% CO2 incubator. The cells were then washed with cold PBS and stained with FRα primary antibody (Invitrogen, USA) followed by secondary staining with Alexa Flour 577 conjugated antibody (R&D System, USA) for 40 mins at room temperature. Subsequently, cells were washed with PBS, and nuclei were counterstained with DAPI (Invitrogen, USA). To identify the internalization of FR from cell membrane to cytoplasm, PC3 and DTX resistant (PC3-R) cells were seeded in 48 well plate overnight and then treated with a known concentration of folate conjugated RES(3μM), and RES+DTX (3μM+0.01μM) nanoparticles combination for 1 h 30 min. Next, the cells were washed thrice with PBS and incubated with primary antibody FRα at 4°C overnight, washed and stained with Alexa Flour 577 (secondary) for 40 mins at room temperature. Post-staining, cells were washed with PBS, and nuclei were counterstained with DAPI (Invitrogen, USA). Similar immunofluorescence staining was performed for ABCB1, ABCC1 and ABCG2 (Cell Signaling, MA, USA) using PC3 and PC3-R, and for F-actin cytoskeleton, cells were stained with Phalloidin™ Red 594 solution (1:30) (BioLegend, USA) for 20 mins at room temperature. Images were captured using a fluorescent microscope with DAPI, FITC/GFP and RFP filter using EVOS FL microscope (Thermo Scientific, USA). ImageJ analysis software was used for the orthogonal views of NPs treated PC3-R cells.
2.11 Transmission electron microscope (TEM)
In addition to the immunofluorescence studies, uptake and cellular internalizations of NPs were examined by transmission electron microscope (TEM). PC3-R cells were treated with FA-RES-NP for 48h followed by washing with PBS to remove unbound NPs. The cells were then fixed with 2.5% glutaraldehyde in cacodylate buffer (pH 7.2) for 20 mins followed by washing twice with PBS and dehydrated in acetone. Subsequently, the samples were treated with spur’s low viscosity resin (Sigma Aldrich, USA) and polymerized at 60°C for 48 hours. The ultrathin sections of 60nm thickness were cut with an ultramicrotome, stained with 1% (w/v) uranyl acetate in alcoholic solution and analyzed by a transmission electron microscope (TEM) JEOL 1200EX (Tokyo, Japan)
2.12 Western blot analysis
To determine the expression of ABC transporters in PC3 and PC3-R cells, the protein’s isolation method was followed to our previous publication [15]. However, to see the effect of nanoparticles, first cells were treated with folic acid conjugated resveratrol and combination (RES+DTX) nanoparticles for 48h. Cells collected were washed with cold phosphate-buffered saline (PBS) followed by lysis buffer. Briefly, harvested cells were lysed with RIPA buffer containing 1X protease inhibitor cocktail (Thermo Scientific, Rockford, IL) and centrifuge for 10 minutes at 11000 rpm to collect the supernatants. The protein concentration of the samples was determined using the BCA protein assay kit (Thermo Scientific, Rockford, IL). An equal amount of cell lysate containing 30 μg of protein was analyzed on 4-12% polyacrylamide gels (Life Technologies, Carlsbad, CA). As with our previous protocol, transferred PVDF membranes were blocked in TBS-T (20 mM TRIS-HCl pH 7.6, 150 mMNaCl, 0.1% Tween 20) (Fisher Scientific, Pittsburgh, PA) and 5% non-fat dry milk (Biorad, USA) for 30 minutes at room temperature, then probed with primary antibodies overnight at 4°C followed by horseradish peroxidase (HRP) labelled secondary antibodies (1:2000 dilution) in TBS-T for 2 h at room temperature. The primary and secondary antibody for FRα was purchased from R&D Biosystem (Minneapolis, MN). The primary and secondary antibody for pro-apoptotic (BAX, BAK), Cleaved Caspase-3, anti-apoptotic (BCL-2, BCL-XL, Survivin), NF-kB p65, COX-2, GAPDH, ABCB1, ABCC1, ABCG2, were procured from Cell Signaling Technology (MA, USA). The signal detection was performed using chemiluminescence (ECL) and Prime Western Blotting detection reagent (Fisher Scientific, Pittsburgh, PA). Protein bands were acquired with ImageQuant LAS4000 (GE Healthcare-Biosciences, Pittsburgh, PA).
2.13 Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
The method of RNA isolation was followed per our previous publication [18]. Briefly, Cells were treated for 48h with a known concentration of folic acid-conjugated nanoparticles as described above and lysed with Trizol reagent (Invitrogen, Paisley, UK) followed by the standard protocol for RNA extraction. RNA was precipitated and resuspended in nuclease-free water and quantified at 260 nm wavelength. cDNA was synthesized using 1 μg of RNA and reverse transcription super mix for RT-qPCR reagent (Biorad, USA) and followed by Bio-Rad PCR condition protocol. Primers for ATP-binding cassette transporter markers were synthesized from National Center for Biotechnology Information (NCBI) gene bank database. The following sequences of the primers were used; ABCB1: 5′-TGACATTTATTCAAAGTTAAAAGCA-3′ and 5′-TAGACACTTTATGCAAACATTTCAA-3′; ABCC1: 5′-AGTGGAACCCCTCTCTGTTTAAG-3 and 5′-CCTGATACGTCTTGGTCTTCATC-3′; ABCG2: 5′-CCGCGACAGTTTCCAATGACCT-3′ and 5′-GCCGAAGAGCTGCTGAGAACTGTA-3′. RT-PCR was performed using SYBR® Green PCR master mix reagents (Biorad, USA) and gene expression analyzed by CFX-manager software (CFX96 Real-Time System-Biorad), 18S primer (5′-GGCCCTGTAATTGGAATGAGTC-3′ and 5′-CCAAGATCCAACTACGAGCTT-3′) was used as endogenous control, and the experiments were repeated three times.
2.14 Statistical analysis
FRα expression intensities in TMAs were tested for normality assumptions using the Shapiro-Wilk test. The experimental data were compared using a two-tailed Student’s t-test and expressed in the mean ±SEM. The results were analyzed using the Stat view II program (Abacus Concepts, Inc.) and were labeled statistically significant if p values < 0.01. Using the flow Jo Software, the Kolmogorov-Smirnov (K-S) two-sample test was used to calculate the statistical significance of the FRα.
3. Results
3.1 PBM Nanoparticle size and zeta potential distribution
Nanoparticles drug delivery depends on the size, zeta potential and other physiochemical properties of the drug formulation. As shown in the Fig. 1A, we determined the size of the PBM nanoparticle (RES+DTX-NP) either coated with/without biodegradable PCL-PEG copolymer. The size-based nanoparticle is related to the ligand-receptor interaction. According to previous reports, nanoparticles with 50 nm size were found optimal for their intracellular uptake and were significantly altered when coated with different targeting to the same receptor [19]. After coating, the size of FA-RES+DTX-NP combination was found to be 36.6 nm while the uncoated was 22.3 nm. Positively charged NPs have been reported to have a strong affinity for negatively charged cell membrane and decrease the non-specific binding of the cell membrane and PBM NPs [20]. Thus, zeta potential was performed to evaluate the surface charge and stability of the nanoparticle that measures the charge repulsion or attraction between the particles. Our results indicated that, after coating with PCL-PEG, the zeta potential of RES+DTX-NP was reduced to 0.6 mV while it was −40.5 mV without coating (Fig. 1B). A recent study has shown that positively charged NPs rapidly clear from circulation [21]. However, in contrast, neutral NPs or those with insignificant negative charge were found to have prolonged circulating half-lifes [21]. As we aim to treat tumor cells in which low oxygen tension is associated with increased metastasis, the NPs were coated with PCL-PEG to enhance the accumulation time of the functionalized NPs in tumors. Moreover, as the reduced zeta potential determines the encapsulation efficiency of charged material [22], the result from this study indicated encapsulation competency of RES and DTX in the starch composite. In addition, our NPs were spherical in shape (Fig. 1C). Experimental evidence showed that the spherical shape of the NPs has extended cellular uptake via endocytosis compared to the needle-shaped [23].
Fig. 1. Characterization of prostate cancer cell-specific PBM nanoparticles for charge, size, morphology and surface chemistry.
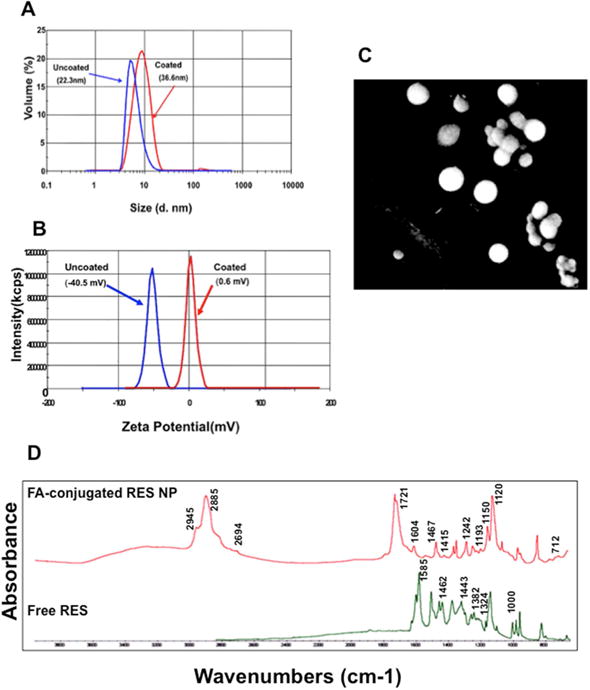
Resveratrol and docetaxel encapsulated; folic acid conjugated PCL-PEG coated and uncoated nanoparticles (A) size and (B) zeta potential (measured using a Malvern Zeta Sizer NS instrument). (C) Scanning electron microscope showing surface morphology of PBM nanoparticles coat with platinum–palladium layer. (D) Fourier transform infrared spectrum of synthesized resveratrol PBM nanoparticle and free resveratrol. The data were collected in absorption mode with 64 background scans and the wave number ranging from 4000 to 500 cm−1.
FTIR spectroscopy techniques provide structural and functional information based on vibration corresponding to the single chemical groups involved in specific reactions [24]. In order to confirm, whether or not RES were successfully coated in PCL-PEG, we analyzed the vibrational frequencies of chemical groups involved in nanoparticles preparation through FTIR. The FTIR spectra of free RES, and FA-RES-NP (Fig. 1D) showed a change in the spectral region between 1800 to 1000 cm−1 with a major peak at 1585 cm−1 observed in the spectra of free RES. However, FA-RES-NP represents the strong peak at 2885 cm−1 due to C-H stretching vibration of starch. Moreover, benzene ring of folic acid represents the peak at 1604 cm−1. The sharp peak at 1721 cm−1 corresponding to C=O stretching and the presence within the amide group that represents slightly shift of RES when conjugated with NHS-folate. These finding indicated that minor structural changes occurred when PCL-PEG attached to the RES.
3.2 Folate receptor expression in human prostate cancer tissue and cells
A membrane-bound protein, folate receptor α (FRα), has a high-affinity binding and expression in epithelial origin malignant tumor tissue compared to normal tissue. Although the expression of FRα is dependent and associated with different stages and grades of tumor tissue [25], it’s functionality in PCa remains indefinite in terms of tumor etiology and progression. Thus, in order to test the significance of FRα in PCa, we employed tissue microarrays (TMAs) that included 80 cases of PCa patients. Normal, matched, moderately, poorly and well-differentiated carcinomas were stained and analyzed for FRα. Furthermore, using the AperioScanScope software, hematoxylin (based on nuclear detection) and DAB staining, the expression levels of FRα were found to be higher in prostate tumor tissue compared to normal matched section. Poorly differentiated carcinomas (stage-IV) were diagnosed in 39 cases that displayed comparatively higher expression of FRα than moderately differentiated n=12 (stage-II) and n=24 (stage III) carcinomas (Fig. 2A).
Fig. 2. Folate receptor expression in human prostate cancer tissue and cells.
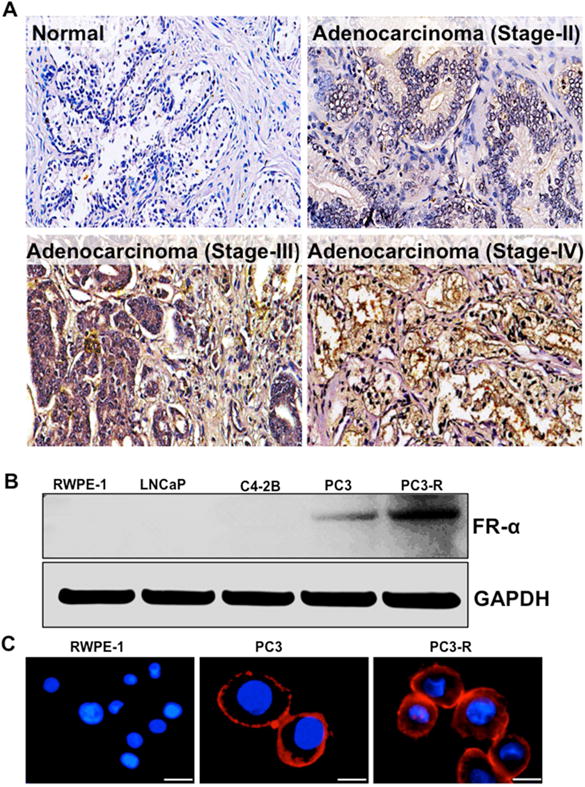
(A) Prostate tissues from normal, matched, moderately, poorly and well-differentiated carcinoma stained with anti-folate receptor-α (FRα) antibody and DAB staining. An Aperio ScanScope CS system with a 40× objective was used to capture digital images of each tissue. (B) Immunoblot expression of the anti-FRα antibody in RWPE-1, LNCaP, C4-2B, PC3 and PC3-R cells. GAPDH antibody was used as a loading control for all the samples. (C) Expression of folate receptor in PCa cells (immunofluorescence staining using anti-FRα antibody); Red: indicates the expression of FRα; Blue: Nuclei counterstained with DAPI, and images captured at 40×. Scale bar, 50 μm.
Based on the results obtained with clinical PCa tissues, we investigated the expression levels of FRα in PCa cell lines (RWPE-1, LNCaP, C4-2b, PC3 and docetaxel-resistant PC3-R) using western blot analysis. Among the PCa cells analyzed, PC3-R showed higher expression of FRα as compared to PC3 cells (Fig. 2B). This was further confirmed by immunofluorescence studies (Fig. 2C) and these observations conveyed that membrane-bound FRα could be a potential target for FA-conjugated nanoparticles.
3.3 Effect of resveratrol and combination with docetaxel PBM-NPs on cell cytotoxicity and viability
In order to investigate the chemotherapeutic effect of FA-RES-NPs and RES-DTX combination (FA-RES+DTX-NPs), cell viability assay (MTT assay) was performed (using PC3 and PC3-R cells) at different time intervals (24, 48 and 72 h) and compared with free drugs RES and DTX that served as control. The IC50 values of the free drugs, DTX and RES against PC3-R cells were found to be 280nM and 120μM, respectively. These results showed that PC3-R cells were highly resistant and the obtained IC50 values were nearly 28- and 4-fold higher compared to the parental PC3 cells. However, PC3-R cells had IC50 values, 3μM (RES) and 10nM (DTX) when treated with conjugated FA-PBM-NP. To test the collective effects of RES and DTX NPs for synergy, the combination index (CI) was calculated according to the Chou and Talalay median effect principle [17]. The combination index was found to be 0.35 in PC3-R cells when treated with 3μM RES and 10nM DTX after 48 h of treatment. Our data suggest that the synergistic effect of RES+DTX was more efficient in a PC3-R cell line after 48 h of treatment. These results showed that the chemotherapeutic effect of FA-conjugated DTX and RES in nanoparticles was enhanced by 28- and 40-fold, respectively compared to the activity of their free drugs. These results indicate dose and time dependency of PC3-R cells, and RES could re-sensitize these DTX resistants PCa cells either alone or in combination with DTX.
3.4 PBM nanoparticles induce apoptosis in docetaxel-resistant prostate tumor cells
As our cell viability study suggested that FA-RES-NP and FA-RES+DTX-NPs perform at a time and dose-dependent manner on PC3-R cells, we sought to determine if the designed NPs could possibly lead to induction of apoptosis. Thus, PC3 and PC3-R cells were subjected to effective drug concentrations obtained with MTT assay and subjected to flow cytometry analysis after 48 h of treatment. As shown in Fig. 3, the early and late apoptotic cells were increased by 47.3% and 18.6% when treated with FA-RES+DTX-NP (3μM +0.01μM), compared to 21.3% and 9.62% with FA-RES-NP alone in PC3-R cells. Further in PC3 cells, FA-RES+DTX-NP could lead to 50.6% apoptosis (early and late) while it remained at 21.09% (early and late apoptosis) with RES-NP. However, in control, only a total of 8.9% cells were found to be apoptotic when treated with empty starch nanoparticles. These results clearly indicate that FA-conjugated RES-NP induces apoptosis in DTX-resistant PC3-R cells similar to that of the parental PC3 cells.
Fig. 3. PBM Nanoparticle-mediated apoptosis of prostate tumor cells.
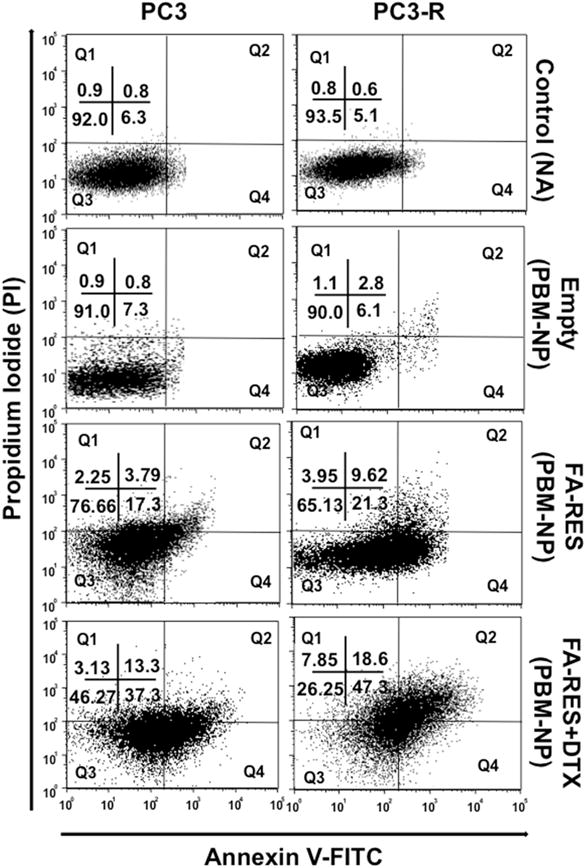
Flow cytometric analysis of Annexin V-FITC and PI-stained PC3 and PC3-R cells treated with FA-conjugated RES-NP (3μM) and RES+DTX-NP (3μM+0.01μM) for 48 h. The numbers in quadrant indicate the percentage of Q1 (necrotic): (Annexin(−)/(PI(+), Q2 (late apoptotic): (Annexin(+)/(PI(+), Q3 (viable): Annexin(−)/(PI(−) and Q4 (early): (Annexin(+)/(PI(−) cells respectively. Data shown are the representative mean of three independent experiments.
3.5 Immunofluorescence staining of targeted PBM nanoparticle in PCa cells
To investigate the cellular uptake and effluxes of FA-conjugated PBM nanoparticles in PCa cells were treated with FA-RES-NP (3μM) and FA-RES+DTX-NP (3μM, 0.01μM) for 1 h 30 mins and subjected to immunofluorescence studies. As shown in Fig. 4A, orthogonal projections (Z-stack) indicated the presence of PBM nanoparticles inside the cells. These results demonstrated that the FA-conjugated formulation of RES and DTX NPs could increase the intracellular concentration of the drugs from the cell membrane to the cytoplasm probably due to the receptor-mediated endocytic pathway. From these observations, it could be inferred that the FA-conjugated RES could counteract the MDR efflux pumps and thus enhance the internalization of the drugs and could be the best approach to treat patients with PCa resistant to DTX therapy.
Fig. 4. Z-stack orthogonal projections images to evaluate the targeted nanoparticle in prostate cancer cells.
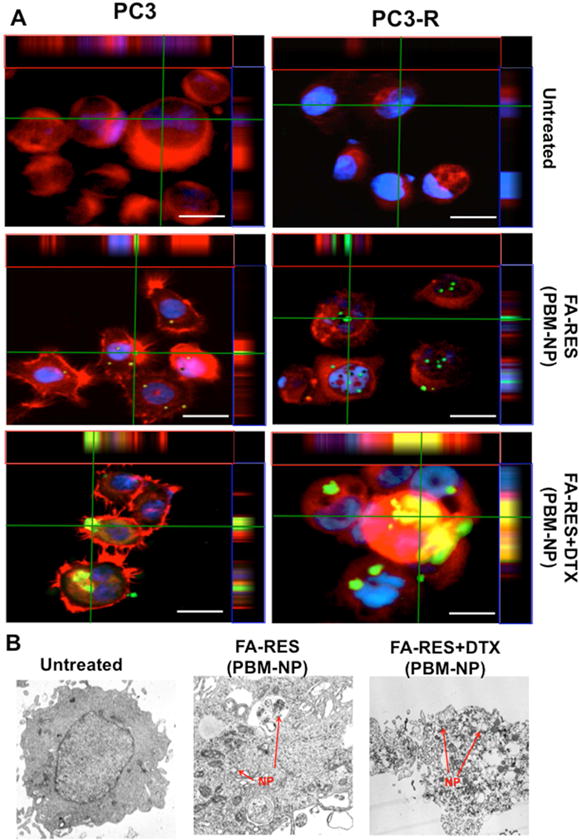
(A) Immunofluorescent staining of PCa cells treated with different dosages of green fluorescein labeled FA-conjugated RES-NP (3μM) and RES+DTX-NP (3μM+0.01μM) (Cells were immunostained with the anti-FRα antibody). Cells treated with NPs were detected by GFP and RFP (FRα), represented as green and red colors respectively. Nuclei were counterstained with DAPI (Blue). The orthogonal projections showed an abundant number of internalized combined nanoparticle (green) through folate receptor in PCa cells. Scale bar, 50 μm. (B) Internalization of PBM nanoparticles in docetaxel-resistant PC3-R cells treated with FA-conjugated RES-NP (3μM) and RES+DTX-NP (3μM+0.01μM), for 48 h and analyzed by TEM. The red arrow heads indicate the PBM nanoparticles within the cells.
Using transmission electron microscopy (TEM), the morphologies of the NPs were further confirmed that the desired morphologies were obtained. In addition to this, TEM images of the cells treated with NPs conjugated with RES and DTX as well as control cells were taken and compared. As shown in the Fig. 4B, the cells treated with NPs had irregularly shaped morphology compared to the control cells which had a smooth surface.
3.6 Folic acid conjugated PBM nanoparticles modulate pro- and anti-apoptotic, pro-survival and inflammatory markers in DTX resistance PC3-R cells
To evaluate the effect of FA-conjugated RES and DTX-RES on apoptosis, the expressions of pro-apoptotic and anti-apoptotic markers in drug resistance cells (PC3-R) were determined using a western blot assay. As shown in the Fig. 5A and 5B, cells treated with FA-RES+DTX-NP (3μM, 0.01μM) for 48 h showed down regulation of anti-apoptotic markers (BCL-2, and survivin) compared to RES-NP alone while BCL-XL was marginally downregulated. Further, the expression of pro-apoptotic markers (BAX, BAK) and cleaved caspase-3 was upregulated when treated with FA-RES+DTX-NP. These results clearly demonstrate that FA-conjugated-RES+DTX-NP is effective in targeting the FRα and induce apoptosis.
Fig. 5. Immunoblot detection of prostate cancer cells targeted by using PBM nanoparticle and the expression of pro- and anti-apoptotic, pro-survival and proliferation markers.
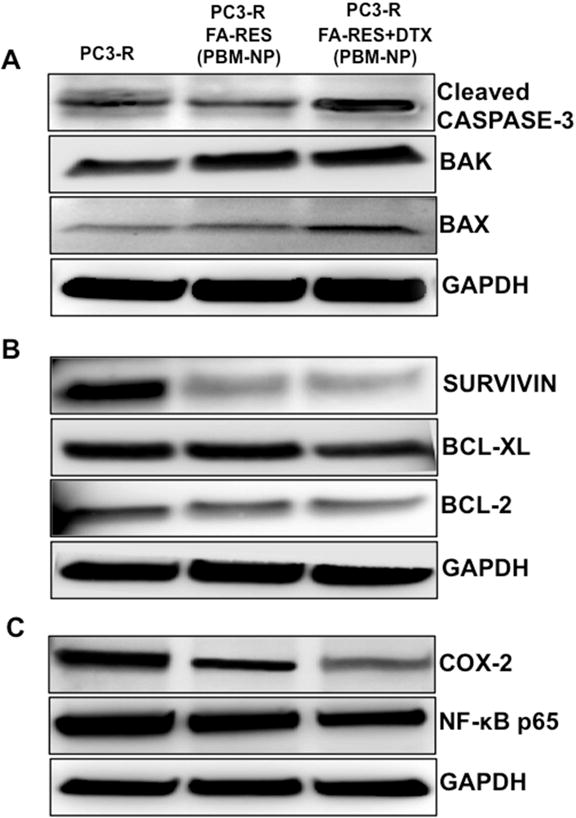
Expression of (A) pro-apoptotic (BAX, BAK) and Cleaved Caspase 3 proteins (B) anti-apoptotic (BCL-2, BCL-XL) and survivin proteins (C) pro-survival and inflammatory markers (NF-kB p65, COX-2) in PC3 and PC3-R cells treated with FA-conjugated (RES or RES+DTX) for 48 h. As an internal standard for equal loading blots was probed with a GAPDH antibody for each sample.
Further, to confirm the synergistic effect of combination (RES+DTX), the treated cells were analyzed for the expression NF-kB p65, a marker that is known to contribute to cell survival, progression, and proliferation of the tumor cells by regulating the expression of various genes. Our results showed that the combined treatment of FA-conjugated RES and DTX nanoparticles effectively downregulated the expression of NF-kB p65, thereby affecting the expression of proliferation and inflammatory markers (COX-2) associated with NF-kB pathway (Fig. 5C).
3.7 Expression of ATP-binding cassette transporters in PC3-R cells
Acquired drug resistance is the major challenge in PCa cells. Current evidence suggests that the overexpression of ABC-transporters is responsible for increased drug efflux of various chemotherapeutic drugs [5]. As anticipated, western blot analysis and immunofluorescence staining showed elevated expression levels of ABCB1 (MDR1 or P-glycoprotein), ABCC1 (MRP1) and ABCG2 (BCRP or MXR) transporters in DTX resistance cells (PC3-R) compared to PC3 (Fig. 6A and 6B). Upon treatment of the PC3-R cells for 48 h with FA-conjugated RES and FA-RES+DTX-NP, the expression levels of these marker genes were reversed (Fig. 6C). We found that FA-RES+DTX-NP could interact with ATP-binding cassette and inhibit their activity by blocking the efflux pump. These results strongly suggested that NPs coated drugs acted as inhibitors of drug efflux and effectually enhanced the intracellular concentration of the drug through the alteration of the MDR activity and re-sensitizes the PC3-R cells to DTX treatment.
Fig. 6. Down-regulation of ATP-binding transporter reverses docetaxel resistance in PC3-R cells.
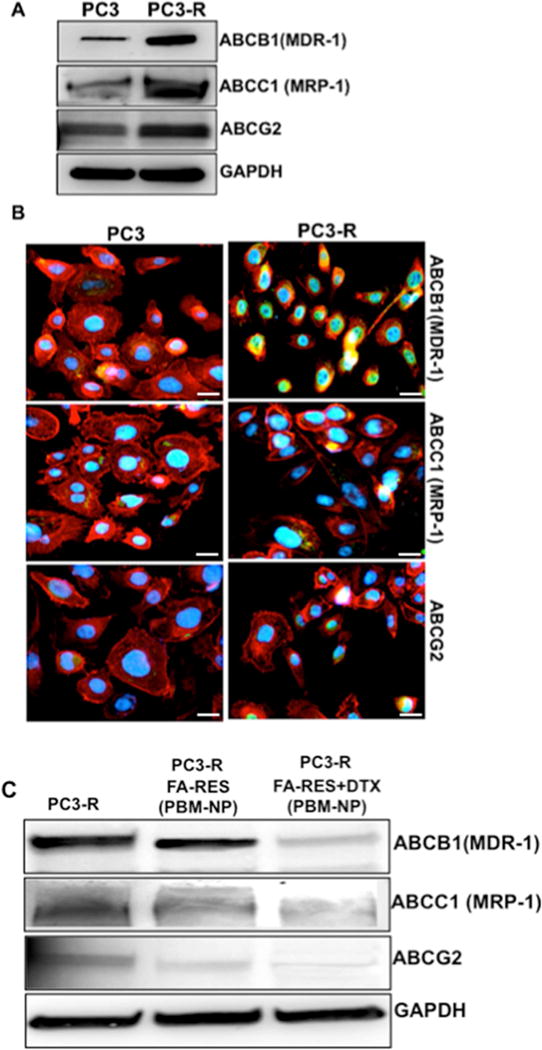
(A) Immunoblots (B) immunostaining for ABCB1(MDR-1), ABCC1(MRP-1) and ABCG2 expression in PC3 and PC3-R cells. Cells were detected by a microscope using GFP and Texas Red filters, represented as green (ATP-binding transporter) and red (Phalladoin) colors respectively. Nuclei were counterstained with DAPI (Blue), detected by DAPI filter. Scale bar, 50 μm. (C) Expression levels of ABCB1(MDR-1), ABCC1(MRP-1) and ABCG2 protein in PCa cells treated with FA-conjugated RES (3μM) and RES+DTX-NP (3μM+0.01μM) for 48 h. As an internal standard for equal loading blots were probed with a GAPDH antibody for each sample.
3.8 Validation of altered ATP-binding cassette transporter expression at the mRNA level in docetaxel resistance PC3 cells
Several cell survival and transcriptional factors are involved in DTX resistance in PCa that alters the MDR gene and mediates the drug efflux [11, 26]. To identify the expression of MDR that belongs to ATP-binding cassette (ABC) transporters, RT-PCR was conducted in PCa cells. As shown in the Fig. 7, the ABCB1, ABCC1 and ABCG2 gene was found to be the highly upregulated in DTX resistance (PC3-R) cells. However, upon treatment with FA-conjugated PBM nanoparticles expression was significantly downregulated in ABCB1 (1.87and 3.0), ABCC1 (1.42 and 2.7) and ABCG2 (2.78 and 3.70) respectively in FA-RES-NP, FA-RES+DTX-NP for 48 h as compared to PC3-R cells. Our data strongly suggested that ABC markers were overexpressed in DTX resistant PC3-R cells and were blocked when used FA-conjugated-NPs. Collectively, our nanoparticles downregulate MDR genes and reverse docetaxel resistance in PC3-R cells.
Fig. 7. Validation of ATP-binding transporters on mRNA in prostate cancer cells.
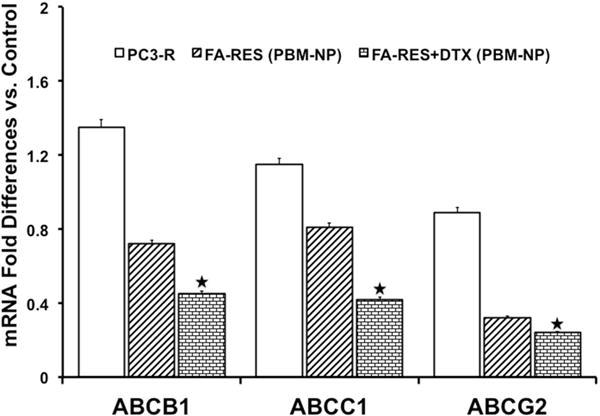
mRNA expression levels of the indicated genes in PC3-R cells quantitated by real-time RT-PCR treated with nanoparticles for 48 h. The expression levels of ABC transporter genes are represented by a fold change relative to control cells. Data were normalized to the levels of the housekeeping gene 18S expression, and the experiments were done in triplicates and repeated three times. Data are presented as the Mean±SEM, and asterisks indicate significance determined by student t-test (*P < 0.01).
4. Discussion
Presently, prostate cancer (PCa) is the aggressive malignant tumor and ranks in the second most common and the sixth leading cause of cancer deaths in men worldwide [27]. Although PCa initially responds to hormonal therapy, its resistance to these is inevitable. Among the chemotherapeutic agents for cancer treatment, docetaxel (DTX) was the mainstay and has contributed to the improved survival of men with metastatic castration-resistant PCa [4]. However, its effectiveness has been limited due to the resistance development to this drug whereby 50% of the men do not respond to the DTX therapy [4, 28]. It is, therefore, reasonable to expect further improvement in enhancing the efficacy of this potential drug (docetaxel) when it is combined with other therapeutics to treat PCa [29]. Due to differences in the pharmacokinetics of the combined drugs, it’s therapy remains unsatisfactory. Nevertheless, a promising approach to circumvent the drug efflux system and improve the effects of the drug combination is the use of a nanocarrier to deliver the drugs to the targets in cancer cells [30]. We recently reported the resveratrol-docetaxel combined modulatory effects on several apoptosis-related proteins in PCa [15]. Consider for the combined chemotherapeutic effect of these two compounds, we used the receptor-based targeted delivery approach using planetary ball milling (PBM) nanoparticle, that could enhance the bioavailability of resveratrol (RES) and DTX to treat advanced metastatic PCa [16]. As shown in the results, neither DTX nor RES alone could cause significant inhibition of the drug-resistant PCa cells. By contrast, the composite nanoparticle with both DTX and RES could overcome MDR and significantly inhibit cell growth.
Resveratrol (3,4,5-trihydroxystilbene), a polyphenolic phytoalexin commonly available through several plant sources has been shown to possess antitumor effects by inducing apoptosis, cell cycle arrest, and the suppression of certain transcription factors in cancer cells [30]. Moreover, RES was found to be a promising chemopreventive molecule due to its antioxidant, anti-inflammatory and growth inhibitory effects [31]. Thus, combining RES with other drugs such as DTX can be advantageous by reducing its cytotoxicity (of DTX) without additional side effects in vivo[30]. However, the efficacy and appropriateness of RES remained inconsistent because of its bioavailability due to quick absorption and metabolism in the in vivo system [32]. Such issues could be solved by encapsulating the drug with nanocarrier coated with PCL-PEG copolymer for its sustained release and enhance its bioavailability. Being used as devices for delivery of drugs, polymers such as PCL and PEG in combination are used in tissue engineering applications [33]. In addition, it is also evident from the cell culture studies that PCL-PEG copolymer exhibited higher hydrophilicity and degradability compared to PCL polymer[33]. Based on this evidence, a coating of RES and DTX with PCL-PEG can enhance the bioavailability of the sustained action of these anti-cancer drugs.
Among the various unique proteins (expressed by cancer cells) that were used as targets in cancer, we aimed at the folate receptor (FRα) for the delivery of nanoparticles, which is overexpressed on the plasma membrane of PCa cells in addition to other types of cancer [13]. In our study on PCa cells, we observed that PC3 and PC3-R had a higher expression of FRα in contrast to LNCaP, C4-2B, and RWPE-1 cells, as a result PC3 and DTX resistant PC3-R cells were selected for targeted delivery of folic acid (FA)-conjugated nanoparticles. A similar finding of FRα expression in PC3 cells was reported in PCa [34]. Despite the many nanocarriers have been employed to treat FRα[35], none of them have considered FA as the target to deliver such chemotherapeutic agents such as DTX and/or RES in PCa. In this regard, we generated the FA conjugated PBM-nanoparticles of either RES/DTX individually and in a combination with both. Immunofluorescence staining of the cells revealed internalization of the FA-conjugated nanoparticles from cell membrane to the cytoplasm. To our knowledge, this is the first study to employ FA as a receptor in using the combination of DTX and RES PBM nanoparticles.
In cancer, multidrug resistance (MDR) is a phenomenon that occurs when cells become resistant to structurally-unrelated chemotherapeutic compounds [36]. Among the various mechanisms that are responsible for MDR, active effluxes of the chemotherapeutic drugs through ABC transporters play a major role in cancer cells in resisting chemotherapy [37]. Several studies conducted on over-expression of ATP-binding cassette transporter genes, ABCB1 (P-gp or MDR1) and ABCC1 (MRP1) were found to be responsible for chemotherapeutic drug resistance in PCa[5]. Similar observations were made in the current study where ABCB1, ABCC1, and ABCG2 were found to be overexpressed in DTX resistance cells (PC3-R) that we generated.
The drug resistance mechanism through ABC transporters (ATP-binding cassette) induces several signaling molecule in PCa is shown in Fig. 8. Tremendous efforts have been made in discovering and synthesizing inhibitors for efflux pumps to re-sensitize resistant cancer cells to chemotherapeutic drugs. One of the few strategies that were successful in inhibiting the active efflux of ABC transporters is the encapsulation of drugs with nanoparticles [30, 38, 39]. In the present study, we, interestingly, found that the combined treatment inhibited the growth of the DTX resistant PC3 cells by down-regulating the ABCB1, ABCC1 and ABCG2 genes at both protein and mRNA levels. As RES combined with paclitaxel was found to modulate the expression of MDR in drug-resistant MCF-7 breast tumor cells [30], we hypothesize that the anticancer and inhibitor activity of RES associated with the cytotoxic activity of DTX has led to increased cell death in DTX resistant PC3 cells.
Fig. 8. Drug resistance mechanism through ABC transporters (ATP-binding cassette) induces different signaling molecule in prostate cancer.
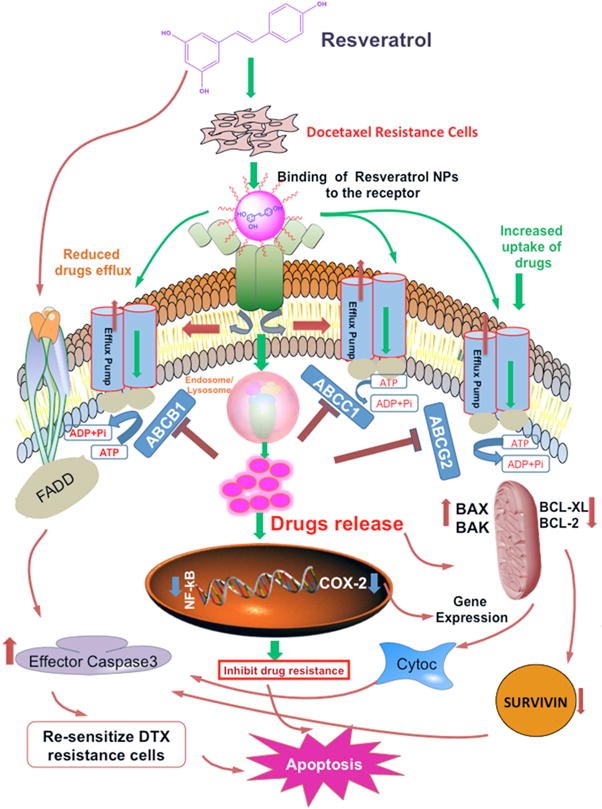
The active efflux of the chemotherapeutic drugs through ABC transporters plays a major role in cancer cells in resisting chemotherapy. The figure summarizes the mechanism by which ATP-binding cassette transporter genes, ABCB1 (P-gp or MDR1), ABCC1 (MRP1) and ABCG2 were overexpressed in docetaxel (DTX) drug resistance PCa cells. The encapsulation of resveratrol in nanoparticles can accumulate within the cells without being recognized by ABC transporters and release the drugs by lysosomal degradation modulating apoptotic pathway with the up-regulation of pro-(BAX, BAK) and down-regulation of anti-apoptotic (BCL-2, BCL-XL) molecules in the tumor cells. Inhibiting the expression of survivin (an attractive target in cancer therapy) activates effector Caspases (Caspase-3) that results in re-sensitizing the cells to apoptosis. Moreover, the binding of NPs to the transcriptional factor such as NF-kB inhibits the expression of various genes contributing cell survival, progression, and proliferation of the tumor cells. This, in turn, affects the expression of proliferation and inflammatory marker (COX-2) associated with NF-kB pathway and induces apoptosis by inhibiting drug resistance PCa cells.
Strategies for effective delivery of the drugs combined with NPs to target cells could be achieved by studying the interaction mechanisms between the cancer cells and NPs [8]. Among the several approaches, targeting of cancer cells through specific interaction between the NPs and the target cells could be more effective. In the present investigation, we selected the folate receptors that are specifically expressed on cancer cells for which were designed the NPs combined with anticancer agents. The successful uptake of the drugs combined with NPs was clearly confirmed through our TEM and FTIR studies. In addition to this, the cytotoxic effect of DTX and RES inducing apoptosis in cancer cells further confirmed the effectiveness and success of targeting folate receptors in eliminating cancer cells.
In our study, we found that the treatment of FA-conjugated-RES-NP and the combination (FA-RES+DTX-NP) significantly down-regulated the expression of anti-apoptotic markers (BCL-2, BCL-XL). In contrast, apoptotic markers such as BAX, and BAK were upregulated. In the present investigation, we showed that the target delivery of RES and DTX-NP combined to suppress the expression of NF-kB p65, which plays a prominent role in promoting apoptosis. It is well established through previous studies that the activation of NF-kB p65 elevates the level of inflammatory markers. The modulation of COX-2 through NF-kB leads to cancer progression, proliferation, angiogenesis, tumor invasion and blocking resistance [12, 40]. The results of the present investigation showed that FA-RES-NP blocked the upregulation of COX-2 in PCa regulated by NF-kB p65, thereby preventing the growth of the prostate cancer cells.
Apoptotic pathways depend upon the expression of caspases-related proteins, inhibitors of apoptosis and BCL-2 family genes. Survivin, a member of the inhibitor of apoptosis (IAP) protein family that inhibits caspases and blocks cell death is highly expressed in most cancers and is associated with a poor clinical outcome [41]. RNA interference studies have shown that elevated expression levels of survivin blocks the cell cycle arrest and inhibit apoptosis by binding to caspase 3 and 7 proteins [42, 43]. It has been previously found that RES inhibit the expression of survivin and elevate the expression of caspase proteins in human gastric cancer cells [44]. Although the apoptosis and its inhibition are regulated through different pathways and mechanism in varied cancers, we found that FA-conjugated RES nanoparticle is effective in promoting apoptosis by inhibiting survivin in prostate cancer. In addition, we also found that combination of RES with DTX-NP could promote the DTX resistant PCa cells susceptible. This evidence provides new insight into treating DTX resistance cancer cells in which the cell growth is regulated through survivin inhibition of apoptosis. This study has been supported by several reports [45–47].
In conclusion, this study found that several clinical PCa tissues display elevated expression of folate receptor. In addition, we also made a novel observation that folate receptor is expressed differentially in PC3 cells and to specify to a higher extent in DTX resistant PCa cells, which needs to be explored further. Mechanistically, we showed that FA-conjugated RES-DTX nanoparticle formulation targeting the folate receptor could decrease PCa cell proliferation by regulating apoptosis and anti-apoptotic pathways as well as the MDR efflux proteins. The findings from the study showed that the FA-RES+DTX nanoparticle combination could be effective in treating DTX-resistant and folate receptor expressing cancers. This evidence provides a new perspective for the clinical application of nanoparticle-mediated RES and DTX combination for cancer prevention.
Highlights.
Clinical samples of prostate cancer (PCa) express higher levels of folate receptor
Docetaxel resistance prostate cancer cells overexpress folate receptor and ATP-binding cassette transporters.
Folic acid conjugated resveratrol and docetaxel nanoparticles downregulate BCL-2, BCLXL and survivin while inducing BAX, BAK, and Caspase-3
Folic acid nanoparticle drugs are recommended for treating docetaxel resistance PCa.
Acknowledgments
We thank Ms. Angela Wimes for reviewing the manuscript. This study was supported in part by the National Cancer Institute of the National Institute of Health under Award Number SC1CA193758, U54CA118638 and from National Institute of Health under Award Number 5G12MD007602
Abbreviations
- FR
folate receptor
- PCa
prostate cancer
- NPs
nanoparticles
- RES
resveratrol
- DTX
docetaxel
- PBM
planetary ball milled
- FA
folic acid
- FA-RES-NPs
folic acid conjugated resveratrol nanoparticles
- FA-RES+DTX-NP
folic acid conjugated resveratrol and docetaxel nanoparticle
- AR
androgen receptor
- ADT
androgen deprivation therapies
- MDR
multidrug resistance
- EPR
enhanced permeability and retention
- MTT
3-(4,5-dimethylthiazol-2yl)2,5-diphenyltetrazolium bromide
- PCL
polycaprolactone
- PEG
polyethylene glycol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Zhu Y, Liu C, Armstrong C, Lou W, Sandher A, Gao AC. Antiandrogens Inhibit ABCB1 Efflux and ATPase Activity and Reverse Docetaxel Resistance in Advanced Prostate Cancer. Clin Cancer Res. 2015;21:4133–4142. doi: 10.1158/1078-0432.CCR-15-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong CM, Gao AC. Drug resistance in castration resistant prostate cancer: resistance mechanisms and emerging treatment strategies. Am J Clin Exp Urol. 2015;3:64–76. [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang C. Overcoming docetaxel resistance in prostate cancer: a perspective review. Ther Adv Med Oncol. 2012;4:329–340. doi: 10.1177/1758834012449685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Demidenko R, Razanauskas D, Daniunaite K, Lazutka JR, Jankevicius F, Jarmalaite S. Frequent down-regulation of ABC transporter genes in prostate cancer. BMC Cancer. 2015;15:683. doi: 10.1186/s12885-015-1689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakshmanan VK. Therapeutic efficacy of nanomedicines for prostate cancer: An update. Investig Clin Urol. 2016;57:21–29. doi: 10.4111/icu.2016.57.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1:16014. [Google Scholar]
- 9.Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5:1909–1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 10.Deckert PM. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr Drug Targets. 2009;10:158–175. doi: 10.2174/138945009787354502. [DOI] [PubMed] [Google Scholar]
- 11.Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8:12–23. doi: 10.1038/nrclinonc.2010.136. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S, Singh SK, Chowdhury I, Lillard JW, Jr, Singh R. Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front Biosci (Elite Ed) 2017;9:235–245. doi: 10.2741/e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwicke GL, Mansoori GA, Jeffery CJ. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012;3 doi: 10.3402/nano.v3i0.18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller C, Schibli R. Folic acid conjugates for nuclear imaging of folate receptor-positive cancer. J Nucl Med. 2011;52:1–4. doi: 10.2967/jnumed.110.076018. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Banerjee S, Acosta EP, Lillard JW, Singh R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget. 2017;8:17216–17228. doi: 10.18632/oncotarget.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lillard JWJ, Singh S, Singh R. Delivery system for specifically targeting cancer cells and method of use thereof. 2013 Feb 21; [Google Scholar]
- 17.Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma. 2008;49:2059–2080. doi: 10.1080/10428190802353591. [DOI] [PubMed] [Google Scholar]
- 18.Singh SK, Mishra MK, Eltoum IA, Bae S, Lillard JW, Jr, Singh R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Scientific reports. 2018;8:1323. doi: 10.1038/s41598-018-19643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 20.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 21.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature biotechnology. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Experimental and molecular pathology. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Lung PSai, Zhao S, Chu Z, Chrzanowski W, Li Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Scientific reports. 2017;7:7315. doi: 10.1038/s41598-017-07588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berthomieu C, Hienerwadel R. Fourier transform infrared (FTIR) spectroscopy. Photosynthesis research. 2009;101:157–170. doi: 10.1007/s11120-009-9439-x. [DOI] [PubMed] [Google Scholar]
- 25.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119:243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP, Gao AC. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Mol Cancer Ther. 2013;12:1829–1836. doi: 10.1158/1535-7163.MCT-13-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Wang Z, Chong T, Chen H, Li H, Li G, Zhai X, Li Y. Over-expression of a poor prognostic marker in prostate cancer: AQP5 promotes cells growth and local invasion. World J Surg Oncol. 2014;12:284. doi: 10.1186/1477-7819-12-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomura T, Yamasaki M, Hirai K, Inoue T, Sato R, Matsuura K, Moriyama M, Sato F, Mimata H. Targeting the Vav3 oncogene enhances docetaxel-induced apoptosis through the inhibition of androgen receptor phosphorylation in LNCaP prostate cancer cells under chronic hypoxia. Mol Cancer. 2013;12:27. doi: 10.1186/1476-4598-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng J, Guo F, Xu H, Liang W, Wang C, Yang XD. Combination Therapy using Co-encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Scientific reports. 2016;6:22390. doi: 10.1038/srep22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Li C, Li H, Li M, Shu X. Resveratrol-mediated reversal of tumor multi-drug resistance. Curr Drug Metab. 2014;15:703–710. doi: 10.2174/1389200215666140926153522. [DOI] [PubMed] [Google Scholar]
- 32.Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209–225. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang MH, Li S, Hutmacher DW, Schantz JT, Vacanti CA, Braud C, Vert M. Degradation and cell culture studies on block copolymers prepared by ring opening polymerization of epsilon-caprolactone in the presence of poly(ethylene glycol) Journal of biomedical materials research Part A. 2004;69:417–427. doi: 10.1002/jbm.a.30008. [DOI] [PubMed] [Google Scholar]
- 34.Joshi HC, Vangapandu SN, Aneja R. Conjugates of noscapine and folic acid and their use in treating cancer. 2013 Apr 23; [Google Scholar]
- 35.Shi H, Guo J, Li C, Wang Z. A current review of folate receptor alpha as a potential tumor target in non-small-cell lung cancer. Drug Des Devel Ther. 2015;9:4989–4996. doi: 10.2147/DDDT.S90670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 37.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1:93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67:4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- 39.Singh SK, Singh S, Lillard JW, Jr, Singh R. Drug delivery approaches for breast cancer. International journal of nanomedicine. 2017;12:6205–6218. doi: 10.2147/IJN.S140325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 41.Chuwa AH, Sone K, Oda K, Ikeda Y, Fukuda T, Wada-Hiraike O, Inaba K, Makii C, Takeuchi M, Oki S, Miyasaka A, Kashiyama T, Arimoto T, Kuramoto H, Kawana K, Yano T, Osuga Y, Fujii T. Significance of survivin as a prognostic factor and a therapeutic target in endometrial cancer. Gynecol Oncol. 2016;141:564–569. doi: 10.1016/j.ygyno.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Miyoshi H, Urabe T, Ohama K, Seyama I. Characteristics of membrane currents in single rat myometrial cells. Jpn J Pharmacol. 1992;58(Suppl 2):402p. [PubMed] [Google Scholar]
- 43.Yan L, Chen WQ, Liang YJ, Dai CL, Wang XH, Shi Z, Chen LM, Fu LW. Study on apoptosis of oral squamous carcinoma cell line KB and KBv200 induced by survivin RNAi. Ai Zheng. 2006;25:933–940. [PubMed] [Google Scholar]
- 44.Liu ML, Zhang SJ. Effects of resveratrol on the protein expression of survivin and cell apoptosis in human gastric cancer cells. J buon. 2014;19:713–717. [PubMed] [Google Scholar]
- 45.Carrasco RA, Stamm NB, Marcusson E, Sandusky G, Iversen P, Patel BK. Antisense inhibition of survivin expression as a cancer therapeutic. Mol Cancer Ther. 2011;10:221–232. doi: 10.1158/1535-7163.MCT-10-0756. [DOI] [PubMed] [Google Scholar]
- 46.Mohammadian J, Sabzichi M, Molavi O, Shanehbandi D, Samadi N. Combined Treatment with Stattic and Docetaxel Alters the Bax/Bcl-2 Gene Expression Ratio in Human Prostate Cancer Cells. Asian Pac J Cancer Prev. 2016;17:5031–5035. doi: 10.22034/APJCP.2016.17.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucukzeybek Y, Gul MK, Cengiz E, Erten C, Karaca B, Gorumlu G, Atmaca H, Uzunoglu S, Karabulut B, Sanli UA, Uslu R. Enhancement of docetaxel-induced cytotoxicity and apoptosis by all-trans retinoic acid (ATRA) through downregulation of survivin (BIRC5), MCL-1 and LTbeta-R in hormone- and drug resistant prostate cancer cell line, DU-145. J Exp Clin Cancer Res. 2008;27:37. doi: 10.1186/1756-9966-27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]


