Abstract
Secretory carcinoma (SC) is a recently described salivary gland carcinoma with characteristic ETV6-NTRK3 fusion. In this case report, we described a SC of the maxillary sinus that underwent high grade transformation in a 61-year-old patient. The diagnosis was confirmed by the presence of ETV6 translocation. Within the sinonasal tract, SC is an important differential diagnosis especially of sinonasal adenocarcinoma, non-intestinal type (non-ITAC), as these two entities bears histologic and immunophenotypic similarity. Distinction between these two tumors can be challenging based on the morphology alone and may require additional immunohistochemical and molecular studies. It is important to recognize that SC can occur in the sinonasal tract as correctly diagnosing SC may be prognostic relevant and may provide new targeted therapeutic avenues for these patients.
Keywords: Secretory carcinoma, Sinonasal non-intestinal type adenocarcinoma, High grade transformation, ETV6-NTRK3 fusion
Introduction
Salivary gland neoplasms are histologically diverse, with more than 30 distinct histologic types recognized by the current World Health Organization (WHO) tumor classification [1]. In the past decade, several new entities have been described and/or redefined based on their unique molecular signatures, including secretory carcinoma (SC) with t(12,15) ETV6-NTRK3 fusion [2], clear cell carcinoma with t(12,22) ATF1-EWSR1 fusion [3], and polymorphous adenocarcinoma with PRKD1 mutation [4]. Salivary gland tumors, malignant or benign, can occur in the sinonasal tract and should be considered in the differential diagnosis of an epithelial tumor in this location.
Primary adenocarcinoma of the sinonasal tract can be non-salivary or salivary gland type. Non-salivary-type sinonasal adenocarcinoma is a rare malignant tumor of presumed surface epithelium/mucoserous gland origin, accounting for less than 1% of head and neck cancers [1, 5]. This tumor can be classified into two broad categories: the intestinal-type adenocarcinoma (ITAC) and the non-intestinal-type adenocarcinoma (non-ITAC). The non-ITACs encompass a group of tumors with marked morphologic and cytologic heterogeneity. Moreover, these tumors show a non-specific immunohistochemical profile. Hence, non-ITACs are often rendered as a diagnostic category of exclusion after salivary gland-type carcinomas or ITACs are excluded. Indeed, a range of architectural and cytologic features have been reported in this type of tumors, including cystic, tubulocystic, papillary, acinar, and solid growth patterns, as well as clear cell, oncocytic, and cuboidal/columnar cytologic features [1]. High grade non-intestinal adenocarcinoma shows marked nuclear pleomorphism, high mitotic activity, and/or necrosis, and is associated with a dismal prognosis of a 3-year survival rate of approximately 20% [1].
As salivary gland SC and non-ITAC are both histologically diverse, it may be diagnostically challenging yet therapeutically and prognostically relevant to make a definite distinction between these two tumors, especially when they are high grade. Here we described a case of SC of the maxillary sinus in association with high grade transformation (HGT). The diagnosis was confirmed by the presence of ETV6 fusion.
Case Report
The patient is a 61-year-old male patient who was found to have a 4.2 cm solid mass in the left maxillary sinus. He underwent a left maxillectomy, fibular free flap reconstruction, orbital reconstruction, vincryl mesh reconstruction of maxilla, free bone graft, and skin graft. He subsequently received adjuvant radiation therapy, mostly due to the concern of a positive posterior maxillary resection margin. Patient decided to discontinue radiation therapy after 34 greys (in 17 fractions) because of the excessive toxicity. Subsequently, he was presented to the radiation oncology department for a second opinion in regard to adjuvant radiation therapy.
He was a former smoker of 30 pack.years; otherwise, his past medical and family history was unremarkable without notable occupational or environmental exposure.
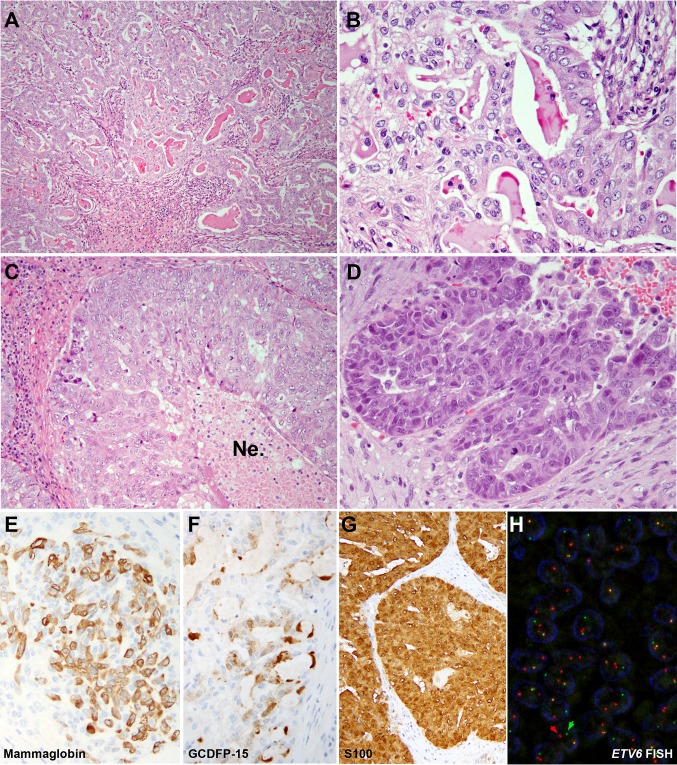
Histologically, the tumor was infiltrative, showing predominant acinar, tubular and microcystic growth patterns (Fig. 1a, b). Intraluminal dense eosinophilic secretions were noted. The tumor cells had relatively uniform round to oval nuclei with small nucleoli and vesicular chromatin. The cytoplasm was eosinophilic granular to clear vacuolated. Multifocally, the tumor exhibited a higher grade component characterized by solid growth, extensive central comedo-type necrosis, elevated mitotic activity with a mitotic index of 10 per 10 high power fields (×400, total field size 2.4 mm2), marked nuclear pleomorphism, hyperchromasia, and prominent nucleoli (Fig. 1c, d). These morphologic features were consistent with high grade transformation. There was no evidence of perineural invasion, lymphovascular invasion, or bone invasion. A single level I neck lymph node removed at the time of the initial resection was negative for metastatic disease.
Fig. 1.
Secretory carcinoma (SC) of maxillary sinus (hematoxylin and eosin, a ×100 and b ×400) with high grade transformation (c ×200, d ×400). The maxillectomy contained an infiltrative carcinoma with predominant microcystic pattern. Eosinophilic material was noted within the cystic lumen. The tumor cells were relatively uniform with eosinophilic to clear vacuolated cytoplasm, oval nuclei, vesicular chromatin, and small nucleoli. Areas of solid growth with comedo type necrosis (Ne) and elevated mitotic activity were present multifocally within this tumor, diagnostic of high grade transformation. e–g Immunophemotypically, this tumor was positive for S100, mammaglobin and gross cystic disease fluid protein-15 (GCDFP-15). h Fluorescence in situ hybridization (FISH) demonstrated ETV6 rearrangement (red and green arrows) using ETV6 break-apart probe
Immunohistochemical stains showed that the carcinoma was positive for CK7 (DAKO OV-TL-12/30, 1:600, Agilent technologies, Santa Clara, CA, US), S100 (DAKO polyclonal antibody, 1:8000, Agilent Technologies, Santa Clara, CA, US), mammaglobin (Ventana 31A5 clone, ready to use, Ventana Medical Systems Inc., Tucson, AZ, US) and gross cystic disease fluid protein-15 (GCDFP-15, Biolegend D6 clone, 1:100, Biolegend, San Diego, CA, US). Fluorescence in-situ hybridization (FISH) using a commercial ETV6 break-apart probe (Abbott Molecular, Des Plaines, IL, US) demonstrated ETV6 rearrangement (Fig. 1, panel H) in this tumor, including the areas of HGT.
The diagnosis of the pathologist in the outside institution was non-salivary -type non-ITAC. However, based on the histologic features, the immunoprofile and the presence of ETV6 translocation, the diagnosis was revised to secretory carcinoma of salivary gland origin with high grade transformation.
The patient had no evidence of disease at his last follow up, eight months after the initial resection.
Discussion
Glandular malignancies may arise anywhere in the sinonasal tract and can be broadly divided into three categories: salivary-type adenocarcinoma, ITAC and non-ITAC [1]. ITACs which are the most common type of sinonasal adenocarcinoma have a distinct microscopic appearance, immunophenotypic features and molecular profile. These tumors histologically resemble malignant intestinal epithelium, are positive for CK20 and CDX2, and are often associated with KRAS or BRAF mutations or EGFR overexpression [1, 6, 7]. On the other hand, salivary-type adenocarcinomas and non-ITACs are histologically diverse and bear an immunohistochemical similarity, which may result in diagnostic uncertainty and impose a challenge for the practicing pathologists.
Non-ITACs are typically positive for CK7, while negative for CK20 and CDX2 [1, 8]. These tumors are known for their marked architectural and cytologic heterogeneity. By the WHO definition, a diagnosis of non-ITAC may only be rendered as a diagnosis of exclusion when an ITAC or a specific type of salivary gland carcinoma is excluded [1]. Non-ITACs can be further divided into low and high grade. The high grade non-ITACs are classified based on the presence of marked cellular pleomorphism, frequent mitoses including atypical forms, and tumor necrosis [1].
With the exception of Warthin tumor and sebaceous tumors, most of the benign and malignant salivary gland tumors have been described in the sinonasal tract with the pleomorphic adenoma and adenoid cystic carcinoma being the most common benign and malignant tumors respectively [9]. Salivary-type adenocarcinomas of sinonasal tract are rare, comprising 5–10% of all sinonasal adenocarcinomas. Secretory carcinoma, which is the new designation of mammary analog secretory carcinoma in the 2017 WHO classification [1], was first described by Skalova et al. as a distinct type of salivary carcinoma with a signature fusion affecting ETV6 locus [2]. The estimated frequency of SC among all salivary gland carcinomas is approximately 4% [10]. Although the majority of SCs affect major salivary glands, roughly 30% may arise in minor salivary glands, predominantly in the oral cavity [11]. To date, only one case of sinonasal SC has been reported, which occurred in the ethmoid sinus in a 67-year-old woman. The tumor diagnosis was confirmed by ETV6 rearrangements detected using FISH [12]. Interestingly, just like in our case, the tumor was also initially diagnosed as non-ITAC. However, HGT was not reported in that case.
Histologically, SC typically exhibits papillary-cystic, microcystic, tubular or solid architectures, and is composed of uniform cells with bland vesicular nuclei and eosinophilic to vacuolated cytoplasm [10, 13–16]. Immunophenotypically, SC is usually positive for GCDFP-15 mammaglobin, S100 and CK7, and negative for DOG1 [13, 14, 17].
In the sinonasal tract, SC can be easily mistaken for non-ITACs, as both tumor types have been associated with cystic-papillary/microcystic architectures, an oncocytic cytomorphology, and a diffuse and strong CK7 and S100 immunoreactivity. Hence, it is important to realize that SC may occur in this location and that the appropriate additional ancillary studies (e.g. immunohistochemistry for GCDFP-15 and mammaglobin, as well as ETV6 FISH) may be initiated to differentiate these two entities. Andreasen et al. have recently described three cases of non-ITACs with ETV-NTRK3 or ETV-X fusion [18]. Unlike the current case which showed typical histology of SC, these reported ETV6-fusion-positive non-ITACs seemed to have different architectural and immunophenotypic features with a prominent tubular or tubulotrabecular architecture, and very focal S100 immunopositivity.
The distinction between SC and non-ITACs is not a trivial one as the correct diagnosis of SC may have prognostic value and may open doors to novel targeted therapies, especially in high grade or advanced tumors which are often resistant to chemotherapy. The reported 5-year overall survival, risk of local recurrence and distant metastases for non-ITAC are 66, 29 and 8% respectively [5]. In comparison, SC is associated with a seemingly better prognosis, i.e. 4% risk of disease specific death, 14% risk of local recurrence and 3% risk of distant metastasis [19, 20]. Recently, TRK inhibitors, e.g. entrectinib and LOXO-101, have been introduced in multiple clinical trials, targeting carcinomas or sarcomas harboring NTRK fusions with promising clinical results [21–25]. For example, Drilion et al. [22] have shown a dramatic and durable response to entrectinib in a patient with metastatic SC with proven ETV6-NTRK3 fusion.
High grade transformation is a rare phenomenon that has been described in an expanding list of salivary gland carcinomas [26], including SCs. To date (including the current case), HGT has been reported in seven patients of SCs [10, 20, 27–29]. The sites of origin of these cases were parotid gland (n = 3), palate (n = 1), buccal space (n = 1), sinonasal tract (n = 1, the current case), and unspecified (n = 1). Histologically, the high grade tumor component is characterized by trabecular or solid growth, nuclear anaplasia/pleomorphism and large geographic comedo necrosis and is usually identified juxtapose to the conventional SC component. Although, only a small number of cases have been described in the literature, it appeared that SC with HGT is associated with adverse clinical outcomes. Four patients with parotid and buccal HGT-SC died of disseminated distant metastases (n = 3) and/or local recurrence (n = 1) within 4 months to 6 years after their initial diagnosis and two (one with palate tumor and one with parotid tumor) had lymph node metastasis at the time of the initial resection [20, 27]. In this case, the patient had no evidence of disease at his last follow up. However, the follow up was short (8 months). A longer follow up may provide a better assessment of the behavior of this tumor.
Conclusion
We report a case of SC with high grade transformation in the maxillary sinus. SC can occur in sinonasal tract and should be recognized as a distinct diagnostic entity. The main differential diagnosis of SC in this location includes sinonasal non-ITAC. Distinction between these two tumors can be challenging based on the morphology alone and may require additional immunohistochemical and molecular studies. Correctly recognizing sinonasal SC may provide prognosis-relevant information and potential avenues for new targeted therapies for these patients.
Funding
This study was funded in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflict of interest
All authors declare that he/she has no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Bin Xu, Email: Bin1.xu@sunnybrook.ca.
Ruth Aryeequaye, Email: rutha@mskcc.org.
Lu Wang, Email: huluwawa2002@gmail.com.
Nora Katabi, Phone: 212-639-3349, Email: katabin@mskcc.org.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. 4. Lyon: IARC Press; 2017. [Google Scholar]
- 2.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 3.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50(7):559–70. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb I, Piscuoglio S, Martelotto LG, Waggott D, Ng CK, Perez-Ordonez B, et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat Genet. 2014;46(11):1166–1169. doi: 10.1038/ng.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhayani MK, Yilmaz T, Sweeney A, Calzada G, Roberts DB, Levine NB, et al. Sinonasal adenocarcinoma: a 16-year experience at a single institution. Head Neck. 2014;36(10):1490–1496. doi: 10.1002/hed.23485. [DOI] [PubMed] [Google Scholar]
- 6.Szablewski V, Solassol J, Poizat F, Larrieux M, Crampette L, Mange A, et al. EGFR expression and KRAS and BRAF mutational status in intestinal-type sinonasal adenocarcinoma. Int J Mol Sci. 2013;14(3):5170–5181. doi: 10.3390/ijms14035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Inclan C, Lopez F, Perez-Escuredo J, Cuesta-Albalad MP, Vivanco B, Centeno I, et al. EGFR status and KRAS/BRAF mutations in intestinal-type sinonasal adenocarcinomas. Cell Oncol. 2012;35(6):443–50. doi: 10.1007/s13402-012-0103-7. [DOI] [PubMed] [Google Scholar]
- 8.Bhaijee F, Carron J, Bell D. Low-grade nonintestinal sinonasal adenocarcinoma: a diagnosis of exclusion. Ann Diagn Pathol. 2011;15(3):181–184. doi: 10.1016/j.anndiagpath.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Leivo I. Sinonasal adenocarcinoma: update on classification, immunophenotype and molecular features. Head Neck Pathol. 2016;10(1):68–74. doi: 10.1007/s12105-016-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majewska H, Skalova A, Stodulski D, Klimkova A, Steiner P, Stankiewicz C, et al. Mammary analogue secretory carcinoma of salivary glands: a new entity associated with ETV6 gene rearrangement. Virchows Arch. 2015;466(3):245–54. doi: 10.1007/s00428-014-1701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skalova A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: report of 4 cases harboring ETV6-X gene fusion. Am J Surg Pathol. 2016;40(1):3–13. doi: 10.1097/PAS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 12.Lurquin E, Jorissen M, Debiec-Rychter M, Hermans R, Hauben E. Mammary analogue secretory carcinoma of the sinus ethmoidalis. Histopathology. 2015;67(5):749–51. doi: 10.1111/his.12702. [DOI] [PubMed] [Google Scholar]
- 13.Skalova A. Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol. 2013;7(Suppl 1):S30-6. doi: 10.1007/s12105-013-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36(1):27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 15.Pinto A, Nose V, Rojas C, Fan YS, Gomez-Fernandez C. Searching for mammary analogue [corrected] secretory carcinoma of salivary gland among its mimics. Mod Pathol. 2014;27(1):30–7. doi: 10.1038/modpathol.2013.84. [DOI] [PubMed] [Google Scholar]
- 16.Stevens TM, Kovalovsky AO, Velosa C, Shi Q, Dai Q, Owen RP, et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol. 2015;28(8):1084–100. doi: 10.1038/modpathol.2015.64. [DOI] [PubMed] [Google Scholar]
- 17.Projetti F, Lacroix-Triki M, Serrano E, Vergez S, Barres BH, Meilleroux J, et al. A comparative immunohistochemistry study of diagnostic tools in salivary gland tumors: usefulness of mammaglobin, gross cystic disease fluid protein 15, and p63 cytoplasmic staining for the diagnosis of mammary analog secretory carcinoma? J Oral Pathol Med. 2015;44(4):244–51. doi: 10.1111/jop.12226. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen S, Skalova A, Agaimy A, Bishop JA, Laco J, Leivo I, et al. ETV6 gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: a novel translocation-associated carcinoma restricted to the sinonasal tract. Am J Surg Pathol. 2017 doi: 10.1097/PAS.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 19.Sethi R, Kozin E, Remenschneider A, Meier J, VanderLaan P, Faquin W, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope. 2014;124(1):188–95. doi: 10.1002/lary.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo W, Lindley SW, Lindley PH, Krempl GA, Seethala RR, Fung KM. Mammary analog secretory carcinoma of salivary gland with high-grade histology arising in hard palate, report of a case and review of literature. Int J Clin Exp Pathol. 2014;7(12):9008–9022. [PMC free article] [PubMed] [Google Scholar]
- 21.Nagasubramanian R, Wei J, Gordon P, Rastatter JC, Cox MC, Pappo A. Infantile fibrosarcoma with NTRK3-ETV6 fusion successfully treated with the tropomyosin-related kinase inhibitor LOXO-101. Pediatr Blood Cancer. 2016;63(8):1468–1470. doi: 10.1002/pbc.26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann Oncol. 2016;27(5):920–926. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ardini E, Menichincheri M, Banfi P, Bosotti R, De Ponti C, Pulci R, et al. Entrectinib, a Pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther. 2016;15(4):628–39. doi: 10.1158/1535-7163.MCT-15-0758. [DOI] [PubMed] [Google Scholar]
- 24.Rolfo C, Ruiz R, Giovannetti E, Gil-Bazo I, Russo A, Passiglia F, et al. Entrectinib: a potent new TRK, ROS1, and ALK inhibitor. Expert Opin Investig Drugs. 2015;24(11):1493–500. doi: 10.1517/13543784.2015.1096344. [DOI] [PubMed] [Google Scholar]
- 25.Doebele RC, Davis LE, Vaishnavi A, Le AT, Estrada-Bernal A, Keysar S, et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 2015;5(10):1049–1057. doi: 10.1158/2159-8290.CD-15-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagao T. “Dedifferentiation” and high-grade transformation in salivary gland carcinomas. Head Neck Pathol. 2013;7(Suppl 1):S37-47. doi: 10.1007/s12105-013-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skalova A, Vanecek T, Majewska H, Laco J, Grossmann P, Simpson RH, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J Surg Pathol. 2014;38(1):23–33. doi: 10.1097/PAS.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 28.Jung MJ, Song JS, Kim SY, Nam SY, Roh JL, Choi SH, et al. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J Pathol. 2013;47(1):36–43. doi: 10.4132/KoreanJPathol.2013.47.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipriani NA, Blair EA, Finkle J, Kraninger JL, Straus CM, Villaflor VM, et al. Salivary gland secretory carcinoma with high-grade transformation, CDKN2A/B loss, distant metastasis, and lack of sustained response to crizotinib. Int J Surg Pathol. 2017. doi:10.1177/1066896917709350. [DOI] [PubMed]