Abstract
The purpose of this proof-of-principle study was to develop a rapid and approachable method to analyse bone resection margins in patients with oral squamous cell carcinoma (OSCC) in an intraoperative setting, similar to assessing frozen sections of soft tissue. Bone excision and risk of remaining tumour cells could be minimised, thus improving reconstruction measures and facilitating convalescence. Frozen, sawed wafers of porcine bone artificially combined with porcine skin (simulating OSCC properties) were used to develop and evaluate a new molecular method: protein transfer from non-decalcified, sawed wafers onto a membrane stained by immunofluorescence (Tissue-ProtTrans). Tissue-ProtTrans was based on the detection of keratin 5/6 as a marker of tumour cells. The results were compared to standard immunohistochemistry (IHC) and H&E results of the same wafers after decalcification. Tissue-ProtTrans resulted in a total assay time of 3.5 h using the Trans-Blot® Turbo™ Transfer System (Bio-Rad) for protein transfer. Amersham Protran® Premium Nitrocellulose Membranes 0.2 µm (GE Healthcare) were stained with a primary antibody to keratin 5/6 (Dako Agilent) and a secondary antibody labelled with IRDye® 800CW (LI-COR). Visualisation was performed with an infrared laser scanner (Odyssey). Upon comparison, five independent experiments on porcine specimens processed with the Tissue-ProtTrans showed similar results to standard IHC and H&E analysis. In comparison to standard IHC results (requiring several days due to decalcification) Tissue-ProtTrans provided similar results, but was much faster (3.5 h). This highly promising method has good potential for further time reduction and will be suitable for intraoperative assessment.
Keywords: Oral squamous cell carcinoma, Head and neck, Immunofluorescence, Intraoperative care, Frozen section, Bone, Surgical margin
Introduction
Neoplasms of the oral cavity are among the top ten malignant neoplasms and a major concern of global health care [1, 2]. About 95% of these tumours are oral squamous cell carcinomas (OSCC) [3]. OSCC has still a poor prognosis, which has not improved in recent decades [4]. The overall 5-year survival rate is approximately 57% [5].
A curative treatment of a patient with OSCC is achieved by complete surgical excision of the tumour depending on the resectability of the tumour and the health status of the patient [4, 6]. The surgeon typically removes the tumour tissue upon macroscopic judgement with a safety margin, which should result in at least 3–5 mm of tumour-free margin in formalin-fixed tissue [3]. If the tumour exceeds tissue boundaries, e.g. the tumour grows next to the mandible or has already invaded the bone of the mandible, a segmental resection of the mandible is necessary in order to remove the tumour completely [3]. It is generally accepted that the surgical margin status is one of the main predictors of survival [7, 8]. Therefore, assurance of tumour-free bone margins is crucial due to possible fatal outcome for patients with residual carcinoma.
The absence of a rapid assay for the evaluation of bone resection margins during surgery on patients with OSCC similar to frozen section analysis (FSA) means that in sano resection of the mandible/maxilla infiltrated by the tumour cannot be surely guaranteed during resection. While operating, the surgeon must rely on pre-operative imaging and clinical experience as well as FSA of gingival mucosa. Few clinics and pathologists support FSA of the infra-alveolar nerve and/or bone marrow analysis [7, 8]. However, these techniques are not reliable enough [7, 9].
In about 7%, as estimated in our own experience and as shown in [8], a positive bone margin is reported in the final histological result after the operation is finished and an overall OSCC recurrence rate of 36% was observed in one study [8]. These unfortunate patients have to undergo a second operation, if possible [7]. Furthermore, during the operation of tumours like ameloblastoma and osteogenic sarcomas, which are associated with the mandible, surgeons have to deal with the same problems as described above [7].
There are two options for surgeons: (a) a two-step procedure (first tumour removal and in a second intervention a reconstruction operation) to ensure tumour-free margins before reconstruction with a bone graft or (b) primary reconstruction with a bone graft without an adequate bone margin assessment. The primary reconstruction seems to be internationally accepted because of a better outcome [8, 10–12], improving the risk of remaining intraosseous tumour cells and accepting an extensive bone loss of more than 2 cm in each direction in addition to the macroscopic tumour expansion [8].
The German guidelines for head and neck surgeons recommend the first procedure (two-step approach) and other studies recommend this procedure in some cases [6, 13, 14]. The German guidelines suggest using a metallic bridge plate as a provisional measure for primary care due to a considerable risk of remaining tumour cells in case of a primary reconstruction by a bone graft. If the resection margins of the mandible turn out to contain tumour cells, a re-resection must be performed, if possible. A final reconstruction consisting of tedious bone grafting is only advisable once tumour-free resection margins have been histologically confirmed [3]. In the ‘Clinical Practice Guidelines in Head and Neck Cancers’ (published by The National Comprehensive Cancer Network of the USA) it is stated that “primary closure [of surgical defects] is recommended when appropriate but should not be pursued at the expense of obtaining wide, tumor free margins” [6].
For surgeons the situation is unsatisfying: A primary reconstruction has principally a better outcome and is favourable to reduce risks of a secondary operation. Since primary reconstruction is becoming more and more common practice in medical centres it is important to improve the outcome further. In order to diminish the disadvantages associated with primary reconstruction without guidance of a histopathological confirmation of a tumour-free bone margin there is the urgent need for an intraoperative evaluation of bone margins. Using a rapid intraoperative bone margin analysis, tumour free primary reconstruction could be improved by saving healthy bone tissue, ensuring in sano resection and favour patients by better reconstruction possibilities.
To our knowledge, none of previously published intraoperative methods (Table 1) have been established in intraoperative routine. Therefore, there is a need for a novel testing procedure that is easy to establish and enables a rapid intraoperative analysis of bone resection margins to overcome this present disadvantage. Such a method may result primarily in minimising bone excision, and secondarily allow for safe, immediate reconstruction thus improving convalescence and outcome in patients after removing OSCC.
Table 1.
Approaches to intraoperative bone margin assessments, adapted from [27]
| Type of method | References | Method | Draw backs |
|---|---|---|---|
| Frozen section analysis | Forrest et al. [21, 22] | Frozen sections of bone marrow chips obtained by a large curette | No full cross-section analysis, risk of missing infiltrated areas, cutting and staining artefacts due to calcified crystals |
| Oxford et al. [23] | Frozen sections of cortical bone scrapings obtained by an curved osteotome | ||
| Bilodeau et al. [24] | Frozen sections of cancellous bone + inferior alveolar nerve specimens | ||
| Wysluch et al. [25] | Frozen sections of cancellous and cortical bone specimens obtained by a trephine drill | ||
| Cytological analysis | Mahmood et al. [26] | Cytological analysis of smear preparation obtained by scraping with a scalpel | No full cross-section analysis, risk of missing infiltrated areas, presence of scattered tumour cells due to operation |
| Nieberler et al. [27] | Cytological analysis cells obtained by a cytobrush | ||
| Accelerated fixation and decalcification | Weisberger et al. [28] | Accelerated fixation and decalcification of sawed bone wafers by microwave technology | Special technical equipment possible toxic fumes artefacts due to partial decalcification |
| Mayer et al. [7] | Frozen section analysis of burred bone dust after short-term decalcification with EDTA | ||
| Elastic scattering spectroscopy | Jerjes et al. [29] | Elastic scattering spectroscopy of formalin fixed, decalcified bone margins | cost-intensive equipment, no testing of frozen tissue |
The aim of this study was to develop and evaluate the possibility of a protein visualisation (Tissue-ProtTrans) using a tissue model. Tissue-ProtTrans is based on the detection of keratins as the gold standard protein markers in the immunohistochemical diagnosis of carcinomas [15]. Tissue-ProtTrans forms the basis for a rapid assay for the detection of tumour cells in bone margins. Moreover, this rapid method does not hamper the subsequent standard histological analysis by preserving the anatomic topology of the tissue.
Methods
Tissue Model
Tissue from pigs was purchased from a local butcher. In this study, skin tissue and tail vertebrae were used.
To simulate a tumour infiltrated mandible, an artificial composite tissue model was set up. This consisted of a pigtail vertebra, i.e. central sections of cortex and marrow mimicking the mandible, with a deposition of porcine skin (mimicking an OSCC tumour tissue containing keratin) placed in a central hole in the vertebra. In the first step, the porcine tails were prepared by removing as much adherent soft tissue as possible (e.g. skin, muscle, nerves and fibrous tissue) using surgical instruments. The necessary hole was drilled into the porcine vertebra (approx. 15 mm, ∅ 3 mm) with a SKIL 450 W drill (Skil Europe B.V., Breda, NL). Subsequently, narrow strips of porcine skin were plugged into the drilled holes until they were firmly fixed (Fig. 1). Composited vertebrae were then sawed after freezing at −20 °C into 2–3 mm thin sections using an Exakt saw comparable to type 300 (Exakt Advanced Technologies GmbH, Norderstedt, Germany).
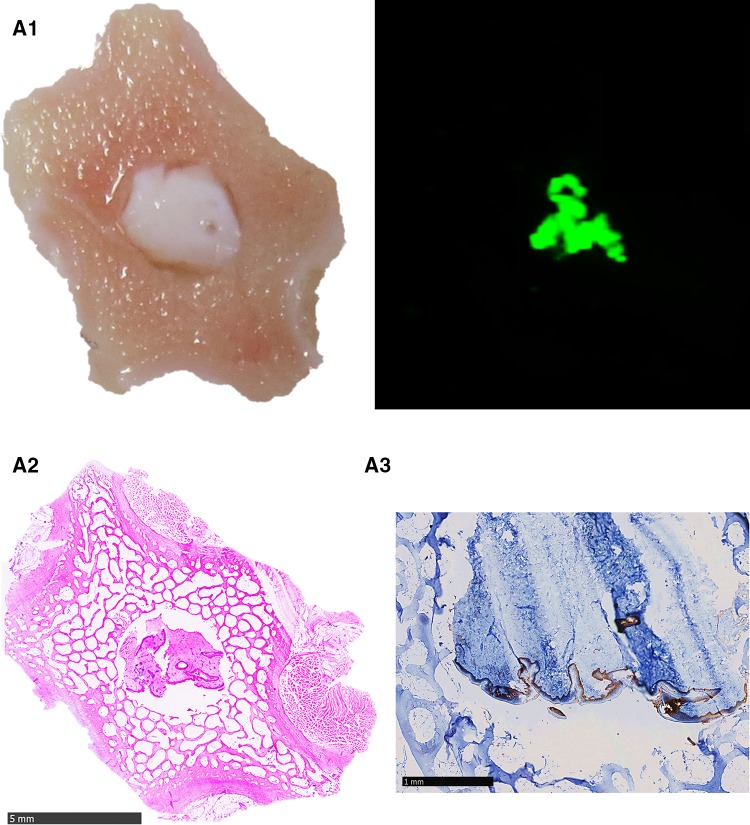
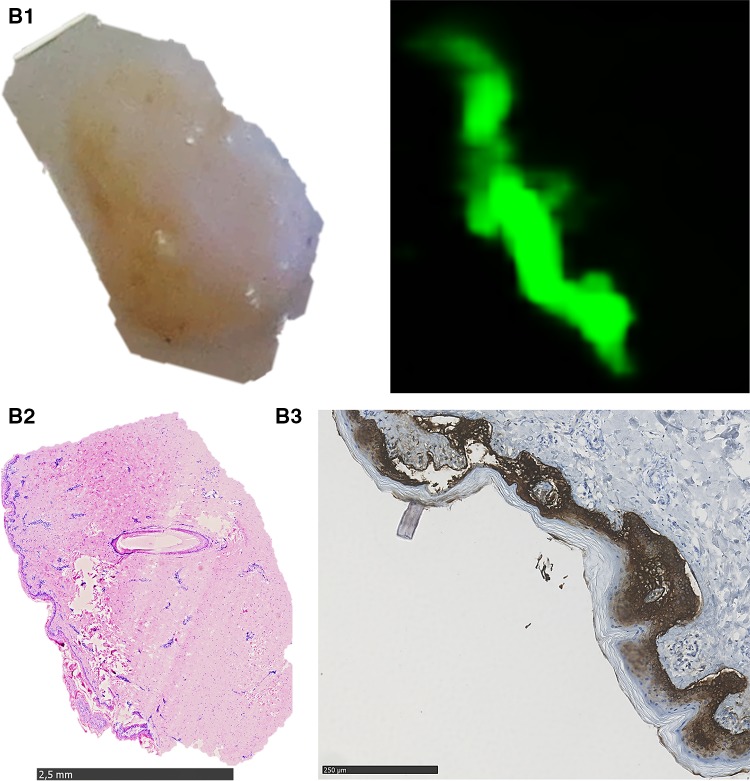
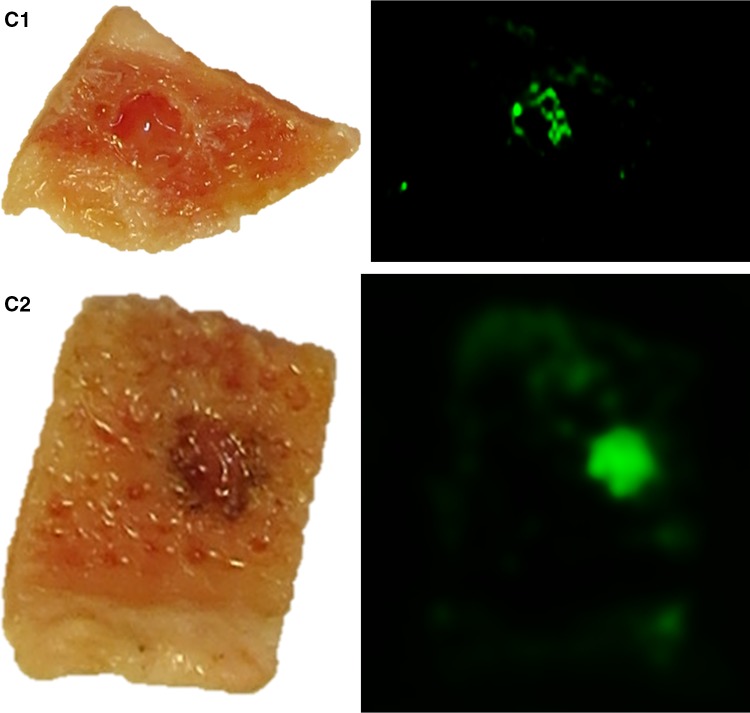
Fig. 1.
Representative results of the Tissue-ProtTrans method with composited bone wafers compared to the results of standard histological staining technique. A1 left part shows an image of native porcine bone wafer with skin placed on NC membrane immediately before protein transfer, right part shows the scanned membrane (800 nm laser beam) of this area after the Tissue-ProtTrans method; A2 H&E stain of the composite bone wafer after protein transfer, fixation, decalcification and paraffinisation; A3 consecutive IHC staining against CK 5/6 (5× fold magnified, selected part), B1 left part shows an image of porcine skin placed on NC membrane immediately before protein transfer, right part shows the scanned membrane (800 nm laser beam) of this area after Tissue-ProtTrans method, B2 H&E stain of the skin tissue after protein transfer, fixation, and paraffinisation. B3 consecutive IHC staining against CK 5/6 (5× fold magnified, selected part), C1 left part shows an image of human bone wafer with tumour tissue ø 4 mm placed on NC membrane right before protein transfer, right part shows the scanned membrane (800 nm laser beam) of this areal after the Tissue-ProtTrans method, C2 left part shows an image of human bone wafer with tumour tissue ø 2 mm placed on NC membrane right before protein transfer, right part shows the scanned membrane (800 nm laser beam) of this area after the Tissue-ProtTrans method
Frozen human squamous cell carcinoma tissue from the oral cavity was provided by the RWTH Biobank. Archival frozen human pelvic bone was provided by the Institute of Pathology.
Immunohistochemistry (IHC) Staining Keratin 5/6
H&E staining for the analysis of tumour-suspicious tissue and immunohistochemical staining against keratin to confirm OSCC are the gold standard methods [15]. IHC was performed to compare the results of newly developed Tissue-ProtTrans method (described in “Newly Developed ‘Tissue-ProtTrans’ Method”) with the results of standard IHC. IHC was conducted consecutively on tissue previously used for Tissue-ProtTrans experiments.
After the protein transfer performed as part of the Tissue-ProtTrans method, tissues (bone wafers and soft tissue) were fixed with formalin (4% v/v) for at least 24 h at room temperature, prior to decalcification [16]. The tissue was decalcified with EDTA solution (containing aqua distilled (10 L), Triplex III (1 kg), Tris(hydroxymethyl)aminomethane (340 g), pH 7.2 (Merck KGaA, Darmstadt, Germany) for 4 days (up to 10 days), depending on the thickness of bone and the amount of compact bone. When the specimens were cuttable with a scalpel, paraffin embedding was performed overnight on a Thermo Scientific Shandon Tissue Processor (Thermo Fisher Scientific, Pittsburgh, USA). Paraffin embedded tissue was cut with a microtome pfm Slide 4003 E (pfm medical, Köln, Germany) in 4 µm sections and mounted on FLEX IHC adhesive microscope slides (Dako Agilent, Agilent Technologies Deutschland GmbH, Waldbronn, Germany). The sections were heat-fixed onto the glass slides for 24 h in an incubator at 37 °C (Heraeus Kelvitron t LUT 6050, Hereaus, Hanau, Germany). Deparaffinisation and rehydration of tissue sections were done manually by serial immersion of the slides in xylol, decreasing alcohol concentrations [100% (v/v) ethanol, 96% (v/v) ethanol, 70% (v/v) ethanol] and distilled aqua [16]. Antigen retrieval of the tissue was performed using a target retrieval solution at pH 9 (Target Retrieval solution pH 9 10× concentrated, Dako Agilent). The slides were placed in a Coplin jar placed in a water bath (heated up to 97 °C) according to the manufacturer’s specification for the primary antibody [16]. After manual antigen retrieval, sections were cooled down to room temperature for 30 min. Then, sections were washed with PBS buffer [Gibco® PBS Tablet (5 g), Thermo Fisher Scientific] (3 × 5 min). The following steps of primary and secondary antibody binding as well as labelling were performed using the Dako Agilent Autostainer (according to manual instructions).
The primary antibody Monoclonal Mouse Anti-Human Keratin 5/6 Clone D5/16 B4 (Dako Agilent) was diluted to 1:100 with Dako Agilent Real Antibody Diluent. In the first step, the sections were blocked with EnVision FLEX Peroxidase Blocking Reagent (Dako Agilent), 100 µL for 5 min followed by one washing cycle with PBS-T. Secondly, the primary antibody was incubated for 30 min followed by one washing cycle with PBS-T. Thirdly, the secondary antibody Flex/HRP (Dako Agilent, EnVision Detections Systems Peroxidase/DAB, Rabbit/Mouse) was incubated for 20 min, followed by two washing cycles of 5 min with PBS-T. Visualisation was done by diaminobenzidine (DAB) chromogen (same kit), which was diluted with HRP Substrate Buffer (same kit). After 10 min of incubation a last washing step with PBS-T solution was performed, followed by counterstaining with hematoxylin.
Finally, the slides were dehydrated with increasing alcohol concentrations [70% (v/v) ethanol, 96% (v/v) ethanol, 100% (v/v) ethanol] and xylol and covered with Vitro-Clud® (Langenbrinck, Emmendingen, Germany) and Tissue-Tek Coverslipping Film (Sakura Finetek Europe B.V., Alphen aan den Rijn, NL).
Haematoxylin-Eosin Staining (H&E)
Rehydrated tissue sections were incubated twice for 4 min with haematoxylin (Merck), washed with H2O, followed by incubation of calcium carbonate (calcium carbonate precipitated pure, pharma grade C, PanReac AppliChem, Darmstadt, Germany) for 5 min. Then, the samples were incubated with eosin (Merck) for 1 min and washed with H2O.
Finally, the slides were dehydrated with increasing alcohol concentrations [70% (v/v) ethanol, 96% (v/v) ethanol, 100% (v/v) ethanol] and xylol and covered with Vitro-Clud® (Langenbrinck) and Tissue-Tek Coverslipping Film (Sakura Finetek Europe B.V.).
Trans-Blot® Turbo™ Transfer System
Proceeding followed in essence the ‘Turbo Protocol’ given in the manual of Trans-Blot® Turbo™ Transfer System (Bio-Rad Laboratories GmbH, München, Germany) [17]. This high-performance western blotting transfer system is designed for a very rapid protein transfer, classically from a gel to a membrane. The protein transfer from the tissue/bone wafer (max. thickness 3 mm) to a membrane in this study was performed in the main instrument in a blotting cassette consisting of a base and a lid where the sandwich stack (filter paper, tissue wafer, membrane, filter paper) was placed. The Trans-Blot® Turbo™ transfer buffer (20% v/v 5× Trans-Blot® Turbo™ Transfer System, 20% v/v methanol, 60% ddH2O) was used for wetting the sandwich stack. The following parameters were set: (a) 7 min of blotting time, (b) set-up ‘mini gel’ was chosen, 1.3 mA (circa 25 V). Immunofluorescence/immunological staining was performed for the detection of keratin 5/6 on the membrane (see below).
Newly Developed ‘Tissue-ProtTrans’ Method
To make tissue proteins available, a digestion step was first performed with the composited wafers (bone combined with skin), skin only (as a positive control) and bone wafers only (as a negative control): Gibco™ trypsin–EDTA solution (0.25% v/v) (Thermo Fisher Scientific) was warmed up for 5 min in an incubator at 37 °C to activate the enzyme. Ready-to-use Proteinase K (Dako Agilent) was warmed up for 5 min at room temperature. Proteinase K (100 µL/wafer) and trypsin (100 µL/wafer) were used for incubation of the composite wafer on a glass slide for 10 min at room temperature. Wafers were turned after 5 min to incubate both sides equally. Rinsing the specimen with 1 mL distilled water thrice stopped digestion.
In each experiment, right before the transfer procedure, filter papers and the Amersham Protran® Premium Nitrocellulose Membranes 0.2 µm (GE Healthcare) were soaked in Trans-Blot® Turbo™ Transfer buffer (Bio-Rad Laboratories GmbH). A transfer sandwich was built up in the cassette base consisting of one filter paper on the bottom, the membrane on top of it, the wafers on top of the membrane and the second filter paper on the top of all (no additional buffer necessary after wetting all sandwich parts). The cassette was closed with the lid and placed in the cassette bay of the main instrument. The protein transfer was acquired with the settings 7 min and 1.3 mA. Afterwards, the cassette lid was opened and the stack was enfolded carefully. Wafers and skin tissue were stored in pre-numbered capsules and fixed in formalin (4% v/v) for further processing with standard IHC. The membrane was placed on a fresh third filter paper soaked in PBS and three dot blots with 2 µL of recombinant human keratin 5 (KRT5) (BIOTREND Chemikalien GmbH, Köln, Germany) diluted 1:10 with PBS were applied in a sketched out circle on the membrane as a positive control for the following subsequent staining process. Immunofluorescence staining of keratin 5/6 (CK5/6) was (see below) done after separating the membrane into three pieces and was carried out in three separate chambers.
Immunofluorescence Staining of Keratin 5/6 on the NC Membrane
After protein transfer from the tissue onto an Amersham Protran® Premium Nitrocellulose Membrane 0.2 µm (GE Healthcare), the membrane was equilibrated in PBS for 5 min, followed by a blocking process with 5% (w/v) milk solution (prepared with skim milk powder for microbiology, Merck Millipore, Darmstadt, Germany) on a shaker for 30 min. Incubation with a primary mouse anti-human CK 5/6 antibody (Dako Agilent, diluted 1:50 in 1% (w/v) milk solution) was performed for 60 min at room temperature on a shaker. Membranes were then washed with PBS-T for 5 min thrice. Afterwards, the membranes were incubated with secondary antibody IRDye® 800CW donkey anti-mouse IgG (H + L), highly crossed adsorbed [LI-COR, diluted with 1% (w/v) milk solution] solution for 30 min at room temperature. Then membranes were washed with PBS-T for 10 min thrice. A negative control membrane without the primary antibody and a blank control membrane without any antibody were included in each run.
After the staining procedure the membranes were dried in an incubator at 37 °C for 30 min. The membranes were then scanned with an infrared laser scanner (Odyssey).
Scanning
In this study, an Odyssey Sa IFred scanner (LI-COR, Bad Homburg, Germany) was used. This infrared laser scanner was equipped with Imaging Studio (Analysis Software Version 4.0). Settings of the infrared laser scanner (Odyssey) were as follows: the scan was done with the 800 nm laser only to obtain specific signal. The resolution was set at 200 µm and the focal plane was at + 3 mm. The laser intensity was adjusted according to the signal. In most cases, an intensity of 2 provided the best result.
Tissue slides stained with H&E or by IHC were scanned using a NanoZoomer Digital slide scanner (Hamamatsu, Herrsching am Ammersee, Germany) and edited with the NDP.view2 software provided by Hamamatsu.
Software
Images were edited using the Microsoft Picture Application (Microsoft Corporation, Redmond, USA) and saved as file format .jpeg or .tiff.
Results
The Tissue-ProtTrans protocol was applied in five independent experiments to assess the reproducibility of the procedure. Example results of one run are shown in Fig. 1.
In all five runs, the imprint of the skin tissue on the membrane (Fig. 1, B1 left) as a positive control for the protein transfer procedure showed a specific fluorescent signal (Fig. 1, B1 right). Additionally, the dot blot of recombinant keratin 5 as a positive control showed a specific fluorescent signal. An excellent result was achieved with the imprint of the composited bone wafer on the membrane (Fig. 1, A1 left), which showed a specific fluorescence signal in the area where the skin was located after applying the novel transfer method (Fig. 1, A2 right). Negative and blank control membranes after protein transfer were negative (results not shown).
After protein transfer onto the membrane, tissues were processed as described in the methods for standard histological examination (immunohistochemistry and H&E staining). Complete concordance of the immunofluorescent signal on the scanned membrane (Fig. 1, A1, A2) and the position of the epidermis could be found by direct comparison of the H&E histology (Fig. 1, A2, B2) and IHC staining results against CK 5/6 (Fig. 1, A3, B3).
We also tested other primary antibodies in preliminary experiments, including Monoclonal Mouse Anti-Human Keratin Clone AE1/AE3 (Dako Agilent) for a broad spectrum of keratins, referred as pan-keratin, Monoclonal Mouse Anti-Human p63 Protein Clone 4A4 (Dako Agilent) and Mouse anti-p40 (∆Np63) (ZYTOMED Systems GmbH, Berlin, Germany). However, best results with respect to the signal-to-background ratio were obtained with the mouse anti-human CK 5/6 primary antibody showing cross reactivity for porcine skin.
Furthermore, we tested other secondary antibodies in preliminary experiments such as Alexa Fluor® 488 conjugate Goat Anti-Mouse IgG (H + L) (ThermoFisher Scientific) and Alexa Fluor® 633 conjugate Goat Anti-Mouse IgG (H + L) (ThermoFisher Scientific) in combination with the laser scanner system Typhoon 9410 variable mode imager (GE Healthcare Life Science, München, Germany) for visualisation. The results were comparable, if a PVDF membrane was used instead of a nitrocellulose membrane as the blotting membrane. However, with the Odyssey Sa IFred scanner (LI-COR, Bad Homburg, Germany) in combination with the secondary antibody IRDye® 800CW donkey anti-mouse IgG (H + L) highly crossed adsorbed (LI-COR) the signal-to-background ratio was better and the scanning software had more image contrasting ability.
Sensitivity Test of Tissue-ProtTrans
To test this platform in human tissue and to obtain insight into the sensitivity of the method, frozen human squamous cell carcinoma tissue of the oral cavity was identified by frozen section analysis. It was then tested with the Tissue-ProtTrans method alone and was found to provide a specific fluorescence signal. In a second step, frozen human pelvic bone wafers were combined with the latter human tumour tissue in different sizes. Drilled holes had sizes of 4 mm ∅ and 2 mm ∅ and were filled with tumour tissue to evaluate the detection limit of the Tissue-ProtTrans method. The experiment showed specific signals, indicating the detection of 4 mm (Fig. 1, C1) and 2 mm diameter tumour tissue (Fig. 1, C2).
Discussion
In our present study, it was shown that the simple technique of tissue printing, which was first developed in the field of Botany [18], and second in printing animal tissue onto a membrane by Reid et al. [19] could be further developed to allow rapid protein transfer from a human (bone) tissue section to a membrane utilising a blotting system. Similar approaches were described in other fields as in sections of paraffin embedded tissue specimens [20].
The Trans-Blot® Turbo™ Transfer System (Bio-Rad) technique is very recent (personal communication with Dr. Hoevel, Bio-Rad) [17]. To date, the classical protein transfer process is more time consuming (about 1–3 h) and the immunofluorescence staining process also takes time (about additional 3 h). Hence, the classical proteomic approach using protein transfer was not suitable for intraoperative evaluation of bone margins. The situation has been changed by the availability of this new rapid protein transfer technique within 3–7 min, using the Trans-Blot® Turbo™ Transfer System (Bio-Rad).
Experiments performed using the Tissue-ProtTrans method showed the successful transfer of keratin from the tissue section and subsequent identification of this protein. This procedure took 3:35 h starting from the superficial digestion of the tissue section, followed by transfer, immunofluorescence staining and finally scanning.
In our study, tissue fixation with formalin was performed for 24 h followed by a decalcification step with EDTA lasting at least 4 days. We used this kind of decalcification process since we wanted to achieve a good cutting result and wanted to stain the obtained sections immunohistochemically afterwards.
In order to achieve a faster decalcification in clinical routine, stronger organic acids (e.g. formic acid) can be used but with the consequence that immunohistological staining cannot be performed afterwards. However, even in the case of achieving a result within 24 h there is no intraoperative result obtainable and no intraoperative action possible in contrast to the use of Tissue-ProtTrans method.
It is an advantage to have flat, smooth bone surfaces available for the blotting procedure. In the optimal case the surgeons deliver a rather flat bone margin wafer directly from the operation room as done with soft tissue for FSA. Surgeons and pathologists have to work interactively in order to address this point properly.
Tissue-ProtTrans method was primarily developed for segmental mandibulectomies. We are also elaborating to implement Tissue-ProtTrans method in marginal mandibular resection. An expansion for maxillectomy is conceivable, especially the intraoperative evaluation of parts of the alveolar ridge and the paranasal and lateral zygomatic pillars which are usually thicker due to their load bearing functions.
A possible expansion of Tissue-ProtTrans method for bone resections of other tumours is conceivable. The blotting process could be used for bone margins of other tumours, only the immunofluorescence and immunohistochemistry would have to be adapted to the special tumour characteristics (target structures).
No routinely used method has yet been established for analysing bone resection margins intraoperatively. Several approaches had been developed and published addressing this problem.
A method developed and refined by Forrest et al. [21, 22] was established for analysing cancellous bone, leaving out cortical parts of the bone margin. Their approach consisted of curettage of the cancellous bone proximally 1 cm from the resection with a “large curette”. Samples were processed using a standard microtome after freezing. The author compared this method with the assay time of frozen section analysis. Oxford et al. [23] described a method for cortical bone in which the bone material was obtained using a curved osteotome and also analysed like frozen sections. Bilodeau et al. [24] described a method of frozen section analysis of cancellous bone chips and in addition frozen section analysis of the inferior alveolar nerve as far as possible (nerve is not available in the mesial parts of the mandible). Wysluch et al. [25] evaluated trephine drill specimens of cancellous and cortical bone like frozen section (30 min on average). These three studies can be summarised as frozen section analyses of bone chips with no decalcification.
Other approaches include cytological analyses by Mahmood et al. [26] and Nieberler et al. [27]. In these studies, material from the cancellous part or both parts of the bone were harvested by scraping or brushing cells from the margin surface using a cytobrush thereby circumventing a cutting process of non-decalcified bone tissue. Afterwards, the cells were mounted onto glass slides, fixed, stained and cytologically analysed (2 h −7 min, depending on the study) [27].
In a study by Mayer et al. [7], a procedure was described for shortening the decalcification time of bone chips using strong organic acid solutions such as formic acid. They evaluated partial decalcification of bone biopsies and bone dust for about 15–75 min using different decalcification solutions. Artefacts are described as ‘minor’ and the results obtained are described as ‘acceptable’. However, experiments with such shortened decalcification procedures in our laboratory exhibited very poor histological quality, insufficient for a valid examination.
Weisberger et al. [28] was the first to suggest a procedure allowing an entire cross-sectional analysis comparable to standard histology. The suggested method included acceleration of the fixation and decalcification process by microwave irradiation. This procedure should allow analysis within three hours with thin samples. Fixation of the surgical specimen was done by incubation in formalin at a temperature of 40 °C for 10 min in a microwave oven to accelerate this process. Then, 1–2 mm sections were collected with a diamond-bladed band saw and the obtained sections were decalcified in a decalcification solution consisting of hydrochloric acid and formalin using a microwave oven. The sections were embedded in paraffin using rapid cycles followed by cutting and evaluation.
Elastic scattering spectroscopy (ESS) [29] represents an alternative method of analysis and also allows for examination of the entire cross-sectional surface. The needed technical equipment is cost intensive and to our knowledge not available as a ready to use instrument. However, since its publication in 2005 describing the analysis of paraffin-embedded tissue with ESS, no further report has been published using this method for the examination of fresh tissue specimens. Two limitations of the ESS are mentioned: (a) the absence of data with respect to oral tissue and (b) the lack of correlation between ESS observations and surgical in vivo observations [30].
We consider frozen section analysis of bone chips to be very time-saving, requiring about 20 min for sectioning, staining and evaluation using relatively cheap, standard pathology equipment. Sensitivity has been indicated between 50% [24] and 89.9% [22, 23], with and a specificity of 100%. However, in two studies [21, 24], the cortical parts of the bone margin were left out of the analysis and bone marrow was analysed only. Typically, the bone marrow exhibits hard areas consisting of bone trabeculae which cannot be scratched out. Furthermore, in a frozen section analysis (FSA) of a bone marrow biopsy small OSCC cell clusters can be missed. In other studies [23, 25], the bone marrow was not analysed. None of the analyses with bone chips guaranteed a full cross-section analysis, thereby incurring the risk of missing infiltrated areas. Furthermore, an additional problem with the problems of frozen section analyses described above is that microtomes are typically not designed for processing calcified tissue specimens. As previously reported, the cutting process is hampered by cutting artefacts as well as abnormal abrasion of the cutting blade [21, 22]. Moreover, during the staining of non-decalcified specimens, further artefacts due to the presence of calcified crystals can interfere with a proper diagnostic evaluation [27].
The two studies employing cytological analyses mentioned an assay time of 2 h [26] down to 7 min [25], which is the fastest assay time of all published methods and may also be cheap due to the use of standard cytology equipment. Sensitivity was stated between 80 [27] and 100% [26] and specificity was about 100% in both studies with 7 [26] and 73 [27] cases. Nevertheless, there is no guarantee of a full cross-sectional analysis. Additionally, the presence of scattered tumour cells due to the operation process, sticking ‘artificially’ to the mandible margin, may result in a false positive evaluation.
The assay time with heating the hydrochloric acid and formalin to accelerate the fixation and decalcification process to gain a full cross-section analysis was about 3 h [28]. The values of sensitivity (100%) and specificity (100%) are the best of all described methods. An estimation of the costs is difficult, as no information was given in the study. However, special technical equipment is necessary as the instruments used are not standard. There are also problems associated with safety in this method as technicians must handle hot solutions that may produce toxic fumes. The duration of this process of up to 3 h (including accelerated paraffinisation for 45 min) seemed to be too long in the view of other authors [7, 27]. Potential measures for shortening this procedure were not proposed in their discussion.
In summary, the shortest assay suggested is the cytological assay requiring 7 min [27]. However, the best results with respect to sensitivity and specificity were obtained with the full cross-section analysis by Weisberger et al. [28].
The method developed in this present study, Tissue-ProtTrans, requires 3.5 h with realistic options for shorting the time down to maybe 1 h. It allows for the analysis of the entire cross-sectional surface of a resection margin with the purpose of obtaining high sensitivity and specificity. However, in contrast to the study by Weisberger et al. [28], the Tissue-ProtTrans method is safe because decalcification and fixation with dangerous chemicals are not necessary for protein transfer. Tissue-ProtTrans is simple (sawing, blotting, staining, scanning) with standard instruments (water-cooled diamond band saw) and easily accessible and cost efficient instruments (blotter, scanner). An additional highly important advantage of this method is that it preserves the morphology of the sectioned tissue, an aspect not fulfilled by most of the other methods discussed above. It opens up the opportunity of subsequently performing histological analyses on the same tissue.
The Tissue-ProtTrans method could be potentially further improved by incubating the antibodies at a higher temperature, e.g. 37 °C. This holds the potential of a significant reduction of the assay time (e.g. down to 2 h instead of 3.5 h). For a further reduction, direct immunofluorescent staining using an antibody binding to keratin directly labelled with a fluorochrome could be considered. In this case, the incubation step with the secondary antibody could be dropped (30 min) as well as the respective washing steps (30 min), so that it would reduce assay time to 2.5 h instead of 3.5 h. The combination of both reductions could result in an assay time of 1 h. However, a decrease in signal should be taken into account and it might affect the specificity of the assay depending o n the antibodies used. Additionally, a test on the sensitivity and specificity of the Tissue-ProtTrans should be performed on human mandible surgical specimens.
In summary, by applying the proposed Tissue-ProtTrans method the bone resection status can be evaluated intraoperatively hence reducing the risk of remaining tumour cells. This intraoperative analysis facilitates safe primary reconstruction in one session. If the bone margins turn out positive for tumour cells, a possibly more extensive resection can be made immediately. In comparison to a secondary operation substantial stress for the patient and the involved surgeons can be avoided. Furthermore, Tissue-ProtTrans method can be of help to reduce overall recurrence rate and a too extensive bone resection can be diminished, which helps patients’ outcome and convalescence. Therefore patients and surgeons benefit from primary bone reconstruction without the potential disadvantages. Even a replacement of the segmental resection of the mandible (which results in a discontinuity) by marginal resection thereby conserving continuity of the mandible might be achievable in one operation by applying the Tissue-ProtTrans method.
Conclusion
In conclusion using the Tissue-ProtTrans method, OSCC marker proteins, including keratin 5/6 could be transferred successfully onto a nitrocellulose membrane and could be distinguished visually by immunofluorescence. Additionally, with Tissue-ProtTrans the anatomic topology of the tissue sample is completely preserved for further investigations, i.e. standard histology. This method is able to overcome the serious limitations of previously described approaches of analysing bone resection margins, including (a) the problem of artefacts due to calcified crystals, (b) the evaluation of selected parts of the surface instead of the entire cross-sectional analysis and (c) the inherent problems of fast decalcification. There is an excellent potential for this approach, worth further investigation.
The Tissue-ProtTrans method represents an absolutely new and promising approach for a reliable and rapid assessment of bone resection margins in squamous cell carcinoma—a problem that has not been solved so far.
Acknowledgements
We want to give special thanks to Sandra Krämer, PhD, Clinic for Thoracic and Cardiovascular Surgery for her help in employing the Trans-Blot® Turbo™ Transfer System (Bio-Rad).
Funding
No funding was obtained.
Compliance with Ethical Standards
Conflict of interest
There is no conflict of interest to disclose.
Ethical Approval
We used porcine tissue in this study. All applicable international, national and institutional guidelines for the care and use of animals were followed. We used archival human tissue in this study. (1) Use of archival human pelvic bone was reviewed and approved by the Ethics Committee of the Medical Faculty of the RWTH Aachen University (No. ek-173/06). (2) Use of human oral squamous cell carcinoma tissue obtained from RWTH Aachen centralised Biomaterial Bank (RWTH cBMB). The cBMB was reviewed and approved by the Ethics Committee of the Medical Faculty of the RWTH Aachen University. A mandatory prerequisite for incorporation of a biomaterial sample into RWTH cBMB is the written consent of the donor. Before signing, the donor is informed by a medical doctor about the research project and the intended storage of donated samples and associated data. The important contribution of the donor to biomedical research is addressed (quoted from https://www.cbmb.rwth-aachen.de/en/data-privacy). All procedures performed involving human tissue were in accordance with the ethical standards of the institutional research committee which are comparable with the 1964 Helsinki declaration and its later amendments.
References
- 1.American Cancer Society . Global cancer facts and figs. 3. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8(9):11884–11894. [PMC free article] [PubMed] [Google Scholar]
- 3.Deutsche Gesellschaft für Mund-, Kiefer- und Gesichtschirurgie. S3-Leitlinie Mundhöhlenkarzinom: “Diagnostik und Therapie des Mundhöhlenkarzinoms”, in Leitlinie (Langversion). Leitlinienprogramm Onkologie der AWMF, Deutschen Krebsgesellschaft e.V. und Deutschen Krebshilfe e.V.: AWMF online; 2012. p. 119.
- 4.Hausamen J-E, et al. Mund-, Kiefer- und Gesichtschirurgie: Operationslehre und -atlas 4. New York, NY: Springer Medizin; 2012. [Google Scholar]
- 5.Oral Cancer Facts: Available from http://www.oralcancerfoundation.org/facts/ (2016). Accessed 11 July 2017.
- 6.Pfister DG, et al. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) head and neck cancers. Washington, DC: National Comprehensive Cancer Network; 2013. [DOI] [PubMed] [Google Scholar]
- 7.Mayer A, et al. Rapid mandible margins for intraoperative assessment. Am J Otolaryngol. 2015;36(3):324–329. doi: 10.1016/j.amjoto.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Camuzard O, et al. Primary radical ablative surgery and fibula free-flap reconstruction for T4 oral cavity squamous cell carcinoma with mandibular invasion: oncologic and functional results and their predictive factors. Eur Arch Otorhinolaryngol. 2017;274(1):441–449. doi: 10.1007/s00405-016-4219-7. [DOI] [PubMed] [Google Scholar]
- 9.Weitz J, et al. Can the inferior alveolar nerve be used as a marker in frozen section for free margin control after segmental mandibulectomy in tumour ablation? Int J Oral Maxillofac Surg. 2016;45(11):1366–1371. doi: 10.1016/j.ijom.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Santamaria E, de la Concha E. Lessons learned from delayed versus immediate microsurgical reconstruction of complex maxillectomy and midfacial defects: experience in a tertiary center in Mexico. Clin Plast Surg. 2016;43(4):719–727. doi: 10.1016/j.cps.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira JJ, et al. Mandible reconstruction: history, state of the art and persistent problems. Prosthet Orthot Int. 2015;39(3):182–189. doi: 10.1177/0309364613520032. [DOI] [PubMed] [Google Scholar]
- 12.Bak M, et al. Contemporary reconstruction of the mandible. Oral Oncol. 2010;46(2):71–76. doi: 10.1016/j.oraloncology.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Shockley WW. Immediate mandibular replacement using reconstruction plates. Arch Otolaryngol Head neck Surg. 1991;117(7):745–9. [DOI] [PubMed]
- 14.Gellrich N-C, et al. Comparative study of locking plates in mandibular reconstruction after ablative tumor surgery: THORP versus UniLOCK system. J Oral Maxillofac Surg. 2004;62(2):186–193. doi: 10.1016/j.joms.2003.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Rao RS, Patil S, Ganavi BS. Oral cytokeratins in health and disease. J Contemp Dent Pract. 2014;15(1):127–136. doi: 10.5005/jp-journals-10024-1502. [DOI] [PubMed] [Google Scholar]
- 16.Education Guide . Immunohistochemical staining methods. 5. Carpinteria: Dako North America; 2009. [Google Scholar]
- 17.Trans-Blot® TurboTM Blotting System: Instruction manual, B.-R. Laboratories, editor. 2010. p. 40.
- 18.Taylor R, Inamine G, Anderson J. Tissue printing as a tool for observing immunological and protein profiles in young and mature celery petioles. Plant Physiol. 1993;102:1027–1031. doi: 10.1104/pp.102.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid PD, et al. Tissue printing: tools for the study of anatomy, histochemistry, and gene expression. San Diego: Academic Press, Inc; 1992. [Google Scholar]
- 20.Chung JY, Hewitt SM. Proteomic expressional profiling of a paraffin-embedded tissue by multiplex tissue immunoblotting. Methods Mol Biol. 2015;1312:175–184. doi: 10.1007/978-1-4939-2694-7_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrest AL, et al. Update on intraoperative analysis of mandibular margins. Am J Ortolaryngol. 1997;18(6):396–399. doi: 10.1016/S0196-0709(97)90060-0. [DOI] [PubMed] [Google Scholar]
- 22.Forrest AL, et al. Rapid analysis of mandibular margins. Laryngoscope. 1995;105(5):475–477. doi: 10.1288/00005537-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Oxford LE, Ducic Y. Intraoperative evaluation of cortical bony margins with frozen-section analysis. Otolaryngol Head Neck Surg. 2006;134(1):138–141. doi: 10.1016/j.otohns.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Bilodeau EA, Chiosea S. Oral squamous cell carcinoma with mandibular bone invasion: intraoperative evaluation of bone margins by routine frozen section. Head Neck Pathol. 2011;5(3):216–220. doi: 10.1007/s12105-011-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wysluch A, et al. Intraoperative evaluation of bony margins with frozen-section analysis and trephine drill extraction technique: a preliminary study. Head Neck. 2010;32(11):1473–1478. doi: 10.1002/hed.21350. [DOI] [PubMed] [Google Scholar]
- 26.Mahmood S, Conway D, Ramesar KC. Use of intraoperative cytologic assessment of mandibular marrow scrapings to predict resection margin status in patients with squamous cell carcinoma. J Oral Maxillofac Surg. 2001;59(10):1138–1141. doi: 10.1053/joms.2001.26710. [DOI] [PubMed] [Google Scholar]
- 27.Nieberler M, et al. Evaluation of intraoperative cytological assessment of bone resection margins in patients with oral squamous cell carcinoma. Cancer Cytopathol. 2014;122(9):646–656. doi: 10.1002/cncy.21428. [DOI] [PubMed] [Google Scholar]
- 28.Weisberger EC, et al. Intraoperative microwave processing of bone margins during resection of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2001;127:790–793. [PubMed] [Google Scholar]
- 29.Jerjes W, et al. Assessment of bony resection margins in oral cancer using elastic scattering spectroscopy: a study on archival material. Arch Oral Biol. 2005;50(3):361–366. doi: 10.1016/j.archoralbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Green B, et al. Optical diagnostic techniques for use in lesions of the head and neck: review of the latest developments. Br J Oral Maxillofac Surg. 2014;52(8):675–680. doi: 10.1016/j.bjoms.2014.06.010. [DOI] [PubMed] [Google Scholar]