Abstract
Beta-ketothiolase (mitochondrial acetoacetyl-CoA thiolase) deficiency is a genetic disorder characterized by impaired isoleucine catabolism and ketone body utilization that predisposes to episodic ketoacidosis. It results from biallelic pathogenic variants in the ACAT1 gene, encoding mitochondrial beta-ketothiolase. We report two cases of beta-ketothiolase deficiency presenting with acute ketoacidosis and “metabolic stroke.” The first patient presented at 28 months of age with metabolic acidosis and pallidal stroke in the setting of a febrile gastrointestinal illness. Although 2-methyl-3-hydroxybutyric acid and trace quantities of tiglylglycine were present in urine, a diagnosis of glutaric acidemia type I was initially suspected due to the presence of glutaric and 3-hydroxyglutaric acids. A diagnosis of beta-ketothiolase deficiency was ultimately made through whole exome sequencing which revealed compound heterozygous variants in ACAT1. Fibroblast studies for beta-ketothiolase enzyme activity were confirmatory. The second patient presented at 6 months of age with ketoacidosis, and was found to have elevations of urinary 2-methyl-3-hydroxybutyric acid, 2-methylacetoacetic acid, and tiglylglycine. Sequencing of ACAT1 demonstrated compound heterozygous presumed causative variants. The patient exhibited choreoathethosis 2 months after the acute metabolic decompensation. These cases highlight that, similar to a number of other organic acidemias and mitochondrial disorders, beta-ketothiolase deficiency can present with metabolic stroke. They also illustrate the variability in clinical presentation, imaging, and biochemical evaluation that make screening for and diagnosis of this rare disorder challenging, and further demonstrate the value of whole exome sequencing in the diagnosis of metabolic disorders.
Keywords: 3-Ketothiolase, 3-Oxothiolase, Beta-ketothiolase, Ketoacidosis, Metabolic stroke, Mitochondrial acetoacetyl-coenzyme A thiolase, Organic acidemia, T2
Introduction
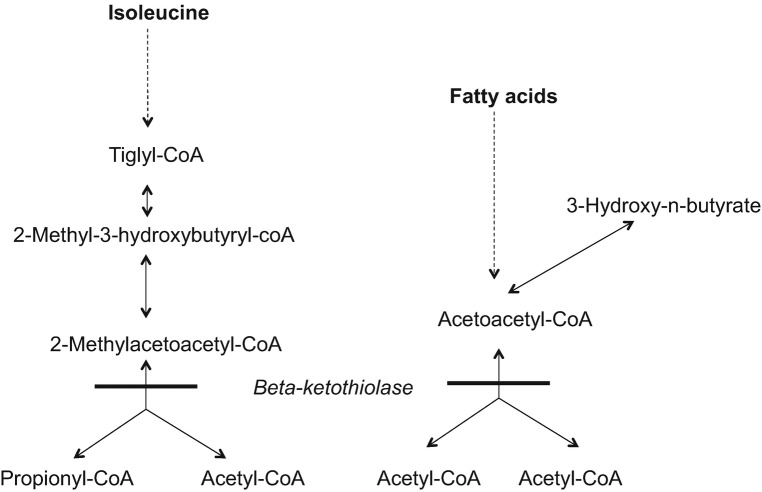
Beta-ketothiolase deficiency is a rare disorder of ketone body and isoleucine metabolism (Fig. 1). This disorder was first reported in a patient with recurrent metabolic acidosis (Daum et al. 1971). Another early report of a patient with “ketotic hyperglycinemia syndrome” identified a defect in the reaction converting 2-methylacetoacetyl-CoA to propionyl-CoA and acetyl-CoA with increased amounts of 2-methyl-3-hydroxybutyric acid, 2-methylacetoacetic acid, and butanone excreted in urine (Hillman and Keating 1974). Beta-ketothiolase has since been identified as the only enzyme able to convert 2-methylacetoacetyl-CoA to propionyl-CoA and acetyl-CoA (Middleton and Bartlett 1983). The beta-ketothiolase enzyme is encoded by ACAT1, and the disorder follows autosomal recessive inheritance.
Fig. 1.
Beta-ketothiolase deficiency. The location of the metabolic defect is indicated by the solid bar
Patients with beta-ketothiolase deficiency typically present in early childhood with acute and recurrent ketoacidosis following an illness (Korman 2006). They are typically asymptomatic between episodes of metabolic decompensation (Fukao et al. 2014), which generally decrease in frequency with age. Similar to a number of other organic acidemias and mitochondrial disorders, beta-ketothiolase deficiency can present with metabolic stroke and associated neurological sequelae (Ozand et al. 1994; Buhas et al. 2013; O’Neill et al. 2014; Yalcinkaya et al. 2001; Shiasi Arani and Soltani 2014; Akella et al. 2014). Acute treatment of ketoacidosis in this disorder typically consists of a high dextrose intravenous (IV) infusion, correction of the metabolic acidosis with bicarbonate, and carnitine supplementation if deficient. Chronic management involves avoiding prolonged periods of fasting and potential dietary modifications including mild protein restriction and carnitine supplementation, if deficient.
We present two patients with beta-ketothiolase deficiency both presenting with ketoacidosis and acute neurological events. Neither case was detected on routine newborn screening. These cases highlight variability in the clinical presentation, imaging, and biochemical evaluation that make screening for and diagnosis of this disorder challenging.
Materials and Methods
Patient One
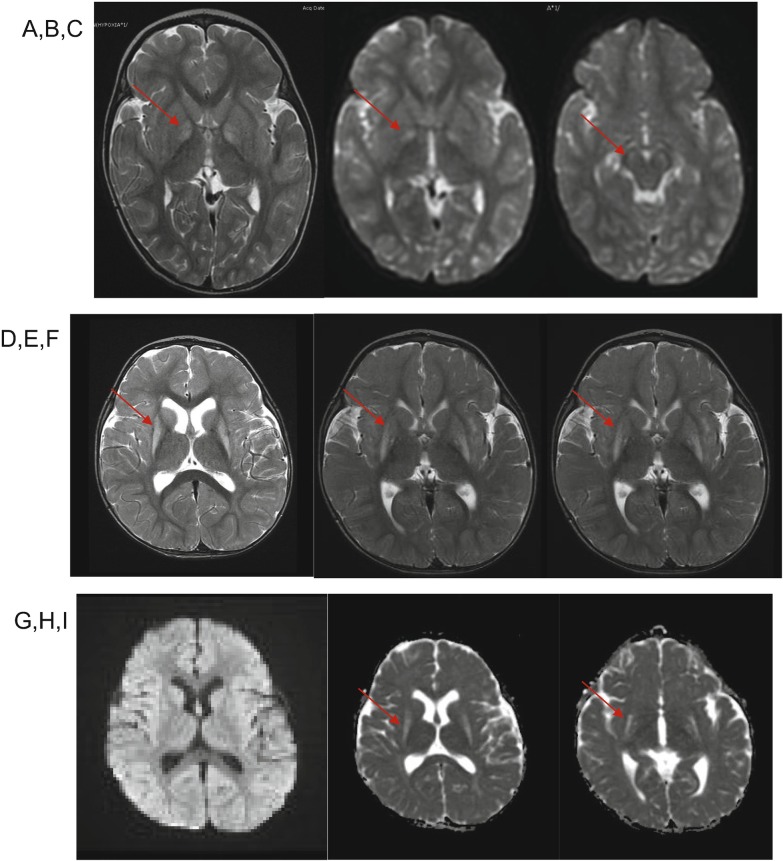
A 28-month-old girl presented with a decreased level of consciousness, hypoglycemia, and profound metabolic acidosis in the setting of an infectious gastrointestinal illness with 2 days of emesis and decreased oral intake. On the day of admission, she was found unresponsive by her parents. Initial blood glucose was 42 mg/dL (reference interval: 40–100). Venous blood gas was notable for pH of 6.87 (reference interval: 7.35–7.45), pCO2 of 23 mmHg (reference interval: 35–45), and measured bicarbonate of less than 5 mmol/L (reference interval: 20–33). Urine ketones were estimated to be greater than 80 mg/dL by dipstick. She was intubated and administered IV boluses of dextrose, normal saline, and sodium bicarbonate and was admitted to the intensive care unit. Brain magnetic resonance imaging (MRI) showed bilateral T2 hyperintensity and reduced diffusivity in the globus pallidi and substantia nigra (Fig. 2).
Fig. 2.
MRI for patient 1 (a–c) and patient 2 (d–i). Patient 1, images obtained at 2 years of age: (a) Axial T2-weighted MR demonstrates T2 hyperintensity of bilateral globus pallidi. (b, c) Diffusion-weighted imaging (DWI) demonstrates bilaterally restricted diffusion in globus pallidi and substantia nigra (arrows). Patient 2, images obtained at 14 months of age: (d–f) Axial T2-weighted MRI demonstrates T2 hyperintensity of bilateral putamina, and to a lesser degree globus pallidi (arrows). (g) Diffusion-weighted imaging (DWI) and (h, i) apparent diffusion coefficient (ADC) sequences demonstrate that regions of signal change in putamina and globus pallidi lack restricted diffusion
Prior to this admission, the child had been healthy and developing normally. She had a history of preterm birth at 32 weeks gestational age. Two newborn screening (NBS) specimens had been collected from the infant on day of life (DOL) 3 and 13 and both were reported as normal (Table 1).
Table 1.
Concentration of acylcarnitines and ratios relevant for profiling beta-ketothiolase in the newborn screening specimens from patient 1
| Relevant acylcarnitines/ratios | Specimen 1–DOL 3 | Specimen 2–DOL 13 | MA | CLR tools | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original | Retest | Original | Retest | cut-off | 99th%ile of normal | 1st%ile of BKT | 5th%ile of BKT | Target cut-offs | |||||
| Value | Z-score | Value | Z-score | Value | Z-score | Value | Z-score | ||||||
| C5:1 | 0.04 | 3.34 | 0.05 | 3.98 | 0.05 | 3.98 | 0.04 | 3.34 | 0.08 | 0.06 | 0.07 | 0.15 | 0.06–0.15 |
| C5OH | 0.18 | 1.44 | 0.16 | 1.09 | 0.14 | 0.7 | 0.18 | 1.44 | 0.8 | 0.36 | 0.49 | 0.65 | 0.36–0.82 |
| C4OH | – | – | 0.1 | −0.48 | – | – | 0.1 | −0.48 | 0.75 | 0.47 | 0.58 | 0.6 | 0.47–0.81 |
| C0 | 29.41 | 0.74 | 55.52 | 2.59 | 26.01 | 0.38 | 28.27 | 0.63 | – | 50.55 | 9.3 | 11.19 | – |
| C5 | 0.37 | 2.5 | 0.27 | 1.52 | 0.33 | 2.14 | 0.38 | 2.58 | – | 0.38 | 0.1 | 0.13 | – |
| C8 | 0.06 | −1.21 | 0.05 | −1.86 | 0.1 | 0.61 | 0.09 | 0.24 | – | 0.17 | 0.03 | 0.04 | – |
| C16 | 1.88 | −1.44 | 1.72 | −1.7 | 0.74 | −4.18 | 0.63 | −4.65 | – | 5.73 | 1.13 | 1.32 | – |
| C5OH × C5:1 | 0.007 | 3.08 | 0.008 | 3.28 | 0.007 | 3.08 | 0.007 | 3.08 | 0.02 | – | – | – | – |
| C5 × C5:1/C5OH | 0.082 | 2.83 | 0.084 | 2.88 | 0.118 | 3.52 | 0.084 | 2.88 | 0.05 | – | – | – | – |
| C5OH/C8 | 2.74 | 1.77 | 3.16 | 2.12 | 1.40 | 0.22 | 2.00 | 1.01 | 10 | 7.81 | 3.68 | 4.95 | 7.81–10 |
| C5OH/C0 | 0.006 | – | 0.002 | – | 0.005 | – | 0.006 | – | – | 0.02 | 0.02 | 0.02 | 0.018–0.026 |
| C4OH/C16 | – | – | 0.06 | – | – | – | 0.16 | – | – | 0.13 | 0.27 | 0.28 | 0.13–0.28 |
BKT beta-ketothiolase deficiency. Concentrations and values shown are those reported originally and after retesting after diagnosis (at time of retest additional markers including C4OH were also being analyzed for routine screening). Values for the 99th percentile of the normal population, and the 1st & 5th percentiles in confirmed BKT cases, and recommended cut-offs for the relevant markers and ratios in the CLIR-R4S database are shown [http://www.clir-r4s.org/; accessed 05/22/2017]. C5:1, C5OH and C4OH are expected to be high in BKT. C0, C5, C8, and C16 are utilized for calculating ratios and scores to risks of BKT by the NBS Analytical Tools in the CLIR-R4S databases and by the New England Newborn Screening Program (NENSP). C5OH × C5:1, [C5 × C5:1/C5OH] and C5OH/C8 are utilized by NENSP for evaluating BKT profiles/risk of disorder. C5OH/C8, C5OH/C0 and C4OH/C16 are required by calculating a score/risk of disorder by the CLIR-R4S in the current BKT Post-Analytic Tool [BKT 009 2012-01-08 Single-D]. The score for patient 1 calculated using the BKT Post-Analytic Tool was “0” in both specimens with the interpretation “Score Profile is not informative for BKT”
The metabolism service was consulted and recommended IV 10% dextrose with half-normal sodium acetate and sodium bicarbonate boluses. Based on an initial suspicon for an organic acidemia, she was empirically treated with intramuscular cyanocobalamin and enteral levocarnitine and riboflavin.
Initial qualitative assessment of urine organic acids was notable for marked ketosis, with large peaks of 3-hydroxybutyric and acetoacetic acids predominating. There was also a medium-chain dicarboxylic aciduria, with significant quantities of adipic acid together with smaller amounts of suberic, sebacic, and 3-hydroxysebacic acids in a pattern consistent with physiologic ketosis. Also notable were the presence of significant quantities of 2-methyl-3-hydroxbutyric and glutaric acids, together with trace quantities of both tiglylglycine and 3-hydroxyglutaric acid. Plasma acylcarnitine analysis demonstrated increases in acetylcarnitine (C2) and 3-hydroxybutyrylcarnitine (C4OH), a pattern consistent with physiologic ketosis. Notably, concentrations of 2-methyl-3-hydroxybutyrylcarnitine (C5OH) and tiglylcarnitine (C5:1) were within the reference intervals. Plasma free carnitine was mildly decreased (22.4 μmol/L; reference interval: 26–60) with a free/total carnitine ratio of 0.31% (reference interval: 0.50–0.90). Plasma amino acid analysis produced a pattern suggestive of an acute catabolic state, with moderate increases in the branched chain amino acids. A plasma glutaric acid level, collected on the day of admission, was elevated at 1,088 ng/mL (reference interval: <250). Plasma 3-hydroxyglutaric acid was also elevated at 198 ng/mL (reference interval: <65). Cerebrospinal fluid (CSF) glutaric acid was normal at 98 ng/mL (reference interval: <250) with increased CSF 3-hydroxyglutaric acid at 121 ng/mL (reference interval: <65). Based on these results, a presumptive diagnosis of glutaric acidemia type I (GA I) was made.
The patient’s metabolic acidosis and ketosis improved over the first hospital day on high-dextrose containing fluids, though recurred during an interruption in her IV fluids and enteral nutrition. Repeat plasma and urine glutaric acid and 3-hydroxyglutaric acid levels obtained during the hospitalization were normal.
Clinically, her level of consciousness improved and she was extubated on hospital day four. She was noted to have generalized athetosis and dystonia, particularly affecting her left leg. She was started on baclofen, trihexyphenidyl, gabapentin, melatonin, and clonazepam. Due to the suspicion for (GA I), a diet low in tryptophan and lysine was initiated.
Enzyme analysis of glutaryl-CoA dehydrogenase was performed in cultured skin fibroblasts and was normal. Sequencing of GCDH for GA I was negative. Additionally, mitochondrial genome screening and sequencing of SURF1, SCO2, and COX10 to evaluate for mitochondrial disorders were unremarkable. She was transitioned back to a regular diet and riboflavin and levocarnitine supplements were discontinued. A follow-up urine collected approximately 1 week later was essentially normal, with only trace levels of 2-methyl-3-hydroxybutyric and 3-hydroxybutyric acids and the medium-chain dicarboxylic acids noted. During subsequent hospital presentations for fever and emesis, she was found to have normal or mildly elevated plasma and urine glutaric and 3-hydroxyglutaric acids, and qualitative urine organic acid profiles that were either normal or consistent with ketosis with trace levels of 2-methyl-3-hydroxybutyric acid.
Her dystonia gradually improved and she was ultimately weaned off baclofen, gabapentin, trihexyphenidyl, clonazepam, and melatonin. At present, she is developmentally normal with spasticity of the lower extremities and occasional dyskinesia of the upper extremities.
Patient 2
A 6-month-old male infant was seen by his pediatrician in the setting of 2 days of nasal congestion and otitis media. The following day, he presented to the emergency department with lethargy and Kussmaul respirations and was found to have severe ketoacidosis, with a blood gas notable for a pH of 7.07, calculated bicarbonate of 3 mmol/L, and base excess −27. Urinalysis showed 4+ ketones by dipstick. He was given an IV fluid bolus and admitted to the intensive care unit.
Prior to this presentation, the patient had been developing normally. At 3 months of age, he had a focal seizure of unclear etiology. Electroencephalogram, head CT, and brain MRI prior to his metabolic decompensation were normal.
Infectious studies returned positive for adenovirus. Urine organic acids showed marked excretion of 3-hydroxybutyric acid, acetoacetic acid, lactic acid, 2-methyl-3-hydroxybutyric acid and 2-methylacetoacetic acid, and trace excretion of tiglylglycine. A plasma acylcarnitine profile revealed elevated C2 and markedly elevated C4OH with normal C5OH and C5:1. Plasma-free carnitine was decreased at 15 μmol/L and total carnitine was normal. Plasma amino acids and urine acylglycines were unremarkable.
He was administered IV levocarnitine while inpatient, and switched to oral levocarnitine for home. He also received high-dextrose IV fluids containing sodium bicarbonate. His severe ketoacidosis persisted for 24 h and then slowly improved, normalizing on the third hospital day.
At a follow-up visit 2 months after his hospitalization, it was noted that the patient had not attained any further developmental milestones. Three months after his hospitalization, at 8 months of age, he was found to have near continuous choreoathetoid movements of his extremities. Brain MRI at 14 months of age demonstrated interval development of increased T2 signal of the bilateral lentiform nuclei, as well as scattered foci of increased T2 signal in the periventricular and subcortical white matter (Fig. 2).
The patient has not since presented with any episodes of ketoacidosis. He continues to receive physical, occupational, and speech therapy and his oral intake remains poor. Clonazepam was tried, but appeared to decrease his appetite even more and did not improve his movement disorder.
Whole Exome Sequencing (Patient 1)
Total genomic DNA was extracted from peripheral blood mononuclear cells using QIAmp DNA Mini Kit (Qiagen). DNA from the proband and both biological parents was sent for whole exome sequencing (WES). WES was provided by the Yale University Center for Mendelian Genomics on an Illumina HiSeq 2000 instrument with blood samples pooled 6 per lane. Libraries (TruSeq DNA v2 Sample Preparation kit; Illumina, San Diego, CA) and whole exome capture (EZ Exome 2.0, Roche) were performed according to manufacturer protocols. FASTQs were filtered, aligned, and variants were filtered and annotated by Codified Genomics (proprietary algorithm, Houston, TX). Sanger confirmation of the candidate variant was performed at the Boston Children’s Hospital Manton Center Gene Discovery Core. Likely pathogenic variants were selected to include nonsynonymous, splice site, and indel variants with an allele frequency <1% in the NHLBI exome variant server database (http://evs.gs.washington.edu/EVS/) or 1000 genomes project (http://www.1000genomes.org) and were evaluated in the ExAC database (http://www.exac.broadinstitute.org). The pathogenicity of the variants was evaluated in silico using Polyphen-2, SIFT, and MutationTaster.
ACAT1 Gene Sequencing (Patient 2)
ACAT1 was clinically sequenced using standard PCR and sequencing methods.
Beta-Ketothiolase Enzyme Activity Measurement (Patient 1)
Beta-ketothiolase enzyme activity was measured in a fibroblast homogenate prepared in phosphate buffered saline by sonication. 2-Methylacetoacetyl-CoA was used as substrate in the reaction in the presence of CoA. After termination of the reaction, substrate and product (propionyl-CoA) were separated by ultra-high pressure liquid chromatography.
Results
Patient 1
WES revealed 470 variants that satisfied the frequency filtration criteria. Of these, 26 were either de novo dominant or recessive (homozygous or compound heterozygous in trans). Genes overlapping those variants were further evaluated for human disease or animal models consistent with the phenotype and only one gene, ACAT1, fulfilled the criteria. A splicing variant, c.1006-1G>C, was maternally inherited while a missense variant, c.1160T>C, p.Ile387Thr, was paternally inherited. The splicing variant has been previously reported as pathogenic (Fukao et al. 1992a) and is not seen in ExAC database (Lek 2016). The missense variant c.1160T>C occurs at a highly conserved position, is seen in only four individuals in ExAC, all heterozygous (mean allele frequency or MAF <0.003%), and is predicted to be pathogenic by several in silico methods including Polyphen-2, SIFT, and MutationTaster.
Analysis of beta-ketothiolase activity in cultured skin fibroblasts from patient 1 confirmed the diagnosis, as the enzyme activity was markedly reduced to below the limit of quantitation of the assay at <1 nmol/(min.mg of protein) [reference interval: 23–74 nmol/(min.mg of protein)].
Patient 2
ACAT1 sequencing revealed bi-allelic mutations, one of which, c.1006-2A>C, has been previously reported as pathogenic (Fukao et al. 1992b) and is seen in only four individuals in ExAC, all heterozygous (MAF <0.003%). The other, c.299G>A, p.Gly100Glu, is not previously reported but occurs at a highly conserved position, is predicted to be pathogenic by SIFT, Polyphen-2 and MutationTaster, and is not seen in ExAC.
Discussion
Beta-ketothiolase deficiency is an autosomal recessive disorder impairing isoleucine catabolism and ketone body utilization that predisposes to episodic ketoacidosis (Fig. 1). We present two patients with beta-ketothiolase deficiency manifesting with ketoacidosis and neurological injury. The diagnosis in the first patient was not suspected clinically, and in fact biochemical evaluation misdirected the work-up towards GA I, despite the presence of 2-methyl-3-hydroxybutyric acid and trace quantities of tiglylglycine on initial qualitative urine organic acid analysis (although these metabolites were absent from subsequent urine organic acid analyses). “Hypothesis-free” testing with WES was required to make the diagnosis, confirmed by enzyme analysis. The second case showed the classic metabolic profile of beta-ketothiolase deficiency, directing clinicians to the appropriate diagnosis. The dichotomy of these cases serves to highlight the challenges in the biochemical diagnosis of beta-ketothiolase deficiency. The classic metabolic perturbations seen in this disorder, namely elevations of 2-methyl-3-hydroxybutyric acid, 2-methylacetoacetic acid, and tiglylglycine in urine, with C5OH and C5:1 in plasma, may not all be present. This is even more problematic in patients with residual enzyme activity, and during periods of clinical stability (Fukao et al. 2003, 2012; Sarafoglou et al. 2011). In particular, 2-methylacetoacetic acid is unstable and can be difficult to detect with routinely used methods for organic acid analysis (Korman 2006; Catanzano et al. 2010).
Patient 1 was initially suspected to have GA I based on elevations of glutaric acid and 3-hydroxyglutaric acid in urine and 3-hydroxyglutaric acid in CSF during her initial presentation. Both enzymatic and genetic testing for this disorder were entirely normal, arguing against even a carrier state. The explanation for these transient elevations is not clear, but could relate to a generalized mitochondrial dysfunction in the context of her severe metabolic decompensation. It is also worth noting that metabolic stroke in GA I typically involves the striatum, which was entirely spared in our first patient.
Neither case was detected through NBS, which has been previously observed in this disorder (Sarafoglou et al. 2011; Estrella et al. 2014). Rather, both patients were diagnosed after an acute decompensation had already occurred, highlighting the need for improved early diagnosis. Beta-ketothiolase deficiency is diagnosed in many NBS programs through demonstration of increased C5OH and C5:1 levels on a dried blood spot specimen. However, the concentrations of these markers may be normal in affected patients. While the incidence of this disorder has been reported at approximately 1 in 1,000,000, this is widely considered to be an underestimate, and significantly more cases are likely to be detected through expanded NBS programs in the United States. Data from two NBS programs suggest an incidence closer to 1 in 200,000–300,000 (Sarafoglou et al. 2011; Frazier et al. 2006; Abdelkreem et al. 2016). The NBS results for patient 1 were scrutinized closely when she presented clinically, and it was noted that the concentration of C5:1 in the two specimens was in the high normal range in both at 0.04 and 0.05 (reference range <0.08), which at the time was attributed to her prematurity. The concentration of C5OH was normal. C4OH, now routinely analyzed and utilized to evaluate the metabolic profile for beta-ketothiolase deficiency, was not part of the analyses when this infant was originally screened. The original screening specimens were retrieved after the diagnosis of beta-ketothiolase deficiency was confirmed and reanalyzed to evaluate these additional markers (Table 1). Although C5:1 was in high range of normal, the metabolic profile was not suggestive of beta-ketothiolase deficiency. The results were also compared and scored using the Post Analytic Interpretative Tools of the CLIR-R4S collaborative database (Marquardt et al. 2012). The score for patient 1 was “0” in both specimens with the interpretation “Score Profile is not informative for BKT.” Notably, the C5:1 concentration in both specimens was below the 1st percentile of C5:1 concentration in confirmed beta-ketothiolase deficiency cases in the database.
Both of the patients that we present developed metabolic stroke, one affecting the globus pallidi and the other the striatum. This variability in the location of metabolic stroke associated with beta-ketothiolase deficiency is consistent with what has been previously described in the literature (Table 2) and is unique amongst organic acidemias since metabolic stroke in these disorders typically shows a striking selective vulnerability for specific structures. Interestingly, patients with 2-methyl-3-hydroxybutyrate dehydrogenase deficiency (HSD10 disease), another disorder of isoleucine metabolism in which elevated 2-methyl 3-hydroxybutyric acid and tiglylglycine are observed, but not 2-methylacetoacetic acid, can present with metabolic stroke affecting the basal ganglia in addition to other abnormalities seen on neuroimaging such as cortical atrophy (Cazorla et al. 2007; Zschocke 2012). The explanation for this variability in metabolic stroke pattern could potentially relate to factors such as central nervous system maturity, modifier genes, or relative accumulation of toxic metabolites. This certainly poses a barrier to MRI pattern recognition of metabolic stroke in this disorder.
Table 2.
Brain imaging findings in beta-ketothiolase deficiency
| Author | Clinical description | Biochemical abnormalities | Enzyme assay | Molecular genetic diagnosis | Neurologic abnormalities | MRI/CT findings |
|---|---|---|---|---|---|---|
| O’Neill et al. (2014) | 5-year-old female presenting with a neurologic decompensation and metabolic acidosis | 2-Methylacetoacetate, 2-methyl-3-hydroxybutyrate, tiglylglycine, abnormal acylcarnitine and tiglylcarnitine in urine | Not performed | Homozygous p.I323V | Abnormal posturing | MRI: initially with T2/FLAIR hyperintensities in bilateral globi pallidi with restricted diffusion, repeated 1 year later with no restricted diffusion seen |
| Buhas et al. (2013) | 17-year-old male presenting with hypotonia and abnormal movements | Elevated 2-methyl-3-hydroxybutyrate and 2-methylacetoacetate in urine and elevated plasma C5:1 acylcarnitine | Reduced activity | p.G125A and p.N158D | Developmental delay and neurologic symptoms | MRI: initially with T2 hyperintensities in the bilateral putamen and cerebral peduncles, repeated several years later with atrophy of putamen and T2 hyperintensity of the posterolateral putamen and hyperintensity of the putamen and caudate nuclei on diffusion-weighted imaging |
| Ozand et al. (1994) | Patient 1: 7-month-old female presenting with ketoacidosis | 2-Methyl-acetoacetate, 2-methyl-3-hydroxybutyrate, and tiglylglycine in urine | Absent activity | Not available | Developmental delay | MRI: T2 hyperintensity in bilateral posterolateral putamen into the lower part of the corona radiata |
| Patient 2: 7-year-old female evaluated due to diagnosis of BKT deficiency in sibling (patient 1) | 2-Methyl-acetoacetate, 2-methyl-3-hydroxybutyrate, and tiglylglycine in urine | Absent activity | Not available | Developmental delay, history of febrile seizure | MRI: lesions in bilateral lentiform nuclei, similar to patient 1 | |
| Patient 3: 8-month-old male presenting with ketoacidosis | 2-Methyl-acetoacetate, 2-methyl-3-hydroxybutyrate, and tiglylglycine in urine | Absent activity | Not available | Developmental delay, febrile seizures, and hypotonia | MRI: lesions in external capsule, similar to patient 1 | |
| Shiasi Arani and Soltani (2014) | 6-month-old male with ketoacidosis | 2-Methyl-3-hydroxybutyrate, and tiglylglycine in urine | Not available | Not available | Developmental regression, abnormal tongue movements, dystonia | MRI: hypomyelination of white matter |
| Akella et al. (2014) | 11-month-old male presenting with ketoacidosis | Elevated C5OH and C5:1 on acylcarnitine profile, elevated 2-methyl-3-hydroxybutyrate, 2-methylacetoacetate, and tiglylglycine in urine | Absent activity | Homozygous p.M193R | Developmental regression, dystonia, seizures | CT: hypodensities in bilateral lentiform nuclei and caudate head, later repeated with basal ganglia calcification seen |
| Yalcinkaya et al. (2001) | 7-year-old male presenting with ketoacidosis | 2-Methyl-3-hydroxybutyrate and tiglylglycine in urine | Decreased activity | Not available | Dystonia | CT: hypodensities in bilateral globi pallidi |
| MRI: T2 hyperintensity and T1 hypointensity in bilateral globi pallidi |
In conclusion, we present two individuals with beta-ketothiolase deficiency, illustrating the wide variability in clinical presentation, imaging, and biochemical studies that make diagnosis challenging. The first patient also highlights the utility of WES in the diagnosis of rare inborn errors of metabolism, particularly when biochemical evaluation is non-contributory or incongruent with the clinical picture. A high index of clinical suspicion in the infant or young child presenting with severe ketoacidosis is required for prompt diagnosis and potentially life-saving medical management.
Synopsis
Beta-ketothiolase deficiency, causing episodic, severe ketoacidosis, can present with metabolic stroke and may be difficult to diagnose biochemically.
Monica H. Wojcik conceptualized this case report, contributed to the writing of the manuscript, and provided clinical care for one of the patients reported.
Klaas J. Wierenga provided clinical care for one of the patients reported and contributed to the writing of the manuscript.
Lance H. Rodan provided clinical care for one of the patients reported and contributed to the writing of the manuscript.
Inderneel Sahai performed the newborn screen analysis for one of the patients reported and critically reviewed the manuscript.
Sacha Ferdinandusse performed the fibroblast enzyme assay for one of the patients reported and critically reviewed the manuscript.
Casie A. Genetti contributed to the whole exome sequencing for one of the patients reported.
Meghan C. Towne contributed to the whole exome sequencing for one of the patients reported.
Roy W.A. Peake aided in the biochemical laboratory interpretation for one of the patients reported and critically reviewed the manuscript.
Philip James provided clinical care for one of the patients reported and critically reviewed the manuscript.
Alan H. Beggs contributed to the whole exome sequencing for one of the patients reported and critically reviewed the manuscript.
Catherine A. Brownstein contributed to the whole exome sequencing for one of the patients reported.
Gerard T. Berry provided clinical care for one of the patients reported and critically reviewed the manuscript.
Pankaj B. Agrawal conceptualized this case report, contributed to the whole exome sequencing for one of the patients reported, and contributed to the writing of the manuscript.
Corresponding Author
Pankaj B. Agrawal, who serves as guarantor for the chapter, accepts full responsibility for the work, had access to the data, and controlled the decision to publish.
Competing Interests
No competing interests are reported by the authors.
Funding
MHW is supported by training grant T32 HD07466 through the National Institutes of Health (NIH). PBA was supported by R01 AR068429 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH and U19 HD077671 from the National Institute of Child Health and Human Development/National Human Genome Research Institute/NIH. The Gene Discovery Core of The Manton Center for Orphan Disease Research, Boston Children’s Hospital also supported the work. Whole exome sequencing was performed by the Yale Center for Mendelian Genomics, supported by NIH grant 2UM1HG006504. Sanger sequencing was performed by the Molecular Genetics Core Facility of the Intellectual and Developmental Disabilities Research Center (IDDRC) at Boston Children’s Hospital, supported by NIH grant U54 HD090255.
Ethics Approval
As these are retrospective case reports, approval by an Institutional Review Board was not required. Patient one and the patient’s parents had whole exome sequencing performed on a research basis through an IRB-approved protocol with the Manton Center for Orphan Disease Research at Boston Children’s Hospital.
Patient Consent Statement
As these are retrospective case reports without identifiable information, patient consent was not obtained.
Contributor Information
Monica H. Wojcik, Email: monica.wojcik@childrens.harvard.edu
Pankaj B. Agrawal, Email: pagrawal@enders.tch.harvard.edu
References
- Abdelkreem E, Otsuka H, Sasai H, et al. Beta-ketothiolase deficiency: resolving challenges in diagnosis. J Inborn Errors Metab Screen. 2016;4:1–9. [Google Scholar]
- Akella RRD, Aoyama C, Mori C, Lingappa L, Cariappa R, Fukao T. Metabolic encephalopathy in beta-ketothiolase deficiency: the first report from India. Brain Dev. 2014;36:537–540. doi: 10.1016/j.braindev.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Buhas D, Bernard G, Fukao T, Decarie JC, Chouinard S, Mitchell GA. A treatable new cause of chorea: beta-ketothiolase deficiency. Mov Disord. 2013;28:1054–1056. doi: 10.1002/mds.25538. [DOI] [PubMed] [Google Scholar]
- Catanzano F, Ombrone D, Di Stefano C, et al. The first case of mitochondrial acetoacetyl-CoA thiolase deficiency identified by expanded newborn metabolic screening in Italy: the importance of an integrated diagnostic approach. J Inherit Metab Dis. 2010;33:91. doi: 10.1007/s10545-009-9028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla MR, Verdu A, Perez-Cerda C, Ribes A. Neuroimage findings in 2-methyl-3-hydroxybutytyl-CoA dehydrogenase deficiency. Pediatr Neurol. 2007;36:264–267. doi: 10.1016/j.pediatrneurol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Daum RS, Lamm PH, Mamer OA, Scriver CR. A “new” disorder of isoleucine catabolism. Lancet. 1971;2:1289–1290. doi: 10.1016/S0140-6736(71)90605-2. [DOI] [PubMed] [Google Scholar]
- Estrella J, Wilcken B, Carpenter K, Bhattacharya K, Tchan M, Wiley V. Expanded newborn screening in New South Wales: missed cases. J Inherit Metab Dis. 2014;37:881–887. doi: 10.1007/s10545-014-9727-2. [DOI] [PubMed] [Google Scholar]
- Frazier DM, Millington DS, McCandless SE, et al. The tandem mass spectrometry newborn screening experience in North Carolina: 1997-2005. J Inherit Metab Dis. 2006;29:76–85. doi: 10.1007/s10545-006-0228-9. [DOI] [PubMed] [Google Scholar]
- Fukao T, Yamaguchi S, Orii T, Osumi T, Hashimoto T. Molecular basis of 3-ketothiolase deficiency: identification of an AG to AC substitution at the splice acceptor site of intron 10 causing exon 11 skipping. Biochim Biophys Acta. 1992;1139:184–188. doi: 10.1016/0925-4439(92)90132-7. [DOI] [PubMed] [Google Scholar]
- Fukao T, Yamaguchi S, Orii T, Schutgens RB, Osumi T, Hashimoto T. Identification of three mutant alleles of the gene for mitochondrial acetoacetyl-coenzyme A thiolase. A complete analysis of two generations of a family with 3-ketothiolase deficiency. J Clin Invest. 1992;89:474–479. doi: 10.1172/JCI115608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Zhang GX, Sakura N, et al. The mitochondrial acetoacetyl-CoA thiolase (T2) deficiency in Japanese patients: urinary organic acid and blood acylcarnitine profiles under stable conditions have subtle abnormalities in T2-deficient patients with some residual T2 activity. J Inherit Metab Dis. 2003;26:423–431. doi: 10.1023/A:1025117226051. [DOI] [PubMed] [Google Scholar]
- Fukao T, Maruyama S, Ohura T, et al. Three Japanese patients with beta-ketothiolase deficiency who share a mutation, C.431A>C (H144P) in ACAT1: subtle abnormality in urinary organic acid analysis and blood acylcarnitine analysis using tandem mass spectrometry. JIMD Reports. 2012;3:107–115. doi: 10.1007/8904_2011_72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014;37:541–551. doi: 10.1007/s10545-014-9704-9. [DOI] [PubMed] [Google Scholar]
- Hillman RE, Keating JP. Beta-ketothiolase deficiency as a cause of the “ketotic hyperglycinemia syndrome”. Pediatrics. 1974;53:221–225. [PubMed] [Google Scholar]
- Korman SH. Inborn errors of isoleucine degradation: a review. Mol Genet Metab. 2006;89:289–299. doi: 10.1016/j.ymgme.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Lek M. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt G, Currier R, McHugh DM, et al. Enhanced interpretation of newborn screening results without analyte cutoff values. Genet Med. 2012;14:648–655. doi: 10.1038/gim.2012.2. [DOI] [PubMed] [Google Scholar]
- Middleton B, Bartlett K. The synthesis and characterisation of 2-methylacetoacetyl coenzyme A and its use in the identification of the site of the defect in 2-methylacetoacetic and 2-methyl-3-hydroxybutyric aciduria. Clin Chim Acta. 1983;128:291–305. doi: 10.1016/0009-8981(83)90329-7. [DOI] [PubMed] [Google Scholar]
- O’Neill ML, Kuo F, Saigal G. MRI of pallidal involvement in beta-ketothiolase deficiency. J Neuroimaging. 2014;24:414–417. doi: 10.1111/j.1552-6569.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- Ozand PT, Rashed M, Gascon GG, et al. 3-Ketothiolase deficiency: a review and four new patients with neurologic symptoms. Brain Dev. 1994;16:38–45. doi: 10.1016/0387-7604(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Sarafoglou K, Matern D, Redlinger-Grosse K, et al. Siblings with mitochondrial acetoacetyl-CoA thiolase deficiency not identified by newborn screening. Pediatrics. 2011;128:e246–e250. doi: 10.1542/peds.2010-3918. [DOI] [PubMed] [Google Scholar]
- Shiasi Arani K, Soltani B. First report of 3-oxothiolase deficiency in Iran. Int J Endocrinol Metab. 2014;12:e10960. doi: 10.5812/ijem.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcinkaya C, Apaydin H, Ozekmekci S, Gibson KM. Delayed-onset dystonia associated with 3-oxothiolase deficiency. Mov Disord. 2001;16:372–375. doi: 10.1002/mds.1060. [DOI] [PubMed] [Google Scholar]
- Zschocke J. HSD10 disease: clinical consequences of mutations in the HSD17B10 gene. J Inherit Metab Dis. 2012;25:81–89. doi: 10.1007/s10545-011-9415-4. [DOI] [PubMed] [Google Scholar]