Abstract
Objective
The automated breast ultrasound system (ABUS) is a potential method for breast cancer detection; however, its diagnostic performance remains unclear. We conducted a hospital-based multicenter diagnostic study to evaluate the clinical performance of the ABUS for breast cancer detection by comparing it to handheld ultrasound (HHUS) and mammography (MG).
Methods
Eligible participants underwent HHUS and ABUS testing; women aged 40–69 years additionally underwent MG. Images were interpreted using the Breast Imaging Reporting and Data System (BI-RADS). Women in the BI-RADS categories 1–2 were considered negative. Women classified as BI-RADS 3 underwent magnetic resonance imaging to distinguish true- and false-negative results. Core aspiration or surgical biopsy was performed in women classified as BI-RADS 4–5, followed by a pathological diagnosis. Kappa values and agreement rates were calculated between ABUS, HHUS and MG.
Results
A total of 1,973 women were included in the final analysis. Of these, 1,353 (68.6%) and 620 (31.4%) were classified as BI-RADS categories 1–3 and 4–5, respectively. In the older age group, the agreement rate and Kappa value between the ABUS and HHUS were 94.0% and 0.860 (P<0.001), respectively; they were 89.2% and 0.735 (P<0.001) between the ABUS and MG, respectively. Regarding consistency between imaging and pathology results, 78.6% of women classified as BI-RADS 4–5 based on the ABUS were diagnosed with precancerous lesions or cancer; which was 7.2% higher than that of women based on HHUS. For BI-RADS 1–2, the false-negative rates of the ABUS and HHUS were almost identical and were much lower than those of MG.
Conclusions
We observed a good diagnostic reliability for the ABUS. Considering its performance for breast cancer detection in women with high-density breasts and its lower operator dependence, the ABUS is a promising option for breast cancer detection in China.
Keywords: Automated breast ultrasound system, breast neoplasms, China
Introduction
The global burden of breast cancer is high and continues to increase, with an estimated 1.67 million new diagnoses and 0.52 million deaths in 2017 (1). Although North America and Europe remain the regions with the highest incidence of breast cancer worldwide, the largest contributors to the global burden are East and South Asia, including countries such as China and India (2,3). In these regions, a high proportion of women present with an advanced stage of the disease, leading to a poor prognosis (3-5).
Early detection and treatment of breast cancer, which can be achieved by the clinical diagnosis of symptomatic breast cancer and by screening asymptomatic women, can reduce mortality and other severe consequences of advanced-staged disease. Substantial evidence from extensive high-quality observational studies and randomized controlled trials (RCTs) has shown that mammography (MG)-based screening can decrease mortality by detecting breast cancer during the early stage (6,7). Although MG is widely used in Western countries for breast cancer screening, its performance declines among young women because of its low sensitivity and the exposure to radiation. More importantly, its performance is lower among women with heterogeneously dense (50%–74% dense tissue) or extremely dense (≥75% dense tissue) breasts. Previous studies reported that having dense breasts is a risk factor for breast cancer (8-10). Chinese women, like other Asian women, typically have smaller and denser breasts than Caucasian women (4). Meanwhile, their average age at the diagnosis of breast cancer is approximately ten years younger than that of Western women (4). Thus, MG may not be the optimal breast cancer screening test for Chinese women.
Recent studies found that handheld ultrasound (HHUS) can detect small and node-negative breast cancer in young women and in those with dense breasts (11). Compared to MG, HHUS is non-invasive, non-radioactive, and inexpensive, making it an attractive method for breast cancer detection. At present, HHUS is regarded as a supplemental tool to MG in the United States and other Western countries. In some Asian countries, such as China and Japan, ultrasound is the primary tool for breast cancer screening in women with denser breasts due to its lower cost, even though evidence showing its effectiveness is inadequate (12,13). The diagnostic accuracy of HHUS heavily depends on the operators’ ability of image acquiring and interpretation, which constrains its broad applications in developing countries.
In recent years, the automated breast ultrasound system (ABUS), an emerging and promising technology for the clinical diagnosis of breast cancer in women, has received increasing attention (14,15). Compared to HHUS, the ABUS depends less on the operator for image selection and his/her ability of image interpretation as it allows radiologists to review the entire dataset and to interpret the images remotely. A study conducted in the United States showed that adding the ABUS to MG screening in women with heterogeneously and extremely dense breasts contributed to better breast cancer detection (16). However, the diagnostic value of the ABUS alone has not been rigorously evaluated so far. Therefore, we designed a multicenter hospital-based study to evaluate the clinical performance of the ABUS for breast cancer detection in Chinese women.
Materials and methods
Study design and sample size calculations
This study was a hospital-based multicenter diagnostic research that evaluated the diagnostic performance of the ABUS for breast cancer detection by comparing it to HHUS and MG in Chinese women.
We estimated the sample sizes to ensure that a sufficient number of women with breast cancer were included to evaluate the performance of the ABUS, HHUS and MG. According to estimated sensitivities from previous studies (17,18), a power of 0.8 and non-inferiority value of 0.06, approximately 60 and 188 breast cancer cases were required for the younger (30–39 years) and older (40–69 years) age groups, respectively. Considering the prevalence of breast cancer in China, this study planned to include 450 women aged 30–39 years and 1,050 women aged 40–69 years.
Given a possibly high variability of breast cancer prevalence across hospitals, we defined specific sample sizes for each Breast Imaging Reporting and Data System (BI-RADS) category (19). According to the American College of Radiology (ACR) BI-RADS distribution, the following specific sample size proportions were allocated to the BI-RADS categories: 40%, 35%, 12.5% and 12.5% for BI-RADS categories 1–2, 3, 4 and 5, respectively. Accordingly, the estimated required sample sizes for BI-RADS 1–2, 3, 4 and 5 were 180, 158, 56 and 56 women, respectively, in the younger age group; in the older age group, they were 420, 368, 131 and 131 women, respectively.
Participants
This study was conducted at five high-level tertiary hospitals in China between February 2016 and March 2017. The hospitals included the Cancer Hospital, Chinese Academy of Medical Sciences in Beijing (BJ), Sun Yat-sen University Cancer Center in Guangzhou (GZ), Tianjin Medical University Cancer Institute and Hospital (TJ), Xin Hua Hospital in Shanghai (SH), and First People’s Hospital of Hangzhou (HZ).
Female outpatients who visited these hospitals with breast-related complaints were proportionally selected and included in this study. We excluded women aged <30 and ≥70 years. We also excluded those who had previously received a diagnosis of or treatment for breast cancer; had undergone surgical or percutaneous breast procedures in the past 12 months; had a history of lumpectomy, contralateral mastectomy, or breast augmentation; and those who were currently pregnant, breastfeeding, or planning to become pregnant.
The study was approved by the institutional review board of the Cancer Institute, Chinese Academy of Medical Sciences (IRB approval number 15-061/988) and the institutional review boards of all participating hospitals. Informed consent was obtained from all study participants.
Flow of the study
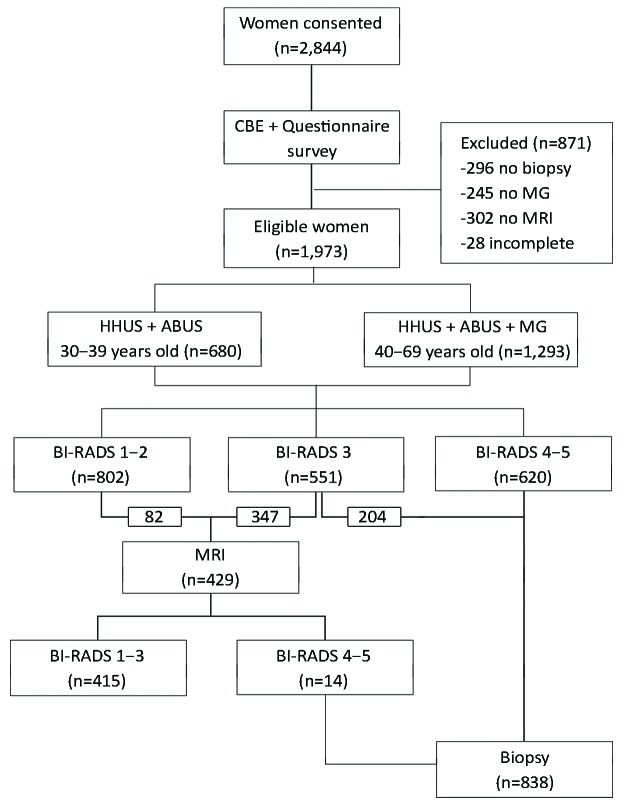
One-on-one questionnaire interviews including items on the participants’ sociodemographic characteristics and potential breast cancer risk factors were conducted by trained healthcare staff. Then, all enrolled participants underwent successive HHUS and ABUS examinations. The women in the older age group also underwent MG; those in the younger age group did not undergo MG due to concerns of radiation (20,21) (Figure 1).
1.
Study flowchart.
According to the ACR BI-RADS classification system, the BI-RADS assessment results for HHUS, ABUS and MG were classified into 6 categories: 0 = incomplete, 1 = normal, 2 = benign, 3 = probably benign, 4 = suspicious, and 5 = highly suggestive of malignancy. In this study, the highest BI-RADS result among HHUS, ABUS and MG was selected as the referral reference for further magnetic resonance imaging (MRI) and/or biopsy testing (e.g., if a woman was classified as BI-RADS categories 1, 2, and 4 by HHUS, ABUS and MG, respectively, she was classified as BI-RADS category 4). Women categorized as BI-RADS 3 underwent MRI or biopsy to distinguish true-negative and false-negative results. For BI-RADS categories 1 and 2, no referral was provided, based on the expectation that 10% of these women were randomly selected for an MRI examination. For women with BI-RADS categories 4 or 5, core aspiration or surgical biopsy was performed, and a pathological diagnosis was made.
We also assessed the MRI-based BI-RADS categories. Women with BI-RADS categories 1–3 in the MRI were considered negative. Those with MRI-based BI-RADS categories 4–5 underwent a biopsy and pathological diagnosis.
ABUS, HHUS and MG
The ABUS (Invenia ABUS, GE Healthcare, Sunnyvale, CA, USA) is a computer-based system for evaluating the complete breast. Each breast was imaged in three views: lateral (LAT), anteroposterior (AP), and medial (MED), with an automated 6–14 MHz linear array transducer attached to a rigid compression plate (covering areas of 15.4 cm × 17.0 cm × 5.0 cm). Each view acquired up to about 300 2D images and reconstructed the breast in the coronal plane, from the skin to the chest wall. The standardized review process involves using a patented thick-slice coronal plane for quick navigation through the breast and the use of the “survey mode”, which is similar to cine and allows the radiologist to rapidly interpret many images. The acquisition time for each view was approximately 60 seconds, with about 3 to 4 min per breast.
According to the clinical routine, HHUS was performed in the supine position by board-certified radiologists who were experienced in breast imaging. The devices used for the examinations included the GE LOGIQ9 (GE Medical Systems, Milwaukee, WI, USA), Aixplorer system (Supersonic Imagine, Aix en Provence, France), iU22 Ultrasound System (Philips Medical Systems, Bothell, WA, USA), and s2000 (Siemens Medical Solutions, Mountain View, CA, USA).
The devices used for obtaining MG images included the GE Sengraphe DS (GE Medical Systems, Milwaukee, WI, USA), Hologic Selenia (Hologic, Bedford, MA, USA), and Fujifilm FDR MS-2500 (Fujifilm Corp, Tokyo, Japan). All procedures strictly followed the clinical routine.
Statistical analysis
Double entry checks using EpiData version 3.1 (EpiData Association, Odense, Denmark) were independently conducted by each of the five hospitals. The Cancer Hospital, Chinese Academy of Medical Sciences was responsible for qualifying and pooling the collected data from the five hospitals into the final dataset. To exclude verification bias, the biopsy results were considered as the gold standard for the diagnostic outcome. Women with no biopsy results who tested negative in all available tests were deemed to be true negatives in this study.
The distributions of the BI-RADS categories and pathology result are shown as specific numbers and proportions. The participants’ sociodemographic characteristics are described using means and standard deviations for continuous variables and percentages for categorical variables. Differences in sociodemographic characteristics by BI-RADS categories (1–3 vs. 4–5) and between malignant and benign findings were compared using the chi-square test for categorical variables and the Student’s t-test for continuous variables. Kappa coefficients test was calculated to compare the diagnostic results among the ABUS, HHUS and MG.
SPSS software (Version 17.0; SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses. Statistical significance was assessed by two-tailed tests with an α level of 0.05.
Results
A total of 2,844 women were recruited, all of whom signed a written informed consent form at 1 of the 5 hospitals. Of these, 296 were excluded because they declined a biopsy. In the older age group, 245 women were excluded because they declined to undergo MG. We also excluded 302 women with BI-RADS category 3 because they refused to undergo MRI or biopsy and 28 women with incomplete information. Therefore, the sample size for the final analysis included 1,973 women (375, 542, 315, 325 and 416 from the BJ, GZ, TJ, SH and HZ, respectively).
Of the 1,973 women that were included in the final analysis, 680 were in the younger age group (30–39 years) and therefore underwent both HHUS and ABUS testing. The remaining 1,293 women were in the older age group (40–69 years); thus, they underwent HHUS, ABUS and MG testing. Based on the BI-RADS results, 429 women underwent further MRI examinations, and 838 women had a biopsy. The distribution of the BI-RADS results confirmed that the sample size of this study had enough power to compare the performances of HHUS, ABUS and MG.
Participant characteristics
The mean age of the 1,973 participants was 45.4±9.7 years. The majority (1,432/1,973; 72.6%) of the women had a normal body mass index (BMI). Of all women, 75.7% (959/1,267) had heterogeneously dense or extremely dense breasts, 71.8% (1,416/1,973) were pre-menopausal, and 81.1% (1,600/1,973) had breastfed. Furthermore, 92.8% (1,831/1,973), 90.5% (1,786/1,973), 97.1% (1,916/1,973), and 90.7% (1,789/1,973) of the women never used oral contraceptives, had no family history of breast cancer, and had no smoking history, and had no history of alcohol consumption, respectively (Table 1).
1.
Demographic, clinical and pathological characteristics at enrollment
| Characteristics | Total (N=1,973) | BI-RADS category | P | Pathology result | P | ||
| Type 1−3
(n=1,353) |
Type 4−5
(n=620) |
Malignant
(n=417) |
Benign
(n=1,556) |
||||
| BMI, body mass index; BI-RADS, Breast Imaging Reporting and Data System. | |||||||
| Age (year) | |||||||
|
45.4±9.7 | 44.3±9.5 | 48.0±9.8 | <0.001 | 49.4±9.7 | 44.4±9.5 | <0.001 |
| 30−39 | 680 (34.5) | 528 (39.0) | 152 (24.5) | <0.001 | 86 (20.6) | 594 (38.2) | <0.001 |
| 40−69 | 1,293 (65.5) | 825 (61.0) | 468 (75.5) | 331 (79.4) | 962 (61.8) | ||
| BMI (kg/m2) | |||||||
|
23.0±3.1 | 22.8±3.0 | 23.4±3.3 | <0.001 | 23.6±3.3 | 22.9±3.0 | <0.001 |
| Under weight
(≤18.49) |
97 (4.9) | 69 (5.1) | 28 (4.5) | 0.029 | 16 (3.8) | 81 (5.2) | 0.023 |
| Normal weight
(18.50−24.99) |
1,432 (72.6) | 1,003 (74.1) | 429 (69.2) | 287 (68.8) | 1,145 (73.6) | ||
| Overweight
(25.00−29.99) |
392 (19.9) | 251 (18.6) | 140 (22.6) | 97 (23.3) | 295 (19.0) | ||
| Obesity (≥30.00) | 52 (2.6) | 30 (2.2) | 23 (3.7) | 17 (4.1) | 35 (2.2) | ||
| Breast density | |||||||
| <25% | 26/1,267 (2.1) | 20/801 (2.5) | 6/466 (1.3) | 0.022 | 5/329 (1.5) | 21/938 (2.2) | <0.001 |
| 25%−50% | 282/1,267 (22.3) | 168/801 (21.0) | 114/466 (24.5) | 96/329 (29.2) | 186/938 (19.8) | ||
| 51%−75% | 731/1,267 (57.7) | 452/801 (56.4) | 278/466 (59.7) | 189/329 (57.4) | 542/938 (57.8) | ||
| >75% | 228/1,267 (18.0) | 161/801 (20.1) | 68/466 (14.6) | 39/329 (11.9) | 189/938 (20.1) | ||
| Menopausal status | |||||||
| Pre-menopausal | 1,416 (71.8) | 1,026 (75.8) | 390 (62.9) | <0.001 | 238 (57.1) | 1,178 (75.7) | <0.001 |
| Menopausal | 557 (28.2) | 327 (24.2) | 230 (37.1) | 179 (42.9) | 378 (24.3) | ||
| Breastfeeding | |||||||
| Yes | 1,600 (81.1) | 1,091 (80.6) | 509 (82.1) | 0.489 | 351 (84.2) | 1,249 (80.3) | 0.109 |
| No | 364 (18.4) | 255 (18.8) | 109 (17.6) | 66 (15.8) | 298 (19.2) | ||
| Unknown | 9 (0.5) | 7 (0.5) | 2 (0.3) | 0 (0) | 9 (0.6) | ||
| Use of oral
contraceptives |
|||||||
| Yes | 137 (6.9) | 93 (6.9) | 44 (7.1) | 0.379 | 29 (7.0) | 108 (6.9) | 0.103 |
| No | 1,831 (92.8) | 1,258 (93.0) | 573 (92.4) | 385 (92.3) | 1,446 (92.9) | ||
| Unknown | 5 (0.3) | 2 (0.1) | 3 (0.5) | 3 (0.7) | 2 (0.1) | ||
| Family history
of breast cancer |
|||||||
| Yes | 182 (9.2) | 125 (9.2) | 57 (9.2) | 0.387 | 41 (9.8) | 141 (9.1) | 0.517 |
| No | 1,786 (90.5) | 1,226 (90.6) | 560 (90.3) | 374 (89.7) | 1,412 (90.7) | ||
| Unknown | 5 (0.3) | 2 (0.1) | 3 (0.5) | 2 (0.5) | 3 (0.2) | ||
| Smoking history | |||||||
| Never | 1,916 (97.1) | 1,321 (97.6) | 595 (96.0) | 0.080 | 399 (95.7) | 1,517 (97.5) | 0.128 |
| Used | 31 (1.6) | 19 (1.4) | 12 (1.9) | 9 (2.2) | 22 (1.4) | ||
| Current | 26 (1.3) | 13 (1.0) | 13 (2.1) | 9 (2.2) | 17 (1.1) | ||
| Alcohol drinking history | |||||||
| Never | 1,789 (90.7) | 1,230 (90.9) | 559 (90.2) | 0.212 | 371 (89.0) | 1,418 (91.1) | 0.195 |
| Used | 132 (6.7) | 93 (6.9) | 39 (6.3) | 30 (7.2) | 102 (6.6) | ||
| Current | 52 (2.6) | 30 (2.2) | 22 (3.5) | 16 (3.8) | 36 (2.3) | ||
A total of 1,353 women (68.6%) were classified as BI-RADS categories 1, 2, or 3; they had a mean age of 44.3±9.5 years. In contrast, 620 participants (31.4%) were defined as BI-RADS categories 4 or 5; they had a mean age of 48.0±9.8 years. A total of 417 women (21.1%) women were diagnosed with a malignancy, whereas the remaining 1,556 women (78.9%) women had benign test results (Table 1).
We then analyzed the sociodemographic and clinical characteristics of the participants by BI-RADS group (BI-RADS 1–3 vs. BI-RADS 4–5). The proportions of women with overweight and obesity were significantly higher in the BI-RADS 4–5 group than in the BI-RADS 1–3 group (P=0.029). We also detected differences for breast density (P=0.022) and menopausal status (P<0.001) between the two groups. However, we found no differences for breastfeeding, the use of oral contraceptives, a family history of breast cancer, smoking history, and a history of alcohol consumption (Table 1).
Then, the participants’ characteristics were compared by malignant and benign findings. The proportions of women with overweight and obesity (P=0.023), dense breasts (P<0.001), and those who were menopausal (P<0.001) were different among women with malignant findings and in those with benign findings. Again, we found no differences for breastfeeding, the use of oral contraceptives, a family history of breast cancer, smoking history, and a history of alcohol consumption between the two groups (Table 1).
Agreement rates
Table 2, 3 , 4 depict the agreement rates between HHUS, ABUS and MG for the older age group. The agreement rate and Kappa value between the ABUS and HHUS were 94.0% and 0.860 (P<0.001), respectively (Table 2). Between the ABUS and MG, they were 89.2% and 0.735 (P<0.001), respectively (Table 3). Last, the agreement rate and Kappa value between HHUS and MG were 89.6% and 0.752 (P<0.001), respectively (Table 4).
2.
Agreement rate between HHUS and ABUS in women aged 40–69 years
| ABUS | HHUS | Total | Agreement
rate (%) |
κ | P | |
| Positive | Negative | |||||
| HHUS, handheld ultrasound; ABUS, automated breast ultrasound system; Positive, BI-RADS 4 or BI-RADS 5; Negative, BI-RADS 1, BI-RADS 2 or BI-RADS 3. | ||||||
| Positive | 356 | 19 | 375 | 94.0 | 0.860 | <0.001 |
| Negative | 58 | 860 | 918 | |||
| Total | 414 | 879 | 1,293 | |||
3.
Agreement rate between ABUS and MG in women aged 40–69 years
| ABUS | MG | Total | Agreement
rate (%) |
κ | P | |
| Positive | Negative | |||||
| ABUS, automated breast ultrasound system; MG, mammography; Positive, BI-RADS 4 or BI-RADS 5; Negative, BI-RADS 1, BI-RADS 2 or BI-RADS 3. | ||||||
| Positive | 299 | 76 | 375 | 89.2 | 0.735 | <0.001 |
| Negative | 64 | 854 | 918 | |||
| Total | 363 | 930 | 1,293 | |||
4.
Agreement rate between HHUS and MG in women aged 40–69 years
| HHUS | MG | Total | Agreement
rate (%) |
κ | P | |
| Positive | Negative | |||||
| HHUS, handheld ultrasound; MG, mammography; Positive, BI-RADS 4 or BI-RADS 5; Negative, BI-RADS 1, BI-RADS 2 or BI-RADS 3. | ||||||
| Positive | 321 | 93 | 414 | 89.6 | 0.752 | <0.001 |
| Negative | 42 | 837 | 879 | |||
| Total | 363 | 930 | 1,293 | |||
Pathology results
Almost half of the pathology results were precancerous lesions or cancer in this study. Among them, non-special type invasive carcinoma (NTIC) was the dominant pathologic subtype (316/417; 75.8%), followed by special-type invasive carcinoma (STIC; 40/417; 9.6%), ductal carcinoma in situ (DCIS; 38/417; 9.1%), and atypical ductal hyperplasia (ADH; 9/417; 2.2%). Among the subtypes of benign lesions, fibroadenoma (FA) accounted for the highest proportion of diagnoses (218/421; 51.8%) (Table 5).
5.
Joint distribution of pathology results and imaging diagnostic results
| Method | Benign lesions [n (%)] | Precancerous lesions or cancer [n (%)] | ||||||||||
| DH | AD | FA | Others | Total | DCIS | ADH | NTIC | STIC | Others | Total | ||
| HHUS, handheld ultrasound; ABUS, automated breast ultrasound system; MG, mammography; DH, ductal hyperplasia; AD, adenosis; FA, fibroadenoma; DCIS, ductal carcinoma in situ; ADH, atypical ductal hyperplasia; NTIC, non-special type invasive carcinoma; STIC, special-type invasive carcinoma. | ||||||||||||
| HHUS | ||||||||||||
| No. 1,2 | 1 (4.0) | 9 (36.0) | 6 (24.0) | 5 (20.0) | 21 (84.0) | 1 (4.0) | 1 (4.0) | 1 (4.0) | 0 (0) | 1 (4.0) | 4 (16.0) | |
| No. 3 | 11 (4.2) | 48 (18.5) | 160 (61.5) | 23 (8.8) | 242 (93.1) | 7 (2.7) | 5 (1.9) | 5 (1.9) | 0 (0) | 1 (0.4) | 18 (6.9) | |
| No. 4,5 | 7 (1.3) | 50 (9.0) | 52 (9.4) | 49 (8.9) | 158 (28.6) | 30 (5.4) | 3 (0.5) | 310 (56.1) | 40 (7.2) | 12 (2.2) | 395 (71.4) | |
| Total | 19 | 107 | 218 | 77 | 421 | 38 | 9 | 316 | 40 | 14 | 417 | |
| ABUS | ||||||||||||
| No. 1,2 | 2 (4.3) | 19 (40.4) | 11 (23.4) | 7 (14.9) | 39 (83.0) | 2 (4.3) | 1 (2.1) | 5 (10.6) | 0 (0) | 0 (0) | 8 (17.0) | |
| No. 3 | 14 (4.6) | 59 (19.4) | 170 (55.9) | 35 (11.5) | 278 (91.4) | 4 (1.3) | 4 (1.3) | 12 (3.9) | 3 (1.0) | 3 (1.0) | 26 (8.6) | |
| No. 4,5 | 3 (0.6) | 29 (6.0) | 37 (7.6) | 35 (7.2) | 104 (21.4) | 32 (6.6) | 4 (0.8) | 299 (61.4) | 37 (7.6) | 11 (2.3) | 383 (78.6) | |
| Total | 19 | 107 | 218 | 77 | 421 | 38 | 9 | 316 | 40 | 14 | 417 | |
| MG | ||||||||||||
| No. 1,2 | 6 (5.5) | 28 (25.7) | 34 (31.2) | 11 (10.1) | 79 (72.5) | 3 (2.8) | 2 (1.8) | 17 (15.6) | 4 (3.7) | 4 (3.7) | 30 (27.5) | |
| No. 3 | 6 (5.4) | 21 (18.8) | 58 (51.8) | 11 (9.8) | 96 (85.7) | 2 (1.8) | 2 (1.8) | 9 (8.0) | 0 (0) | 3 (2.7) | 16 (14.3) | |
| No. 4,5 | 3 (0.8) | 21 (5.8) | 26 (7.2) | 26 (7.2) | 76 (21.1) | 21 (5.8) | 1 (0.3) | 229 (63.4) | 28 (7.8) | 6 (1.7) | 285 (78.9) | |
| Total | 15 | 70 | 118 | 48 | 251 | 26 | 5 | 255 | 32 | 13 | 331 | |
Table 5 shows the joint distribution of pathology and diagnostic imaging results, providing essential information on the consistency between testing and pathology results (the gold standard of the diagnostic outcome). For HHUS, 84.0% (21/25) and 93.1% (242/260) of cases classified as BI-RADS 1–2 and BI-RADS-3, respectively, were benign lesions based on pathology. For BI-RADS 4–5, 71.4% (395/553) of the cases had precancerous lesions or cancer. For ABUS, 83.0% (39/47) and 91.4% (278/304) of cases identified as BI-RADS 1–2 and BI-RADS-3, respectively, were benign lesions. For BI-RADS 4–5, 78.6% (383/487) of the cases had precancerous lesions or cancer. For MG, 72.5% (79/109) and 85.7% (96/112) of cases categorized as BI-RADS 1–2 and BI-RADS 3, respectively, were benign lesions. For BI-RADS 4–5, 78.9% (285/361) of the women had precancerous lesions or cancer (Table 5).
Discussion
This study, to the best of our knowledge, is the first multicenter hospital-based study to evaluate the clinical performance of the ABUS for breast cancer detection by comparing it to HHUS and MG among Chinese women.
MG has been widely used for the early detection of breast cancer worldwide. However, MG has some limitations, including exposure to radiation and a relatively high rate of false-positive results (22-24). More importantly, its performance is worse in young women and in women with heterogeneously dense or extremely dense breasts, which are highly prevalent among women from Asian countries such as Malaysia, Korea, and China (25-27). Therefore, researchers in these countries have been searching for new methods to substitute MG.
HHUS is the primary tool for breast cancer screening in some Asian countries; however, little evidence has shown its effectiveness and accuracy (13). Moreover, HHUS is operator-dependent and has poor repeatability, constraining its use in developing countries. Recently, researchers have been paying considerable attention to the ABUS because of its good performance in detecting breast cancer in women with high-density breasts and because it is less dependent on the operator than HHUS. These features make the ABUS a promising breast cancer screening tool for women in Asian countries.
The results of this study show that the ABUS had a good diagnostic performance. In the comparisons between the ABUS and HHUS, we found that the results of the ABUS were more in line with the pathology results in the BI-RADS 4–5 groups. Specifically, 78.6% of women who were classified as BI-RADS 4 or 5 based on the ABUS were diagnosed with precancerous lesions or cancer; this was 7.2% higher than the proportion of women who were classified in the same BI-RADS categories based on HHUS. For the BI-RADS 1–2 group, the false-negative rate of the ABUS was almost identical to that of HHUS. The heterogeneity in the operator skills across the different hospitals of this study might have been the main reason for the inferior performance of HHUS.
The false-negative rate of the ABUS was much lower than that of MG (17.0% vs. 27.5%, respectively), while the detection rates of the ABUS and MG were similar (78.6% vs. 78.9%, respectively). Moreover, the sensitivity and specificity of ABUS were higher than that of MG; ABUS had less false positive findings (high specificity) with a slightly decease in sensitivity. These findings imply that the ABUS is a promising method for breast cancer detection among women with breast-related complaints. Further studies should determine whether the ABUS can be used in large-scale population-based screening programs in China and other Asian countries.
In this study, we were also able to identify the risk factors for breast cancer in Chinese women. A higher BMI and post-menopausal status were associated with higher BI-RADS scores and malignant findings, while a smoking history and a history of alcohol consumption were not correlated to higher BI-RADS scores or malignant findings. These results are consistent with previous studies on the risk factors of breast cancer among Chinese women (4,28,29) and in agreement with relevant studies from other Asian countries (30,31). Interestingly, breastfeeding, a family history of breast cancer, and the use of oral contraceptives were not associated with the BI-RADS category in this study; this is inconsistent with previous findings (29-31). The reasons for these differences remain obscure. They might be attributed to the limited sample size of this study or selection bias.
The findings of this study need to be considered in light of its limitations. First, selection bias is possible because the participants were all recruited at high-level hospitals located in well-developed regions in China, which might not be representative of all hospitals across China. Second, 871 women were excluded from this study for different reasons. We cannot do a test to determine whether or not these excluded women have significant difference with women recruited in this study. However, we predefined the proportions of sample sizes according to BI-RADS categories to avoid disproportionate enrollment. We recruited another woman who has the same BI-RADS classification given that one woman did not comply with the study procedure. Hence, we believe that non-compliance is likely to have limited effects on the results of this study. Last, data quality depends on the experience of the radiologists, pathologists, and healthcare staff who interviewed the participants.
Conclusions
We observed a good reliability for the ABUS when compared to HHUS and MG in our analysis. Considering its reliable performance for detecting breast cancer among women with high-density breasts, its lower operator dependence, and its feasibility (radiologists only require training for a short period to become proficient in lesion interpretation), the ABUS is a promising diagnostic method for detecting breast cancer among large populations in Asian and developing countries such as China.
Acknowledgements
This work was supported by GE Healthcare (No. CH-EPI-027). The opinions expressed in this paper are those of the authors and do not necessarily represent those of GE Healthcare. The authors wish to thank the hospitals involved in this study and the women who participated in this study. The authors assume full responsibility for database creation, analyses and interpretations of the data.
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
References
- 1.Ervik M, Lam F, Ferlay J, et al. Cancer Today. Lyon: International Agency of Research on Cancer, 2017. Available online: http://gco.iarc.fr/today
- 2.Chen W, Zheng R, Zhang S, et al Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29:1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res 2017;29:1-10. <DOI: 10.21147/j.issn.1000-9604.2017.01.01> <PMID: 28373748>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sankaranarayanan R, Swaminathan R, Brenner H, et al Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11:165–73. doi: 10.1016/S1470-2045(09)70335-3. [Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol 2010;11:165-73. <DOI: 10.1016/S1470-2045(09)70335-3> <PMID: 20005175>] [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhang BN, Fan JH, et al A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011;11:364. doi: 10.1186/1471-2407-11-364. [Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011;11:364. <DOI: 10.1186/1471-2407-11-364> <PMID: 21859480>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan L, Strasser-Weippl K, Li JJ, et al Breast cancer in China. Lancet Oncol. 2014;15:e279–89. doi: 10.1016/S1470-2045(13)70567-9. [Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. <DOI: 10.1016/S1470-2045(13)70567-9> <PMID: 24872111>] [DOI] [PubMed] [Google Scholar]
- 6.Nelson HD, Tyne K, Naik A, et al Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–37, W237-42. doi: 10.7326/0003-4819-151-10-200911170-00009. [Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151:727-37, W237-42. <DOI: 10.7326/0003-4819-151-10-200911170-00009> <PMID: 19920273>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding C, Pompei F, Burmistrov D, et al Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. 2015;175:1483–9. doi: 10.1001/jamainternmed.2015.3043. [Harding C, Pompei F, Burmistrov D, et al. Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med 2015;175:1483-9. <DOI: 10.1001/jamainternmed.2015.3043> <PMID: 26147578>] [DOI] [PubMed] [Google Scholar]
- 8.Pace LE, Keating NL A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311:1327–35. doi: 10.1001/jama.2014.1398. [Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014;311:1327-35. <DOI: 10.1001/jama.2014.1398> <PMID: 24691608>] [DOI] [PubMed] [Google Scholar]
- 9.Yaghjyan L, Colditz GA, Rosner B, et al Mammographic breast density and breast cancer risk: interactions of percent density, absolute dense, and non-dense areas with breast cancer risk factors. Breast Cancer Res Treat. 2015;150:181–9. doi: 10.1007/s10549-015-3286-6. [Yaghjyan L, Colditz GA, Rosner B, et al. Mammographic breast density and breast cancer risk: interactions of percent density, absolute dense, and non-dense areas with breast cancer risk factors. Breast Cancer Res Treat 2015;150:181-9. <DOI: 10.1007/s10549-015-3286-6> <PMID: 25677739>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd NF, Guo H, Martin LJ, et al Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356:227-36. <DOI: 10.1056/NEJMoa062790> <PMID: 17229950>] [DOI] [PubMed] [Google Scholar]
- 11.Lee WK, Chung J, Cha ES, et al Digital breast tomosynthesis and breast ultrasound: Additional roles in dense breasts with category 0 at conventional digital mammography. Eur J Radiol. 2016;85:291–6. doi: 10.1016/j.ejrad.2015.09.026. [Lee WK, Chung J, Cha ES, et al. Digital breast tomosynthesis and breast ultrasound: Additional roles in dense breasts with category 0 at conventional digital mammography. Eur J Radiol 2016;85:291-6. <DOI: 10.1016/j.ejrad.2015.09.026> <PMID: 26499000>] [DOI] [PubMed] [Google Scholar]
- 12.Berg WA, Bandos AI, Mendelson EB, et al Ultrasound as the primary screening test for breast cancer: Analysis from ACRIN 6666. J Natl Cancer Inst. 2015;108:djv367. doi: 10.1093/jnci/djv367. [Berg WA, Bandos AI, Mendelson EB, et al. Ultrasound as the primary screening test for breast cancer: Analysis from ACRIN 6666. J Natl Cancer Inst 2015;108:djv367. <DOI: 10.1093/jnci/djv367> <PMID: 26712110>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauby-Secretan B, Scoccianti C, Loomis D, et al Breast-cancer screening — viewpoint of the IARC Working Group. N Engl J Med. 2015;372:2353–8. doi: 10.1056/NEJMsr1504363. [Lauby-Secretan B, Scoccianti C, Loomis D, et al. Breast-cancer screening — viewpoint of the IARC Working Group. N Engl J Med 2015;372:2353-8. <DOI: 10.1056/NEJMsr1504363> <PMID: 26039523>] [DOI] [PubMed] [Google Scholar]
- 14.Chang JM, Cha JH, Park JS, et al Automated breast ultrasound system (ABUS): reproducibility of mass localization, size measurement, and characterization on serial examinations. Acta Radiol. 2015;56:1163–70. doi: 10.1177/0284185114551565. [Chang JM, Cha JH, Park JS, et al. Automated breast ultrasound system (ABUS): reproducibility of mass localization, size measurement, and characterization on serial examinations. Acta Radiol 2015;56:1163-70. <DOI: 10.1177/0284185114551565> <PMID: 25280476>] [DOI] [PubMed] [Google Scholar]
- 15.Drukker K, Sennett CA, Giger ML Computerized detection of breast cancer on automated breast ultrasound imaging of women with dense breasts. Med Phys. 2014;41:012901. doi: 10.1118/1.4837196. [Drukker K, Sennett CA, Giger ML. Computerized detection of breast cancer on automated breast ultrasound imaging of women with dense breasts. Med Phys 2014;41:012901. <DOI: 10.1118/1.4837196> <PMID: 24387528>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilczek B, Wilczek HE, Rasouliyan L, et al Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol. 2016;85:1554–63. doi: 10.1016/j.ejrad.2016.06.004. [Wilczek B, Wilczek HE, Rasouliyan L, et al. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur J Radiol 2016;85:1554-63. <DOI: 10.1016/j.ejrad.2016.06.004> <PMID: 27501888>] [DOI] [PubMed] [Google Scholar]
- 17.Brem RF, Tabár L, Duffy SW, et al Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology. 2015;274:663–73. doi: 10.1148/radiol.14132832. [Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight Study. Radiology 2015;274:663-73. <DOI: 10.1148/radiol.14132832> <PMID: 25329763>] [DOI] [PubMed] [Google Scholar]
- 18.Mehnati P, Tirtash MJ Comparative efficacy of four imaging instruments for breast cancer screening. Asian Pac J Cancer Prev. 2015;16:6177–86. doi: 10.7314/apjcp.2015.16.15.6177. [Mehnati P, Tirtash MJ. Comparative efficacy of four imaging instruments for breast cancer screening. Asian Pac J Cancer Prev 2015;16:6177-86. <PMID: 26434814>] [DOI] [PubMed] [Google Scholar]
- 19.D’Orsi CJ, Bassett LW, Berg WA, et al. Breast imaging reporting and data systems, BI-RADS: mammography. 4th edition. Reston: American College of Radiology, 2003.
- 20.Miglioretti DL, Lange J, van den Broek JJ, et al Radiation-induced breast cancer incidence and mortality from digital mammography screening: A modeling study. Ann Intern Med. 2016;164:205–14. doi: 10.7326/M15-1241. [Miglioretti DL, Lange J, van den Broek JJ, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening: A modeling study. Ann Intern Med 2016;164:205-14. <DOI: 10.7326/M15-1241> <PMID: 26756460>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yahalom J Evidence-based breast cancer screening guidelines for women who received chest irradiation at a young age. J Clin Oncol. 2013;31:2240–2. doi: 10.1200/JCO.2013.48.7652. [Yahalom J. Evidence-based breast cancer screening guidelines for women who received chest irradiation at a young age. J Clin Oncol 2013;31:2240-2. <DOI: 10.1200/JCO.2013.48.7652> <PMID: 23610108>] [DOI] [PubMed] [Google Scholar]
- 22.Jacklyn G, Howard K, Irwig L, et al Impact of extending screening mammography to older women Information to support informed choices. Int J Cancer. 2017;141:1540–50. doi: 10.1002/ijc.30858. [Jacklyn G, Howard K, Irwig L, et al. Impact of extending screening mammography to older women Information to support informed choices. Int J Cancer 2017;141:1540-50. <DOI: 10.1002/ijc.30858> <PMID: 28662267>] [DOI] [PubMed] [Google Scholar]
- 23.Elmore JG, Barton MB, Moceri VM, et al Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338:1089–96. doi: 10.1056/NEJM199804163381601. [Elmore JG, Barton MB, Moceri VM, et al. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med 1998;338:1089-96. <DOI: 10.1056/NEJM199804163381601> <PMID: 9545356>] [DOI] [PubMed] [Google Scholar]
- 24.Nelson HD, Pappas M, Cantor A, et al Harms of breast cancer screening: Systematic review to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164:256–67. doi: 10.7326/M15-0970. [Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: Systematic review to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 2016;164:256-67. <DOI: 10.7326/M15-0970> <PMID: 26756737>] [DOI] [PubMed] [Google Scholar]
- 25.Zulfiqar M, Rohazly I, Rahmah M Do the majority of Malaysian women have dense breasts on mammogram? Biomed Imaging Interv J. 2011;7:e14. doi: 10.2349/biij.7.2.e14. [Zulfiqar M, Rohazly I, Rahmah M. Do the majority of Malaysian women have dense breasts on mammogram? Biomed Imaging Interv J 2011;7:e14. <DOI: 10.2349/biij.7.2.e14> <PMID: 22291859>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae JM, Shin SY, Kim EH, et al Distribution of dense breasts using screening mammography in Korean women: a retrospective observational study. Epidemiol Health. 2014;36:e2014027. doi: 10.4178/epih/e2014027. [Bae JM, Shin SY, Kim EH, et al. Distribution of dense breasts using screening mammography in Korean women: a retrospective observational study. Epidemiol Health 2014;36:e2014027. <DOI: 10.4178/epih/e2014027> <PMID: 25381996>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai H, Yan Y, Wang P, et al Distribution of mammographic density and its influential factors among Chinese women. Int J Epidemiol. 2014;43:1240–51. doi: 10.1093/ije/dyu042. [Dai H, Yan Y, Wang P, et al. Distribution of mammographic density and its influential factors among Chinese women. Int J Epidemiol 2014;43:1240-51. <DOI: 10.1093/ije/dyu042> <PMID: 24639441>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Li JY, Fan JH, et al Risk factors for breast cancer among Chinese women: a 10-year nationwide multicenter cross-sectional study. J Epidemiol. 2014;24:67–76. doi: 10.2188/jea.JE20120217. [Lee H, Li JY, Fan JH, et al. Risk factors for breast cancer among Chinese women: a 10-year nationwide multicenter cross-sectional study. J Epidemiol 2014;24:67-76. <DOI: 10.2188/jea.JE20120217> <PMID: 24270059>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Ji J, Wang JB, et al Attributable causes of breast cancer and ovarian cancer in China: reproductive factors, oral contraceptives and hormone replacement therapy. Chin J Cancer Res. 2012;24:9–17. doi: 10.1007/s11670-012-0009-y. [Li L, Ji J, Wang JB, et al. Attributable causes of breast cancer and ovarian cancer in China: reproductive factors, oral contraceptives and hormone replacement therapy. Chin J Cancer Res 2012;24:9-17. <DOI: 10.1007/s11670-012-0009-y> <PMID: 23359757>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang EJ, Seo JH, Kim LY, et al Pregnancy-associated risk factors of postpartum breast cancer in Korea: a nationwide health insurance database study. PLoS One. 2016;11:e0168469. doi: 10.1371/journal.pone.0168469. [Kang EJ, Seo JH, Kim LY, et al. Pregnancy-associated risk factors of postpartum breast cancer in Korea: a nationwide health insurance database study. PLoS One 2016;11:e0168469. <DOI: 10.1371/journal.pone.0168469> <PMID: 27977789>] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada K, Nagata C, Tamakoshi A, et al Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol. 2014;25:519–24. doi: 10.1093/annonc/mdt542. [Wada K, Nagata C, Tamakoshi A, et al. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol 2014;25:519-24. <DOI: 10.1093/annonc/mdt542> <PMID: 24412821>] [DOI] [PubMed] [Google Scholar]