Abstract
Campylobacter jejuni is a leading cause of foodborne illness worldwide, mainly due to consumption and handling of contaminated raw chicken. Rapid detection methods for C. jejuni are vital for monitoring contamination levels in chicken products and reducing human Campylobacteriosis cases. The ‘gold standard’ culture-based method of Campylobacter detection takes 3–5 days and is too slow to permit effective intervention. Immuno-based methods are faster, but usually necessitate use of animals or hybridoma technology to produce antibodies; making them difficult and expensive to produce. Here, we report the generation and characterization of recombinant single-chain variable fragment (scFv) antibodies specific for C. jejuni cells, and evaluation of one scFv antibody for an immunomagnetic separation-quantitative PCR (IMS-qPCR) method to rapidly, sensitively, and specifically detect low numbers of C. jejuni. An scFv antibody phage-display library was constructed using spleen mRNA derived from a rabbit immunized with gamma-irradiated C. jejuni cells. This library was screened by surface biopanning against C. jejuni whole cells. Enriched clones were analyzed by enzyme-linked immunosorbent assay (ELISA). Two scFv antibodies that strongly and specifically recognized C. jejuni cell were expressed in Escherichia coli. Western blot analysis showed that one antibody, scFv80, was expressed as a soluble protein and retained its specific and strong binding to C. jejuni cells. This recombinant monoclonal scFv antibody was purified and used to covalently coat paramagnetic beads to be used for IMS-qPCR. The IMS-qPCR method was able to specifically and sensitively detect C. jejuni in mixed cultures within 3 h.
Electronic supplementary material
The online version of this article (10.1007/s00253-018-8949-x) contains supplementary material, which is available to authorized users.
Keywords: Campylobacter jejuni, Recombinant scFv antibody, Immunomagnetic separation (IMS), Detection specificity, Phage display
Introduction
Campylobacter, especially C. jejuni, is one of the leading causes of bacterial foodborne illnesses worldwide (Silva et al. 2011; Tram et al. 2012). In the industrialized countries, Campylobacter spp. are the most common cause of foodborne illness in humans, ahead of Salmonella (Food Standards Agency 2009; Dominguez 2012). Data on Campylobacteriosis from developing countries is scarce, but there is a suggestion that the human burden due to Campylobacter infection is considerable (World Health Organization 2012; Platts-Mills and Kosek 2014).
Mishandling of contaminated raw chicken and consumption of undercooked poultry have been incriminated as the most significant source of C. jejuni infections for humans (Tam et al. 2009; Sheppard et al. 2009; Dominguez 2012). Campylobacter is a harmless commensal intestinal inhabitant of chicken (Doyle 1994), which makes it difficult to spot early infection of Campylobacter in a flock. It spreads quickly and 95% of a flock of 20,000 chickens can be infected by Campylobacter within 4 to 7 days after colonization of the first bird (Van Gerwe et al. 2009). Cross contamination of broiler carcasses can also occur in the slaughterhouse (Hermani et al. 2003; Tram et al. 2012). A significant correlation between the contamination of the broilers during rearing and the carcasses after processing has been observed (Hermani et al. 2003).
There is a need to monitor Campylobacter during the life of the chickens in broiler houses, to spot the infection as early as possible and to determine effectiveness of interventions. In the slaughter house, Campylobacter infection status needs to be checked in order to schedule flocks for slaughtering and prevent cross contamination (Tram et al. 2012). However, such monitoring has been hampered by the current ‘gold standard’ culture-based detection method, ISO/TS/EN 10272-2:2006 (International Standards Organization 2006). This method generally require 24–48 h enrichment culture before isolation of C. jejuni on selective culture media. Enrichment is followed by confirmatory methods such as biochemical and serological tests (Goossens and Butzler 1992), DNA/RNA hybridizations (Cudjoe et al. 1991; Ransom et al. 1994), enzyme-linked immunosorbent (ELISA) assay (Haas 1997), randomly amplified polymorphic DNA (RAPD) analysis (Mazurier et al. 1992; Hilton et al. 1997), polymerase chain reaction (PCR) or quantitative PCR (qPCR) assays (Giesendorf et al. 1992; Sails et al. 2003), and loop-mediated isothermal amplification (LAMP) of DNA (Yamazaki et al. 2009). Combination of culture enrichment procedure with these confirmatory methods leads to 3–5 day detection time, which is too long, bearing in mind the 4–7 day flock infection rate. By the time the results become available, the status of the flock may have changed, which may result in a false negative flock status. Another limitation of culture enrichment-dependent methods is their inability to detect viable but non-culturable (VBNC) Campylobacter cells that may be able to regenerate and become infectious again (Rollins and Colwell 1986; Silva et al. 2011). Direct detection of Campylobacter by PCR and qPCR methods without culture enrichment has been published (Rasmussen et al. 1996; Bang et al. 2001; Vondrakova et al. 2014). However, sensitive, specific, and rapid PCR detection can be hampered by inhibitors present in the crude sample matrix leading to false negative results (Tram et al. 2012). The other limitation of all the described culture-based methods is that only a very small portion (10–500 μl) of the considerable volume of biological samples (50–250 ml) is actually tested; this may also lead to false negative results, given that the abundance of Campylobacter spp. in biological samples may be low. Detection sensitivity of tests for Campylobacter spp. could be substantially improved if most of the Campylobacter cells in the large initial volume of sample could be concentrated into a small volume prior to testing.
To by-pass culture enrichment, immunomagnetic separation (IMS) methods have previously been developed using polyclonal (pAb) or monoclonal (mAb) antibodies to capture Campylobacter cells from milk, chicken meat, and chicken feces (Docherty et al. 1996; Lamoureux et al. 1997; Yu et al. 2001; Tram et al. 2012). IMS concentrates the target bacterial cells, thus reducing the sample volume, and removes sample constituents that may interfere with subsequent PCR; hence, the sensitivity of the assay is increased. However, production of appropriate antibodies is often time consuming, laborious, and expensive. pAbs can be only generated in limited amounts, dependent on animal size. The advent of hybridoma technology ushered in production of mAbs with defined antigen specificity and enables long-term antibody supply (Köhler and Milstein 1975; Lipman et al. 2005). However, the conventional hybridoma approach, which requires animal cell culture, is every expensive (Nelson et al. 2000). The clones produced also tend to lose antibody-secreting ability over time (Singh et al. 2010), limiting continuous supply.
Recombinant DNA technology has made it possible to generate monoclonal single-chain variable fragment (scFv) antibodies as recombinant proteins in Escherichia coli (Skerra and Pluckthun 1988). The scFv antibodies are a minimal form of functional antibodies which preserve the binding specificity of the parental IgG antibodies (Hagemeyer et al. 2009). A large repertoire of diverse scFv-libraries is constructed from RNA isolated from spleenocytes or the bone marrow of a host previously immunized with an antigen (Barbas et al. 2001). cDNA fragments coding variable heavy (VH) and variable light (VL) chains are amplified from the RNA by RT-PCR and randomly joined by overlap PCR. The resulting full-length scFv cDNA fragments are then cloned into a suitable expression vector to express the scFv antibodies. Their small size makes cloning into E. coli expression vectors easy, resulting in the ability to produce large quantities of monoclonal scFv antibodies quickly in bacterial cultures at a relatively low cost. The cloned fragments can be genetically engineered, if desired, e.g., to further optimize specificity and affinity (Hagemeyer et al. 2009).
Phage display is the most efficient system of expressing and screening recombinant scFv antibodies (Barbas et al. 2001). To this end, the full-length scFv cDNA fragments are cloned into phagemid vectors and introduced into E. coli cells to produce phage particles. The resultant display of the scFv antibody on the surface of the bacteriophage particle has been utilized as a means for effective screening for monoclonal scFv antibodies of desired specificity by phage biopanning (Barbas et al. 2001). Biopanning of scFv phage display libraries has been used to provide scFv antibodies against many antigens including foodborne contaminants Listeria monocytogenes (Paoli et al. 2007), staphylococcal enterotoxin B (SEB) (Singh et al. 2010), Salmonella Typhimurium (Meyer et al. 2012), and T3SS needle of Vibrio parahaemolyticus (Wang et al. 2014). However, to our knowledge, no recombinant anti-Campylobacter scFv antibodies have been reported to date.
In this study, recombinant rabbit scFv antibodies exhibiting specific binding to C. jejuni cells were constructed and selected by phage display technology. One of these anti-C. jejuni recombinant scFv antibodies was expressed in E. coli and purified. It was then coupled to tosylactivated paramagnetic beads to successfully capture C. jejuni cells in a specific and sensitive manner by IMS.
Material and methods
Preparation of irradiated C. jejuni cells
Campylobacter jejuni ATCC 29428 was cultured on Columbia Blood agar with 5% horse blood (Oxoid, Basingstoke, England) for 48 h at 42 °C under microaerophilic conditions. Cells were harvested and washed three times in phosphate buffer saline (PBS). The final pellet was resuspended in PBS to OD600 1.0. Aliquoted cells were then gamma (γ)-irradiated with a dose of 10 kGy using a Gammabeam 650 cobalt irradiator (located at Agri-Food and Biosciences Institute for Northern Ireland, Newforge Lane, Belfast, Northern Ireland) and stored at − 80 °C to be used as the whole cell antigen (WCA) for antibody production and phage display biopanning purposes.
Rabbit immunizations
Two New Zealand White rabbits (Q1 and Q2) were hyper-immunized with 109 cfu/ml of γ-irradiated C. jejuni WCA. In total, 1 ml of WCA was mixed with 1 ml of mineral oil and administered to each rabbit in six injections over a 4-month period with 2-week intervals between the first and second injections, and then 4-week intervals subsequently. Blood was taken 10 days after each of the first five immunizations and titered by indirect enzyme-linked immunosorbent assay (ELISA) to determine presence of C. jejuni-specific antibodies.
Total RNA extraction and cDNA synthesis
Fourteen days after the final immunization, rabbit Q1 was euthanized and exsanguinated. Spleen was harvested into RNALater (Thermo Scientific). Total RNA was extracted using 3 ml of TRI-reagent (Thermo Scientific) per 100 mg of tissue, following the manufacturer’s instructions. The RNA samples were then treated with DNase using TURBO DNA-free™ Kit (Ambion) to remove traces of DNA. Concentration of the RNA samples was quantified using NanoDrop™-2000, and 500 ng of RNA was checked by agarose gel electrophoresis to confirm no degradation of RNA. Five micrograms of total RNA purified from the rabbit spleen was used to synthesize first-strand cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions.
Generation of rabbit scFv cDNA library
First-strand cDNA derived from immune rabbit spleen was used to amplify scFv cDNA fragments with an overlap, 7-amino-acid (GGSSRSS), short linker (SL) sequence for diabody formation in two PCR steps using a procedure adapted from Barbas et al. (2001). In the primary PCR, cDNA fragments encoding the variable light (VL) chains (VK and Vλ) and the variable heavy (VH) chains were amplified separately. Using primers in Table 1, nine reactions were performed by pairing the three VK 5′ forward primers with each of the three VK 3′ reverse primers, to amplify the 350 bp VK cDNA fragments; one reaction with Vλ 5′ forward primer RSCλ1 and Vλ 3′ reverse primer RJλo-B to amplify the 350 bp Vλ cDNA; and four reactions by paring each of the four VH 5′ forward primers with the VH 3′ reverse primer RSCG-B to amplify the 450 bp VH cDNA. After PCR, VL and VH cDNA pools were separately made by combining the 10 VL and 4 VH PCR products. After primary purification using ethanol precipitation, the cDNA fragments encoding the VL and the VH, respectively, were purified from a 1.5% agarose electrophoresis gel. These two fragments were mixed in equal molar ratios and used as the template in the secondary overlap extension PCR to amplify the full-length scFv cDNA fragments using primers RSC-F and RSC-R (Table 1). The assembled 800 bp full-length scFv cDNA fragment was purified by ethanol precipitation, resolved in a 1% agarose gel, and purified from gel bands.
Table 1.
Details of PCR primers employed for construction of rabbit scFv fragments. Short linker sequences (GGSSRSS), underlined, are based on Protocol 9.7, Barbas et al. (2001)
| Primer type | Primer name | Primer ID | Primer sequence |
|---|---|---|---|
| Variable Kappa light chain | Vκ5′ Forward | RSCVK1 | 5′ GGG CCC AGG CGG CCG AGC TCG TGM TGA CCC AGA CTC CA 3′ |
| RSCVK2 | 5′GGG CCC AGG CGG CCG AGC TCG ATM TGA CCC AGA CTC CA 3′ | ||
| RSCVK3 | 5′ GGG CCC AGG CGG CCG AGC TCG TGA TGA CCC AGA CTG AA 3′ | ||
| Vκ 3′ Reverse | RKB9J1o-B | 5′ GGA AGA TCT AGA GGA ACC ACC TTT GAT TTC CAC ATT GGT GCC 3′ | |
| RKB9Jo-B | 5′ GGA AGA TCT AGA GGA ACC ACC TAG GAT CTC CAG CTC GGT CCC 3′ | ||
| RKB42Jo-B | 5′ GGA AGA TCT AGA GGA ACC ACC TTT GAC SAC CAC CTC GGT CCC 3′ | ||
| Variable Lambda light chain | Vλ 5′ Forward | RSCλ1 | 5′ GGG CCC AGG CGG CCG AGC TCG TGC TGA CTC AGT CGC CCT C 3′ |
| Vλ 3′ Reverse | RJλo-B | 5′ GGA AGA TCT AGA GGA ACC ACC GCC TGT GAC GGT CAG CTG GGT CCC 3′ | |
| Variable heavy chain | VH 5′ Forward | RSCVH1 | 5′ GGT GGT TCC TCT AGA TCT TCC CAG TCG GTG GAG GAG TCC RGG 3′ |
| RSCVH2 | 5′ GGT GGT TCC TCT AGA TCT TCC CAG TCG GTG AAG GAG TCC GAG 3′ | ||
| RSCVH3 | 5′ GGT GGT TCC TCT AGA TCT TCC CAG TCG YTG GAG GAG TCC GGG 3′ | ||
| RSCVH4 | 5′ GGT GGT TCC TCT AGA TCT TCC CAG SAG CAG CTG RTG GAG TCC GG 3′ | ||
| VH 3′ Reverse | RSCG-B | 5´ CCT GGC CGG CCT GGC CAC TAG TGA CTG AYG GAG CCT TAG GTT GCC C 3′ | |
| Overlap | Overlap Forward | RSC-F | 5′ GAG GAG GAG GAG GAG GAG GCG GGG CCC AGG CGG CCG AGC TC 3´ |
| Overlap Reverse | RSC-B | 5′ GAG GAG GAG GAG GAG GAG CCT GGC CGG CCT GGC CAC TAG TG 3′ |
pComb3XSS phagemid vector (obtained from Barbas lab, The Scripps Research Institute, CA; http://www.scripps.edu/barbas/) and the full-length scFv cDNA fragment were digested with SfiI (Thermo Scientific), gel purified, and ligated using T4 DNA ligase, after dephosphorylation of the vector. Ligation product was purified by ethanol precipitation and resuspended in 15 μl water. Then 15 electroporations were performed; each transformed 1 μl of the purified ligation product into 25 μl E. coli ER2738 electrocompetent cells (Lucigen Corporation, USA). The transformed E. coli ER2738 cells were titered on Luria-Bertani (LB) agar plates with 100 mg/L carbenicillin to determine the number of independent clones in the library.
Production of scFv displaying phage particles
To produce a library of phage particles displaying scFv, the transformed E. coli ER2738 scFv cDNA library was amplified and infected with helper phage M13K07 (Barbas et al. 2001). After overnight infection, the phage particles were harvested by polyethylene glycol (PEG) precipitation and resuspended in 2 ml 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS) (50 mM Tris-HCl (pH 7.5), 150 mM NaCl), supplemented with 0.02% sodium azide (NaN3), to kill residual E. coli and prevent microbial growth, then filtered through a 0.2 μm syringe filter. The concentration of the phages in library was determined by titering, before storage at 4 °C. Prior to biopanning, the phage library was re-amplified as per Barbas et al. (2001). Only phage library freshly prepared on the same day was used for biopanning.
Biopanning of phage displayed antibody library
The phage library was biopanned against irradiated C. jejuni WCA, in order to select anti-C. jejuni scFv displaying phages, that bound to C. jejuni cells with high affinity, using surface panning. Eight wells of a 96-well Nunc Maxisorp round-bottom microtiter plate (Thermo Scientific) were each coated with 100 μl C. jejuni WCA (1 × 109 cfu/ml) in 0.1 M NaHCO3 buffer pH 8.6. The coated wells were blocked with 300 μl PBS containing 5% skimmed milk (5% PBSM) and washed twice with TBS with 0.05% Tween 20. Then, 100 μl freshly prepared starting phage library containing 6.8 × 107 phages was added into each well and the plate was incubated at 37 °C for 2 h with gentle rocking. After decanting the phage solution, each well was washed five times with 200 μl TBS containing 0.5% Tween 20. The bound phages in each well were eluted using 100 μl of 100 mM Glycine–HCl pH 2.2 with 1 mg/ml BSA, and neutralized with 6 μl 2 M Tris (pH 9.1). The eluted phages were added into wells coated with C. jejuni WCA on the second plate and biopanning repeated as above (a procedure called double recognition, only applied to this first round biopanning). Eluted phage solutions were neutralized, titered to determine the output, and the rest of the phages amplified by infecting E. coli ER2738 cells before being rescued using helper phage M13K07 (Barbas et al. 2001). The amplified phages were titered and used as inputs for next round of biopanning as above, but with single recognition for all subsequent rounds. In total, six biopanning rounds were carried out against C. jejuni WCA to enrich the binding phages.
C. jejuni-binding by the enriched phages from rounds 3 to 6 was analyzed by ELISA. Wells of Nunc-Maxisorp microtiter plate were coated with irradiated C. jejuni WCA (1 × 107 cfu/well) and 2.8 × 105 phages were added into each well. Final anti-serum from rabbit Q1, parental phage that did not display a scFv, and unpanned phage display library were included as controls. Uncoated wells were included to assess the background binding. Bound phages were detected by horseradish peroxide (HRP)-conjugated anti-M13 monoclonal antibody (GE Healthcare) and its substrate 2,2′-azino-di[3-ethyl-benzthiazolin-sulfonate] (ABTS). The bound polyclonal antibody from the anti-serum was detected by HRP-conjugated anti-rabbit IgG (GE Healthcare) and its substrate TMB (3,3′,5,5′-tetramethybenzidine).
Screening, selection, and characterization of scFv antibody-display phage clones
The phage clones eluted after round 6 biopanning were titered and colony PCR was performed to amplify the insert of scFv cDNA from 135 individual colonies using primers omseq (5′ AAGACAGCTATCGCGATTGCAG 3′) and gback (5′ GCCCCCTTATTAGCGTTTGCCATC 3′) which are located either side of the insert. PCR products were subsequently digested by Mva I and resolved in a 3% agarose gel. The clones were grouped according to their cleavage patterns.
The scFv antibodies were targeted against surface epitopes of C. jejuni whole cells, so representative clones from each group observed were analyzed for binding capacity to irradiated C. jejuni WCA by phage-binding ELISA. The individual phage clones were firstly amplified as described above. Concentration of amplified phage samples was quantified by quantitative PCR using primers pComb3xss_qPCR.F (TTTCCGTGTCGCCCTTATTC) and pComb3xss_qPCR.R (CCCAACTGATCTTCAGCATCTT) and Roche LightCycler ® 480 SYBR Green I Master. A 96-well Nunc-Maxisorp microtiter plate was coated with C. jejuni WCA (1 × 108 cfu/well) and 2.8 × 105 phages were used in each well. Bound phages were detected by HRP-conjugated anti-M13 monoclonal antibody (GE Healthcare) and its substrate ABTS. Phage clones which showed high binding activity to C. jejuni cells were also tested for reaction with non-irradiated C. jejuni, Campylobacter coli, and Campylobacter lari and three other unrelated foodborne pathogens, L. monocytogenes, S. Typhimurium, and E. coli by ELISA, as described above.
Expression and purification of anti-C. jejuni scFv monoclonal antibodies
Clones scFv61 and scFv80, shown by ELISA to have the highest binding specificity, were chosen to express soluble anti-C. jejuni scFv antibodies. The phagemid DNA was purified from E. coli ER2738 and transformed into non-suppressor E. coli TOP10F′ cells (Invitrogen). Transformants were confirmed by colony PCR, as described above.
A single colony was inoculated into 10 ml pre-warmed super broth (SB) medium containing 50 μg/ml of carbenicillin and cultured overnight at 37 °C. The overnight culture was diluted 1:50 into fresh SB supplemented with 50 μg/ml carbenicillin and 40 mM MgCl2. The culture was incubated for 6 h at 37 °C with shaking at 250 rpm. Expression of the scFv antibodies was induced by adding isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. The expression was induced for 18 h at 37 °C with shaking at 250 rpm. The culture was harvested by centrifuging at 3000 g for 30 min at 4 °C. The pellet was resuspended in 30–40 ml ice-cold extraction buffer containing 1× PBS, 1× Complete Cocktail Protease Inhibitor, 6.4 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM MgCl2, 0.05 mg/ml DNase 1, and 0.05% β-mercaptoethanol. Cells were lysed by six French Press cycles at 1000 psi at 4 °C. The cleared lysate was centrifuged at 20,100 g for 30 min at 4 °C. The His-tagged scFv antibody in the supernatant was purified by adding 2 ml HisPur Ni-NTA Resin (Thermo Scientific), in the presence of imidazole. Binding was performed at 4 °C for 2 h with slow rotation. The beads were harvested by centrifuging at 2000 g for 5 min at 4 °C, washed twice in 50 ml ice-cold PBS containing imidazole for 10 min at 4 °C. The washed beads were resuspended into 2 ml of ice-cold PBS with high concentrations of imidazole and incubated for 15 min at 4 °C with gentle shaking. After a final centrifugation at 2000 g for 5 min at 4 °C, the supernatant containing the eluted antibody was aliquoted and stored at − 80 °C.
Presence of the scFv antibodies was checked by Western blotting. Protein samples were resolved by 15% SDS-PAGE before they were transferred onto Protran 0.2 NC nitrocellulose blotting membrane (Amersham). The membrane was blocked with a buffer consisting of 25 mM Tris pH 7.2, 0.15 M NaCl, 0.025% Tween-20, and 1% BSA for 1 h before being washed twice in TBS with 0.025% Tween 20. The His-tagged scFv was detected by incubating the membrane in 1:5000 dilution of India HisProbe-HRP (Thermo Scientific) in blocking buffer. After four washes with TBS containing 0.025% Tween 20, the membrane was incubated in SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), exposed to X-ray film, and then developed.
Characterization of soluble scFv80 antibody
The concentration of purified anti-C. jejuni scFv80 antibody was estimated by an ELISA assay, using His-tagged Cardiac Troponin I (cTnI) (GenScript) as the standard. Antigen-recognition capability and specificity of scFv80 for C. jejuni was then assessed by ELISA. A 96-well Nunc-Maxisorp microtiter plate was coated overnight at 4 °C with non-irradiated C. jejuni and five other non-target bacterial cells (108 cfu/well). The plate was washed twice with 200 ml of TBS containing 0.05% Tween 20 (0.05% of PBS/Tween 20) and blocked with 200 ml of 1% BSA in PBS at 37 °C. After washing the plate twice with 0.05% of PBS/Tween 20, 100 μl scFv80 antibody (200 ng/ml) was added to each well, while PBS was added to control wells. The plate was incubated for 2 h at 37 °C with shaking and then washed three times with 0.05% of PBS/Tween 20. Bound scFv80 antibody was detected by adding 100 μl HisProbe-HRP conjugate (1:50,000 diluted in TBS containing 0.05% Tween 20) per well and incubating for 1 h at room temperature with shaking. Following four washes with 0.05% of PBS/Tween 20, 100 μl TMB(E) was added to each well, incubated at room temperature for 5 min, and then stopped with 25 μl 1 M H2SO4. Colorimetric signal was read at 450 nm using a plater reader.
Development of an automated IMS procedure for capturing C. jejuni using anti-C. jejuni scFv80
BcMag™ long-arm and short-arm tosylactivated magnetic beads (10 mg each) (Bioclone Inc., San Diego) were covalently coated with 200 μg of purified scFv80 following the bead manufacturer’s instructions. To determine the capture capability and sensitivity of the coated beads, five ten-fold dilutions of non-irradiated C. jejuni cells (containing 107–103 cfu/ml) were processed by IMS, performed using a Dynal BeadRetriever instrument (Invitrogen). Each 1 ml dilution was mixed with 10 μl of coated beads (containing around 1.7 × 107 beads) for 30 min, followed by two 1-min washes in PBS-0.05% Tween 20. The washed beads were resuspended in 100 μl TE buffer, pH 8.0, and heated at 99 °C for 10 min in a heating block to release DNA from any bound C. jejuni cells. The tubes were centrifuged for 2 min at 21,800 g to sediment beads and cell debris leaving DNA in the supernatant. C. jejuni DNA in the supernatant was quantified by qPCR. Subsequently, to evaluate the specificity of the IMS process, IMS was repeated to capture C. jejuni cells from a mixed sample containing C. jejuni, C. coli, and C. lari (each present at 104 cfu/ml), before quantification of the captured C. jejuni, C. coli, and C. lari by qPCR. C. jejuni was quantified by qPCR targeting the hipOa gene (Keramas et al. 2003) using primers CGTGCAGATATGGATGCTTTG and TGCAGCAAGCAATAAAGAAGTAG. C. coli was quantified by qPCR targeting the glyAb gene (Vondrakova 2014) using primers GCGAGTGCTTATGCTCGTATTA and CCAGCAATGTGTGCAATGTC. C. lari was quantified by qPCR targeting the pepTc gene (Vondrakova 2014) using primers GAGCTGATGATAAAGCTGCTATTG and CACCTCTTAGGCCTTGTTCTT. All of the qPCR reactions were performed in triplicate. Input and captured cells were quantified against standard curves and % recovery of cells by IMS was calculated.
Results
Successful immunization of rabbits with C. jejuni whole cell antigen and construction of scFv phage display library
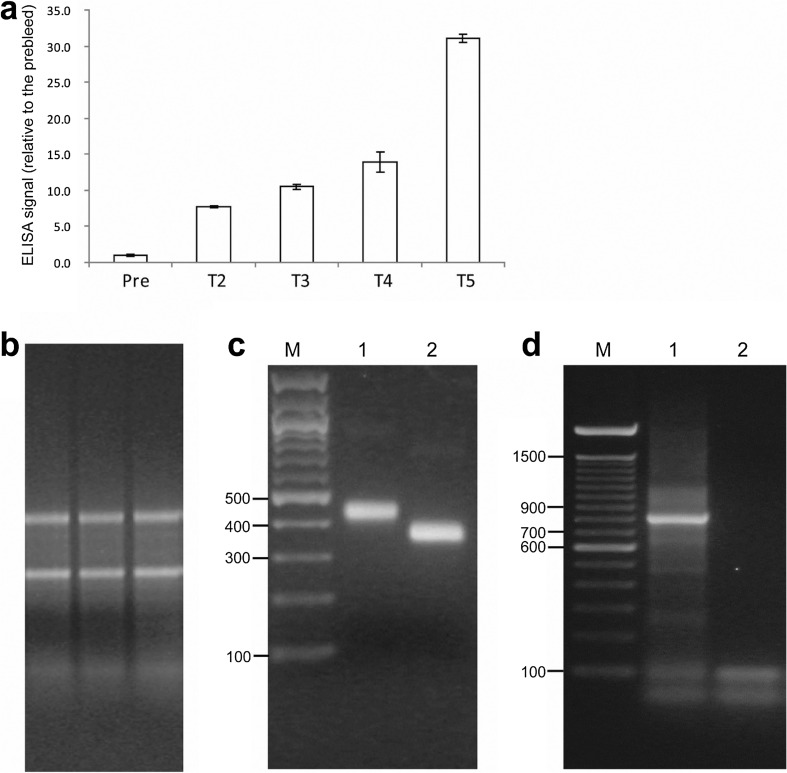
After the immunization course was completed for two New Zealand White rabbits, a substantial increase in anti-C. jejuni IgG production was observed in one of the rabbits (Fig. S1; Fig. 1a) and spleen and bone marrow were harvested from this rabbit. Total RNA extracted from the spleen (Fig. 1b) was used to synthesize the first-strand complementary DNA (cDNA) from which individual VL- and VH-chain region genes of approximately 350 and 400 bp, respectively, were amplified by PCR (Fig. S1, S2; Fig. 1c). The VL and VH chains were randomly joined by overlap extension PCR through a linker sequence to construct the 800 bp cDNA encoding full-length scFv fragments (Fig. S1, Fig. 1d).
Fig. 1.
Successful immunization of a rabbit with C. jejuni WCA and amplification of scFv cDNA fragments from spleen of the rabbit. a ELISA signal against C. jejuni cells, using pre-bleed and antisera from the rabbit after two (T2), three (T3), four (T4), and five (T5) immunizations. Each column represents the mean fold difference in ELISA signal from pre-bleed. The error bars represent standard deviation of the signals in the triplicate wells for each antibody. b Agarose gel image of the three total RNA samples purified from the spleen of the rabbit immunized with C. jejuni WCA. The sharp rRNA bands show that the RNA samples had no degradation. c Agarose gel image of purified cDNA fragments for VH and VL chains amplified from immune rabbit spleen. PCR products using nine pairs of VK and 1 pair of Vλ primers were pooled together to purify the VL fragment. PCR products using four pairs of VH primers were pooled together to purify the VH fragment. Lane M = 100 bp ladder; bp size of some bands is shown on the left of the image. Lane 1 = ~ 450 bp VH cDNA, lane 2 = ~ 350 bp VL cDNA. d Agarose gel image of purified full-length scFv cDNA fragment after merging the VL and VH fragments by overlap extension PCR. Lane M = 100 bp ladder. Lane 1 = ~ 800 bp scFv cDNA fragment, lane 2 = non-template PCR control
Following digestion with SfiI, scFv constructs of approximately 750 bp in size were ligated into the SfiI sites of a 3.4 kb linearized, dephosphorylated pComb3XSS phagemid vector. A library of phage particles displaying scFv on the surface, comprised of 6.8 × 107 independent transformants, was produced by successful transformation of the ligation into E. coli ER2738 electrocompetent cells and rescuing the library by infecting with helper phage M13 K07. The efficiency of library construction, determined by randomly selecting 93 colonies from the titered library and performing colony PCR, showed that the positive rate of transformants containing cDNA in the phagemid was 96%.
Screening and characterization of scFv-displaying particles which can strongly and specifically recognize C. jejuni cells
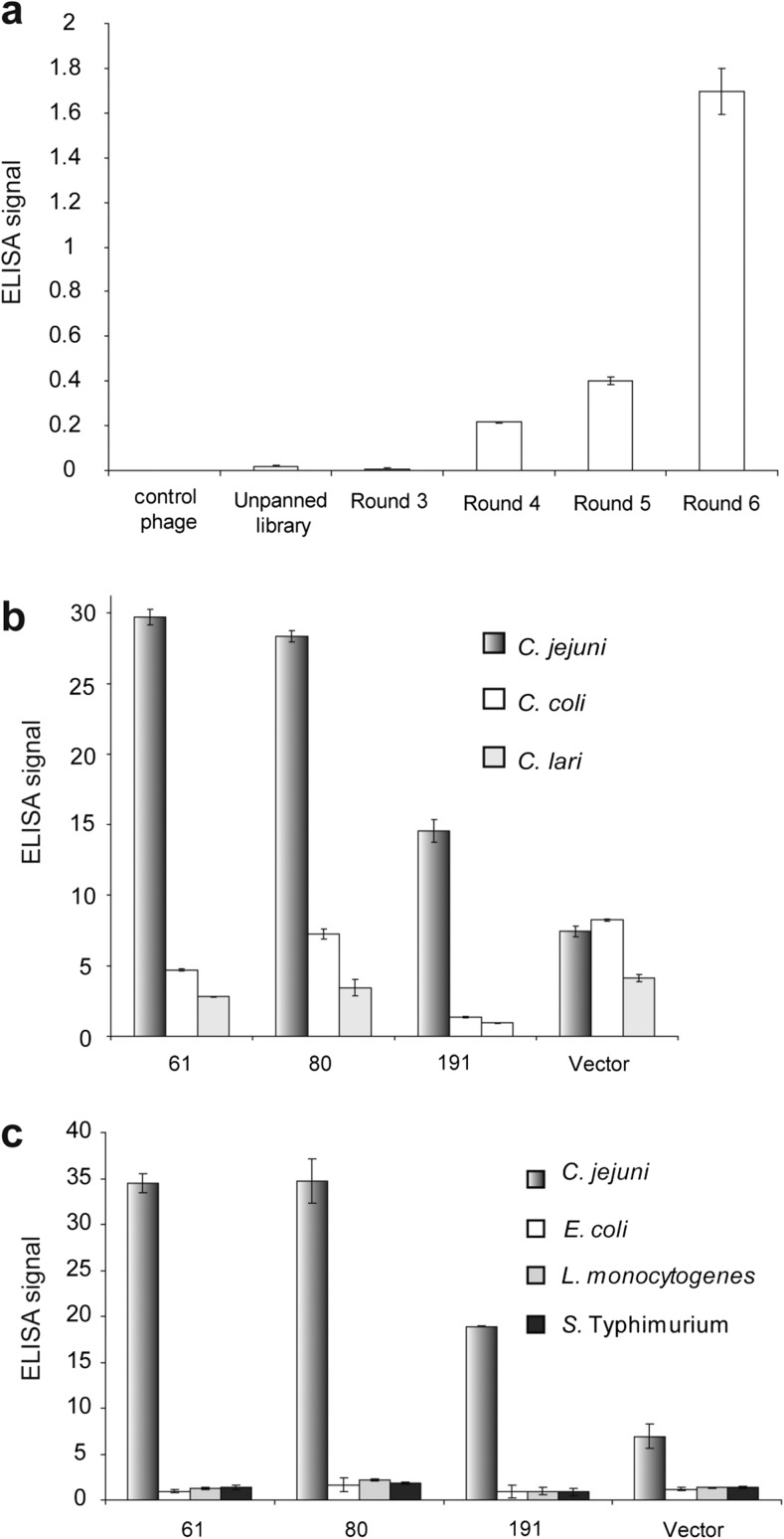
After six rounds of biopanning, including double recognition in first round, the effectiveness of the enrichment in phage pools from different rounds was verified by indirect phage-binding ELISA assay (Fig. 2a). The unpanned library showed slight signal compared to the control phage that did not display scFv, demonstrating that the constructed library contained phage clones that recognized C. jejuni WCA. However, no significant enrichment of antibodies was found in phage pools after three rounds of biopanning. Progression in enrichment of positive phage clones was detected gradually with increase in stringency of washes and once high stringency washing was adopted from round 4 to round 6, an 8.5-fold enrichment of phage particles in phage pools was achieved, demonstrated by an increase in OD415 from 0.2 to1.7.
Fig. 2.
Enrichment of C. jejuni-binding scFv-displaying phages by biopanning and characterization of selected phage clones for binding specificity. In a–c equal amount of cells and phage particle were used in each experiment. The bound phage particles were detected using HRP-conjugated anti-M13 antibody and the substrate ABTS. a ELISA assay to test binding activity to C. jejuni cell, using scFv-displaying phage pools that resulted from different rounds of biopanning. The control phage is the non-displaying phage. Signal reading at 415 nm is shown in the Y-axis. b The three best scFv-displaying phage clones (scFv 61, scFv 80, scFv 191) were tested in ELISA assay for their binding activity to C. jejuni and other Campylobacter spp. c The three best scFv-displaying phage clones were tested in ELISA assay, comparing their binding activity to C. jejuni and three non-Campylobacter bacterial cells. In b, c, the signal reading at 415 nm of the cell-coated wells was normalized against that in the uncoated wells. Each column and error bar in a–c represents the mean and standard deviation of triplicates of reactions
One hundred thirty-five individual clones randomly selected from titered output phages after round 6 were checked for diversity of the scFv cDNA sequences by PCR followed by digestion with restriction enzyme Mva I. Analysis of the digestion patterns revealed 47 unique groups (data not shown). A representative clone from each group was analyzed for binding activity and specificity to freshly prepared (non-irradiated) C. jejuni, C. coli, and C. lari WCAs using phage ELISA. Clones with signals at least two times greater than that of the empty vector control were considered to be binding Campylobacter cells. This testing revealed 12 clones which strongly recognized C. jejuni (Fig. S3); 3 of which (61, 80, and 191, from different Mva I digestion groups) specifically recognized C. jejuni and not the other 2 Campylobacter spp. (C. coli and C. lari) (Fig. 2b) or other unrelated foodborne pathogens (L. monocytogenes, S. Typhimurium, and E. coli) (Fig. 2c) tested.
Expression and purification of anti-C. jejuni scFv monoclonal antibodies
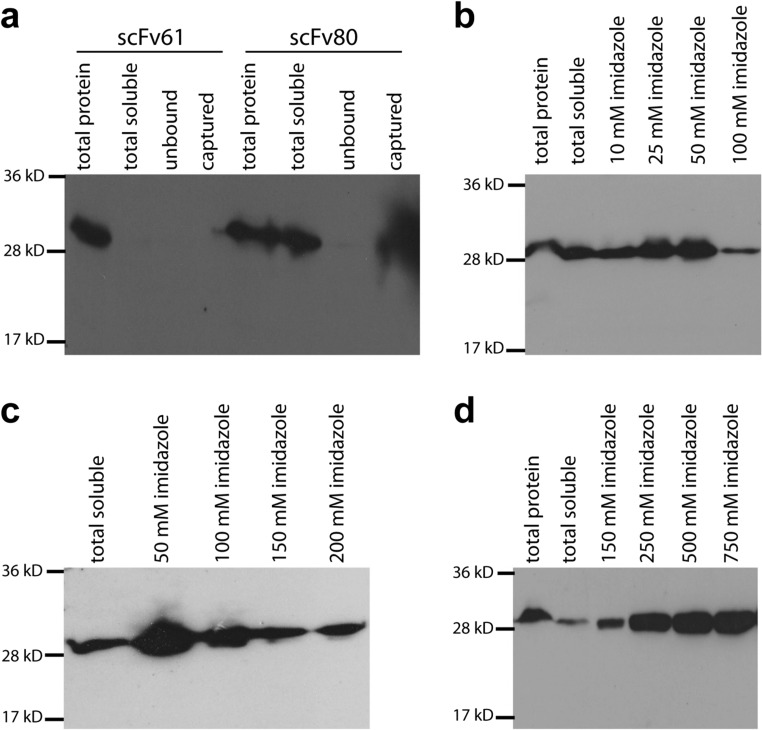
Clones scFv61 and scFv80 were selected for expression of antibody fragments not fused to phage particles. Recombinant pComb3XSS phagemid DNA of scFv61 and scFv80 was isolated from E. coli ER2738 cells, transformed into TOP10F′ cells (a nonsuppressor strain of E. coli) and confirmed by colony PCR. The transformed TOP10F′ cells were induced by IPTG to produce a protein band of ~ 30 kDa in total protein fractions of both scFv61 and scFv80 as revealed by Western blotting (Fig. 3a), demonstrating successful expression of both antibodies. However, only scFv80 was shown to be expressed in a soluble form, as shown by the presence of the ~ 30 kDa protein band in the total soluble fraction of scFv80 but not that of scFv61. While both antibodies were fused with a poly histidine tag (His6-tag) at the C-terminus, the presence of a ~ 30 kDa band in the captured fraction of scFv80 only clearly shows that the soluble scFv80 protein readily bound to Ni-NTA resin and was successfully eluted. In contrast, absence of protein band in the captured fraction of scFv61 confirmed that it was expressed as insoluble protein, which could not be captured by beads.
Fig. 3.
Expression and purification of C. jejuni-specific scFv antibodies. a Western blot of scFv61 and scFv80, expressed in E. coli and captured by affinity beads. Both antibodies have 6xHis tag at their C-terminus and were detected using His-probe. Nickel beads were used to capture the soluble antibodies. b Western blot of scFv80 expressed and purified using different imidazole concentrations in the binding buffer. c Western blot of scFv80 expressed and purified using different imidazole concentrations in the washing buffer. d Western blot of scFv80 expressed and purified using different imidazole concentrations in the elution buffer
To maximize the purity of the scFv80 when using HisPur Ni-NTA affinity Resin, concentration of imidazole in binding, washing, and elution buffers was optimized. Increasing the concentration of imidazole in the binding buffer to 50 mM was observed to give the highest stringency with optimum binding of scFv80 to the beads, while 100 mM of imidazole significantly reduced capturing of the antibody (Fig. 3b). Analysis of the impact of different concentrations of imidazole (50, 100, 150, and 200 mM) in the washing buffer after binding scFv80 antibody in 50 mM of imidazole showed that washes in 50 mM imidazole retained the highest yield of scFv80 on beads, whereas imidazole concentrations above 50 mM significantly washed off the antibody from the beads (Fig. 3c). Elution efficiency of scFv80 antibody in elution buffer containing 150, 250, 500, or 750 mM of imidazole from beads that had been bound and washed with the optimized 50 mM imidazole respectively showed that elution efficiency increased significantly from 150 to 250 mM of imidazole and no further significant increase in elution efficiency was observed beyond 250 mM of imidazole (Fig. 3d). Therefore, the optimal concentrations of imidazole for producing pure levels of scFv80 antibody were 50 mM in binding buffer, 50 mM in washing buffer, and 250 mM in elution buffer.
Binding characteristics of purified anti-C. jejuni scFv80 antibody to bacterial cells
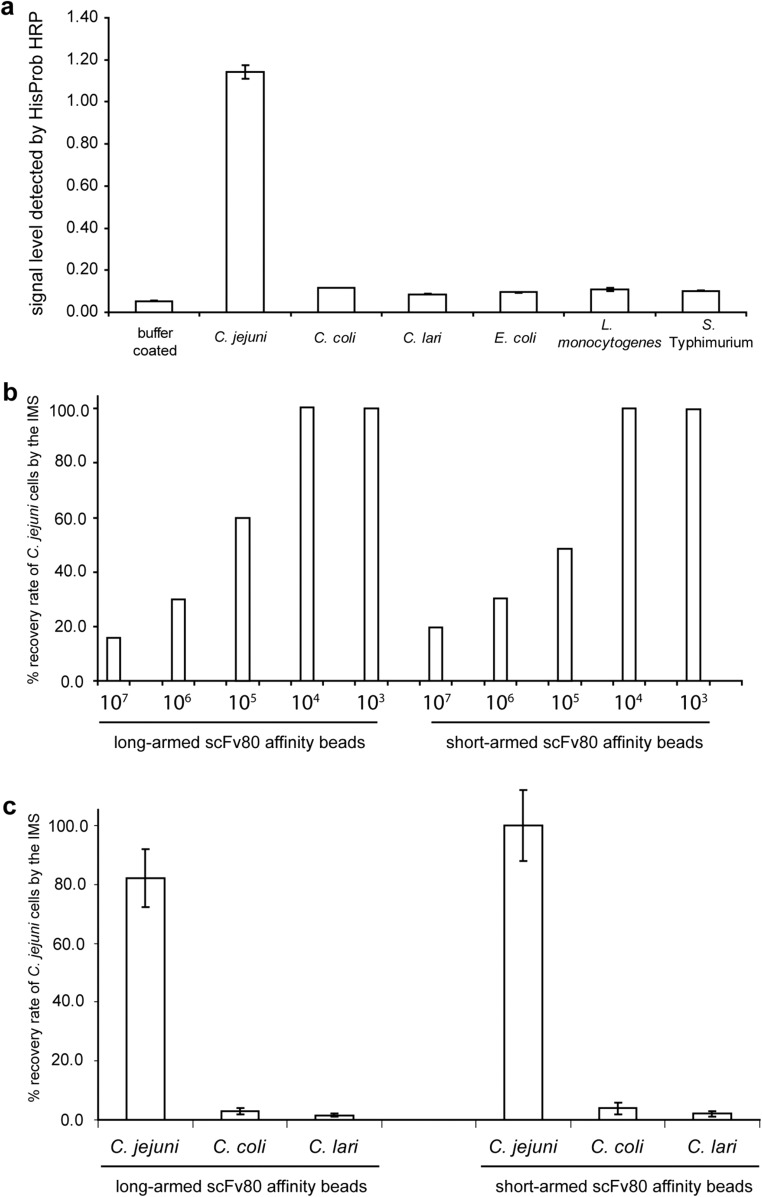
A large-scale culture of scFv80 antibody was purified using the optimized imidazole concentrations. The concentration of purified scFv80 was estimated to be 180 ng/μl by ELISA using commercial His-tagged cTnI as a standard. The purified scFv80 antibody was tested for its binding specificity to C. jejuni and other non-target bacteria in an ELISA assay. This assay clearly demonstrated that the purified scFv80 specifically recognized C. jejuni cells but did not bind to either its relatives (C. coli and C. lari), or the three other unrelated foodborne pathogens (E. coli, S. Typhimurium, or L. monocytogenes), even though these cells were exposed to the antibody at a high concentration of 1 × 108 cells/well (Fig. 4a).
Fig. 4.
Purified scFv80 antibody specifically recognizes C. jejuni cells. a In the ELISA assay, purified scFv80 was used to detect C. jejuni and other foodborne pathogens. The same amount of bacterial cells and scFv80 antibody was used in all the ELISA wells. Bound scFv80 was detected by HisProbe-HRP Conjugate and the substrate TMB (E). Signal read at 450 nm was shown in the Y-axis. Each column represents the mean and standard deviation of signals in three wells. b Recovery of C. jejuni by IMS from solutions containing different cell concentrations (103–107 cfu/ml) using scFv80 coated beads. Cell concentrations are labeled below each column. Recovery was calculated according to qPCR quantification data of the captured and input cells. c Recovery of Campylobacter cells by IMS using scFv80 coated beads from a mixed culture of C. jejuni, C. coli, and C. lari (each present at 104 cfu/ml). qPCR reactions specific to C. jejuni, C. coli, or C. lari were used to quantify the input and captured cells before calculating the recovery rate of each species
Evaluation of purified scFv80 in IMS procedure to capture C. jejuni cells
The capacity of purified scFv80 antibody as a ligand to capture C. jejuni cells by IMS was tested using long-arm and short-arm BcMag™ tosylactivated paramagnetic beads that had been covalently coated with the antibody. The captured cells were quantified by qPCR. When sensitivity of the IMS in recovering C. jejuni cells from samples containing 103 to 107 cells/ml was assessed, both long-arm and short-arm tosylactivated beads coated with scFv80 were found to be able to capture 100% of C. jejuni cells from dilutions containing 103 to 104 cells/ml (Fig. 4b). It was observed that when the cell input was higher than 104 cells/ml, the recovery rate of C. jejuni by the beads decreased, ranging from 16 to 60% (Fig. 4b), which suggested that the coated beads had a maximum binding capacity.
In biological samples, C. jejuni could be present along with other cells, so to test if scFv80-coated beads could specifically recover C. jejuni from mixed cell suspensions, IMS was performed on a sample containing C. jejuni, C. lari, and C. coli, all at 104 cells/ml. Recovered cells were quantified by qPCRs targeting genes specific to C. jejuni, C. lari, and C. coli. Figure 4c shows that in this IMS, the short-arm beads recovered 100% C. jejuni, whereas the long-arm beads recovered 82%. The short-arm scFv80-coated beads performed better than long-arm scFv80-coated beads, although both beads were very specific for C. jejuni, capturing only 1.5–4% of either C. coli or C. lari cells.
Discussion
Campylobacteriosis, especially caused by C. jejuni, is the most common bacterial cause of human gastroenteritis worldwide (Silva et al. 2011; Tram et al. 2012). Poultry is the main reservoir of C. jejuni (Skarp et al. 2016) and most human infections are caused by consuming undercooked poultry or mishandling of contaminated poultry products (Silva et al. 2011). Effective monitoring of C. jejuni in chickens prior to slaughter is crucial to tackling Campylobacteriosis, but better detection methods are urgently needed for this purpose.
Here, we present the results of construction of a scFv-phage displaying library derived from a C. jejuni immunized rabbit, and selection of phage clones displaying scFv antibodies recognizing C. jejuni cell by phage display biopanning. In addition, we describe development of an IMS method employing one of the C. jejuni-specific scFv antibodies produced, to capture and concentrate C. jejuni from test samples. This IMS method was subsequently combined with a qPCR method to achieve rapid detection and quantitation of C. jejuni. We demonstrate that the resultant IMS-qPCR method could potentially be suitable for C. jejuni monitoring purposes, subject to some further optimization.
In order to produce novel recombinant scFv antibodies specific for C. jejuni cells, two rabbits were immunized with C. jejuni whole cells that had been subjected to gamma radiation rather than heat-inactivation before use. Irradiation kills the bacterial cells while maintaining the integrity of cell surface antigens, unlike heating which is liable to denature some or all of these surface antigens (Stewart et al. 2012). Once a high serum antibody titer against C. jejuni had been reached in one of the immunized rabbits, indicating that there was enrichment for antigen-specific antibodies, the rabbit spleen was harvested for RNA extraction. Either spleen or bone marrow from the immune-responsive rabbit could have been selected as the starting point for recombinant antibody production. We selected spleen as our starting material because for high titer animals, like the one in this study, it has been shown that V genes encoding antigen-specific antibodies dominate the large diverse B lymphocytes in the spleen indicating that total splenocytes can be used in place of bone marrow plasma cells for antibody discovery (Saggy et al. 2012). The VL and VH chain genes were separately amplified from the spleen cDNA along with the short linker sequence through which the individual cDNA fragments were randomly joined by overlap extension PCR to produce full-length scFv cDNA fragments (Barbas et al. 2001). The short linker joining VH and VL in scFv maintains the antigen-binding sites, with enhanced avidity for the specific antigen, as previously noted by Turner et al. (1997) and Singh et al. (2010). According to Barbas et al. (2001), the isolation of specific antibodies from a cloned immunological repertoire requires a large, diverse, and complex library in the range of 107 to 108 independent transformants, as well as an efficient selection process. In this study, when cloned into the phagemid vector pComb3XSS which had been dephosphorylated in order to increase the proportion of transformants containing the cDNA fragments, the scFv genes resulted in a combinatorial library with 6.8 × 107 clones. This was considered to have sufficient complexity to proceed to phage display. The scFv antibodies were expressed as fusions to the gene III coat protein (pIII) of the phage surface, as first described by Smith (1985), which made it possible for them to be screened by successive rounds of biopanning against the C. jejuni WCA (Barbas et al. 2001) to enrich clones expressing scFv antibodies strongly recognizing C. jejuni cells.
ELISA analysis of the outcome phages of the first three rounds of biopanning did not show significant enrichment of C. jejuni binders (Fig. 2a). However, some evidence of enrichment began to be observed after round 4, doubled after round 5, and then showed a marked increase after round 6 (Fig. 2a). As the stringency of the selection conditions increased with successive rounds of biopanning, more scFv-displaying phage clones with better binding capabilities were successfully selected, resulting in library enrichment at round 6 of biopanning. After round 6, 135 clones were randomly selected to amplify the scFv cDNA and subjected to Mva I restriction digestion; the digestion patterns were used to quickly test sequence diversity of scFv cDNA among the large numbers of clones (Barbas et al. 2001).
Two phage clones, 61 and 80, were selected to express scFv antibodies without pIII. pComb3XSS contains the amber codon such that when transformed into a non-suppressor bacterial strain, it can express soluble protein without coat protein III (Barbas et al. 2001). These scFv antibodies are fused with a poly histidine tag (His6-tag) and hemagglutinin tag (HA-tag) at their C-terminus which aids affinity purification and immuno-detection of the antibody proteins by Western blotting. Phagemid DNA isolated from clones 61 and 80 was transformed into the non-suppressor E. coli TOP10F′ cells. Protein expression was induced by IPTG at 37 °C for 18 h. scFv80 was expressed as a soluble protein (Fig. 3a); a pure, single scFv80 protein band, of size ~ 30 kDa, was observed on SDS-PAGE gel and by Western blotting. In contrast, scFv61 was expressed as an insoluble protein. The high-level expression of scFv61 in TOP10F′ cells may have led to formation of highly aggregated protein commonly referred to as inclusion bodies (Palmer and Wingfield 2012), resulting in it being insoluble. Several alternative strategies to generate soluble scFv61 were tried, including expressing the protein at varying temperatures in TOP10F′ cells, supplementing with glucose and sorbitol, and expression in a different non-suppressor strain, E. coli BL-21-A in the presence of 0.2% (w/v) arabinose, but all have proved unsuccessful to date.
The specificity of the purified, soluble C. jejuni-specific scFv80 antibody was assessed by ELISA. The recombinant antibody was confirmed to have retained its high binding capability for C. jejuni WCA when not expressed on the phage and it still did not cross-react with two other Campylobacter spp. or three other unrelated foodborne bacterial pathogens (Fig. 4a). These results indicated that scFv80 was very specific for C. jejuni and, hence, potentially suitable to be incorporated into a novel detection approach for this foodborne pathogen. IMS linked with qPCR was our novel detection approach of choice because IMS offers the possibility of selectively extracting C. jejuni cells from heterogeneous suspensions, such as those that are encountered in food, clinical specimens, and feces, and qPCR offers the possibility of rapid, specific, and quantitative detection of the captured C. jejuni cells assuming appropriate primers are selected. Therefore, purified scFv80 antibody was coated onto tosylactivated magnetic beads and suitable primers were selected/designed for qPCR detection; targeting the HipO gene, encoding hippuricase, that is absent in all Campylobacter species except C. jejuni (Keramas et al. 2003), and the glyA and pepT genes in C. coli and C. lari, respectively (Vondrakova 2014).
Immunomagnetic separation has been successfully used to capture foodborne Salmonella spp. and Listeria monocytogenes using beads coated with phage display-derived peptides (Carnazza et al. 2007; Morton et al., 2013a, b), or beads coated with a phage display-derived single-chain antibody for Listeria monocytogenes (Paoli et al. 2007). IMS methods for C. jejuni involving polyclonal and monoclonal antibodies have previously been reported (Yu et al. 2001; Tram et al. 2012). To our knowledge, this study is the first to assess the IMS potential of a recombinant scFv antibody specific for C. jejuni. The novel recombinant scFv80 antibody was evaluated for IMS by coating two types of tosylactivated paramagnetic beads (‘short-arm’ and ‘long arm’); these were both shown to be capable of capturing 100% C. jejuni cells from a pure culture containing 1000 to 10,000 cfu/ml (Fig. 4b). We did not test recovery of cells below 1000 cfu/ml. However, we have tested the sensitivity of the qPCR step and found that it can detect as low as five cells (data not shown). Therefore, we are confident the limit of detection (LOD) of the IMS can be further reduced. Percentage recovery from high concentrations of C. jejuni by the IMS was low (Fig. 4b). However, this is not an issue in terms of potential application of the beads in real life sample because rarely would biological samples have such high levels of C. jejuni present. The long- and short-arm beads both specifically and selectively captured 82 and 100% C. jejuni cells, respectively, from a mixed suspension containing 10,000 cfu/ml of each of C. jejuni, C. coli, and C. lari (Fig. 4c). In this study, 1 ml of cell samples was used in IMS. However, real biological samples are often in volumes of 50 to 250 ml. A centrifugation step could be introduced to concentrate the cells into 1 ml before applying the IMS to this concentrated sample. This experiment demonstrated that the IMS procedure using recombinant monoclonal scFv80 antibody derived from the spleen of a C. jejuni immunized rabbit specifically captured C. jejuni. To our knowledge, our work represents the first attempt to use recombinant scFv antibodies in IMS for capturing Campylobacter spp. to date. The IMS-qPCR method was able to specifically detect 1000 cfu/ml C. jejuni in just under 3 h. With some further optimization of the IMS, it may be possible to improve on the detection sensitivity.
In conclusion, a novel anti-C. jejuni recombinant scFv antibody, scFv80, demonstrating strong and specific binding to C. jejuni cells was successfully generated from an immune spleen combinatorial library by phage display. When this novel recombinant monoclonal scFv80 antibody was coated onto BcMag™ long-arm and short-arm tosylactivated magnetic beads, and IMS was carried out in combination with qPCR as the endpoint detection method, the resultant IMS-qPCR assay indicated the specific capture of 82–100% C. jejuni cells, from a mixed culture containing C. jejuni, C. coli, and C. lari. The scFv80 antibody-based IMS-qPCR method merits further development and optimization, as it could potentially reduce the current detection time for C. jejuni from 3 to 5 days to just under 3 h without the need for a culture enrichment step.
Electronic supplementary material
(PDF 412 kb)
Acknowledgments
We wish to thank Dr. Terence Fodey and colleagues at the Veterinary Sciences Division, Agri–Food and Biosciences Institute for Northern Ireland for immunizing the rabbits, and The Barbas Laboratory—The Scripps Research Institute, CA. USA, for generously providing the pComb3XSS phagemid vector.
Funding
This research was undertaken while Dr. Ruramayi M. Nzuma was in receipt of a Daphne Jackson Fellowship funded by the UK Society of Chemical Industry; consumables budget was provided by Drs Grant and Liu.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
None of the authors of this article performed any studies with animals. Rabbit immunizations were performed, under license, by staff at the Agri-Food and Biosciences Institute (AFBI) for Northern Ireland, Stormont, Belfast, in accordance with the Animals (Scientific Procedures) Act 1986, UK. The project license was approved by AFBI Animal welfare and Ethical Review Body and issued by the Department of Health, Social Services and Public Safety for Northern Ireland.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00253-018-8949-x) contains supplementary material, which is available to authorized users.
Contributor Information
Fuquan Liu, Phone: +44 28 9097 2477, Email: f.liu@qub.ac.uk.
Irene R. Grant, Phone: +44 28 9097 2109, Email: i.grant@qub.ac.uk
References
- Bang DD, Pedersen K, Madsen M. Development of a PCR assay suitable for Campylobacter spp. mass screening programs in broiler production. J Rapid Meth Automat Microbiol. 2001;9:97–113. doi: 10.1111/j.1745-4581.2001.tb00233.x. [DOI] [Google Scholar]
- Barbas CFI, III, Burton DR, Scott JK, Silverman GJ. Phage display: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Carnazza S, Gioffre G, Felici F, Guglielmino S. Recombinant phage probes for Listeria monocytogenes. J Phys Condens Matter. 2007;19:395011. doi: 10.1088/0953-8984/19/39/395011. [DOI] [Google Scholar]
- Cudjoe KS, Kapperud G, Olsvik Ø. Identification of thermotolerant Campylobacter species from poultry using an enzyme-labelled oligonucleotide DNA probe. Int J Food Microbiol. 1991;12:271–274. doi: 10.1016/0168-1605(91)90078-4. [DOI] [PubMed] [Google Scholar]
- Docherty L, Adams MR, Patel P, McFadden J. The magnetic immuno-polymerase chain reaction assay for the detection of Campylobacter in milk and poultry. Lett Appl Microbiol. 1996;22:288–292. doi: 10.1111/j.1472-765X.1996.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Dominguez J (2012) Modernisation of meat inspection: the UK experience. http://wwwsalutegovit/imgs/C_17_notizie_606_listaFile_itemName0_filepdf Accessed 22 Nov 2017
- Doyle MP. The emergence of new agents of foodborne disease in the 1980s. Food Res Int. 1994;27:219–226. doi: 10.1016/0963-9969(94)90087-6. [DOI] [Google Scholar]
- Food Standards Agency (2009) FSA publishes survey of Campylobacter and Salmonella in chicken. www.food.gov.uk/news/newsarchive/2009/oct/chicksurvey Accessed 22 November 2017
- Giesendorf BAJ, Quint WGV, Henkens MHC, Stegeman H, Huf FA, Niesters HGM. Rapid and sensitive detection of Campylobacter spp. in chicken products by using the polymerase chain reaction. Appl Environ Microbiol. 1992;58:3804–3808. doi: 10.1128/aem.58.12.3804-3808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H, Butzler JP. Isolation and identification of Campylobacter spp. In: Nachamkins I, Blaser MJ, Tompkins LS, editors. Campylobacter jejuni: current status and future trends. Washington DC: ASM Press; 1992. p. 93. [Google Scholar]
- Hagemeyer CE, von Zur Muhlen C, von Elverfeldt D, Peter K. Single-chain antibodies as diagnostic tools and therapeutic agents. Thromb Haemost. 2009;101:1012–1019. [PubMed] [Google Scholar]
- Haas B (1997) Biotin–streptavidin-amplified enzyme-linked immunosorbent assay for the detection of antibodies to Campylobacter jejuni and coli in chickens. In Proceedings of Poultry and Food Safety, 20-22 August 1997, Budapest, Hungary, p 261
- Hermani L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rolliers I, De Zutter L. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect. 2003;131:1169–1180. doi: 10.1017/S0950268803001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton AC, Mortiboy D, Banks JG, Penn CW. RAPD analysis of environmental, food and clinical isolates of Campylobacter spp. FEMS Immunol Med Microbiol. 1997;18:119–124. doi: 10.1111/j.1574-695X.1997.tb01036.x. [DOI] [PubMed] [Google Scholar]
- International Standards Organisation . ISO/TS 10272–2:2006 microbiology of food and animal feeding stuffs–horizontal method for detection and enumeration of Campylobacter spp. part. Geneva: Colony Count Technique. International Organization for Standardization; 2006. p. 2. [Google Scholar]
- Keramas G, Bang DD, Lund M, Madsen M, Rasmussen SE, Bunkenborg H. Development of a sensitive DNA microarray suitable for rapid detection of Campylobacter spp. Mol Cell Probes. 2003;17:187–196. doi: 10.1016/S0890-8508(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lamoureux M, MacKay A, Messier S, Fliss I, Blais BW, Holley RA, Simard RE. Detection of Campylobacter jejuni in food and poultry viscera using immunomagnetic separation and microtiter hybridization. Appl Microbiol. 1997;83:641–651. doi: 10.1046/j.1365-2672.1997.00273.x. [DOI] [PubMed] [Google Scholar]
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J. 2005;46(3):258–268. doi: 10.1093/ilar.46.3.258. [DOI] [PubMed] [Google Scholar]
- Mazurier S, van de Giessen A, Heuvelman K, Wernars K. RAPD analysis of Campylobacter isolates: DNA fingerprinting without the need to purify DNA. Lett Appl Microbiol. 1992;14:260–262. doi: 10.1111/j.1472-765X.1992.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Meyer T, Schirrmann T, Frenzel A, Miethe S, Stratmann-Selke J, Gerlach GF, Strutzberg-Minder K, Dubel S, Hust M. Identification of immunogenic proteins and generation of antibodies against Salmonella Typhimurium using phage display. BMC Biotechnol. 2012;12:29. doi: 10.1186/1472-6750-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J, Karoonuthaisiri N, Stewart LD, Oplatowska M, Elliott CT, Grant IR. Production and evaluation of the utility of novel phage display-derived peptide ligands to Salmonella spp. for magnetic separation. J Appl Microbiol. 2013;115:271–281. doi: 10.1111/jam.12207. [DOI] [PubMed] [Google Scholar]
- Morton J, Karoonuthaisiri N, Charlermroj R, Stewart LD, Elliott CT, Grant IR. Phage display-derived binders able to distinguish Listeria monocytogenes from other Listeria species. PLoS One. 2013;8:e74312. doi: 10.1371/journal.pone.0074312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PN, Reynolds GM, Waldron EE, Ward E, Giannopoulos K, Murray PG. Monoclonal antibodies. Mol Pathol. 2000;53:111–117. doi: 10.1136/mp.53.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer I, Wingfield PT. Preparation and extraction of insoluble (inclusion-body) proteins from Escherichia coli. Curr Protoc Protein Sci. 2012;70:6.3:6.3.1–6.36.3.20. doi: 10.1002/0471140864.ps0603s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli GC, Kleina LG, Brewster JD. Development of Listeria monocytogenes-specific immunomagnetic beads using a single-chain antibody fragment. Foodborne Pathog Dis. 2007;4:74–83. doi: 10.1089/fpd.2006.65. [DOI] [PubMed] [Google Scholar]
- Platts-Mills JA, Kosek M. Update on the burden of Campylobacter in developing countries. Curr Opin Infect Dis. 2014;27:444–450. doi: 10.1097/QCO.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom GM, Dreesen DW, Rose BE, Lattuada CP. Assessment of three nucleic acid hybridization systems for detection of Campylobacter spp. in poultry products. J Food Prot. 1994;57:703–709. doi: 10.4315/0362-028X-57.8.703. [DOI] [PubMed] [Google Scholar]
- Rasmussen HN, Olsen JE, Jorgensen K, Rasmussen OF Detection of Campylobacter jejuni and Camp. coli in chicken faecal samples by PCR. Lett Appl Microbiol. 1996;23:363–366. doi: 10.1111/j.1472-765X.1996.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Rollins DM, Colwell RR. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggy I, Wine Y, Shefet-Carasso L, Nahary L, Georgiou G, Benhar I. Antibody isolation from immunized animals: comparison of phage display and antibody discovery via V gene repertoire mining. Protein Eng Des Sel. 2012;25:539–549. doi: 10.1093/protein/gzs060. [DOI] [PubMed] [Google Scholar]
- Sails AD, Fox AJ, Bolton FJ, Wareing DRA, Greenway DLA. A real-time PCR assay for the detection of Campylobacter jejuni in foods after enrichment culture. Appl Environ Microbiol. 2003;69:1383–1390. doi: 10.1128/AEM.69.3.1383-1390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK, Dallas JF, Strachan NJC, MacRae M, McCarthy ND, Wilson DJ, Gormley FJ, Falush D, Ogden ID, Maiden MCJ, Forbes KJ. Campylobacter genotyping to determine the source of human infection. Clin Infect Dis. 2009;48:1072–1078. doi: 10.1086/597402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front Microbiol. 2011;2:200. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Agrawal R, Kamboj DV, Gupta G, Boopathi M, Goel AK, Singh L. Construction of a single-chain variable-fragment antibody against the superantigen staphylococcal enterotoxin B. Appl Environ Microbiol. 2010;76:8184–8191. doi: 10.1128/AEM.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarp CPA, Hänninen ML, Rautelin HIK. Campylobacteriosis: the role of poultry meat. Clin Micro Infect. 2016;22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Skerra A, Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Sci. 1988;240:1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Sci. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- Stewart LD, McNair J, McCallan L, Thompson S, Kulakov LA, Grant IR. Production and evaluation of antibodies and phage display-derived peptide ligands for immunomagnetic separation of Mycobacterium bovis. Clin Microbiol. 2012;50:1598–1605. doi: 10.1128/JCM.05747-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam CC, Higgins CD, Neal KR, Rodrigues LC, Millership SE, O’Brien SJ, on behalf of the Campylobacter Case–Control Study Group Chicken consumption and use of acid-suppressing medications as risk factors for Campylobacter enteritis, England. Emerg Infect Dis. 2009;15:1402–1408. doi: 10.3201/eid1509.080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tram LLT, Cao C, Hogberg J, Wolff A, Bang DD. Isolation and detection of Campylobacter jejuni from chicken fecal samples by immunomagnetic separation–PCR. Food Cont. 2012;24:23–28. doi: 10.1016/j.foodcont.2011.08.030. [DOI] [Google Scholar]
- Turner DJ, Ritter MA, George AJ. Importance of the linker in expression of single-chain Fv antibody fragments: optimization of peptide sequence using phage display technology. J Immunol Meth. 1997;205:43–54. doi: 10.1016/S0022-1759(97)00057-4. [DOI] [PubMed] [Google Scholar]
- Van Gerwe T, Miflin JK, Templeton JM, Bouma A, Wagenaar JA, Jacobs-Reitsma WF, Stegeman A, Klinkenberg D. Quantifying transmission of Campylobacter jejuni in commercial broiler flocks. Appl Environ Microbiol. 2009;75:625–628. doi: 10.1128/AEM.01912-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondrakova L, Pazlarova J, Demnerova K. Detection, identification and quantification of Campylobacter jejuni, coli and lari in food matrices all at once using multiplex qPCR. Gut Pathog. 2014;6:12. doi: 10.1186/1757-4749-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Fang S, Xiang S, Ling S, Yuan J, Wang S. Generation and characterization of a scFv antibody against T3SS needle of Vibrio parahaemolyticus. Indian J Microbiol. 2014;54(2):143–150. doi: 10.1007/s12088-013-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (2012) in collaboration with World Organisation of Animal Health, Food and Agriculture of the United Nations, Universiteit Utrecht. The Global View of Campylobacteriosis: Report of an Expert Consultation. Utrecht, Netherlands, 9–11 July 2012. http://apps.who.int/iris/bitstream/10665/80751/1/9789241564601_eng.pdf Accessed 22 November 2017
- Yamazaki W, Taguchi M, Kawai T, Kawatsu K, Sakata J, Inoue K, Misawa N. Comparison of loop-mediated isothermal amplification assay and conventional culture methods for detection of Campylobacter jejuni and Campylobacter coli in naturally contaminated chicken meat samples. Appl Environ Microbiol. 2009;75:1597–1603. doi: 10.1128/AEM.02004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu lSL, Uknalis J, Tu S-I. Immunomagnetic separation methods for the isolation of Campylobacter jejuni from ground poultry meats. Immunol Meth. 2001;256:11–18. doi: 10.1016/S0022-1759(01)00372-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 412 kb)