Abstract
The aim of this study was to test the effects of five different concentrations (0, 10−3, 10−4, 10−5, and 10−6 M) of resveratrol (Res) supplementation in bull sperm washing and fertilisation medium on levels of reactive oxygen species (ROS), phosphatidylserine (PS) externalisation, mitochondrial membrane potential (Δψm), ATP and malondialdehyde (MDA), acrosomal integrity, blastocyst rate, and blastocyst quality after in vitro fertilisation (IVF). The results for sex-sorted sperm from three bulls showed: (1) ROS and MDA levels in 10−3 M and 10−4 M Res groups were significantly lower than those of controls (P < 0.05); (2) the percentage of viable sperm, percentage of sperm with high Δψm, and the ATP content in 10−3 M and 10−4 M Res groups were significantly higher than those of controls (P < 0.05); (3) the percentage of viable sperm with acrosomal integrity, and the blastocyst percentage and quality of the 10−4 M Res group were significantly higher than those of controls (P < 0.05). In conclusion, 10−4 M Res supplementation in washing and fertilisation medium of sex-sorted bull sperm significantly decreased ROS, PS externalisation, and MDA, and protected mitochondrial function and acrosomal integrity, thereby increasing blastocyst percentage and quality following IVF.
Introduction
In the dairy cattle industry, the ability to control the sex of calves presents a significant advantage for maximising profit from cattle breeding and dairy farming. Initially developed by Johnson et al.1, sperm sex sorting by flow cytometry is now widely used to sort X and Y bearing sperm and control the sex of calves by using artificial insemination. At present, artificial insemination of sex-sorted semen can achieve a female calf rate of >90%2. Additionally, more than 40% of embryo transfers across the world use embryos produced in vitro by in vitro fertilisation (IVF), and sex-sorted sperm plays an important role in the commercial production of sexed embryos by IVF3,4.
Both flow cytometry sorting5 and cryopreservation6 greatly increase reactive oxygen species (ROS) levels in sperm. ROS is the main inducer of lipid peroxidation (LPO) in sperm because their plasma membranes are rich in polyunsaturated fatty acids, the principal target for oxidation7, and this decreases sperm motility and viability8. ROS also cause DNA fragmentation, altering cellular communication and enzymatic pathways9, leading to motility loss, disruption of membrane fusion events, poor fertilisation rate, and impaired embryogenesis10. Meanwhile, sperm cytoplasm is mainly restricted to the midpiece, in which very few antioxidant mechanisms operate7,11, and antioxidants are further diminished when freezing spermatozoa due to the large dilution during the cryopreservation procedure5. Furthermore, freeze-thawed bull spermatozoa are more easily peroxidised than fresh spermatozoa12. Thus, lowering ROS levels in sex-sorted sperm remains a priority.
Resveratrol (Res) is a natural grape-derived phytoalexin13 possessing stronger antioxidant activity than vitamins E and C, as well as lower toxicity14. Res acts both in the initiation and propagation of the oxidative process14, and can access peroxidised rigid membranes and increase membrane fluidity12,15,16. Researchers have successfully utilized Res to stimulate energetic metabolism in spermatozoa to improve viability and motility6,16. However, the effects of Res on the quality of sex-sorted bull sperm remain poorly understood, as do the mechanisms involved.
In the present study, five different concentrations (0, 10−3, 10−4, 10−5, and 10−6 M) of Res were supplemented in sex-sorted bull sperm washing and fertilisation medium. After washing twice with washing medium and incubating in fertilisation medium for 1.5 h, sex-sorted sperm from three bulls (005x, 011x, and 056x) were tested for ROS, phosphatidylserine (PS) externalisation, mitochondrial membrane potential (Δψm), ATP and malondialdehyde (MDA), as well as acrosomal integrity, and blastocyst rate and blastocyst quality following IVF. The results contribute to the development of an efficient method for improving the fertilisation capacity of sex-sorted bull sperm, and exploration of the mechanisms involved.
Results
Effect of Res on ROS levels in sex-sorted sperm from three bulls
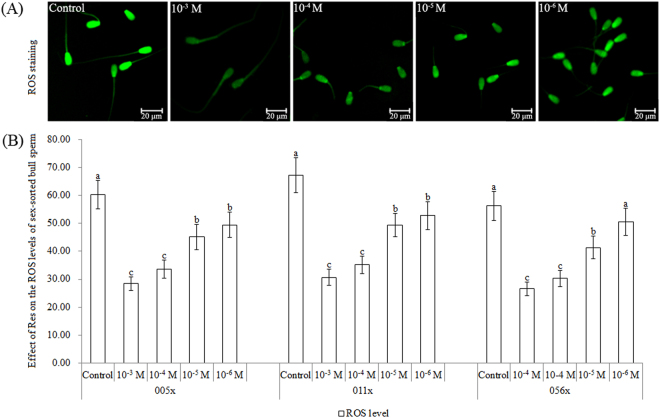
As shown in Fig. 1B, for bulls 005x, 011x, and 056x, ROS levels in the 10−4 M Res group were similar to those in the 10−3 M group, but significantly lower than those in the 10−5 M group (P < 0.05), while ROS levels in these three groups were significantly lower than those in the control group (P < 0.05).
Figure 1.
Effect of Res on ROS levels in sex-sorted bull sperm. (A) Representative images showing ROS staining. Scale bar = 20 μm. (B) a,b,cValues with no common superscript are different (P < 0.05).
Effect of Res on PS externalisation of bull sex-sorted sperm
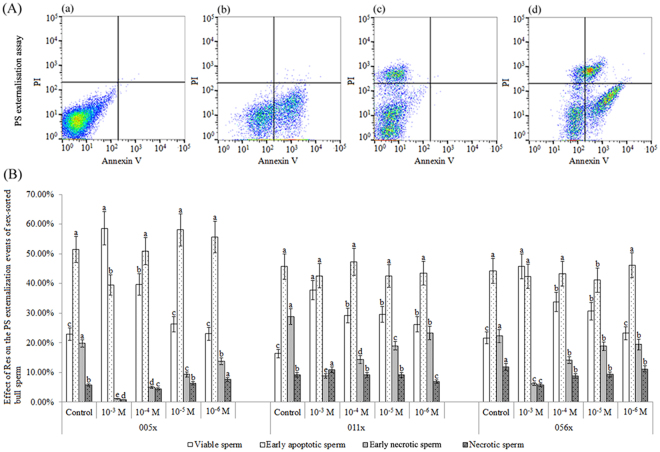
As shown in Fig. 2B, for bulls 005x, 011x, and 056x, the percentage of viable sperm in the 10−4 M Res group (39.69 ± 3.64%, 29.17 ± 2.49%, and 33.71 ± 3.25%, respectively) was significantly lower than in the 10−3 M Res group (58.58 ± 5.62%, 37.80 ± 3.25%, and 45.82 ± 4.16%; P < 0.05), but significantly higher than in controls (22.87 ± 2.15%, 16.38 ± 1.41%, and 21.60 ± 2.01%; P < 0.05).
Figure 2.
Effect of Res on PS externalisation in sex-sorted bull sperm. (A) PS externalisation assay. (a) Negative control. (b) Annexin V FITC staining control. (c) PI staining control. (d) Analysis of sex-sorted bull sperm. Quadrants represent viable sperm (lower-left quadrant), necrotic sperm (upper-left quadrant), early apoptotic sperm (lower-right quadrant), and early necrotic sperm (upper-right quadrant). (B) a,b,c,d,eValues with no common superscript are different (P < 0.05).
Meanwhile, the percentage of early necrotic sperm in the 10−3 M Res group (1.16 ± 0.13%, 8.80 ± 0.81%, and 6.12 ± 0.51%) and the 10−4 M Res group (4.97 ± 0.38%, 14.35 ± 1.42%, and 14.20 ± 1.13%) was significantly lower than that of the control group (19.83 ± 1.27%, 28.81 ± 2.74%, and 22.28 ± 2.14%; P < 0.05), but no significant differences were found between the percentage of early apoptotic sperm in the control groups (44.25 ± 4.16% to 51.49 ± 4.35%) and 10−4 M Res groups (43.31 ± 4.25% to 50.88 ± 4.52%; P > 0.05).
Effect of Res on Δψm in sex-sorted bull sperm membranes
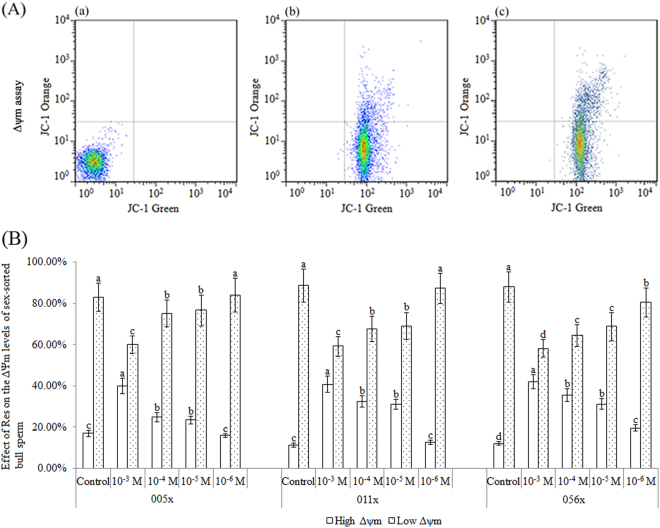
As shown in Fig. 3B, for sex-sorted sperm from bull 005x, the percentage of sperm with a high Δψm value in 10−3 M (40.01 ± 3.82%), 10−4 M (24.80 ± 2.17%), and 10−5 M (23.40 ± 1.83%) Res groups was significantly higher than that of the control group (16.91 ± 1.45%; P < 0.05). The 10−3 M Res group included the highest percentage of sperm with a high Δψm value, and the same results were found for bulls 011x (40.81 ± 3.92%, 32.43 ± 2.81%, and 31.07 ± 2.24% vs. 11.37 ± 1.06%; P < 0.05) and 056x (41.88 ± 3.42%, 35.59 ± 3.06%, and 31.22 ± 2.57% vs. 12.04 ± 0.48%; P < 0.05).
Figure 3.
Effect of Res on Δψm in sex-sorted bull sperm membranes. (A) Δψm assays. (a) Negative control. (b) Positive control. (c) Analysis of sex-sorted bull sperm. Quadrants represent sperm with low Δψm (lower-right quadrant) and high Δψm (upper-right quadrant). (B) a,b,c,dValues with no common superscript are different (P < 0.05).
Meanwhile, for sex-sorted sperm of bull 005x, the percentage of sperm with a low Δψm value in 10−3 M (59.99 ± 4.37%), 10−4 M (75.20 ± 6.54%), and 10−5 M (76.60 ± 7.48%) Res groups was significantly lower than that of the control group (83.06 ± 6.82%; P < 0.05). The 10−3 M Res group included the lowest percentage of sperm with a low Δψm value, and the same results were found for bulls 011x (59.18 ± 4.82%, 67.57 ± 6.15%, and 68.94 ± 6.46% vs. 88.63 ± 8.13%; P < 0.05) and 056x (58.12 ± 4.27%, 64.41 ± 5.41%, and 68.77 ± 6.75% vs. 87.93 ± 7.32%; P < 0.05).
Effect of Res on ATP content in sex-sorted bull sperm
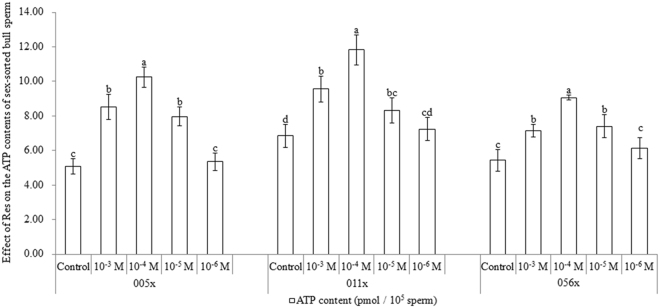
As shown in Fig. 4, for sex-sorted sperm from bulls 005x, 011x, and 056x, the ATP content in 105 sperm from the 10−3 M Res group (8.51 ± 0.73 pmol, 9.56 ± 0.74 pmol, and 7.15 ± 0.35 pmol) was similar to that in the 10−5 M Res group (7.97 ± 0.56 pmol, 8.32 ± 0.74 pmol, and 7.40 ± 0.70 pmol), significantly lower than the 10−4 M Res group (10.24 ± 0.58 pmol, 11.84 ± 0.87 pmol, and 9.07 ± 0.14 pmol), and significantly higher than controls (5.10 ± 0.44 pmol, 6.85 ± 0.66 pmol, and 5.43 ± 0.64 pmol; P < 0.05).
Figure 4.
Effect of Res on ATP content in sex-sorted bull sperm. a,b,c,dValues with no common superscript are different (P < 0.05).
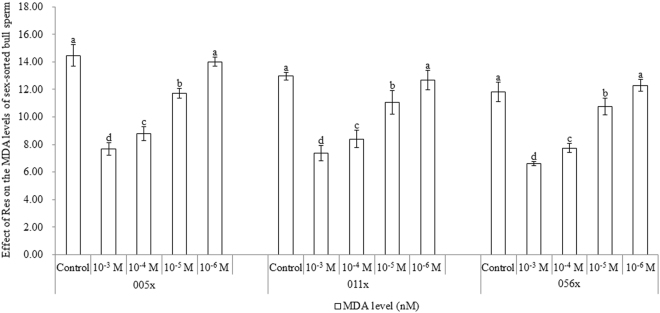
Effect of Res on MDA content in sex-sorted bull sperm
As shown in Fig. 5, for sex-sorted sperm from bulls 005x, 011x, and 056x, the MDA content in the 10−3 M Res group (7.67 ± 0.47 nM, 7.37 ± 0.54 nM, and 6.62 ± 0.14 nM) was significantly lower than that in the 10−4 M Res group (8.79 ± 0.49 nM, 8.41 ± 0.65 nM, and 7.75 ± 0.31 nM; P < 0.05), and they were all significantly lower than controls (14.47 ± 0.79 nM, 12.96 ± 0.29 nM, and 11.83 ± 0.71 nM; P < 0.05).
Figure 5.
Effect of Res on MDA content in sex-sorted bull sperm. a,b,c,dValues with no common superscript are different (P < 0.05).
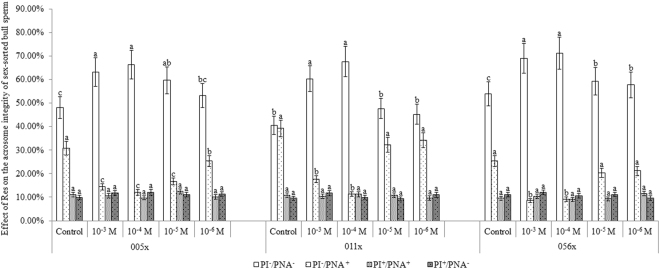
Effect of Res on acrosome integrity in sex-sorted bull sperm
Figure 6 shows representative acrosome staining images. As shown in Fig. 7, for sex-sorted sperm from bulls 005x, 011x, and 056x, the percentage of viable sperm with acrosomal integrity in the 10−4 M Res group (66.27% ± 6.08%, 67.61 ± 6.38%, and 71.21 ± 6.81%) was similar to the 10−3 M Res group (63.16 ± 6.14%, 60.29 ± 5.46%, and 68.97 ± 6.38%), and significantly higher than the control group (48.15 ± 4.62%, 40.54 ± 3.85%, and 53.97 ± 5.06%; P < 0.05). Meanwhile, the percentage of viable sperm without acrosomal integrity in the 10−4 M Res group (12.05 ± 1.16%, 11.27 ± 1.03%, and 9.09 ± 0.89%) was similar to the 10−3 M Res group (14.47 ± 1.32%, 17.65 ± 1.43%, and 8.62 ± 0.82%), and significantly lower than the control group (30.86 ± 2.93%, 39.19 ± 3.61%, and 25.40 ± 2.31%; P < 0.05). No significant differences were found in the percentage of dead sperm with or without acrosomal integrity among all five groups for each bull.
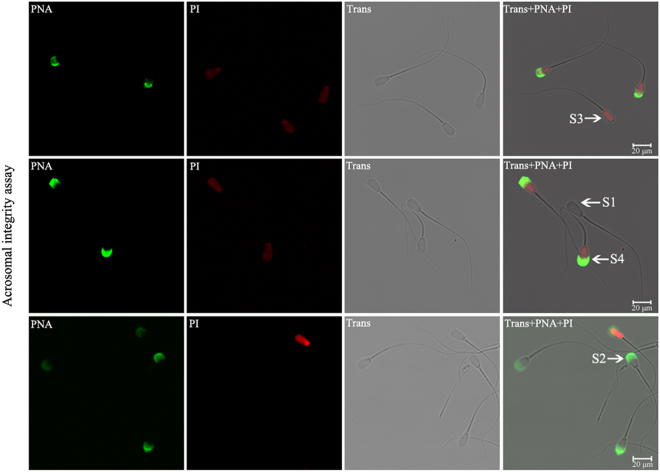
Figure 6.
Representative images of acrosome staining of sex-sorted bull sperm. S1, viable sperm with integral acrosomes (PI−/PNA−). S2, viable sperm with damaged acrosomes (PI−/PNA+). S3, dead sperm with acrosomal integrity (PI+/PNA−). S4, dead sperm with damaged acrosomes (PI+/PNA+). Scale bar = 20 μm.
Figure 7.
Effect of Res on acrosome integrity in sex-sorted bull sperm. a,b,cValues with no common superscript are different (P < 0.05).
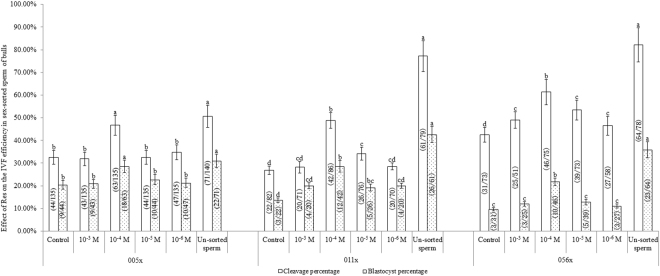
Effect of Res on IVF efficiency in sex-sorted bull sperm
As shown in Fig. 8, for sex-sorted sperm from bull 005x, the cleavage and blastocyst percentages in the 10−4 M Res group (46.67 ± 4.41%, and 28.57 ± 2.68%) were similar to those in un-sorted sperm (50.71 ± 4.89%, and 30.99 ± 2.98%), but significantly higher than the control group (32.59 ± 3.02%, and 20.45 ± 2.01%; P < 0.05).
Figure 8.
Effect of Res on IVF efficiency in sex-sorted bull sperm. a,b,c,dValues with no common superscript are different (P < 0.05).
For sex-sorted sperm from bull 056x, cleavage and blastocyst percentages of the 10−4 M Res group (61.33 ± 5.65%, and 21.74 ± 1.65%) were significantly lower than those of un-sorted sperm (82.05 ± 7.52%, and 35.94 ± 3.52%; P < 0.05), but significantly higher than the control group (42.47 ± 3.26%, and 9.68 ± 0.74%; P < 0.05). The same results were found in the cleavage (48.84 ± 3.49% vs. 77.22 ± 6.82%, and 26.83 ± 1.85%; P < 0.05) and blastocyst (28.57 ± 2.46% vs. 42.62 ± 3.57%, and 13.64 ± 0.96%; P < 0.05) percentages for sex-sorted sperm from bull 011x.
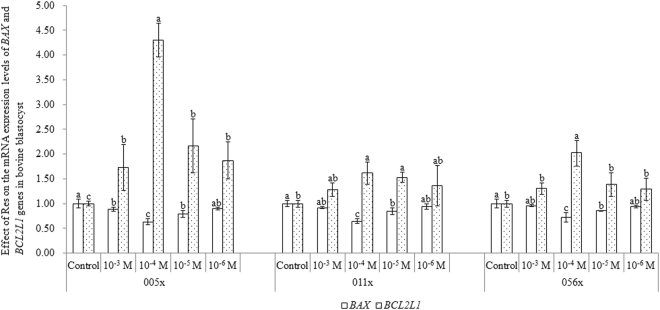
Effect of Res on the expression of BAX and BCL2L1 mRNAs in bovine blastocysts of sex-sorted bull sperm
As shown in Fig. 9, for sex-sorted sperm from bulls 005x, 056x, and 011x, expression levels of BAX mRNA in the 10−4 M and 10−5 M Res groups were significantly lower than controls (P < 0.05). By contrast, BCL2L1 mRNA expression levels in the 10−4 M Res groups were significantly higher than controls (P < 0.05).
Figure 9.
Effect of Res on the expression of BAX and BCL2L1 mRNAs in bovine blastocysts from sex-sorted bull sperm. a,bValues with no common superscript are different (P < 0.05).
Discussion
Effect of Res on ROS levels in sex-sorted bull sperm
It has been reported that Res supplementation in the extender before cryopreservation significantly decreases oxidative stress in buffalo sperm17, and Res treatment significantly decreased ROS levels in mouse sperm11. As shown in Fig. 1, Res supplementation in washing and fertilisation medium significantly decreased ROS levels in sex-sorted bull sperm. Res is a powerful antioxidant due to its ability to inhibit ROS formation by enzymatic and non-enzymatic systems, and its ROS scavenging activity14, which helps to explain these results.
Effect of Res on PS externalisation in sex-sorted bull sperm
PS externalisation is considered an early apoptotic marker. As shown in Fig. 2, 10−3 M or 10−4 M Res treatment significantly increased the percentage of viable sperm. It has been reported that oxidative stress leads to PS translocation in human sperm18,19, which helps to explain the improved percentage of viable sperm in 10−3 M and 10−4 M Res groups containing lower ROS levels (Fig. 1).
Effect of Res on Δψm values in sex-sorted bull sperm
Mitochondria play a significant role in the apoptotic process20, and impaired mitochondrial function results in diminished fertility and motility in sperm from human21 and ram22. The Δψm parameter is an important indicator of mitochondrial function, and its measurement provides useful information on the ability of sperm to fertilise eggs23. Oxidative stress can lower the Δψm value in human sperm24,25, and loss of Δψm decreases the ability of sperm to trigger the acrosome reaction26, impairs ATP synthesis, and increases ROS generation27.
It has been reported that supplementation of bull semen extender with Res increases mitochondrial activity12. As shown in Fig. 3, our results also showed that Res treatment (10−3 M, 10−4 M, and 10−5 M) significantly increased Δψm in sex-sorted bull sperm, presumably due to lower ROS levels in these groups (Fig. 1) because oxidative stress is known to decrease Δψm in human sperm24,25.
Effect of Res on ATP content in sex-sorted bull sperm
An increase in ROS content can target mitochondria and affect ATP production28, and the Δψm value is positively correlated with the ATP content in sperm29. This may help to explain the higher ATP content in the 10−3 M, 10−4 M and 10−5 M Res groups (Fig. 4), given their lower ROS content and higher Δψm values.
Effect of Res on MDA content in sex-sorted bull sperm
Mammalian spermatozoa, especially frozen sperm, are highly sensitive to LPO6, which is formed following oxidation of membrane lipids by ROS30. MDA is the main byproduct of LPO, which aside from its longevity, can easily diffuse across membranes and react with proteins or DNA to form adducts31. Similar to previous reports20,32, our current results also showed that 10−3 M or 10−4 M Res treatment significantly decreased the MDA content in sex-sorted bull sperm (Fig. 5), presumably due to their lower ROS levels, as described above.
Effect of Res on acrosome integrity in sex-sorted bull sperm
ROS impairs the acrosome membrane of sperm33. Moreover, polyunsaturated fatty acids (PUFAs) located in sperm are sensitive to the LPO cascade, resulting in sperm membrane lipid peroxidation injury and changes to the liquidity and integrity of the membrane, which can result in acrosome breakage and leakage of the contents, culminating in sperm-egg recognition errors34. Moreover, it has been proven that Res plays an important role in preventing premature sperm capacitation and, consequently, acrosome reaction35,36. Together, these results can help explain the higher percentage of sex-sorted sperm in which the integrity of the acrosome is preserved in the 10−3 M or 10−4 M Res groups, as shown in Fig. 7.
Effect of Res on IVF efficiency in sex-sorted bull sperm
It has been reported that 50 μM Res supplementation in the cryopreservation extender significantly increases the normal fertilisation rate of buffalo oocytes17, and 2 mM Res supplementation in thawing extender exerts a positive effect on the total efficiency of fertilisation in boar sperm13. Similarly, our experiments showed that 10−4 M Res supplementation in washing and fertilisation medium of sex-sorted bull sperm significantly increased the cleavage and blastocyst percentage in oocytes following IVF (Fig. 8).
ROS can induce LPO and DNA fragmentation in sperm, decreasing sperm motility37 and lowering the Δψm value in human sperm24,25, and diminishing the ability of sperm to trigger the acrosome reaction26. Res can protect bull sperm motility12 and prevent premature capacitation of buffalo sperm by protecting membranes from toxic oxygen metabolites17. In our present study, Res significantly decreased ROS levels (Fig. 1), PS externalisation (Fig. 2), and MDA content (Fig. 5), and increased the Δψm value (Fig. 3) and ATP content (Fig. 4), which helps to explain the increased cleavage and blastocyst percentages of sex-sorted bull sperm following IVF (Fig. 8).
Effect of Res on the expression of BAX and BCL2L1 mRNAs in bovine blastocysts of sex-sorted bull sperm
The BAX protein enhances the release of cytochrome c from mitochondria and thereby promotes apoptosis38, while the BCL2L1 protein inhibits the release of cytochrome c from mitochondria, preventing apoptosis39. In our experiments, Res treatment significantly increased the expression of BCL2L1 mRNAs and decreased the expression of BAX mRNAs (Fig. 9), indicating that Res treatment of sex-sorted sperm inhibits the apoptotic pathway in blastocysts obtained following IVF.
In conclusion, our results showed that treatment with 10−4 M Res significantly decreased the levels of ROS, MDA, and PS externalisation, and protected mitochondrial function and acrosomal integrity in sex-sorted bull sperm, thereby increasing their blastocyst percentage and blastocyst quality following IVF.
Materials and Methods
Chemicals and Ethics statement
Unless otherwise specified, all chemicals and reagents used in the experiments were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All treatment of animals was performed according to the requirements of the Institutional Animal Care and Use Committee of the Chinese Academy of Agricultural Sciences. Before the start of this experiment, the protocol was approved by the same committee, including feeding, management of animals, and so on.
Oocyte recovery and in vitro maturation (IVM)
Bovine ovaries were transported from a local slaughterhouse to the laboratory within 2 h of collection. Cumulus-oocyte complexes (COCs) were aspirated from follicles (2−8 mm in diameter), and only those surrounded with at least three layers of cumulus cells were selected for IVM. IVM medium contained HEPES-buffered TCM-199 (Earle’s salt; Gibco BRL, Grand Island, NY, USA) enriched with 10 μg/mL follicle stimulating hormone (FSH), 10% (v/v) fetal bovine serum (FBS), 10 μg/mL luteinising hormone (LH), 10 μg/mL heparin, and 1 μg/mL estradiol. For IVM, groups of 50 COCs were incubated in four-well plates containing 500 μL IVM-culture drops under mineral oil and incubated in a humidified atmosphere at 38.5 °C in 5% CO2 for 22–24 h.
Preparation of sex-sorted semen
Frozen sex-sorted bovine sperm were purchased from OX Livestock Breeding Co. Ltd (Shandong, China). During the thawing procedure, frozen sex-sorted semen was removed from liquid nitrogen and placed in a water bath at 38 °C for 1 min, then transferred to 15 mL sterile conical centrifuge tubes and mixed with 3 mL pre-heated washing medium (Brackett and Oliphant medium supplemented with 2.5 mM caffeine and different concentrations of Res). Subsequently, they were washed by centrifuging for 5 min at 328 × g. After centrifugation, the sperm pellet was suspended in fertilisation medium (Brackett and Oliphant medium contain 20 mg/mL BSA, 20 μg/mL heparin, 100 IU/mL penicillin, 100 μg/mL streptomycin, and different concentrations of Res) to a final concentration of 1 × 106/mL. Resuspended sperm were then incubated at 38.5 °C in 5% CO2 for 1.5 h before use in subsequent experiments.
Analysis of ROS levels
According to the method described by Barroso et al.40, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was used to analyse ROS levels in sperm. Briefly, each sperm group was incubated in 10 μM H2DCFDA for 20 min at 38.5 °C in the dark. Samples were then washed and centrifuged for 5 min at 328 × g, resuspended in phosphate-buffered saline (PBS), and analysed by a laser confocal microscope (Leica, SP8, Germany) to measure ROS levels within cells.
Analysis of PS externalisation events
An Annexin V-FITC kit (Biovision, Mountain View, CA, USA) was used to detect PS externalisation events in sperm. In brief, a sperm pellet prepared as described above was supplemented with 1 mL of 1× Annexin V binding buffer, then washed by centrifuging for 5 min at 328 × g. Subsequently, the sperm pellet was incubated in 5 μL Annexin V-FITC and 5 μL propidium iodide (PI) at 38.5 °C for 5 min in the dark. Samples were analyzed by flow cytometry (MoFlo XDP, Beckman, USA), and a total of 10,000 sperm were analysed for each group. Sperm stained and unstained with Annexin V-FITC or PI were used as controls.
According to the method described by Anzar et al.41, sperm were separated into four groups based on staining results; viable sperm (Annexin V-FITC− and PI−), early apoptotic sperm (Annexin V-FITC+ and PI−), early necrotic sperm (Annexin V-FITC+ and PI+), and necrotic sperm (Annexin V-FITC− and PI+). Each experiment was repeated at least three times.
Analysis of Δψm using a JC-1 probe
A JC-1 probe (MitoProbe JC-1 assay kit; Invitrogen) was used to evaluate the Δψm value of sex-sorted bull sperm according to the method described previously42. Each sperm group was incubated in 2 μM JC-1 for 30 min at 38.5 °C in the dark, and samples were washed then analysed using flow cytometry. A total of 10,000 sperm were analysed for each group. Meanwhile, unstained sperm were used as negative controls, and sperm exposed to ultraviolet light for 30 min were used as positive controls.
Analysis of ATP content
A bioluminescence assay kit (ATP Bioluminescence Assay Kit HS II, Roche Diagnostics GmbH, Mannheim, Germany) was used to measure the ATP content of sex-sorted sperm. Briefly, sperm from each group were added to extraction medium consisting of 100 mM TRIS-HCl (pH 7.75) and 4 mM EDTA. The sperm suspension was boiled for at 100 °C for 2 min, then centrifuged at 12,000 × g for 20 min. The ATP content of the supernatant was measured using a Bioluminescence Assay Kit. The luminescence was read using a luminometer (InfniteM200, Tecan Group Ltd., Untersbergstrasse, Austria), and data expressed as picomoles (pmol) of ATP per 105 sperm.
Analysis of MDA content
An MDA kit (Nanjing Jiancheng Bioengineering Institute, China) was used to measure the MDA level in sperm samples. For this assay, each sperm group was incubated for 80 min in 0.5% TBA, followed by rapid cooling and acquisition of the supernatant by high-speed centrifugation, and samples were injected directly into a spectrophotometer (Beckman Coulter, Inc., CA, USA). MDA levels are expressed in nM units.
Analysis of acrosomal integrity by FITC-PNA
According to the method described previously43, sperm were stained with 5 µg/mL FITC-PNA and 50 µg/mL PI and incubated at 38.5 °C in the dark for 10 min. Sperm were then washed and observed by confocal microscopy (Nikon, Tokyo, Japan). The percentage of viable sperm with integral acrosomes (PI−/PNA−), viable sperm with damaged acrosomes (PI−/PNA+), dead sperm with acrosomal integrity (PI+/PNA−), and dead sperm with damaged acrosomes (PI+/PNA+) was then determined.
IVF experiments
After IVM, COCs were transferred into 0.1% (w/v) hyaluronidase for 30 s and gently pipetted to remove some of the cumulus cells. Only oocytes with the first poly body and homogenous cytoplasm were selected for IVF. Sex-sorted sperm were washed and diluted to achieve a concentration of 5 × 106/mL as mentioned above. A 20 μL aliquot of this suspension was added to 80 μL droplets of fertilisation medium containing 20–30 oocytes under mineral oil, and incubated at 38.5 °C in air with 5% CO2 for insemination. After 16−18 h, presumptive zygotes were transferred and cultured in CR1aa culture medium (Rosenkrans and First, 1994) for 48 h. Cleaved embryos were then recorded and cultured in CR1aa medium containing 10% FBS for 5 days. The medium was changed every 2 days throughout the culture period. To evaluate the effect of Res on fertilisation, cleavage and blastocyst percentages were recorded on days 2 and 7 after fertilisation.
Analysis mRNA levels of apoptotic genes in blastocysts following IVF
A single Cell-to-CT quantitative real-time PCR kit (Life Technologies, Carlsbad, CA, USA) and the 7900HT system (Applied Biosystems, Foster City, CA, USA) were utilised to measure mRNA levels of apoptotic genes in blastocysts as described previously44. The PCR procedure was carried out in 10 μL mixtures containing 2 μL complementary DNA, 5 μL Fast SYBR Green Master Mix (Invitrogen), and 0.2 μL of each primer (10 μM). Primers are listed in Table 144. The results were analysed by the comparative Ct (2−ΔΔCt) method with Β-ACTIN as the reference gene.
Table 1.
Sequences of primers used to amplify the selected blastocyst genes44.
| Gene | Primers (5′-3′) | GeneBank accession No. |
|---|---|---|
| BAX | F: TTCTGACGGCAACTTCAACTG | NM_173894.1 |
| R: CTCTCGAAGGAAGTCCAATGTC | ||
| BCL2L1 | F: AGGAGATGCAGGTATTGGTGA | NM_001077486.2 |
| R: CATTGTTCCCGTAGAGTTCCA | ||
| R: CATCTCCTGAGGAAGACCAAA | ||
| Β-ACTIN | F: GGGAAATCGTCCGTGACATCA | NM_173979 |
| R: GATGGTGATGACCTGCCCGT |
Experimental design
To investigate the effect of Res on the quality of sex-sorted bull semen and the mechanisms involved, five different concentrations of Res (0, 10−3, 10−4, 10−5, and 10−6 M) were supplemented in the washing medium and fertilisation medium for bulls 005x, 011x, and 056x, and levels of ROS, PS externalisation, Δψm, ATP, MDA, and acrosomal integrity, blastocyst rate, and quality following IVF were determined.
Statistical analysis
Data are expressed as mean ± standard error. All biological experiments were repeated at least three times. All data were analysed using Statistical Analysis System software (SAS Institute Inc., Cary, NC, USA) to perform one-way analysis of variance (ANOVA) using Duncan’s tests. For all analyses, the results were considered statistically significant at P < 0.05.
Acknowledgements
This work was supported by the The Foundation of Chinese Academy of Agricultural Sciences (Y2017JC54), and the Agricultural Science and Technology Innovation Program (ASTIP- 2016-IAS-06) funding. We are very grateful to Prof Evans from University College Dublin (Ireland) for careful reading of the manuscript.
Author Contributions
X.M.Z. designed and performed experiments, Y.-H.Z., H.-S.H., H.-Y.W., J.-M.H., C.-L.Y., P.-P.Z., W.-H.D., Y.L., Y.-W.P. and H.-B.Z. analysed the data and contributed reagents, C.Y.L., P.-P.Z. and X.-M.Z. wrote the manuscript, and all authors read and approved the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Chong-Yang Li and Ya-Han Zhao contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hua-Bin Zhu, Email: zhuhuabin@caas.cn.
Xue-Ming Zhao, Email: zhaoxueming@caas.cn.
References
- 1.Johnson LA, Flook JP, Hawk HW. Sex preselection in rabbits: live births from X and Y sperm separated by DNA and cell sorting. Biol Reprod. 1989;41:199–203. doi: 10.1095/biolreprod41.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Chang LB, et al. Artificial insemination of Holstein heifers with sex-sorted semen during the hot season in a subtropical region. Trop Anim Health Prod. 2017;49:1157–1162. doi: 10.1007/s11250-017-1311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao XM, et al. Apoptosis-like events and in vitro fertilization capacity of sex-sorted bovine sperm. Reprod Domest Anim. 2014;49:543–549. doi: 10.1111/rda.12305. [DOI] [PubMed] [Google Scholar]
- 4.An LY, et al. Significant heparin effect on bovine embryo development during sexed in vitro fertilization. J Reprod Dev. 2017;63:175–183. doi: 10.1262/jrd.2016-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev. 2000;55:282–288. doi: 10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Cheema RS, Bansal AK, Bilaspuri GS. Manganese provides antioxidant protection for sperm cryopreservation that may offer new consideration for clinical fertility. Oxid Med Cell Longev. 2009;2:152–159. doi: 10.4161/oxim.2.3.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tvrda E, et al. Antioxidant efficiency of lycopene on oxidative stress - induced damage in bovine spermatozoa. J Anim Sci Biotechnol. 2016;7:50. doi: 10.1186/s40104-016-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saradha B, Mathur PP. Effect of environmental contaminants on male reproduction. Environ Toxicol Pharmacol. 2006;21:34–41. doi: 10.1016/j.etap.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41:183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 10.Baker MA, Aitken RJ. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod Biol Endocrinol. 2005;3:67. doi: 10.1186/1477-7827-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mojica-Villegas MA, Izquierdo-Vega JA, Chamorro-Cevallos G, Sanchez-Gutierrez M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients. 2014;6:489–503. doi: 10.3390/nu6020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucak MN, et al. Lycopene and resveratrol improve post-thaw bull sperm parameters: sperm motility, mitochondrial activity and DNA integrity. Andrologia. 2015;47:545–552. doi: 10.1111/and.12301. [DOI] [PubMed] [Google Scholar]
- 13.Gadani B, Bucci D, Spinaci M, Tamanini C, Galeati G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology. 2017;90:88–93. doi: 10.1016/j.theriogenology.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Silva EC, Cajueiro JF, Silva SV, Soares PC, Guerra MM. Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology. 2012;77:1722–1726. doi: 10.1016/j.theriogenology.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Brittes J, Lucio M, Nunes C, Lima JL, Reis S. Effects of resveratrol on membrane biophysical properties: relevance for its pharmacological effects. Chem Phys Lipids. 2010;163:747–754. doi: 10.1016/j.chemphyslip.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Eleawa SM, et al. Resveratrol reverses cadmium chloride-induced testicular damage and subfertility by downregulating p53 and Bax and upregulating gonadotropins and Bcl-2 gene expression. J Reprod Dev. 2014;60:115–127. doi: 10.1262/jrd.2013-097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longobardi V, et al. Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology. 2017;88:1–8. doi: 10.1016/j.theriogenology.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Moustafa MH, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004;19:129–138. doi: 10.1093/humrep/deh024. [DOI] [PubMed] [Google Scholar]
- 19.Kotwicka M, Jendraszak M, Jedrzejczak P. Phosphatidylserine membrane translocation in human spermatozoa: topography in membrane domains and relation to cell vitality. J Membr Biol. 2011;240:165–170. doi: 10.1007/s00232-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuluğ E, Türedi S, Alver A, Türedi S, Kahraman C. Ffects of resveratrol on methotrexate-induced testicular damage in rats. ScientificWorldJournal. 2013;2013:489–659. doi: 10.1155/2013/489659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connell M, McClure N, Lewis SE. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod. 2002;17:704–709. doi: 10.1093/humrep/17.3.704. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Pastor F, et al. Use of chromatin stability assay, mitochondrial stain JC-1, and fluorometric assessment of plasma membrane to evaluate frozen-thawed ram semen. Anim Reprod Sci. 2004;84:121–133. doi: 10.1016/j.anireprosci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Espinoza JA, Schulz MA, Sanchez R, Villegas JV. Integrity of mitochondrial membrane potential reflects human sperm quality. Andrologia. 2009;41:51–54. doi: 10.1111/j.1439-0272.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 24.Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, et al. Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003;80(Suppl 2):844–850. doi: 10.1016/S0015-0282(03)00983-X. [DOI] [PubMed] [Google Scholar]
- 26.Gallon F, Marchetti C, Jouy N, Marchetti P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril. 2006;86:1526–1530. doi: 10.1016/j.fertnstert.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Rajender S, Rahul P, Mahdi AA. Mitochondria, spermatogenesis and male infertility. Mitochondrion. 2010;10:419–428. doi: 10.1016/j.mito.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Rexroth S, et al. Reactive oxygen species target specific tryptophan site in the mitochondrial ATP synthase. Biochim Biophys Acta. 2012;1817:381–387. doi: 10.1016/j.bbabio.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Ryu DY, et al. Peroxiredoxin activity is a major landmark of male fertility. Sci Rep. 2017;7:17174. doi: 10.1038/s41598-017-17488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucak MN, et al. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: antioxidants protect DNA integrity against cryodamage. Cryobiology. 2010;61:248–253. doi: 10.1016/j.cryobiol.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360–438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uguralp S, Usta U, Mizrak B. Resveratrol may reduce apoptosis of rat testicular germ cells after experimental testicular torsion. Eur J Pediatr Surg. 2005;15:333–336. doi: 10.1055/s-2005-865757. [DOI] [PubMed] [Google Scholar]
- 33.Rui BR, et al. Impact of induced levels of specific free radicals and malondialdehyde on chicken semen quality and fertility. Theriogenology. 2017;90:11–19. doi: 10.1016/j.theriogenology.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XG, et al. The effects of different levels of superoxide dismutase in Modena on boar semen quality during liquid preservation at 17 degrees C. Anim Sci J. 2017;88:55–62. doi: 10.1111/asj.12574. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, et al. Resveratrol reduces intracellular free calcium concentration in rat ventricular myocytes. Sheng Li Xue Bao. 2005;57:599–604. [PubMed] [Google Scholar]
- 36.McNiven MA, Richardson GF. Effect of quercetin on capacitation status and lipid peroxidation of stallion spermatozoa. Cell Preserv Technol. 2006;4:169–177. doi: 10.1089/cpt.2006.4.169. [DOI] [Google Scholar]
- 37.Ourique GM, et al. Resveratrol improves sperm motility, prevents lipid peroxidation and enhances antioxidant defences in the testes of hyperthyroid rats. Reprod Toxicol. 2013;37:31–39. doi: 10.1016/j.reprotox.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 39.Tan Y, Demeter MR, Ruan H, Comb MJ. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000;275:25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 40.Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000;15:1338–1344. doi: 10.1093/humrep/15.6.1338. [DOI] [PubMed] [Google Scholar]
- 41.Anzar M, He L, Buhr MM, Kroetsch TG, Pauls KP. Sperm apoptosis in fresh and cryopreserved bull semen detected by flow cytometry and its relationship with fertility. Biol Reprod. 2002;66:353–360. doi: 10.1095/biolreprod66.2.354. [DOI] [PubMed] [Google Scholar]
- 42.Gravance CG, Garner DL, Baumber J, Ball BA. Assessment of equine sperm mitochondrial function using JC-1. Theriogenology. 2000;53:1691–1703. doi: 10.1016/S0093-691X(00)00308-3. [DOI] [PubMed] [Google Scholar]
- 43.Purdy PH. Ubiquitination and its influence in boar sperm physiology and cryopreservation. Theriogenology. 2008;70:818–826. doi: 10.1016/j.theriogenology.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 44.Wang N, et al. Calcium ion regulation by BAPTA-AM and ruthenium red improved the fertilisation capacity and developmental ability of vitrified bovine oocytes. Sci Rep. 2017;7:10652. doi: 10.1038/s41598-017-10907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]