Abstract
Past life on Mars will have generated organic remains that may be preserved in present day Mars rocks. The most recent period in the history of Mars that retained widespread surface waters was the late Noachian and early Hesperian and thus possessed the potential to sustain the most evolved and widely distributed martian life. Guidance for investigating late Noachian and early Hesperian rocks is provided by studies of analogous acidic and sulfur-rich environments on Earth. Here we report organic responses for an acid stream containing acidophilic organisms whose post-mortem remains are entombed in iron sulphates and iron oxides. We find that, if life was present in the Hesperian, martian organic records will comprise microbial lipids. Lipids are a potential sizeable reservoir of fossil carbon on Mars, and can be used to distinguish between different domains of life. Concentrations of lipids, and particularly alkanoic or “fatty” acids, are highest in goethite layers that reflect high water-to-rock ratios and thus a greater potential for habitability. Goethite can dehydrate to hematite, which is widespread on Mars. Mars missions should seek to detect fatty acids or their diagenetic products in the oxides and hydroxides of iron associated with sulphur-rich environments.
Introduction
The martian surface environment has changed through time and the rock record has preserved evidence of a series of mineralogically-distinct Eras1–4. Early Noachian Mars displayed wet and more Earth-like conditions that led to the production of widespread hydrated phyllosilicates1,2,5–7. The diminishment of the martian atmosphere during the late Noachian8,9 in conjunction with increasing volcanic activity, led to acidic, sulfur-rich conditions and the regional deposition of sulfate salts in aqueous conditions that continued throughout the Hesperian6,10–12. There then followed an environment characterized by oxidising and dry conditions dominated by iron oxides, which has existed for a majority of Mars history, from 3.0 Ga up to and including the present day1.
It has been suggested that the preservation of life’s organic compounds may be difficult in Fe3+ mineral-containing sedimentary deposits owing to a predisposition for oxidation reactions13,14, yet contrary evidence has been provided by molecular organic extracts of ferruginous sediments from recent15 and ancient16 deposits from natural acid streams on Earth (e.g. Rio Tinto), while another study found that up to 21% of organic carbon in sediment is bound to Fe-bearing phases17. Lipid preservation studies have already been conducted in other Fe-rich environments such as iron hot springs, and suggest that the encrustation of cells by iron oxide minerals can enhance the initial preservation of lipids by rendering them unavailable for enzymatic degradation by heterotrophs18,19.
Acidic streams on Earth are proposed as appropriate mineralogical environments to study as analogues of Mars20–23. We collected samples from an acidic stream (pH 3.5) located in St. Oswald’s Bay, Dorset (Figs 1 and 2), in which ferric sulfate-rich streamwaters derived from the aqueous oxidation of pyrite in surrounding sedimentary rocks have led to the precipitation of jarosite, in a process that is analogous to the provenance of some martian jarosite24. Goethite is also observed that may have been formed together with jarosite24, or produced by the transformation of jarosite, in line with established understanding of reactions in iron-rich sediments25,26.
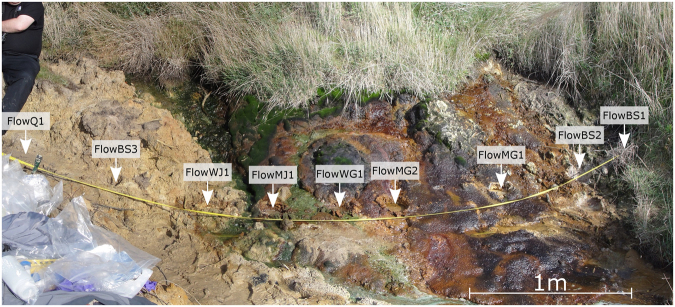
Figure 1.
Overview of a ferric sulfate-rich stream in Dorset, Southern England. The study area is approximately 4 m across, and the locations of each of the sampled cores are shown. Ferric iron and sulfates are derived from the oxidation of pyrite in surrounding sedimentary rocks, giving the water a pH of 3.5.
Figure 2.

A closeup of the mineralogy present in the stream. Jarosite (yellow) is precipitated and where water-to-rock ratios are high and/or the pH increases, jarosite can be converted to goethite (reddish-brown). The stream hosts a distinct microbial community, including acidophilic algae (green) and microbial mats (purple).
Acidophilic algae, purple mat-forming sulfur bacteria and wood fragments from the adjacent bankside represent the primary organic inputs into the acid stream system (Fig. 2). Bacteria and algae contain a range of microbial lipids, including fatty acids, that can be used to consider the possible fate of similar lipids from any microbes on Mars while the wood fragments contain oxidized and degraded organic structures, such as phenols and polycyclic aromatic hydrocarbons (PAHs), which bear some superficial chemical similarities to meteoritic organic matter27 and can represent abiotic organic matter delivered to the martian surface as meteorite infall. The acid stream organic assemblage and its mineralogical context provides us with an opportunity to study the early stage diagenesis of lipids in an acidic, Fe-rich environment that acts as a mineralogical and geochemical analogue of conditions suggested by deposits detected on Mars.
We prepared gram-sized samples by grinding and homogenization in a sterilized, porcelain pestle and mortar. Mineral contents were assessed using X-ray diffraction (XRD) (Table 1). Lipid contents were extracted using sonication and centrifugation and an initial monophase water:methanol:chloroform solvent mixture followed by the addition of more water and chloroform to produce a two phase solution with any lipids isolated in the chloroform layer28. The chloroform extract was reduced by evaporation and derivatized with a boron trifluoride and methanol mixture to form fatty acid methyl esters (FAME). This lipid fraction was then analyzed by gas chromatography-mass spectrometry (GC-MS).
Table 1.
Samples from flowing and dry acidic, ferric sulfate-rich streams. XRD data from47.
| Sample | Code | Mineralogy (wt %) or Biomass type | cm from W bank |
|---|---|---|---|
| Flowing stream | |||
| Acidophilic algae | FlowAL | Algae | |
| Bank sediment (W) | FlowBS1a | Grass | 0 |
| FlowBS1b | Q:69,G:0,J:1,I:21,K:8,M:1 | ||
| FlowBS2a | Wood | 30 | |
| FlowBS2b | Q:64,G:0,J:8,I:12,K:15,M:1 | ||
| Matt over goethite | FlowMG1a | Q:64,G:26,J:0,I:0,K:0,M:0 | 85 |
| FlowMG1b | Q:23,G:72,J:5,I:0,K:0,M:0 | ||
| FlowMG1c | Q:89,G:0,J:5,I:0,K:4,M:2 | ||
| FlowMG2a | Q:40,G:59,J:0,I:0,K:1,M:0 | 150 | |
| FlowMG2b | Q:53,G:47,J:0,I:0,K:0,M:0 | ||
| FlowMG2c | Q:89,G:0,J:7,I:0,K:3,M:1 | ||
| Wood over goethite | FlowWG1a | Wood | 190 |
| FlowWG1b | Q:55,G:45,J:0,I:0,K:0,M:0 | ||
| FlowWG1c | Q:80,G:0,J:0,I:13,K:7,M:0 | ||
| FlowWG1d | Q:96,G:0,J:7,I:0,K:0,M:4 | ||
| Matt over jarosite | FlowMJ1a | Q:56,G:19,J:2,I:18,K:4,M:1 | 225 |
| FlowMJ1b | Q:99,G:0,J:0,I:0,K:0,M:1 | ||
| FlowMJ1c | Q:73,G:0,J:26,I:0,K:0,M:1 | ||
| Wood over jarosite | FlowWJ1a | Wood | 260 |
| FlowWJ1b | Q:65,G:0,J:27,I:0,K:8,M:0 | ||
| FlowWJ1c | Q:63,G:0,J:28,I:0,K:9,M:0 | ||
| Bank sediment (E) | FlowBS3a | Q:81,G:0,J:2,I:9,K:7,M:1 | 325 |
| FlowBS3b | Wood | ||
| FlowBS3c | Q:79,G:0,J:1,I:10,K:9,M:1 | ||
| FlowBS3d | Q:58,G:0,J:41,I:0,K:0,M:1 | ||
| FlowBS3e | Q:72,G:0,J:1,I:15,K:11,M:1 | ||
| Quartz sand | FlowQ1 | Q:87,G:0,J:1,I:7,K:4,M:1 | 380 |
| Dry stream | DryMJ1a | Q:87,G:18,J:6,I:25,K:11,M:0 | |
| DryMJ1b | Q:43,G:38,J:19,I:0,K:0,M:0 | ||
| DryMJ1c | Q:23,G:72,J:5,I:0,K:0,M:0 | ||
| DryMJ1d | Q:32,G:56,J:12,I:0,K:0,M:0 | ||
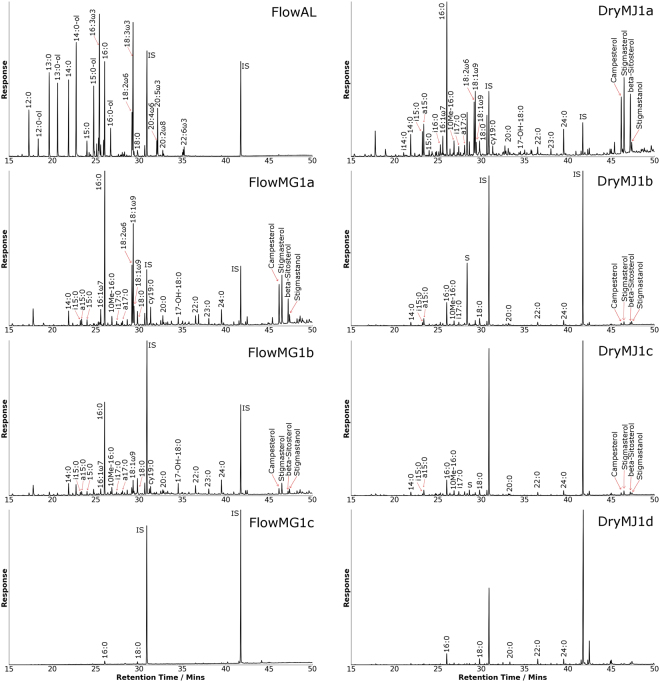
Our acid stream organic matter provided a glimpse into how the general markers of life may be preserved in martian environments of similar chemistries (Fig. 3). In the acid stream biomass, saturated straight-chain fatty acids were by far the most predominant indicators of life, consistent with their recognized high abundance in bacterial membrane phospholipids29–31. The acid stream fatty acids exhibit clearly recognizable even-over-odd (EOP) predominances in carbon-chain length and the C16 and C18 saturated fatty acids were particularly ubiquitous, consistent with the knowledge that these two species of acid are the most common saturated fatty acids in organisms29,31.
Figure 3.
Total ion current chromatograms displaying lipids obtained from solvent extraction of the flowing and dry acidic, ferric sulfate-rich streams, as well as from the algae.
In addition to general indicators of life, some lipids from the acid stream samples were specific to certain types of organisms or biosynthetic pathways. The presence of terminally branched saturated fatty acids, i15:0, a15:0, and a17:0, and mid-chain branched 10Me16:0 detected in FlowMG1a are biomarkers for sulfate-reducing bacteria32–36. Cyclopropyl fatty acids appeared only in the microbial mat samples, consistent with their association with anaerobic bacteria33,34,37. ω-7 monounsaturated fatty acids (with a double bond 7 carbon atoms from the methyl end), such as 11-octadecenoic acid are formed by anaerobic-desaturase pathways, and are thus markers for anaerobic biosynthesis29,35,37,38, though aerobes are capable of using this pathway as well34. In contrast, ω-9 monounsaturated fatty acids such as 9-octadecenoic are produced by an oxygen-mediated biosynthetic pathway38, and are characteristic of cyanobacterial lipid profiles35. If life ever arose on Mars, it is unlikely that its evolutionary development would have extended to land plants; hence the fossil derivatives of ω-7 and cyclopropyl fatty acids would be available as use as microbial markers of anaerobic and aerobic metabolism34. Polyunsaturated fatty acids such as 20:4ω6, 20:5ω3, and 22:6ω3 are considered markers of microalgae39.
Some acid stream compounds are specific to higher plants with the polycyclic terpene phytosteroids such as campesterol, stigmasterol and β-sitosterol detected in the microbial mat samples FlowMG1a, FlowMG2a, FlowMJ1a and DryMJ1a. Goethite samples FlowMG1b, FlowMG2b, FlowWG1b and DryMJ1c also exhibited limited abundances of these compounds. Higher plants never evolved on Mars, but the fate of their steroid remains can suggest how more Mars-relevant polycyclic terpenes, such as bacterial hopanoids, may behave during fossilization under Mars-like conditions. Long-chain alcohols were observed in most of the microbial mat samples, and are derived from wax esters that are not found in purple sulfur bacteria40,41, and are instead markers of plant surface lipids42, which are likely to be of limited relevance to Mars.
Diagenesis is controlled by microbial action or chemical reactions catalyzed by mineral surfaces, and leads to the reduction in abundance and diversity of all lipid compounds with increasing depth43. Almost all acid stream cores in our study display a decrease in fatty acid abundances with depth over centimeter scales (Table 2, Fig. 4). The rate of decrease in fatty acid abundances during diagenesis appears related to the mineralogical host. Goethite layers appear to preserve fatty acids relatively well including the retention of biogenic signatures such as EOP in the saturated fatty acids and the retention of unsaturated fatty acids to some extent. More complex organic compounds such as the polycyclic terpenes preserve less well in our acid stream environment, irrespective of the mineralogical host. Exceptions are DryMJ1c, which experienced repeated instances of stream flow, and the flowing stream bank sediment (FlowBS3d and FlowBS3e) where a wood layer occurred at the middle of the core. Because the acidic stream data can only be confidently extrapolated to the initial stages of diagenesis, it is important to understand how these markers would appear in a fossilized state in order to identify biomarkers in ancient martian sulfate environments. Microbial degradation also causes changes to the chemistry of the lipids, including processes such as defunctionalization. Most of the long-chain fatty acids containing oxygen-functional groups will undergo dehydration and decarboxylation resulting in saturated hydrocarbons30. Thermal diagenesis over long timescales causes the decarboxylation of the saturated fatty acids and their EOP pattern will be converted to a corresponding odd-over-even predominance (OEP) in saturated hydrocarbons44–46. For our acid stream samples, the characteristic C19 cyclopropyl fatty acid would convert to C18 mid-chain mono-methyl alkanes and these compounds have been recognized previously in cyanobacterial mats40.
Table 2.
Fatty acid concentrations (ppm) flowing and dry acidic, ferric sulfate-rich streams.
| Sample | Code | C16 FA | C18 FA | Total FA | cm from W bank |
|---|---|---|---|---|---|
| Flowing stream | |||||
| Acidophilic algae | FlowAL | 176.0 | 17.9 | 1530.8 | |
| Bank sediment (W) | FlowBS1a | 158.3 | 104.3 | 679.3 | 0 |
| FlowBS1b | 35.5 | 21.6 | 93.7 | ||
| FlowBS2a | 42.2 | 16.4 | 575.9 | 30 | |
| FlowBS2b | 6.8 | 4.5 | 36.6 | ||
| Matt over goethite | FlowMG1a | 173.3 | 38.7 | 572.3 | 85 |
| FlowMG1b | 27.7 | 8.2 | 58.9 | ||
| FlowMG1c | 2.0 | 1.4 | 3.3 | ||
| FlowMG2a | 272.7 | 25.2 | 455.1 | 150 | |
| FlowMG2b | 11.3 | 4.3 | 42.6 | ||
| FlowMG2c | 1.9 | 1.2 | 3.1 | ||
| Wood over goethite | FlowWG1a | 60.4 | 41.6 | 196.4 | 190 |
| FlowWG1b | 31.2 | 6.0 | 62.4 | ||
| FlowWG1c | 5.0 | 1.8 | 9.1 | ||
| FlowWG1d | 3.1 | 1.4 | 7.1 | ||
| Matt over jarosite | FlowMJ1a | 62.1 | 16.4 | 226.8 | 225 |
| FlowMJ1b | 3.0 | 2.0 | 5.0 | ||
| FlowMJ1c | 2.3 | 1.4 | 18.7 | ||
| Wood over jarosite | FlowWJ1a | 13.3 | 4.2 | 76.9 | 260 |
| FlowWJ1b | 4.9 | 2.2 | 13.3 | ||
| FlowWJ1c | 2.1 | 1.2 | 4.6 | ||
| Bank sediment (E) | FlowBS3a | 7.0 | 6.9 | 22.9 | 325 |
| FlowBS3b | 5.7 | 3.6 | 103.6 | ||
| FlowBS3c | 2.9 | 1.9 | 75.8 | ||
| FlowBS3d | 6.1 | 4.2 | 11.2 | ||
| FlowBS3e | 3.6 | 3.2 | 25.9 | ||
| Quartz sand | FlowQ1 | 4.1 | 1.7 | 5.8 | 380 |
| Dry stream | DryMJ1a | 91.1 | 22.2 | 338.6 | |
| DryMJ1b | 4.7 | 2.7 | 14.4 | ||
| DryMJ1c | 9.6 | 5.7 | 31.1 | ||
| DryMJ1d | 6.0 | 3.3 | 28.2 | ||
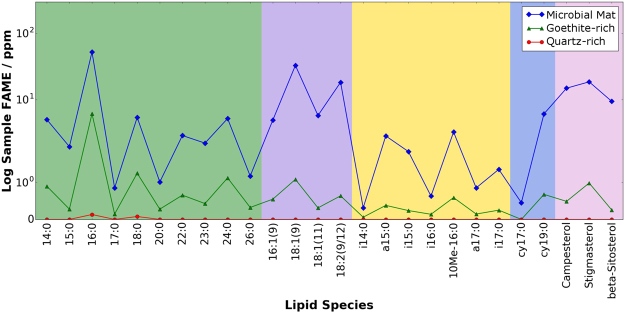
Figure 4.
Lipid abundances (ppm) from three different mineralogies. The core displayed, with increasing depth, a microbial mat, a goethite-rich sediment from the flowing acidic ferric sulfate-rich stream (FlowMG1a – FlowMG1c) and underlying quartz sands and clays (comprised of minor kaolinite and microcline). Lipid classes are indicated by different colored backgrounds and categorised as follows: green, saturated fatty acids; purple, unsaturated fatty acids; yellow, branched fatty acids; blue, cyclopropyl fatty acids; pink, hydroxy-fatty acids; brown, phytosterols. Lipid abundances are much greater within the goethite-rich section compared to the clay-rich section of the core. FAME = Fatty Acid Methyl Esters. Note the logarithmic scale.
There also appears to be a mineralogical influence on the initial preservation of the acid stream organic biosignatures. Concentrations of lipids are highest in goethite layers which represent environments of heightened aqueous activity conducive to habitation, and hence predisposed to receiving heightened microbial fossil inputs. The dependence of lipid records on conditions that promote habitation is further demonstrated by the paucity of lipids in the quartz sand and clay layers immediately beneath the goethite, which are not directly exposed to lipid inputs, as well as the jarosite-rich layers adjacent to the stream, which indicate environments of lower water activity and pH, and thus less favourable conditions for the growth of organisms. Moreover, goethite has been suggested recently as a high priority mineral for sampling because of its ability to originate from the transformation of jarosite25 and host organic matter, yet avoid the negative effects of oxygen generation and organic combustion during thermal extraction47,48. The lipid extract data now reveal that goethite-rich sedimentary materials contain relatively high concentrations of biosignatures irrespective of the analytical extraction methods employed.
A possible concern is that the higher abundance and diversity of lipids found in the goethite and jarosite layers can be attributed to the close association between these sublayers and the extant microbial mat, as opposed to the preservation potential of iron oxides. Contributions from extant biomass can be identified by the presence of intact polar membrane lipids, such as phospholipids or glycolipids, that degrade rapidly in 9–12 hours after cell death49. Analysis of the lipid fraction of the goethite layers revealed no phospholipids or glycolipids to a detection limit of 0.05 ppm and there were, therefore, no significant contributions from living biomass to the lipids detected in the goethite layer. All lipids extracted from the goethite layers must therefore be fossil molecules and not inadvertent contributions from the overlying microbial mat.
Terrestrial microbes contain 1 to 10% fatty acids50 suggesting that a past martian biosphere could have contained substantial amounts of fatty acids. The goethite-containing layers in samples from the acid streams contained between 31.05 to 58.94 ppm of total fatty acids respectively. Non-volcanic Hesperian sediment occupies approximately 16.099 × 106 km2 of the martian surface51. The uppermost centimetre of Hesperian terrain represents a volume of 1.610 × 1011 m3 of material and a mass of 4.857 × 1014 kg. If the fatty acid concentrations of the Hesperian sediments on Mars are equivalent to those in the Hesperian analogue acidic sulfur stream then the most shallow centimetre layer would potentially contain up to 1.50 × 1010 kg (0.015 Gt) to 2.86 × 1010 kg (0.029 Gt) of fatty acids that survive initial diagenesis.
It must be noted that our calculations contain a number of assumptions. Mars may not have supported an equivalent microbial biomass as exhibited at the acid stream, and many of the postulated fatty acids on Mars could have been transformed to alkanes by enzymatic52 or diagenetic30 processes or destroyed by the ionizing effects of radiation53 or the oxidising effects of perchlorates that are abundant on the martian surface54. It is also pertinent to note that Mars would not have had available a similarly efficient aerobic metabolism as found on the present day Earth55 and the lack of martian plate tectonics would have led to different burial processes and associated thermal effects. Differences between diagenesis on Earth and Mars may partly explain why the Sample Analysis at Mars instrument on the Mars Science Laboratory Curiosity rover was able to detect only chlorinated benzenes in surface rocks48. Nevertheless, this data provides conceptual guidance for present and future Mars missions, and suggests focusing the search for evidence of fatty acid monomers and/or their diagenetic products in goethite deposits that are mineralogical signposts of aqueous, and thus more habitable conditions.
Methods
Samples
Samples were collected from a field site located in St. Oswald’s Bay, Dorset, United Kingdom, where a small stream was flowing from slumped Wealden Beds that are rich in pyrite (Figs 1 and 2). The water was acidic (pH 3.5) and jarosite was observed in many of the sedimentary layers surrounding the stream. There were substantial lateral variations across the study site. Where water-to-rock ratios and/or pH were higher the jarosite was closely associated with goethite. Biology was evident in the form of a purple microbial mat that partially covered the goethite layer, and acidophilic algae were observed as green particulates within the stream. To the east of St Oswald’s Bay, at Stair Hole, a locality of similar facies was observed but was found to be mostly dried out except for a small flow of pH 5 water. Stream samples were extracted as cylindrical cores of 10 cm diameter and 10–15 cm depth using a trowel. The samples were packaged in aluminum foil and freeze died. Individual biological and mineral layers were separated using a solvent-cleaned saw (washed with methanol and DCM) to ensure that there was no contamination in the core, and subsequently powdered with a pestle and mortar as described in the literature47 to allow for X-ray diffraction and lipid analysis.
X-Ray diffraction (XRD)
The samples were analysed using a Philips PW 1830 between 2.5 and 90 °2Ө with a step size of 0.02 and two seconds per step. The X’Pert HighScore program was used to analyse the diffraction patterns and perform Rietveld refinements. Quantification of crystalline phases was possible to an accuracy of 1–3 wt%.
Lipid Extraction
Between 0.5 g and 2 g of each sample were loaded into centrifuge tubes and 10 µl of internal standard added (1 mg ml−1 of 5-β cholanic acid and 0.5 mg ml−1 of p-terphenyl). A monophase solvent was prepared (4:10:5 ratio of water:methanol:chloroform; Bligh and Dyer, 1959) and 4 ml added to the sample. The mixture was subjected to sonication (5 min) and centrifugation (3 min, 1800 rpm). The supernatant was transferred to stoppered test tubes and the process repeated three times. Chloroform and water were then added to the test tubes, shaken and left to settle, resulting in immiscible layers of clear water over lipid-rich chloroform. The chloroform was pipetted into round-bottomed flasks, and the process repeated three times, before the combined extracts were reduced by rotary evaporation (40 °C). Dichloromethane (DCM) and anhydrous sodium sulfate were added to the extract to remove any remaining chloroform and water. The resulting solution was pipetted into 4 ml vials for derivatization.
Lipid Derivatization
The DCM extracts were dried down under nitrogen gas. 1 ml of boron trifluoride-methanol (BF3-methanol) solution was added to the vials and heated at 80 °C for 40 min. After cooling, 1 ml of water and 1 ml of hexane were added to the vials forming immiscible layers of hexane over water, with the hexane layer containing the methyl esters of any carboxylic acids present. The hexane layer was collected using a pipette, transferred to a short alumina column and eluted with 1:1 DCM:hexane. The eluate was evaporated under nitrogen gas prior to analysis.
Gas Chromatography-Mass Spectrometry
GC-MS analysis utilised an Agilent Technologies 7890 GC coupled to a 5973 MS. Injection (1 μl) was splitless with helium carrier gas at a constant flow rate of 1.1 mL min−1. Separation was performed on a J&W DB-5MS UI column (approximately 30 m in length, 0.25 mm internal diameter, and a film thickness of 0.25 μm). The GC oven temperature was held at 40 °C for 2 minutes and then ramped at 5 °C min−1 to 310 °C, where it was held for 9 minutes. Mass spectra were acquired in the scan range (45–550 amu). Peak identification was based on retention time and mass spectra comparisons with authenticated standards and by reference to published reports. In particular, we used standards for stearic acid (18:0) and oleic acid (18:1(9)); 18:1(11) was assumed to be the additional peak with identical mass spectra to oleic acid (18:1(9)) owing to its status as the other of the two most common monounsaturated fatty acids in bacterial cell membranes30. The peak assigned as cyclopropyl fatty acid was identified owing to its unique mass spectra; the assignment is restricted to the class and molecular weight with no positional detail relied upon for our interpretations.
Acknowledgements
This work was supported by UK Space Agency grant ST/N000560/1 to MAS, an Imperial College President’s PhD Scholarship to JT and a Science and Technology Facilities Council studentship to JMTL.
Author Contributions
J.M.T.L. and M.A.S. collected the samples, J.T. performed the laboratory work, J.T. and M.A.S. analysed the data, and all authors contributed to the writing process.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bibring J-P, et al. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science. 2005;307:1576–1581. doi: 10.1126/science.1108806. [DOI] [PubMed] [Google Scholar]
- 2.Poulet F, et al. Phyllosilicates on Mars and implications for early martian climate. Nature. 2005;438:623–627. doi: 10.1038/nature04274. [DOI] [PubMed] [Google Scholar]
- 3.Ehlmann BL, et al. Clay minerals in delta deposits and organic preservation potential on Mars. Nat. Geosci. 2008;1:355–358. doi: 10.1038/ngeo207. [DOI] [Google Scholar]
- 4.Milliken RE, Grotzinger JP, Thomson BJ. Paleoclimate of Mars as captured by the stratigraphic record in Gale Crater. Geophys. Res. Lett. 2010;37:L04201. doi: 10.1029/2009GL041870. [DOI] [Google Scholar]
- 5.Ehlmann BL, et al. Subsurface water and clay mineral formation during the early history of Mars. Nature. 2011;479:53–60. doi: 10.1038/nature10582. [DOI] [PubMed] [Google Scholar]
- 6.Gendrin A, et al. Sulfates in Martian layered terrains: The OMEGA/Mars Express view. Science. 2005;307:1587–1591. doi: 10.1126/science.1109087. [DOI] [PubMed] [Google Scholar]
- 7.Grotzinger JP, et al. A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science. 2014;343:1–14. doi: 10.1126/science.1242777. [DOI] [PubMed] [Google Scholar]
- 8.Acuña MH, et al. Global distribution of crustal magnetization discovered by the Mars Global Surveyor MAG/ER experiment. Science. 1999;284:790–793. doi: 10.1126/science.284.5415.790. [DOI] [PubMed] [Google Scholar]
- 9.Chassefière E, Leblanc F. Mars atmospheric escape and evolution; interaction with the solar wind. Planet. Space Sci. 2004;52:1039–1058. doi: 10.1016/j.pss.2004.07.002. [DOI] [Google Scholar]
- 10.Gaillard F, Michalski J, Berger G, Mclennan SM, Scaillet B. Geochemical reservoirs and timing of sulfur cycling on Mars. Space. Sci. Rev. 2013;174:251–300. doi: 10.1007/s11214-012-9947-4. [DOI] [Google Scholar]
- 11.Klingelhöfer G, et al. Jarosite and hematite at Meridiani Planum from Opportunity’s Mössbauer spectrometer. Science. 2004;306:1740–1745. doi: 10.1126/science.1104653. [DOI] [PubMed] [Google Scholar]
- 12.Squyres SW, et al. In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science. 2004;306:1709–1714. doi: 10.1126/science.1104559. [DOI] [PubMed] [Google Scholar]
- 13.Klein C. Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origin. Am. Mineral. 2005;90:1473–1499. doi: 10.2138/am.2005.1871. [DOI] [Google Scholar]
- 14.Sumner DY. Poor preservation potential of organics in Meridiani Planum hematite-bearing sedimentary rocks. J. Geophys. Res. Planet. 2004;109:E12007. doi: 10.1029/2004JE002321. [DOI] [Google Scholar]
- 15.Colín-García M, et al. Detection of peptidic sequences in the ancient acidic sediments of Río Tinto, Spain. Origins Life Evol. B. 2011;41:523–527. doi: 10.1007/s11084-011-9258-x. [DOI] [PubMed] [Google Scholar]
- 16.Preston LJ, et al. The preservation and degradation of filamentous bacteria and biomolecules within iron oxide deposits at Rio Tinto, Spain. Geobiology. 2011;9:233–249. doi: 10.1111/j.1472-4669.2011.00275.x. [DOI] [PubMed] [Google Scholar]
- 17.Lalonde K, Mucci A, Ouellet A, Gelinas Y. Preservation of organic matter in sediments promoted by iron. Nature. 2012;483:198–200. doi: 10.1038/nature10855. [DOI] [PubMed] [Google Scholar]
- 18.Parenteau MN, Cady SL. Microbial biosignatures in iron-mineralized phototrophic mats at Chocolate Pots Hot Springs, Yellowstone National Park, United States. Palaios. 2010;25:97–111. doi: 10.2110/palo.2008.p08-133r. [DOI] [Google Scholar]
- 19.Parenteau MN, Jahnke LL, Farmer JD, Cady SL. Production and early preservation of lipid biomarkers in iron hot springs. Astrobiology. 2014;14:502–521. doi: 10.1089/ast.2013.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amils R, et al. Extreme environments as Mars terrestrial analogs: The Rio Tinto case. Planet. Space Sci. 2007;55:370–381. doi: 10.1016/j.pss.2006.02.006. [DOI] [Google Scholar]
- 21.Burns RG. Gossans on Mars: Spectral features attributed to jarosite. Lunar. Planet. Sci. Conf. 1987;18:141–142. [Google Scholar]
- 22.Fernández-Remolar DC, Morris RV, Gruener JE, Amils R, Knoll AH. The Río Tinto Basin, Spain: Mineralogy, sedimentary geobiology, and implications for interpretation of outcrop rocks at Meridiani Planum, Mars. Earth Planet. Sci. Lett. 2005;240:149–167. doi: 10.1016/j.epsl.2005.09.043. [DOI] [Google Scholar]
- 23.Kaplan HH, et al. Orbital evidence for clay and acidic sulfate assemblages on Mars based on mineralogical analogs from Rio Tinto, Spain. Icarus. 2016;275:45–64. doi: 10.1016/j.icarus.2016.03.019. [DOI] [Google Scholar]
- 24.Zolotov MY, Shock EL. Formation of jarosite-bearing deposits through aqueous oxidation of pyrite at Meridiani Planum, Mars. Geophys. Res. Lett. 2005;32:L21203. doi: 10.1029/2005GL024253. [DOI] [Google Scholar]
- 25.Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta. 1996;60:2111–2121. doi: 10.1016/0016-7037(96)00091-9. [DOI] [Google Scholar]
- 26.Bigham JM, Nordstrom DK. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Mineral. Geochem. 2000;40:847–853. doi: 10.2138/rmg.2000.40.7. [DOI] [Google Scholar]
- 27.Matthewman R, Martins Z, Sephton MA. Type IV kerogens as analogues for organic macromolecular materials in aqueously altered carbonaceous chondrites. Astrobiology. 2013;13:324–333. doi: 10.1089/ast.2012.0820. [DOI] [PubMed] [Google Scholar]
- 28.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 29.Harwood, J. L. & Russel, N. J. Lipids in Plants and Microbes. 162 (1984).
- 30.Killops, S. D. & Killops, V. J. Introduction to Organic Geochemistry. (Blackwell Publishing, 2005).
- 31.Ratledge, C. & Wilkinson, S. G. Microbial lipids, vol. 1. (Academic Press, 1988).
- 32.Boschker HTS, Middelburg JJ. Stable isotopes and biomarkers in microbial ecology. FEMS Microb. Ecol. 2002;40:85–95. doi: 10.1111/j.1574-6941.2002.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 33.Perry GJ, Volkman JK, Johns RB, Bavor HJ. Fatty acids of bacterial origin in contemporary marine sediments. Geochim. Cosmochim. Acta. 1979;43:1715–1725. doi: 10.1016/0016-7037(79)90020-6. [DOI] [Google Scholar]
- 34.Vestal JR, White DC. Lipid analysis in microbial ecology: quantitative approaches to the study of microbial communities. Bioscience. 1989;39:535–541. doi: 10.2307/1310976. [DOI] [PubMed] [Google Scholar]
- 35.Wakeham SG. Lipid biomarkers for heterotrophic alteration of suspended particulate organic matter in oxygenated and anoxic water columns of the ocean. Deep-Sea Res. 1995;42:1749–1771. doi: 10.1016/0967-0637(95)00074-G. [DOI] [Google Scholar]
- 36.Zhang CL, et al. Lipid and carbon isotopic evidence of methane-oxidizing and sulfate-reducing bacteria in association with gas hydrates from the Gulf of Mexico. Geology. 2002;30:239–242. doi: 10.1130/0091-7613(2002)030<0239:LACIEO>2.0.CO;2. [DOI] [Google Scholar]
- 37.Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: A review. Biol. Fert. Soils, 111–129 (1999).
- 38.Bloch K. Enzymatic Synthesis of Monounsaturated Fatty Acids. Acc. Chem. Res. 1969;2:193–202. doi: 10.1021/ar50019a001. [DOI] [Google Scholar]
- 39.Volkman JK, et al. Microalgal biomarkers: A review of recent research developments. Org. Geochem. 1998;29:1163–1179. doi: 10.1016/S0146-6380(98)00062-X. [DOI] [Google Scholar]
- 40.Shiea J, Brassel SC, Ward DM. Comparative analysis of extractable lipids in hot spring microbial mats and their component photosynthetic bacteria. Org. Geochem. 1991;17:309–319. doi: 10.1016/0146-6380(91)90094-Z. [DOI] [Google Scholar]
- 41.Wahlund TM, Woese CR, Castenholz RW, Madigan MT. A thermophilic green sulfur bacterium from New Zealand hot springs, Chlorobium tepidum sp. nov. Arch. Microbiol. 1991;156:81–90. doi: 10.1007/BF00290978. [DOI] [Google Scholar]
- 42.Huang, Y., Street-Perrott, F. A., Perrott, R. A., Metzger, P. & Eglinton, G. Glacial-interglacial environmental changes inferred from molecular and compound-specific δ13C analyses of sediments from Sacred Lake, Mt. Kenya. Geochim. Cosmochim. Acta (1999).
- 43.Summons, R. E., Jahnke, L. L. & Simoneit, B. R. T. Lipid biomarkers for bacterial ecosystems: studies of cultured organisms, hydrothermal environments and ancient sediments. In: Evolution of hydrothermal ecosystems on Earth (and Mars?). CIBA Foundation Symposium, vol. 202, pp 174–194 (Wiley, 1996). [DOI] [PubMed]
- 44.Clark RJ, Blumer M. Distribution of n-paraffins in marine organisms and sediment. Limnol. Oceanogr. 1967;12:79–87. doi: 10.4319/lo.1967.12.1.0079. [DOI] [Google Scholar]
- 45.Eglinton G, Hamilton RJ. Leaf epicuticular waxes. Science. 1967;156:1322–1335. doi: 10.1126/science.156.3780.1322. [DOI] [PubMed] [Google Scholar]
- 46.Schneider H, Gelpi E, Bennett EO, Oró J. Fatty acids of geochemical significance in microscopic algae. Phytochemistry. 1970;9:613–617. doi: 10.1016/S0031-9422(00)85701-5. [DOI] [Google Scholar]
- 47.Lewis, J. M. T., Najorka, J., Watson, J. S. & Sephton, M. A. The search for Hesperian organic matter on Mars: Pyrolysis studies of sediments rich in sulfur and iron. Astrobiology8 (2018). [DOI] [PMC free article] [PubMed]
- 48.Freissinet C, et al. Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J. Geophys. Res. Planet. 2015;120:495–514. doi: 10.1002/2014JE004737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey HR, Fallon RD, Patton JS. The effect of organic matter and oxygen on the degradation of bacterial membrane lipids in marine sediments. Geochim. Cosmochim. Acta. 1986;50:795–804. doi: 10.1016/0016-7037(86)90355-8. [DOI] [Google Scholar]
- 50.O’leary WM. The fatty acids of bacteria. Bacteriol. Rev. 1962;26:421–447. doi: 10.1128/br.26.4.421-447.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka KL, Robbins SJ, Fortezzo CM, Skinner JA, Hare TM. The digital global geologic map of Mars: Chronostratigraphic ages, topographic and crater morphologic characteristics, and updated resurfacing history. Planet. Space Sci. 2014;95:11–24. doi: 10.1016/j.pss.2013.03.006. [DOI] [Google Scholar]
- 52.Schirmer, A., Rude, M. A., Li, X., Popova, E. & Del Cardayre, S. B. Microbial Biosynthesis of Alkanes. Science (2010). [DOI] [PubMed]
- 53.Dartnell LR. Ionizing radiation and life. Astrobiology. 2011;11:551–582. doi: 10.1089/ast.2010.0528. [DOI] [PubMed] [Google Scholar]
- 54.Hecht, M. H. et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science (2009). [DOI] [PubMed]
- 55.Leahy JG, Colwell RR. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]