Abstract
γδ T cells have been implicated in inflammatory diseases as an important link between the innate and adaptive immune responses, however, their role in inflammatory arthritis remain unclear. To define the contribution of γδ T cells in the pathogenesis of inflammatory arthritis, we performed gene transfer of IL-23 in B10.RIII mice to establish joint inflammation in the presence or absence of γδ T cells. We demonstrated that γδ T cell blockade has a protective effect on arthritis incidence and severity by preventing neutrophil accumulation in the blood, spleen and bone marrow as well as by reducing neutrophil infiltration into the joints. Furthermore, our data demonstrate that absence of γδ T cells was associated with an increase of IL-27 levels produced by neutrophils and dendritic cells, and systemic IL-27 expression also prevents IL-23-induced inflammatory arthritis and limits neutrophil expansion. Collectively our findings reveal an immunomodulatory effect of γδ T cells on neutrophils associated with IL-27 synthesis and secretion and indicate a novel link between IL-27 and the modulation of γδ T cells and neutrophils that can be targeted in the treatment of inflammatory arthritis.
Introduction
Gamma delta (γδ) T cells are a minor population of T cells that express the T-cell receptor γδ chains, accounting for less than 5% of the total T cells in the peripheral blood of mice and humans and are more commonly localized in mucosal tissues, such as the gut, skin and lung1,2. These cells exhibit different functional activity with an adaptive potential and an innate-like capacity to respond to pro-inflammatory cytokines in the absence of further antigens3. γδ T cells can produce high levels of interferon-γ (IFN-γ) and tumor necrosis factor (TNF), Interleukin 17 (IL-17) and large amounts of chemokines reflecting their role in the effector phase of immune response4. In this regard, γδ T cells may participate in the early stages of inflammation in synchrony with innate immune cells.
γδ T cells are known to have a strong clinical association with many autoimmune diseases, such as rheumatoid arthritis (RA) but their function in disease activity is not clearly understood. Significantly higher levels of γδ T cells are found in RA patients5,6 associated with enhanced IL-17 secretion7 and hyperplasia of the synovial tissue and progressive destruction of joint structure. The role of γδ T cells has been documented in the collagen-induced arthritis (CIA) animal model of experimental arthritis where γδ T cells depletion prior to disease induction delayed both the onset and severity of the disease. In contrast, depletion of γδ T cells in established arthritic mice accelerated cellular infiltration into the joint and induced bone erosion8. These data suggest that γδ T cells might exhibit different functions depending on other effector cells present in the inflammatory environment of the joint.
A strong link between the proinflammatory IL-23/IL-17 axis and γδ T cells lineage has been established. IL-23 is produced by innate immune cells and is an essential mediator of joint inflammation and is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass9,10. γδ T cells express constitutively high amounts of IL-23 receptor (IL-23R) that drives their expansion and therefore their secretion of IL-1711.
Several studies demonstrated that γδ T cells are a predominant source of IL-17 in the swollen joints of mice with CIA12,13 suggesting that cytokine process may drives the pathogenic effect of γδ T cells. The dependence of arthritis initiation on IL-17 alone seems highly unlikely as we have shown that IL-17 alone is not capable of inducing arthritis in vivo9 although we and others have shown that IL-17 can very well exacerbate established arthritis14,15.
Another potent mechanism that may modulate γδ T cells function is their ability to interact with other innate immune cells. In models of bacterial infection, γδ T cells were found to control neutrophil infiltration16,17. This notion is also consistent with a recent study reporting γδ T cells and neutrophils conspiration to promote breast cancer metastasis18. In inflammatory conditions such as RA, neutrophils play a role in the persistence of inflammation and progression of joint damage. Increased numbers of neutrophils have been found in the synovial fluid of patients with RA19,20. In the arthritis animal model induced by anti-type II collagen (CII) antibodies and lipopolysaccharide (LPS) injection, neutrophils are the major population of infiltrating cells in the joint space and neutrophil depletion using mouse antibody (mAb) against Gr-1 in vivo suppresses the development of arthritis21. Furthermore, neutrophil depletion renders mice resistant to K/B × N serum-induced joint inflammation22. Kim et al. showed that neutrophils were crucial for arthritis generation and chemokine production in the K/BxN mouse model23. The prominent role of neutrophils and γδ T cells in inflammatory arthritis, with regard to their localization, cytokine production and interaction with other immune cells that influence pathogenesis merits further investigation.
In this study, we describe the involvement of γδ T cells to the pathogenesis of IL-23-induced arthritis mice model and further evaluated the impact on myeloid cells. We found that γδ T cell blockade prior IL-23 MC injection significantly reduced both incidence and disease severity score by suppressing neutrophil expansion and increasing IL-27 levels. Furthermore, IL-27 gene transfer prior IL-23 MC injection inhibits arthritis development and both neutrophils and γδ T cell expansion. Collectively our data describe a novel interplay between γδ T cells and neutrophil secretion of IL-27, which negatively regulates inflammatory arthritis.
Results
Protective effect of γδ T cell blockade in IL-23-induced arthritis
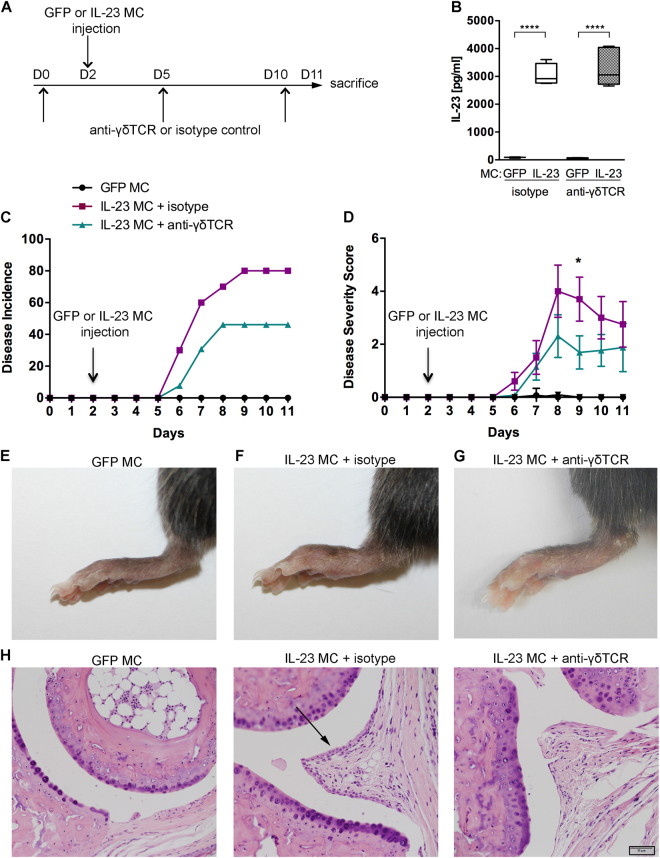
To analyze the functional role of γδ T cells in IL-23-induced arthritis, we performed IL-23 or GFP control in vivo gene transfer in B10.RIII mice as previously described24 to induce inflammatory arthritis in the presence or absence of γδ T cells (Fig. 1A). IL-23 MC injected mice revealed a significant elevation of serum IL-23 whereas GFP MC injected mice did not have detectable levels of IL-23 (Fig. 1B). Blockade of γδ T cells by anti-γδ TCR mAb was performed 2 days prior gene transfer and analyzed by flow cytometry in the spleen and draining lymph nodes. Our data showed that antibody blockade at the selected dose was comparable with TCRδ−/− deficient mice (Supplemental Fig. 1A). Administration of the anti-γδ TCR or isotype mAb did not affect myeloid populations in the blood (Supplemental Fig. 1B,C), spleen (Supplemental Fig. 1D) or bone marrow, as confirmed by flow cytometry (Supplemental Fig. 1E). Our results show that γδ T cell blockade prior to IL-23 gene transfer caused a marked decrease (46.15%) in disease incidence compared to controls (80%) at day 11 post-gene transfer (Fig. 1C). γδ T cell blockade also resulted in a significant decrease of the disease severity score as compared to control mice (Fig. 1D) as shown by reduced paw swelling in the γδ T cells depleted group in our arthritis model (Fig. 1E–G). Histologic assessment of the ankle joints revealed a marked synovial hyperplasia in mice injected with IL-23 MC, which is reduced in anti-γδ TCR mAb-treated mice. Representative sections of the average disease score (mild inflammation) are shown (Fig. 1H). These observations suggest that γδ T cells play a pathogenic role in supporting the development of arthritis in IL-23 gene transfer model of inflammatory arthritis. Next, we examined the potential cellular mechanisms that are responsible for the protective effect of γδ T cell blockade.
Figure 1.
Decrease of IL-23-induced arthritis in anti-γδ TCR mAb treated mice. (A) Schematic illustration of the experimental protocols. B10.RIII mice at the age of 10–12 weeks were treated on days 0, 5 and 10 with anti-γδ TCR or isotype control mAb prior to GFP or IL-23 MC injection at day 2 (n = 10–13 per group). (B) Serum IL-23 levels by ELISA of each group (n = 4–5 per group). Median, interquartile minimum, and maximum range is depicted by box plots, ****p < 0.0001 by one-way ANOVA with Sidak’s multiple comparisons test. (C) Time course of disease incidence and (D) severity score of arthritis in mice injected with GFP or IL-23 MC and treated with anti-γδ TCR or isotype mAb (n = 10–14 per group). *p < 0.05 by using two-tailed Student’s t-test. Representative pictures showing the hind paws of B10.RIII mice injected with GFP MC (E) or IL-23 MC + isotype (F) or IL-23 MC + anti-γδ TCR (G). (H) Representative H&E stained sections of day-11 metatarsophalangeal joint from GFP MC and IL-23 MC injected mice treated with isotype (middle column) or γδ TCR mAb (right column) are shown (20× objective). The black arrow indicates synovial hyperplasia. Scale bars, 50 μm. Data are representative of three independent experiments. All data are shown as mean ± SEM.
γδ T cells regulate the expansion and recruitment of neutrophils
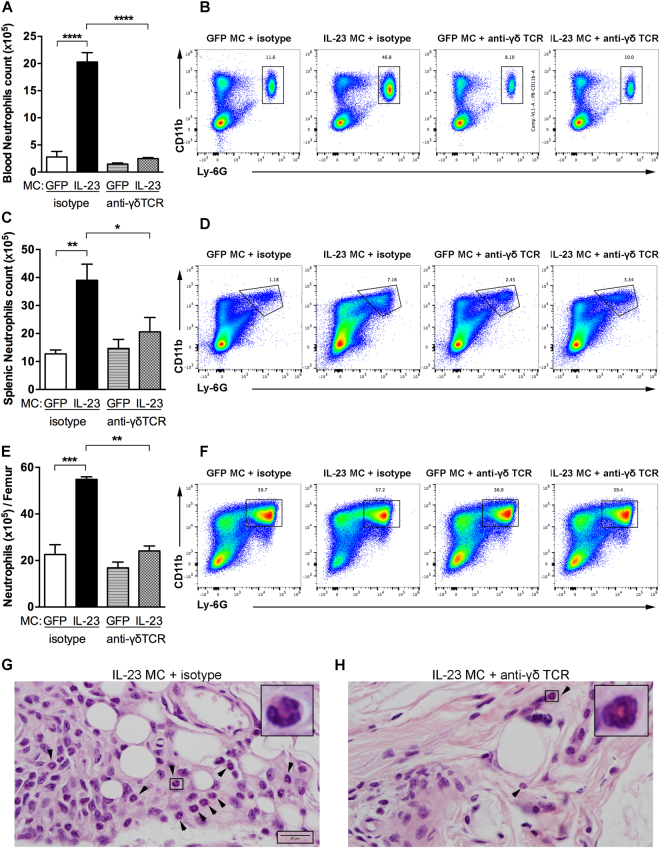
We previously showed that systemic IL-23 exposure induced myelopoiesis in the bone marrow and the spleen9,24. To investigate whether γδ T cells affect IL-23-induced myelopoiesis, myeloid cells were analyzed in B10.RIII mice injected with GFP or IL-23 MC and treated with anti-γδ TCR or isotype mAb. We found that IL-23 MC injection increases neutrophil populations in the blood compared to GFP MC. Interestingly, blood CD11b+ Ly-6G+ neutrophils were significantly reduced in mice injected with IL-23 MC and treated with anti-γδ TCR mAb compared to control mice as shown in the representative FACS-plots and total counts (Fig. 2A,B). Analysis of splenic neutrophils also showed a significant increase in IL-23 injected mice compared to GFP MC, which again was inhibited by γδ T cell blockade (Fig. 2C,D). Splenic CD11b+ CD64+ macrophages as well as CD11chi MHCII+ dendritic cells count remained unchanged (data not shown). Similarly, analysis of bone marrow isolated cells revealed an increase of neutrophils in IL-23 MC injected mice compared to control mice which was again significantly reduced in anti-γδ TCR mAb treated mice as shown in the representative FACS-plots and total counts (Fig. 2E,F). To assess whether γδ T cells promote neutrophil migration into the joint, neutrophils were visualized by H&E staining within the joint capsule. We found that IL-23 MC gene transfer induced a marked neutrophil infiltration into the joint (Fig. 2G), which was reduced by γδ T cell blockade (Fig. 2H). Neutrophil elevation was not detected in the synovium of mice treated with GFP MC (data not shown). These data suggest that γδ T cells are able to modulate the IL-23-induced neutrophil expansion. We next investigated any possible molecular changes that might be affected by γδ T cell blockade.
Figure 2.
Anti-γδ TCR treatment inhibits the expansion of neutrophils. (A) Quantification of CD11b+ Ly-6G+ blood neutrophils in B10.RIII mice injected with GFP or IL-23 MC and treated with anti-γδ TCR or isotype mAb determined by flow cytometry. (B) Representative flow cytometry plots showing the gating strategy for evaluating the numbers of blood neutrophils. (C) Splenic CD11b+ Ly-6G+ neutrophil counts in B10.RIII mice injected with GFP or IL-23 MC and treated with anti-γδ TCR or isotype mAb determined by flow cytometry. (D) Gating strategy for evaluating the percentage and absolute numbers of neutrophils in mouse spleen. (E) Absolute numbers of CD11b+ Ly-6G+ neutrophils per femur. (F) Representative flow cytometry plots showing the gating strategy. Numbers depict the percentage of CD45+ leukocytes. (n = 4–5 per group). (G,H) Representative haematoxylin and eosin-stained sections of day-11 ankles histopathology from mice receiving isotype control or anti-γδ TCR mAb showing neutrophils in joint capsule imaged with 100 × oil-immersion objective lense. Arrows indicate polymorphonuclear neutrophils. Scale bars, 20 μm. Data were obtained from 3 independent experiments (n = 4–5 per group). All data are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparisons test.
γδ T cell deficiency increases IL-27 levels
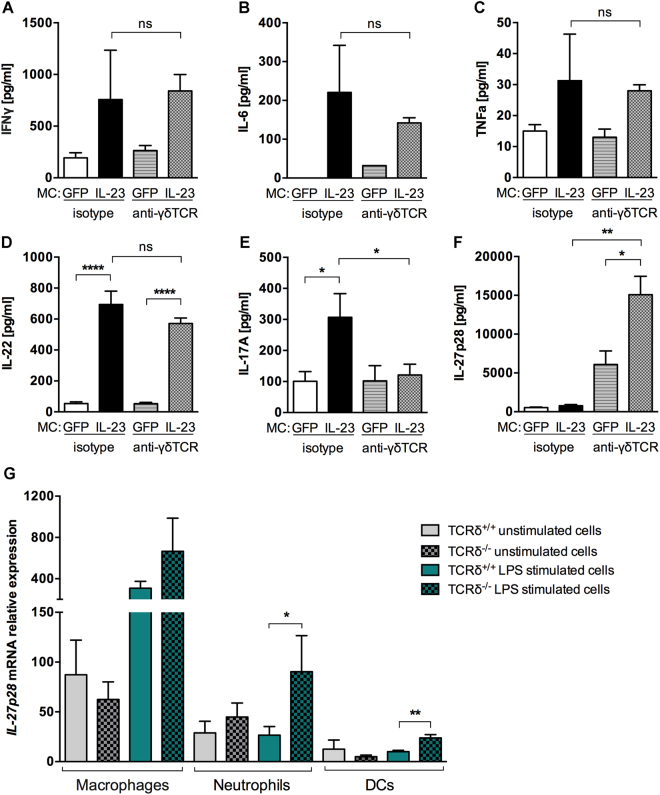
IL-23 MC gene transfer induced the expression of pro-inflammatory cytokines as previously shown9, however treatment with UC7-13D5 mAb did not have a profound effect on the expression of IFNγ, TNF, IL-6, and IL-22 (Fig. 3A–D), with the exception of a marked decrease in IL-17A serum concentration (60.83%) (Fig. 3E).
Figure 3.
γδ T cell blockade increases IL-27 levels. (A–F) Cytokine levels in serum of B10.RIII mice injected with GFP or IL-23 MC and treated with anti-γδ TCR or isotype mAb on day 11 (n = 4–5 per group). (G) qRT–PCR expression analysis of IL-27p28 mRNA expression in sorted macrophages, neutrophils and dendritic cells (DCs) isolated from TCRδ+/+ or TCRδ−/− mice after 5 hours of LPS stimulation. Data are representative of three independent experiments. All data are shown as mean ± SEM. ns, not significant (p > 0.05) *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as determined by using two-tailed Student’s t-test.
In contrast, IL-27p28 serum levels, which were not increased with IL-23 MC injection in isotype mAb treated mice, were markedly increased in the absence of γδ T cells (Fig. 3F). Therefore, we next identified the IL-27p28 producing cells by challenging splenocytes isolated from TCRδ+/+ or TCRδ−/− mice with LPS, a known inducer of IL-27 production25. Then, macrophages, dendritic cells and neutrophils were sorted based on gated strategy used in Supplemental Fig. 2A and we tested their ability to produce IL-27p28. Our data show that IL-27p28 mRNA expression was mainly produced by activated macrophages and neutrophils (Fig. 3G). In addition, we demonstrated that neutrophils and dendritic cells isolated from TCRδ−/− mice express more IL-27p28 mRNA compared to TCRδ+/+ isolated cells as analyzed by qPCR. Taken together our results indicate that γδ T cells have an important regulatory effect on IL-27 synthesis. Next, we investigated whether increased IL-27 production affect the development of IL-23-induced arthritis.
IL-27 gene transfer inhibits IL-23-induced arthritis by negative regulation of neutrophil motility and γδ T cells population
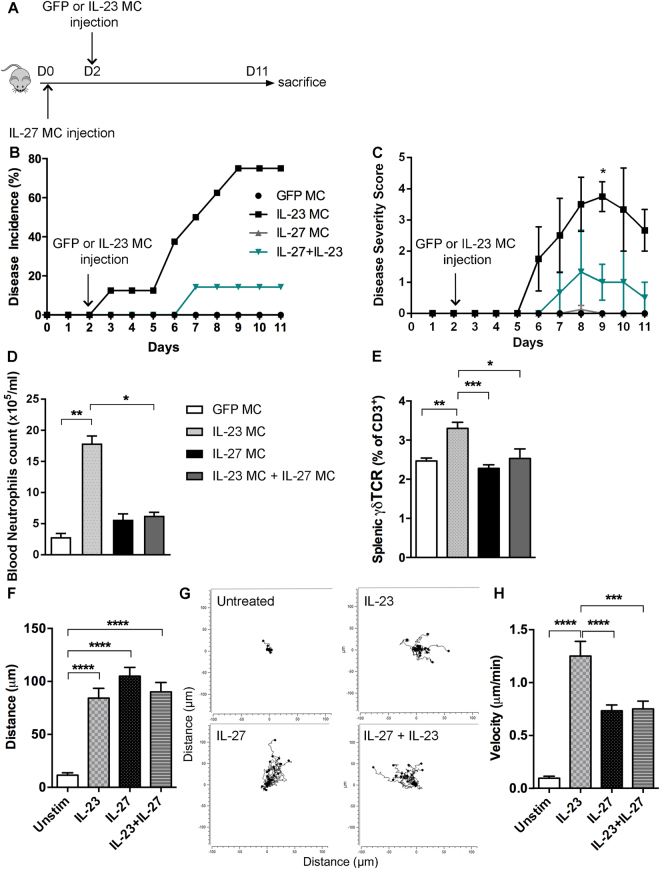
To determine whether IL-27 modulates disease severity in vivo, IL-27 MC alone or prior IL-23 MC gene transfer was administered by hydrodynamic injection into B10.RIII mice (Fig. 4A). Quantification of serum IL-27 taken from periodic tail bleeds demonstrated that IL-27 was stably expressed for a period of at least 11 days (data not shown). We found that IL-27 MC injection is unable to induce disease development. However, IL-27 MC injection prior IL-23 MC gene transfer significantly ameliorated both disease incidence (Fig. 4B) and disease severity (Fig. 4C) in the IL-23-induced arthritis. To determine whether IL-27 MC affects myeloid cell populations, we quantified blood monocytes and neutrophils by flow cytometry. Monocyte numbers were not affected by IL-27 injections (data not shown). However, IL-23 MC injection drastically increased blood neutrophil numbers and IL-27 MC injection suppressed the IL-23-induced neutrophil expansion (Fig. 4D and Supplemental Fig. 2B). Interestingly, we identified by flow cytometric analysis an increase of γδ T cells in the spleens of IL-23 MC injected mice compared to GFP control or IL-27 MC injected mice (Fig. 4E and Supplemental Fig. 2C). Moreover, we found that IL-27 is also able to inhibit IL-23 induced splenic γδ T cell accumulation. We next assessed how IL-27 modulates neutrophil migration in chemotaxis experiments where neutrophils were exposed to a diffusion gradient of IL-27 or IL-23 or both, IL-23 and IL-27. We did not detect any changes in directed displacement in the x or y direction in the absence of chemotactic factors (untreated). Interestingly, our data revealed that IL-23 and IL-27 are both able to increase neutrophils migration (Fig. 4F,G). Moreover, both IL-23 and IL-27 were able to increase the migration velocity of neutrophils. Interestingly, neutrophil stimulation with both IL-23 and IL-27 revealed a reduced migration velocity compared to IL-23 alone (Fig. 4H). Taken together, our data show that IL-27 negatively regulates IL-23-induced arthritis by decreasing the expansion and motility of neutrophils and by reducing γδ T cells during arthritis development.
Figure 4.
IL-27 inhibitory effect on the development of IL-23-induced arthritis. (A) Schematic illustration of the experimental protocols. B10.RIII mice at the age of 10–12 weeks were injected on days 0 with IL-27 MC prior GFP or IL-23 MC injection at day 2. (B) Time course of disease incidence and (C) severity score of arthritis in B10.RIII mice after GFP MC, IL-23 MC, IL-27 MC, or IL-23 + IL-27 MC injection (n = 7–10 per group). Data are representative of three independent experiments. All data are shown as mean ± SEM. *p < 0.05, as determined by using two-tailed Student’s t-test. (D) Quantification of CD11b+ Ly-6G+ blood neutrophils in mice injected with GFP MC, IL-23 MC, IL-27 MC or IL-23 + IL-27 MC. (E) Percentage of splenic γδ T cells analyzed by flow cytometry of the isolated splenocytes of each group. (F) Analysis of neutrophil migratory distance (in μm) under IL-23, IL-27 or IL-23 + IL-27 stimulation. (G) Representative neutrophil trajectory plots over a 2-hour period. (H) Analysis of neutrophils velocity (μm/min) under IL-23, IL-27 or IL-23 + IL-27 stimulation. Data are representative of two independent experiments. All data are shown as mean ± SEM. *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparisons test.
Discussion
This study provides novel molecular insights into the regulation of neutrophils by γδ T cells. We identified a pathogenic role played by γδ T cells on inflammatory arthritis in the IL-23 gene transfer model by regulating the expansion of the neutrophil population as well as IL-27 synthesis and secretion. Furthermore, we reported that IL-27 is able to suppress IL-23-induced arthritis through limiting neutrophil expansion and migration velocity as well as γδ T cell accumulation. By this mechanism, γδ T cell blockade exert a protective effect on IL-23-induced inflammatory arthritis.
Although, there is a single report that treatment with anti-γδ TCR mAb leads to downregulation of the TCR rather than γδ T-cell depletion26, others have shown significant effects of the treatment with anti-γδ TCR mAb on disease incidence and severity indicating at least, that the treatment with UC7-13D5 mAb leads to functional impairment of γδT cells27–29. Furthermore, we found consistent results between B10RIII mice that received the UC7-13D5 mAb and TCRδ−/− mice.
In our study, we demonstrated an increase of IL-17 expression in IL-23 injected mice, which is reduced with anti-γδ TCR treatment. These results suggest that γδ T cells activated by IL-23 are an important source of IL-17, which could subsequently impact arthritis development. Indeed, several observations in animal models point to the importance of IL-17 in driving synovial inflammation and joint destruction15,30. Although γδ T cells are an important source of IL-17, we do not exclude the possibility that other cells types might secrete IL-17 in response to IL-23 gene transfer. Indeed, natural killer (NK) cells, lymphoid-tissue inducer (LTi)-like cells and neutrophils have been described in the literature as an early source of IL-17 in response to IL-23 signalling31,32. However, the signaling pathway (receptors and transcription factors) required for most IL-17-producing cells remain unclear.
Nevertheless, pleiotropic functions of γδ T cells have been described in the literature and our results highlight an alternative mechanism, by which γδ T cells may mediate inflammatory arthritis, by regulating neutrophils. We identified that IL-23 induces the expansion of neutrophils into the blood, spleen, bone marrow and increased neutrophil infiltration into the joint, which was significantly reduced in anti-γδ TCR mAbs treated mice. This finding points to an immunomodulatory effect of γδ T cells on neutrophils, which could subsequently impact arthritis development.
Together, these results show that γδ T cells stimulation by IL-23 enhances the production of IL-17A by both neutrophils and γδ T cells. IL-17A elevates neutrophil counts, and increases their recruitment into the joint leading to inflammatory arthritis. Indeed, critical roles for neutrophils in initiating and maintaining joint inflammatory processes have been described in experimental arthritis mouse models33. Once in the joint, neutrophils perpetuate their own recruitment by releasing chemotactic factors contributing to the chronicity of the disease23,34. In this setting, IL-27p28 is not increased suggesting that macrophage and neutrophil activation by IL-23 and IL-17 alone is not sufficient to induce IL-27p28 expression. However, we show that IL-23 stimulation in absence of γδ T cells and neutrophil activation failed to increase IL-17 secretion and decrease inflammatory arthritis. We hypothesize that γδ T cells might inhibit/downregulate IL-27p28 expression by macrophages and/or neutrophils. This would imply that targeting the factor by which γδ T cells modulate neutrophils might be a successful therapeutic strategy for inflammatory arthritis.
The mechanism of γδ T cells migration into inflammatory sites is poorly understood as well as the heterogeneity of the local γδ T cells. Both Vγ6+ and Vγ4+ γδ T cells has been shown to be recruited to the joints, but only the Vγ6+ subset efficiently produced IL-1735. However, the differences between the pathogenic roles of these subsets, particularly the contribution to inflammatory diseases, remain unclear. Prospective studies should further investigate specific subsets of γδ T cells able to modulate neutrophils.
In this study, we identified a molecular interplay between IL-27, γδ T cells and neutrophils suggesting that IL-27 could be the mechanism by which γδ T cell regulates neutrophil expansion and subsequently impact arthritis development. With a combination of in vivo and in vitro experiments, we demonstrate that IL-27 is significantly increased in the absence of γδ T cells indicating a regulatory role of γδ T cells on IL-27 independently of GFP or IL-23 MC injection. Our data show that IL-27p28 mRNA expression was mainly produced by macrophages and neutrophils.
Our results suggest that despite a reduction of neutrophils count in the blood, the spleen and the bone marrow upon anti-γδ TCR treatment in arthritic mice, those neutrophils are able to increase IL-27 production. Indeed, number of cells and activity of cells are not always related. Furthermore, several studies reported the presence in circulation and tissue of distinct subsets of neutrophils, characterized by the expression of different markers36,37. However, it remains to be shown whether γδ T cell could modulate or switch neutrophils subsets.
Moreover, we found that IL-27 is also able to inhibit IL-23 induced splenic γδ T cell accumulation showing that IL-27 acts as a reciprocal regulator of γδ T cells. In keeping with our data, F. Morandi et al. provided us with the first demonstration that IL-27 modulates human γδ T cell functions in vitro38. Furthermore, IL-27 has been shown to inhibit the differentiation of Th17 cells and reduces the production of IL-17 in both, human and experimental autoimmune encephalomyelitis9,39,40 as well as CIA model41. This is consistent with studies demonstrating that IL-27 reduces the development of CIA and prevents progression of articular damage42,43. Similar to these observations in our data overexpression of IL-27 also inhibited arthritis initiation and progression. Mechanistically this was mainly due to a suppression of the IL-23-induced neutrophil expansion and neutrophil migration velocity. Keeping with our observations, Watzlawick, R. and al. demonstrated that IL-27 treatment inhibits neutrophil accumulation in peritonitis42. We hypothesize that IL-27 might regulate the expression of protein implicates into the leukocyte adhesion cascade (low rolling, adhesion strengthening, and intraluminal crawling). This may be linked with decreased Mac-1 expression in human neutrophils and suppressed neutrophil adhesion as well as LPS-induced ROS production and expression of cytotoxic granule components by IL-2744. While several studies indicate that γδ T cells support neutrophil recruitment, we do not exclude the possibility of the inverse relationship in which neutrophils modulate γδ T cells as it has been shown in human studies suggesting a bi-directional cross talk between γδ T cells and neutrophils43,45. Prospective studies should further investigate the specific subset of γδ T cells able to modulate this relationship.
In conclusion, our results establish a mechanistic connection between γδ T cells and neutrophils via IL-27. IL-27 suppresses the activation of γδ T cells and neutrophil expansion, thereby contributing to the resolution of inflammation. Accordingly, limiting the activities of γδ T cells may provide important insights and new treatment avenues for autoimmune and inflammatory diseases.
Materials and Methods
Reagents and mice
Male B10.RIII-H2r H2-T18b/(71NS)SnJ (B10.RIII), C57BL/6J (TCRδ+/+) and δ-chain TCR−/− mice (TCRδ−/−) mice were purchased from Jackson Laboratories (Sacramento, CA, USA). Sex- and age-matched mice at 12–14 weeks of age were used for each experiment. The University of California at Davis Institutional Animal Care and Use Committee approved all animal protocols. All experiments were performed in accordance with relevant guidelines and regulations. Serum samples were analyzed for cytokine protein levels using either ELISA kits for IL-27p28 (from R&D Systems), IL-17 and IL-23 from BioLegend in accordance with the manufacturer’s instructions or Th17 Bead-Based Multiplex Assays (Millipore). Flow cytometry antibodies were purchased from BioLegend (San Diego, USA). Splenocytes stimulation with LPS were performed in RPMI 1640 medium containing 10% FBS, 2 mM glutamine, penicillin/streptomycin (100 IU/mL) (Life Technologies). LPS (Sigma L4524 from Escherichia Coli 055:B5).
Production and purification of GFP, IL-23 and IL-27 minicircle DNA and hydrodynamic delivery
Minicircle-RSV.Flag.mIL23.elasti.bpA, p2øC31-RSV-PPT-FLAG-mIL-27.Elasti.bpA and RSV.eGFP.bpA was produced as described by Chen et al.46. Briefly, a single isolated colony from a fresh plate was grown for 8 h in 2 ml Luria-Bertani broth with appropriate antibiotic. Eight hundred microliters of this culture was used to inoculate 1 L Terrific broth and grown for an additional 17 h. Overnight cultures were centrifuged at 20 °C, 4000 rpm for 20 min. The pellet was resuspended 4:1 (v/v) in fresh Luria-Bertani broth containing 1% L-arabinose. The bacteria were incubated at 32 °C with constant shaking at 250 rpm for 2 h. After adding half volume of fresh low-salt Luria-Bertani broth (pH 8.0) containing 1% L-arabinose, the incubation temperature was increased to 37 °C and the incubation continued for an additional 2 h. Episomal DNA circles were prepared from bacteria using plasmid purification kits from Endofree Qiagen Megaprep (Chatsworth, CA, USA). Hydrodynamic delivery of MC DNA using the tail vein was performed as previously described9.
In vivo administration of anti-γδ TCR
B10.RIII mice were injected intraperitoneally with 200 μg of anti-mouse γδ TCR Abs every 5 days (clone UC7-13D5; BioLegend). Control mice received equal amounts of armenian hamster IgG isotype control antibodies. Anti-γδ TCR efficacy was tested by FACS analysis of CD3+ TCRδ+ in the spleen and lymph nodes (Supplemental Fig. 1A).
Clinical and Histological Methods
Disease severity for each limb is recorded as follows: 0 = normal; 1 = erythema and swelling of one digit; 2 = erythema and swelling of two digits 3 = erythema and swelling of more than two digits and/or swelling ankle joint. The clinical arthritis score was defined as the sum of the scores for all four paws of each mouse. Incidence was expressed as the percentage of mice with a disease score ≥1. Whole-ankle joints were fixed in 10% formalin decalcified in 10% EDTA and embedded in paraffin. Serial sections (4 μm) were stained with haematoxylin and eosin. Positive identification of neutrophils into the arthritic joints was determined by nuclear morphology and cytoplasmic color. The slides were obtained by Olympus BX61 confocal microscope and were analyzed with cellSens Dimension software.
Flow cytometry
Blood samples were collected in EDTA tubes (Sarstedt) from tail bleeds, were labeled with leucocyte-specific antibodies as previously described47. Spleen was enzymatically digested for 30 min at 37 °C in Hank’s balanced salt solution (Life Technologies) containing 1 mg/ml collagenase D (Sigma) and pushed through a 70-μm cell strainer to obtain a single-cell suspension, which was then stimulated and/or stained. BM was flushed out of femur by use of a 27-gauge needle attached to a 10 ml syringe filled with PBS. Red blood cells were lysed with BD Pharm Lyse (BD Biosciences). Non-specific binding was blocked with TruStain FcX antibody (BioLegend) for 10 min at 4 °C in FACS buffer (Ca2+/Mg2+-free PBS with 2% FBS and 0.5 M EDTA) before staining (30 min) with appropriate antibodies. Abs were purchased from BioLegend and included.
CD45 (30-F11), CD11b (M1/70), Ly-6C (HK1.4), Ly-6G (1A8) CD115 (AFS98), class II major histocompatibility complex (MHC) (IA/IE), CD11c (N418), CD64 (X54-5/7.1), TCRγδ (GL3), CD3ε (145-2C11). Isotype controls Abs were used at the same protein concentrations as their corresponding markers. As a gating strategy we used forward- and side-scatter parameters to exclude cell aggregates and debris from analysis. Cells and beads (polyscience) were counted on an Attune Cytometer (Life Technologies) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Cell sorting
For analysis of IL-27p28 mRNA, splenocytes from 12-weeks-old mice γδ TCR+/+ and γδ TCR−/− mice were stimulated for 5 H with 1 μg/mL LPS. Then, macrophages (CD64+ CD11bint), dendritic cells (CD11chi, MHCII+) and neutrophils (CD11b+ Ly-6G+) were sorted for qPCR analysis (Supplementary Fig. 2A). Dead cells and debris were excluded from the analysis using Zombie NIR™ Fixable Viability kit (Biolegend). Sorting was performed on a FACSAria II (BD Biosciences) and was reliably >90% of target population.
Quantitative real-time RT-PCR
Total RNA from sorted cells was prepared using RNeasy Mini Kit (QIAGEN). cDNA was prepared using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions in a final volume of 20 μL, starting with a 5 min template denaturation step at 95 °C followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C with the following primers: Mouse Gapdh 5′-TGGCCTTCCGTGTTCCTAC-3′ and 5′-GAGTTGCTGTTGAAGTCGCA-3′ Il-27p28 5′-CAGGATTCAAATGTTCAAAGG-3′ and 5′-GGGCAGCTTCTTTTCTTCTT-3′. Relative expression of real-time PCR products was determined by using the ΔΔCt method to compare target gene and GAPDH mRNA expression.
Chemotaxis experiment
Chemotactic migration of sorted blood neutrophils toward 10 ng/ml of IL-23 and IL-27 (from R&D Systems) or culture medium (negative control) was tested using IBIDI u slide chemotaxis. Cells (0.3 × 106 cells in 6 μ) were loaded into the central transversal chamber and incubated at 37 °C for 60 minutes to allow cell attachment. Fresh RPMI was loaded into adjacent reservoirs and a chemotactic gradient was created following the manufacturer’s instructions. Neutrophil migration was monitored by analyzing captured images in 2 min intervals for a total duration of 2 hours with 40X objective using Keyence BZ-9000 microscope. Images were analyzed with the ImageJ software using the Manual Tracking plugin. Chemotaxis plots and migration parameters (distances and velocities) were obtained with the Chemotaxis and Migration tool from Ibidi.
Statistical analysis
All results are expressed as mean ± SEM. Unpaired Student’s t test was used for determination of the significance of differences between two groups. One-way ANOVA was used to determine statistical significances in groups larger than two. A probability value of less than 0.05 is considered significant. Statistical analyses were performed using GraphPad Prism VI software (GraphPad Software, Inc.).
Electronic supplementary material
Acknowledgements
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health Grant AR62173, Shriners Hospitals for Children Grant 250862, and a National Psoriasis Foundation Translational Research Grant (to I.E.A.). EM.G is supported by the Austrian Science Fund (FWF): J3715-B26. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Author Contributions
Conceived and designed the experiments: I.E.A. Performed the experiments: L.B., M.K., E.M.G., I.E.A. Analyzed the data: L.B., M.K., E.M.G., I.E.A. Wrote the paper: L.B., I.E.A. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25988-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nature reviews. Immunology. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 2.Paul S, Shilpi, Lal G. Role of gamma-delta (gammadelta) T cells in autoimmunity. Journal of leukocyte biology. 2015;97:259–271. doi: 10.1189/jlb.3RU0914-443R. [DOI] [PubMed] [Google Scholar]
- 3.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nature reviews. Immunology. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beetz S, et al. Innate immune functions of human gammadelta T cells. Immunobiology. 2008;213:173–182. doi: 10.1016/j.imbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs MR, Haynes BF. Increase in TCR gamma delta T lymphocytes in synovia from rheumatoid arthritis patients with active synovitis. Journal of clinical immunology. 1992;12:130–138. doi: 10.1007/BF00918143. [DOI] [PubMed] [Google Scholar]
- 6.Kjeldsen-Kragh J, et al. T gamma delta cells in juvenile rheumatoid arthritis and rheumatoid arthritis. In the juvenile rheumatoid arthritis synovium the T gamma delta cells express activation antigens and are predominantly V delta 1+, and a significant proportion of these patients have elevated percentages of T gamma delta cells. Scandinavian journal of immunology. 1990;32:651–659. doi: 10.1111/j.1365-3083.1990.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 7.Kenna TJ, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive gamma/delta T cells in patients with active ankylosing spondylitis. Arthritis and rheumatism. 2012;64:1420–1429. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 8.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of gamma delta T cells in murine collagen-induced arthritis. Journal of immunology. 1993;151:6546–6558. [PubMed] [Google Scholar]
- 9.Adamopoulos IE, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. Journal of immunology. 2011;187:951–959. doi: 10.4049/jimmunol.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. The Journal of experimental medicine. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petermann F, et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis and rheumatism. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 13.Roark CL, et al. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. Journal of immunology. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamopoulos IE, et al. IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Annals of the rheumatic diseases. 2015;74:1284–1292. doi: 10.1136/annrheumdis-2013-204782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubberts E, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. Journal of immunology. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 16.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. Journal of immunology. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 17.Cho JS, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. The Journal of clinical investigation. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffelt SB, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohr W, Westerhellweg H, Wessinghage D. Polymorphonuclear granulocytes in rheumatic tissue destruction. III. an electron microscopic study of PMNs at the pannus-cartilage junction in rheumatoid arthritis. Annals of the rheumatic diseases. 1981;40:396–399. doi: 10.1136/ard.40.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittkowski H, et al. Effects of intra-articular corticosteroids and anti-TNF therapy on neutrophil activation in rheumatoid arthritis. Annals of the rheumatic diseases. 2007;66:1020–1025. doi: 10.1136/ard.2006.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119:195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. Journal of immunology. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 23.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. The Journal of experimental medicine. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchareychas L, Grossinger EM, Kang M, Qiu H, Adamopoulos IE. Critical Role of LTB4/BLT1 in IL-23-Induced Synovial Inflammation and Osteoclastogenesis via NF-kappaB. Journal of immunology. 2016 doi: 10.4049/jimmunol.1601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villarino AV, Hunter CA. Biology of recently discovered cytokines: discerning the pro- and anti-inflammatory properties of interleukin-27. Arthritis research & therapy. 2004;6:225–233. doi: 10.1186/ar1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenecke C, et al. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. European journal of immunology. 2009;39:372–379. doi: 10.1002/eji.200838741. [DOI] [PubMed] [Google Scholar]
- 27.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollinger B, et al. Th17 cells, not IL-17+ gammadelta T cells, drive arthritic bone destruction in mice and humans. Journal of immunology. 2011;186:2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 29.Kong X, Sun R, Chen Y, Wei H, Tian Z. gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. Journal of immunology. 2014;193:1645–1653. doi: 10.4049/jimmunol.1303432. [DOI] [PubMed] [Google Scholar]
- 30.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. Journal of immunology. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 31.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nature reviews. Immunology. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 32.Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology. 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. The Journal of experimental medicine. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akitsu A, et al. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vgamma6(+)gammadelta T cells. Nature communications. 2015;6:7464. doi: 10.1038/ncomms8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nature immunology. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morandi F, Prigione I, Airoldi I. Human TCRgammadelta+T cells represent a novel target for IL-27 activity. European journal of immunology. 2012;42:1547–1552. doi: 10.1002/eji.201142241. [DOI] [PubMed] [Google Scholar]
- 39.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature immunology. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 40.Diveu C, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. Journal of immunology. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 41.Moon SJ, et al. In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Experimental & molecular medicine. 2013;45:e46. doi: 10.1038/emm.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watzlawick R, Kenngott EE, Liu FD, Schwab JM, Hamann A. Anti-Inflammatory Effects of IL-27 in Zymosan-Induced Peritonitis: Inhibition of Neutrophil Recruitment Partially Explained by Impaired Mobilization from Bone Marrow and Reduced Chemokine Levels. PloS one. 2015;10:e0137651. doi: 10.1371/journal.pone.0137651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabbione F, et al. Neutrophils suppress gammadelta T-cell function. European journal of immunology. 2014;44:819–830. doi: 10.1002/eji.201343664. [DOI] [PubMed] [Google Scholar]
- 44.Li JP, et al. Interleukin-27 as a negative regulator of human neutrophil function. Scandinavian journal of immunology. 2010;72:284–292. doi: 10.1111/j.1365-3083.2010.02422.x. [DOI] [PubMed] [Google Scholar]
- 45.Bank I, et al. gammadelta T cell subsets in patients with arthritis and chronic neutropenia. Annals of the rheumatic diseases. 2002;61:438–443. doi: 10.1136/ard.61.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen ZY, He CY, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Human gene therapy. 2005;16:126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- 47.Bouchareychas L, et al. Promoting macrophage survival delays progression of pre-existing atherosclerotic lesions through macrophage-derived apoE. Cardiovascular research. 2015;108:111–123. doi: 10.1093/cvr/cvv177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.