ABSTRACT
Retrograde transport (RT) allows cells to retrieve receptors and other cellular cargoes for delivery to the Golgi apparatus, contributing to the maintenance of cellular homeostasis. This transport route is also commonly used by several bacterial toxins to exert their deleterious actions on eukaryotic cells. While the retrograde transport process has been well characterized, the contribution of microRNAs (miRNAs) in regulating this cellular transport mechanism remains unknown. Here, we determined that mir-199a and mir-199b, members of the intronic miRNA family, coordinate genes regulating RT and endosome trafficking. We demonstrate that miR-199a-5p attenuates the expression of Vps26A, Rab9B, and M6PR, thereby controlling RT from endosomes to the trans-Golgi network (TGN). Importantly, we found that overexpression of a Vps26A construct resistant to the inhibitory action of miR-199a-5p abrogates the effect of miR-199a-5p on RT. Finally, we demonstrate that miR-199-5p overexpression attenuates Shiga toxin type 1 (Stx1)-mediated inhibition of protein biosynthesis. In summary, our work identifies the first noncoding RNA that influences RT and reduces the inhibition of protein biosynthesis caused by bacterial toxins.
KEYWORDS: bacterial toxins, dynamin, retrograde transport, miRNAs
INTRODUCTION
Endosomal-system homeostasis is crucial for intracellular functions like development, metabolism, and signaling (1, 2). Endocytic internalization and recycling routes have been studied mechanistically in some detail. For maintenance of proper protein and cargo sorting, cells need to coordinate intracellular trafficking through the functioning of endosomes (3). To this end, the retrograde transport (RT) route allows trafficking of protein and lipid cargoes from endosomes to the trans-Golgi network (TGN) and plasma membrane, thus regulating the abundance and intracellular distribution of cargoes within cells (4–6). Using this pathway, intracellular resident proteins, such as TGN46, furin, or cation-independent mannose-6-phosphate receptor (CI-M6PR), evade degradative trafficking by being retrieved from endosomes to the TGN (7–9). Moreover, numerous viruses and bacterial toxins utilize RT to enter the cell and reach the endoplasmic reticulum (ER). The RT route converges with the forward biosynthetic pathway (exit route) at the trans-Golgi network. Retrograde trafficking can be initiated at different levels of the endosome (early, late, and recycling endosomes) in a process that is termed retrograde sorting (10, 11). Exit of cargo molecules is mediated by different retrograde-sorting proteins for delivery to the TGN and Golgi apparatus, where a diverse set of tethering factors act on acceptor membranes (12, 13).
A number of proteins with previously reported roles in endocytosis and intracellular trafficking have been shown to participate in RT, including clathrin, AP-1, OCRL, and others (10, 14, 15). Interestingly, there is a set of proteins that have specific roles in RT, among them the evolutionarily conserved retromer complex that mediates sorting from endosomes to the TGN. This protein complex is highly selective and involves two protein subcomplexes. The mammalian retromer is a pentameric complex that consists of the vacuolar protein-sorting (VPS) trimer subcomplex VPS26/VPS29/VPS35 and the less-defined sorting nexin (SNX) dimer subcomplex (16, 17). Retromer-mediated RT is initiated by the core trimer responsible for cargo recognition, binding, and selection through binding cytosolic domains of cargo molecules to VPS35 and VPS26 (18). The resulting nucleation complex also interacts with GTP-activated Rab7, and then several SNXs (SNX1, SNX2, SNX5, and SNX6) associate in dimers with this nascent nucleation retromer complex, facilitating endosomal membrane curvature to produce tubules/vesicles (19, 20). Once the cargo carriers are matured, dynamin-2 (DNM2) catalyzes the excision of vesicles. Finally, the fusion of RT intermediates with the TGN requires tethering factors like golgin-97, SNARE complexes, and Rab GTPases (21–23).
Many proteins are known to traffic between endosomes and the TGN, including the acid hydrolase-sorting receptor M6PR. In the biosynthetic/secretory pathway, newly synthesized acid hydrolase precursor proteins bind to M6PR in TGN membranes and are transferred to endocytic pathways. At the endosome compartment, the low acidic pH in the late endosome-lysosome results in the uncoupling of receptor-ligand complexes. M6PR is then recycled back to the TGN by means of RT, in which the retromer acts to initiate a new round of delivery (24). CI-M6PR may follow a more complex route to traffic from the plasma membrane to the TGN. Alternatively, retrieval of CI-M6PR from late endosomes appears to progress through a TIP-/Rab9-dependent pathway that is independent of retromers (9, 25). In addition to endogenous proteins, several pathogens, such as Shigella dysenteriae, secrete extracellular toxins, such as Shiga toxin type 1 (Stx1) and cholera toxin, that exploit the intracellular RT to enter the ER in the host cells and are delivered by retrotranslocation to the cytosol, where they exert their toxic effects (26).
While the molecular machinery that controls RT is well understood, the contribution of noncoding RNAs in regulating this process remains unknown. Here, we identify a family of microRNAs (miRNAs), encoded within the intronic regions of DNM genes (mir-199a and -b [mir-199a/b]), whose members control RT. miR-199a-5p regulates the expression of genes associated with RT, including Vps26A, Rab9B, and M6PR, and protects against Stx1-induced protein biosynthesis inhibition. We also demonstrate that miR-199a-5p expression influences autophagosome formation and lysosomal function. Most importantly, we elucidate a novel molecular mechanism by which miR-199-5p isoforms and the host genes where they are encoded (DNM) jointly regulate different genes/proteins involved in the RT intracellular trafficking pathway.
RESULTS
miR-199a-5p regulates the expression of genes associated with retrograde transport.
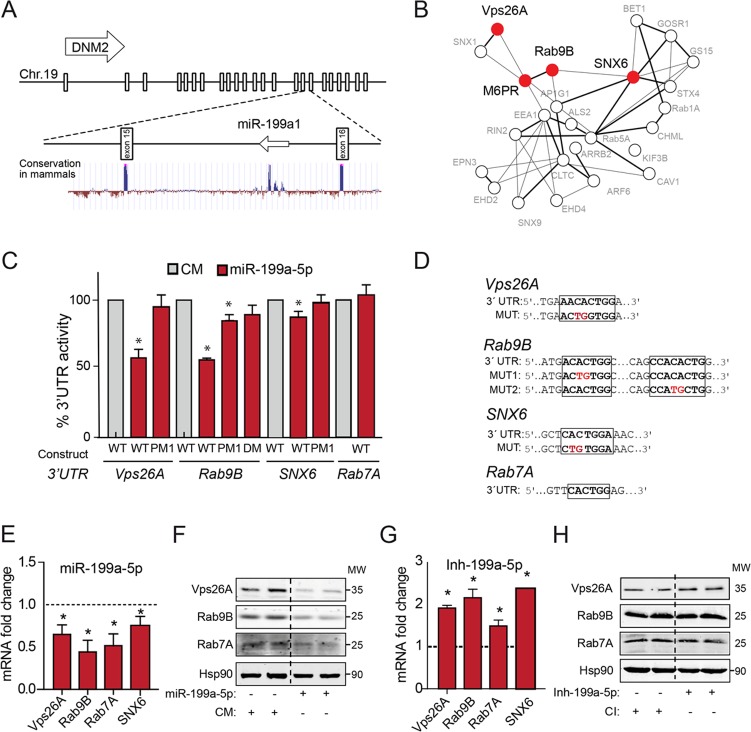
We and others have previously identified the intriguing genomic localization of the mir-199 miRNA family within intronic sequences of DNM genes (Fig. 1A) (27). Since intronic miRNAs are known to regulate cellular functions similar to those of the gene where they are encoded, we performed a number of bioinformatics analyses searching for potential mRNA targets associated with DNM-regulated functions, using Targetscan (http://www.targetscan.org) and miRanda (http://www.microrna.org). Interestingly, we found among the highest-scored predicted miR-199-5p target genes several that encode important regulators of the RT pathway, such as Vps26A, SNX6, Rab9B, and Rab7A, and cargo proteins, including M6PR, whose product mediates the transport of hydrolases to the lysosomes (Fig. 1B). The 3′ untranslated regions (UTRs) of Vps26A, SNX6, Rab9B, and Rab7A have predicted binding sites for miR-199-5p that are highly conserved across species (data not shown). To determine whether miR-199a-5p directly binds Vps26A, SNX6, Rab9B, and Rab7A 3′ UTRs, we generated reporter constructs with the luciferase-coding sequence fused to the 3′ UTRs of these genes. The results show that miR-199a-5p markedly repressed Vps26A, SNX6, and Rab9B 3′-UTR–luciferase gene activity, demonstrating that the expression of these genes is directly regulated by miR-199a-5p (Fig. 1C). Importantly, specific mutations in miR-199a-5p binding sites released the repression of Vps26A and Rab9B 3′-UTR activity by miR-199a-5p overexpression, consistent with a direct interaction of miR-199a-5p with these sites (Fig. 1C and D). Surprisingly, miR-199a-5p did not repress Rab7A 3′-UTR activity despite the presence of a putative specific binding site and the decreased mRNA levels upon transfection with miR-199a-5p mimics (miR-199a-5p mimics are small, chemically modified double-stranded RNAs that mimic endogenous miRNAs and enable miRNA functional analysis by upregulation of miRNA activity) (Fig. 1E). We next determined whether miR-199a-5p levels influence Vps26A, Rab9B, and Rab7A mRNA and protein expression levels. To this end, we transfected HeLa cells with miR-199a-5p mimics or scrambled control mimic (CM) and assessed Vps26A, Rab9B, and Rab7A mRNA and protein expression by quantitative real-time PCR and Western blotting, respectively. As expected from the inhibitory effect of miR-199a-5p on 3′-UTR luciferase activity, miR-199a-5p overexpression significantly attenuated Vps26A and Rab9B mRNA and protein expression (Fig. 1E and F). In addition to its effect on Vps26 and Rab9B expression, we observed that miR-199a-5p overexpression also decreases Rab7A mRNA and protein expression, suggesting that miR-199a-5p might influence Rab7A expression by an indirect mechanism. We further assessed whether inhibition of miR-199a-5p enhances the expression of Vps26A, Rab9B, Rab7A, and SNX6 mRNA. Importantly, we found that miR-199a-5p antagonism in vitro increases the expression of these transcripts (Fig. 1G). Vps26A protein levels were also enhanced in cells in which miR-199-5p was silenced (Fig. 1H). These results strongly suggest that the endogenous miR-199b-5p levels influence the expression of Vps26A, Rab9B, Rab7A, and SNX6. Together, these findings demonstrate that miR-199a-5p might control RT by directly regulating the expression of Vps26A, Rab9B, Rab7A, and SNX6.
FIG 1.
miR-199a is encoded in DNM locus genomic locations and regulates the expression of genes associated with retrograde transport (RT). (A) Schematic representation of genomic locations of DNM2 gene and intronic mir-199a1. (B) Gene ontology analysis of the miR-199a/b target genes predicted using Panther software. A protein-protein interaction analysis scheme of selected predicted miR-199a-5p target genes using STRING 9.1 software and Navigator 2.2 is shown. RT-related protein-coding genes are highlighted in red. (C and D) Luciferase reporter activity (C) in COS7 cells transfected with control mimic (CM) or miR-199a-5p mimic and the indicated human 3′-UTR sequences containing or not containing (wild-type [WT]) the indicated point mutations (PM) in the miR-199a-5p-binding sites (D). DM, double mutation; MUT, mutant construct. (E) Quantitative real-time PCR analysis of Vps26A, Rab9B, Rab7A, and SNX6 mRNA expression in HeLa cells transfected with CM and miR-199a-5p mimic. (F) Western blot analysis of Vps26A, Rab9B, and Rab7A in HeLa cells transfected with CM or miR-199a-5p. Hsp90 was used as a loading control. (G) Quantitative real-time PCR analysis of Vps26A, Rab9B, Rab7A, and SNX6 expression in HeLa cells transfected with control inhibitor or miR-199a-5p inhibitor. (H) Western blot analysis of Vps26A, Rab9B, and Rab7A in HeLa cells transfected with control inhibitor (CI) or miR-199a-5p inhibitor Inh-199a-5p. (C) Data are expressed as percentages of 3′-UTR activity of miR-199a-5p versus that in CM-transfected cells and represent the mean values ± SEM of a representative experiment performed three times in triplicate. (E and G) Data are expressed as mean values ± SEM and are representative of ≥3 independent experiments performed in triplicate. *, P ≤ 0.05. (F and H) MW, molecular weight in thousands.
miR-199a-5p impairs intracellular retrograde transport.
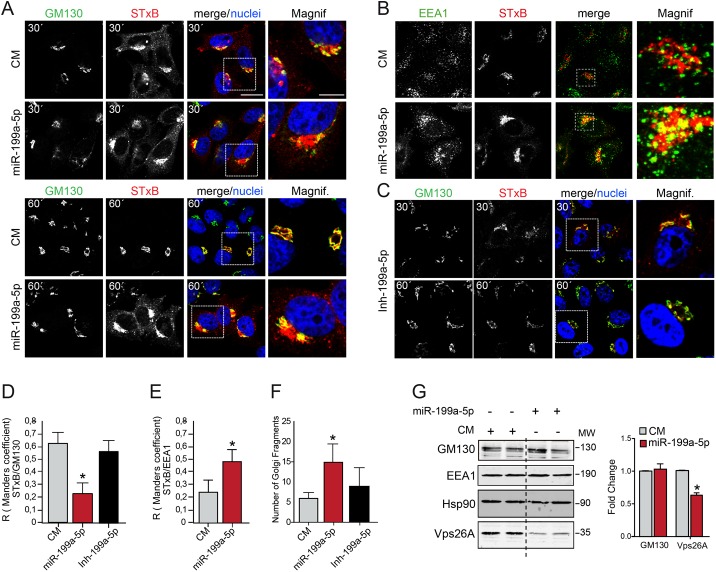
Vps26A and SNX6, as part of the retromer (10, 28), and Rab9B and Rab7, in regulating endosome trafficking, have previously been reported to play a role in RT (29). A number of bacterial toxins, including Stx1, use RT to enter into the cell (30). The Shiga toxins, Stx1 and Stx2, consist of two major subunits, the A and B subunits. The A subunit binds noncovalently to five B subunits as a pentamer complex. While the A subunit (StxA) of the toxin triggers the inhibition of protein biosynthesis in the eukaryotic ribosome, the B subunit (StxB) binds to the glycolipid globotriaosylceramide (Gb3), its cellular receptor, and is further internalized from endosomes to the TGN in a retrograde manner. Thus, Stx1 (A and B subunits) finally reaches the ER to exert the toxic effect. To investigate whether miR-199a-5p controls the RT pathway, we transfected HeLa cells with either miR-199a-5p or CM and assessed the internalization of fluorophore-tagged StxB (Cy3-StxB). As seen by the results in Fig. 2A, top, StxB is efficiently internalized after 30 min of incubation at 37°C in HeLa cells transfected with CM or miR-199a-5p. However, StxB accumulation in the TGN-Golgi apparatus region was markedly reduced in miR-199a-5p-transfected cells, and the protein remained associated with peripheral structures, even after 60 min of incubation (Fig. 2A and D). We next characterized the intracellular localization of StxB in cells transfected with miR-199a-5p mimics and found that miR-199a-5p overexpression enhanced the colocalization of Cy3-StxB with early endosome antigen 1 (EEA1), an early endosome marker, suggesting that miR-199a-5p impairs transport from early endosomes to the Golgi apparatus (Fig. 2B and E). Interestingly, when we analyzed the Golgi apparatus structure in miR-199a-5p-transfected cells, we observed an increased Golgi apparatus fragmentation compared with the results for CM-treated cells (Fig. 2A). This effect was quantified by measuring the number of independent structures that stained for GM130 under each condition (Fig. 2F). We also analyzed the effect on StxB targeting upon miR-199a-5p antagonization and found no differences compared to the results for control-miR-treated cells at either 30 min or 60 min (Fig. 2C, D, and F). The impact of miR-199a-5p levels in regulating intracellular transport and Golgi morphology was not associated with changes in EEA1 and GM130 expression (Fig. 2G).
FIG 2.
miR-199a-5p regulates Shiga toxin internalization in HeLa cells. (A and B) CM- and miR-199a-5p-transfected cells were incubated with Cy3-StxB (5 μg/ml) on ice for 48 h and then shifted to 37°C for 30 min (A, top, and B) and 60 min (A, bottom) Cells were fixed and labeled with anti-GM130 antibody (A), anti-EEA1 antibody (B), and DAPI (4′,6-diamidino-2-phenylindole) (A and B). Z projections of confocal stacks are shown. Note that miR-199a-5p-overexpressing cells accumulated StxB in peripheral membranes. Scale bar = 15 μm. Magnif, magnifications of areas in dotted boxes. Scale bar = 10 μm. (C) HeLa cells treated with Inh-199a-5p were incubated for 30 min and 60 min with Cy3-StxB as described in the legend to panels A and B. (D and E) Quantification of Manders' coefficients for colocalization of Cy3-StxB localized in the Golgi apparatus (GM130, at 60 min) or early endosomes (EEA1, at 30 min) in experiments whose results are shown in panels A and C or B, respectively. (F) Quantification of Golgi apparatus fragments in control mimic (CM)- and miR-199a-5p-transfected cells in experiments whose results are shown in panels A to C. (G) Western blot analysis of GM130, EEA1, and Vps26A in cells transfected with CM or miR-199a-5p mimics. MW, molecular weight in thousands. Right, quantification of the relative amounts of proteins. The mean values ± SEM from three independent experiments are shown. P values were calculated using the t test. *, P ≤ 0.05.
miR-199a-5p overexpression markedly attenuates StxB-mediated inhibition of protein biosynthesis.
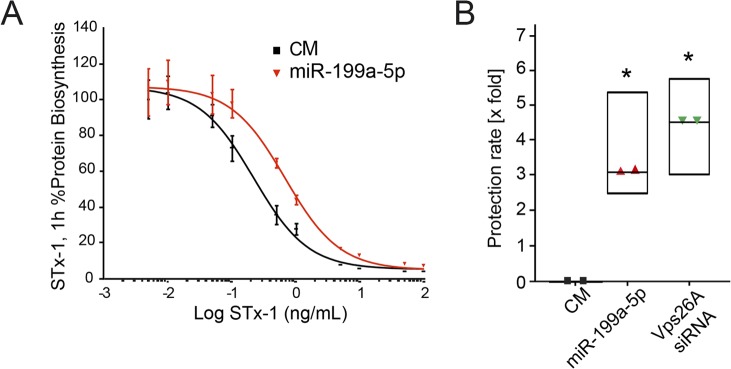
Given that miR-199a-5p impairs trafficking of StxB via the retrograde route, we next tested whether miR-199a-5p expression attenuates the inhibitory effect of Stx1 on protein biosynthesis. To this end, we transfected HeLa cells with CM or miR-199a-5p and then treated them with increasing concentrations of Stx1 and assessed protein biosynthesis by measuring the incorporation of radiolabeled methionine into neosynthesized polypeptides. As seen by the results in Fig. 3A, miR-199a-5p overexpression lessened the Stx1-mediated inhibition of protein synthesis as early as 1 h after toxin exposure. At this time, miR-199a-5p protected cells against Stx1 compared with the toxin's effect on CM-treated cells, with an observed protection factor of 3. This value is in close proximity to that observed when we treated HeLa cells with Vps26A small interfering RNA (siRNA) (Fig. 3B), suggesting that Vps26A could mediate, in part, the effect of miR-199a-5p in RT.
FIG 3.
miR-199a-5p protects against Shiga toxin intoxication in HeLa cells. (A) HeLa cells were transfected for 48 h with control mimic (CM) and miR-199a-5p before the addition of Shiga toxin (StxB) for 1 h. Intoxication of HeLa cells is shown. Each point corresponds to the mean value ± SEM from a representative experiment out of two to three determinations. (B) Protection factors calculated over the indicated number of experiments. Mean values ± SEM are shown. P values were calculated using the t test. *, P ≤ 0.05.
Vps26A expression rescues retrograde transport and maintenance of Golgi apparatus structure in HeLa cells overexpressing miR-199a-5p.
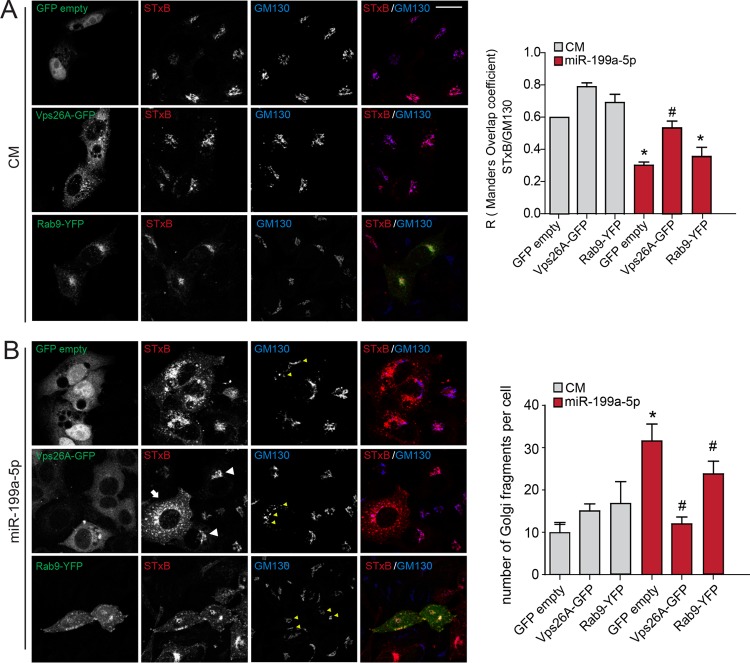
To define the contribution of Vps26A in regulating miR-199a-5p-induced RT suppression, we transfected HeLa cells with vector encoding green fluorescent protein (GFP) only (control) or Vps26A-GFP that lacked the 3′-UTR sequence and, thus, was resistant to the inhibitory action of miR-199a-5p. The results showed that while both GFP- and Vps26A-GFP-expressing cells transfected with CM internalized Cy3-StxB and the toxin accumulated in perinuclear membranes that were labeled by anti-GM130 antibody (Fig. 4A, top and middle), transfection with miR-199-5p resulted in significant impairment of RT (Fig. 4B, top). Of note, Vps26A-GFP transfection rescued the inhibitory effect on RT in miR-199a-5p-overexpressing cells (Fig. 4B, middle). We also noticed that miR-199a-5p-transfected cells that expressed Vps26A-GFP showed a compacted Golgi apparatus structure similar to that in CM-transfected cells, suggesting that restoration with ectopic Vps26A leads to maintenance of a proper Golgi apparatus architecture (Fig. 4B). In contrast, when we transfected miR-199a-5p-overexpressing cells with a Rab9-yellow fluorescent protein (YFP) construct that was defective in its 3′ UTR, we did not observe any restoration effect in either StxB targeting or Golgi structure (Fig. 4B, bottom). These results suggest that Vps26A but not Rab9 expression is a major determinant that mediates the regulation of StxB RT by miR-199a-5p.
FIG 4.
Vps26A is necessary for Shiga toxin internalization and maintenance of Golgi apparatus structure in miR-199a-5p-overexpressing HeLa cells. (A and B) HeLa cells were cotransfected as indicated with control mimic (CM) (A) or miR-199a-5p (B) and GFP (GFP empty), Vps26A-GFP, or Rab9-YFP for 48 h. Live cells were incubated with Cy3-StxB (5 μg/ml) on ice and then shifted to 37°C for 60 min. After Cy3-StxB internalization, cells were fixed and labeled with anti-GM130 antibody and DAPI. Z projections of confocal stacks are shown. The arrow indicates an miR-199a-5p-overexpressing cell with increased accumulation of StxB in peripheral membranes, and white arrowheads show Vps26A-GFP cells that efficiently accumulated StxB in the Golgi apparatus area, as indicated by GM130 labeling. Yellow arrowheads show dispersed Golgi apparatus structures. Scale bar = 15 μm. Right, colocalization coefficients for Cy3-StxB and GM130 (A) and quantification of Golgi apparatus fragments (B) under the indicated conditions in three independent experiments. Data are expressed as mean values ± SEM. *, P ≤ 0.05; #, P > 0.05.
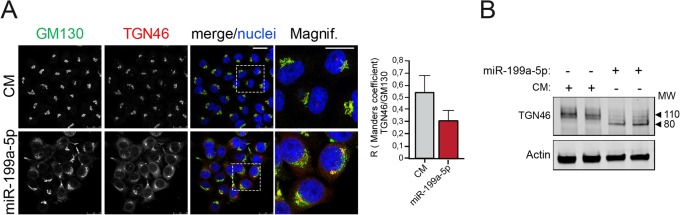
Vps26A regulates the subcellular localization and glycosylation state of TGN46.
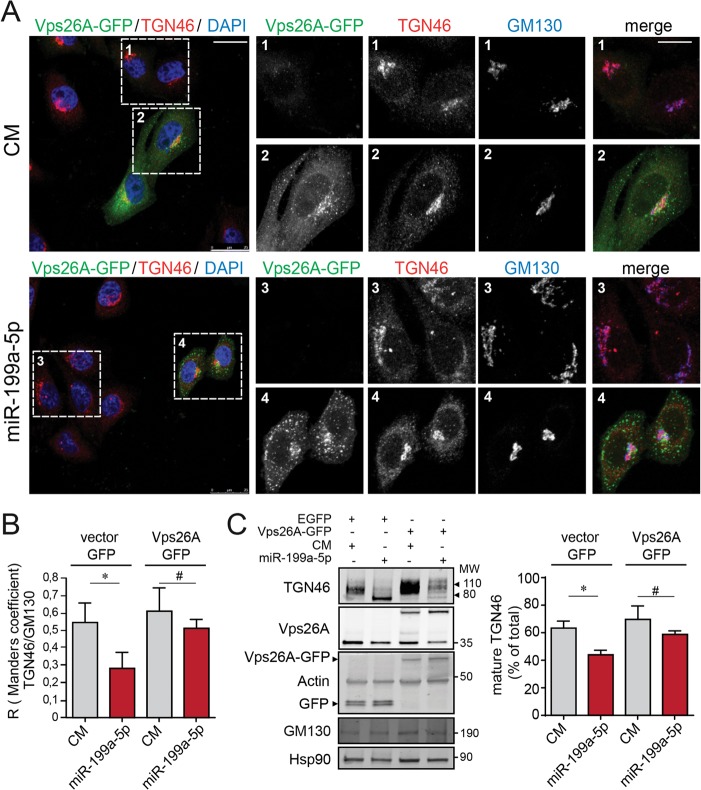
Next, we wondered whether miR-199a-5p might also influence the RT of an endogenous protein. Therefore, we tested whether miR-199a-5p influences the intracellular trafficking of TGN46, a transmembrane glycosylated protein that is localized to the TGN and cycles between the TGN and the plasma membrane (31). At steady state, TGN46 predominantly localized in the perinuclear Golgi apparatus, as evidenced by its being colabeled with GM130 in CM-transfected cells (Fig. 5A, top). In contrast, miR-199a-5p overexpression resulted in TGN46 and GM130 dispersion in the cell periphery, confirming Golgi apparatus fragmentation (Fig. 5A, bottom). TGN46 has a molecular mass of 46 kDa, but as a result of various glycosylation processes occurring at the ER and the Golgi complex (32), the mature protein has an apparent molecular mass of ∼110 kDa (Fig. 5B). As expected based on our previous observations, we found a significant loss of TGN46 glycosylation in cells transfected with miR-199-5p, suggesting that miR-199a-5p impairs TGN46 transport between the ER and Golgi apparatus (Fig. 5B). We also performed rescue-of-function experiments by using a Vps26-GFP construct that is resistant to miR-199a-5p inhibition. As seen by the results in Fig. 6A, TGN46 and GM130 colocalized in the Golgi apparatus (Fig. 6A1). We observed that steady-state localization of ectopic Vps26A-GFP is perinuclear and that it is localized partially in association with Golgi apparatus markers (GM130) but also in endosomes (Fig. 6A2). Of note, we found that transfection of Vps26A-GFP in miR-199a-5p-overexpressing cells restored TGN46 perinuclear localization and Golgi apparatus architecture (Fig. 6A4) compared with the results for cells not transfected with Vps26A-GFP (Fig. 6A3).
FIG 5.
miR-199a-5p regulates the intracellular localization and glycosylation of TGN46. (A) Steady-state intracellular location of TGN46 in HeLa cells transfected with control mimic (CM) and miR-199a-5p for 48 h and costained with GM130. Scale bar = 25 μm. Magnif, magnification of areas in dashed boxes. Scale bar = 10 μm. Right, colocalization coefficients for GM130 and TGN46. Data are from three independent experiments and are expressed as mean values ± SEM. (B) Representative Western blot analysis of TGN46 in HeLa cells transfected with CM or miR-199a-5p mimic after 48 h. MW, molecular weight in thousands.
FIG 6.
Vps26A overexpression leads to recovery from miR-199a-5p inhibition of TGN46 retrograde transport. (A) Immunofluorescence analysis showing HeLa cells cotransfected with control mimic (CM) and Vps26A-GFP (top left) or miR-199a-5p and Vps26A-GFP (bottom left) for 48 h. Cells were fixed and labeled with anti-TGN46 antibody, anti-GM130 antibody, and DAPI, and the steady-state localization of TGN46 and GM130 was observed. Z projections of confocal stacks are shown. Scale bar = 25 μm. Magnified images of areas in dashed boxes are shown to the right. Scale bar = 10 μm. (B) Colocalization coefficients for TGN46 and GM130 under the indicated conditions. (C) Representative Western blot analysis of TNG46, Vps26, Vps26-GFP, actin, GFP, GM130, and Hsp90 in HeLa cells transfected as indicated (CM and EGFP, miR-199a-5p and EGFP, CM and Vps26A-GFP, and miR-199a-5p and Vps26A-GFP). Actin and Hsp90 were used as loading controls. MW, molecular weight in thousands. Right, quantification of mature TGN46 (110 kDa). Data are expressed as mean percentages of the total amount of TGN46 ± SEM. *, P ≤ 0.05; #, P > 0.05.
We also assessed the glycosylation pattern of TGN46 upon rescue with Vsp26-GFP expression. Importantly, the effect of miR-199a-5p overexpression on the glycosylation state of TGN46 was partially restored when we transfected HeLa cells with a Vps26A construct resistant to the inhibitory action of miR-199a-5p (Fig. 6B). Next, we further examined the role of Rab9 GTPase in RT after miR-199a-5p transfection. Together, these results demonstrate that miR-199a-5p controls the RT of endogenous proteins and Golgi apparatus homeostasis mainly by regulating the expression of Vps26A, an essential component of the retromer complex.
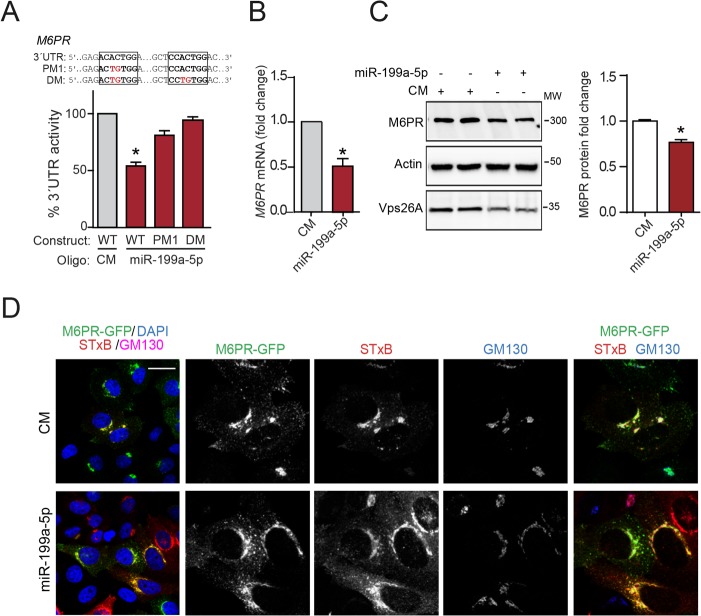
miR-199a-5p controls the expression of M6PR and the lysosome function.
M6PR cycles between the plasma membrane, lysosomes, and TGN/Golgi apparatus; in the latter, M6PR binds to newly synthesized hydrolases that will be transported all the way to the lysosomes (33, 34). It has been well established that this receptor utilizes RT to return from the late endosomes/lysosomes to the TGN and then initiates a new cycle of delivery (35). Interestingly, we found that M6PR was a predicted target for miR-199a-5p in our bioinformatics analysis (Fig. 1C). To directly demonstrate that M6PR is a bona fide miR-199a-5p target gene, we cloned the 3′ UTR of M6PR in a luciferase reporter construct and measured the luciferase activity in HeLa cells transfected with miR-199a-5p or CM. The results showed that miR-199a-5p markedly reduced luciferase activity (Fig. 7A). Most importantly, mutations in the two miR-199a-5p predicted binding sites within the 3′ UTR of M6PR abolished the miR-199a-5p inhibitory action, thus confirming the direct binding of miR-199a-5p to the M6PR 3′ UTR (Fig. 7A). We next evaluated the effect of miR-199a-5p overexpression on M6PR expression and functionality in HeLa cells. As seen by the results in Fig. 7B and C, overexpression of miR-199a-5p significantly downregulated M6PR mRNA and protein expression compared to their levels in CM-transfected cells. We explored the contribution of M6PR restoration to StxB-impaired RT upon the expression of miR-199a-5p. The results showed that transfection of an ectopic miRNA-resistant M6PR-GFP construct failed to rescue the StxB targeting to the TGN independently of the expression of miR-199a-5p, suggesting that M6PR as an endogenous RT cargo does not regulate StxB RT (Fig. 7D).
FIG 7.
miR-199a-5p controls M6PR expression. (A) Luciferase reporter activity in COS7 cells transfected with control mimic (CM) or miR-199a-5p mimic and M6PR 3′ UTR containing or not containing (WT, wild-type) the indicated point mutations (PM) in the miR-199a-5p-binding sites. Data are expressed as percentages of M6PR 3′-UTR activities in miR-199a-5p- versus CM-transfected cells. *, P ≤ 0.05. DM, double mutation. (B) Quantitative real-time PCR analysis of M6PR expression in HeLa cells transfected with control mimic (CM) and miR-199a-5p mimic after 48 h. (C) Representative Western blot analysis of M6PR expression in HeLa cells treated as described in the legend to panel B. (B and C) Data are expressed as mean values ± SEM and are representative of the results of ≥3 experiments in triplicate. *, P ≤ 0.05. (D) Immunofluorescence analysis of Cy3-StxB internalization in HeLa cells cotransfected with CM and M6PR-GFP or miR-199a-5p and M6PR-GFP for 48 h. Steady-state localization is shown in Z projections of confocal stacks. Scale bar = 20 μm.
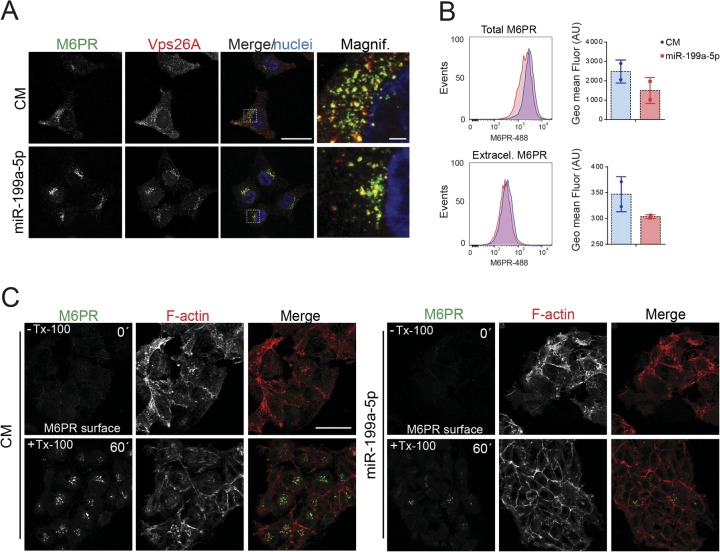
We next examined the steady-state subcellular distribution of M6PR in cells transfected with miR-199a-5p mimics. While M6PR was located both in a perinuclear area and in peripheral vesicles, where it partially localized to retromer component Vps26A in HeLa cells transfected with CM, M6PR and Vps26A localized mainly in the perinuclear region but not in the cellular peripheral area in miR-199a-5p-overexpressing cells (Fig. 8A). We further analyzed the effect of miR-199-5p overexpression on M6PR cellular localization by flow cytometry. As expected, the total M6PR levels (both intracellular and extracellular staining) were significantly reduced in cells transfected with miR-199-5p compared with the levels in CM-overexpressing cells (Fig. 8B, top). Similarly, we also found a modest decrease of M6PR at the plasma membrane (Fig. 8B, bottom). The impairment in M6PR intracellular transport was confirmed by measuring M6PR antibody uptake in living HeLa cells. To this end, both CM- and miR-199a-5p-transfected cells were preincubated at 4°C to decrease plasma membrane endocytosis and exposed to anti-M6PR antibodies that recognize an extracellular epitope of the receptor, and then intracellular transport was induced by shifting to 37°C. As seen by the results in Fig. 8C, right, miR-199a-5p expression prevented the accumulation of M6PR in the TGN after 60 min of internalization, suggesting that miR-199a-5p largely decreased M6PR internalization and intracellular distribution in live HeLa cells.
FIG 8.
miR-199a-5p regulates M6PR plasma membrane expression and M6PR intracellular transport. (A) Immunofluorescence analysis showing steady-state localization of endogenous M6PR and Vps26A after 48 h of transfection with CM and miR-199a-5p. Scale bar = 25 μm. Magnif, magnification of areas in dotted boxes. Scale bar = 5 μm. (B) Representative flow cytometry histograms of total M6PR staining (top) and extracellular M6PR (bottom). Right, quantification of geometric mean fluorescence. AU, arbitrary units. (C) Immunofluorescence analysis showing HeLa cells transfected with CM or miR-199a-5p as indicated and incubated with anti-M6PR antibody for 60 min at 4°C and then either fixed and stained with anti-mouse Alexa Fluor 488-conjugated M6PR antibody without Triton X-100 (Tx-100; top panels) or allowed to internalize antibody complexes for 60 min at 37°C, fixed with PFA, and stained with anti-mouse Alexa Fluor 488-conjugated antibody (bottom panels). The Tx-100 permeabilization step was included to visualize internal compartments. To visualize the F-actin fibers and nuclei, phalloidin red and DAPI were used, respectively. Scale bar = 25 μm.
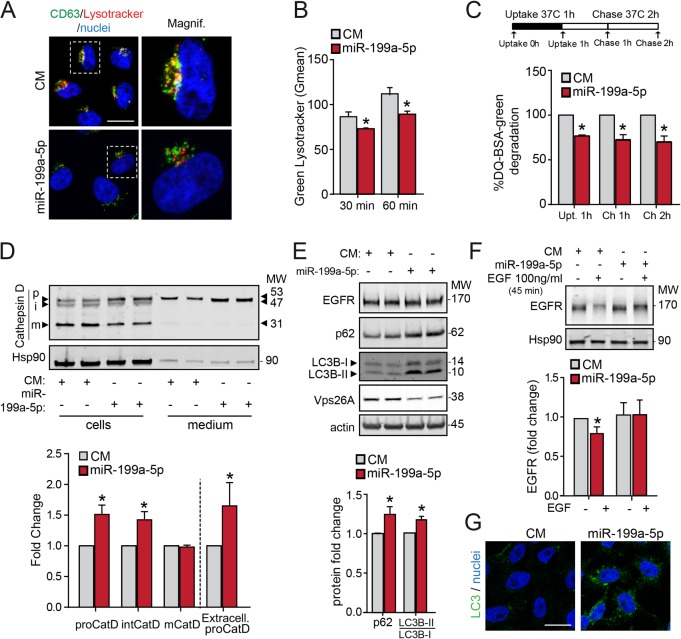
M6PR dissociates from its ligands upstream from lysosomes, allowing the ligands to move to the lysosomes, and M6PR is recycled back to the TGN (36). To assess the potential effect of miR-199a-5p on lysosome function, we assessed the colocalization of CD63, a lysosomal protein, with LysoTracker red, a red fluorescent probe used for labeling acidic organelles in live cells. Interestingly, the results showed that miR-199a-5p attenuated the colocalization between CD63 and LysoTracker compared to the results for CM cells (Fig. 9A). In addition to impaired localization of LysoTracker in lysosomes, we also analyzed the internalization of LysoTracker red fluorescence in control and transfected miR-199a-5p HeLa cells during 30 and 60 min of treatment. As shown by the results in Fig. 9B, miR-199a-5p overexpression lowered intracellular LysoTracker red levels, suggesting inhibition of the lysosome acidification. We further studied the endolysosomal trafficking by assessing DQ green BSA (bovine serum albumin), a fluorogenic probe that traffics through the endosomal pathway and undergoes quenching following proteolytic cleavage in the lysosome. The results showed that HeLa cells transfected with miR-199a-5p mimics had decreased lysosomal DQ green BSA degradation compared with that in CM-transfected cells, suggesting that the endolysosomal trafficking was also affected by miR-199a-5p overexpression (Fig. 9C). Because retromers function in the RT of M6PR from endosomes to the TGN, we wondered whether interfering with RT by enhancing miR-199a-5p expression in HeLa cells would alter the trafficking of the receptors, leading to missorting of newly synthesized acid hydrolases. To test this hypothesis, we examined the expression of the precursor and mature forms of cathepsin D by Western blotting. As expected, we found that in CM-transfected cells, most of the cathepsin D occurred as the 31-kDa mature form, with small amounts of the 53-kDa precursor and 47-kDa intermediate forms (Fig. 9D). We also observed reduced amounts of the precursor forms in the conditional medium isolated from CM-transfected cells (Fig. 9D). This efficient processing and intracellular retention of the mature form of cathepsin D reflects the integrity of the normal mechanism for trafficking of the enzyme from the TGN to lysosomes. In contrast to the results obtained in CM-transfected cells, miR-199a-5p overexpression increased the amounts of intracellular cathepsin D precursor and intermediate forms (Fig. 9D). Moreover, the amount of the precursor form of cathepsin D was markedly increased in the conditional medium (Fig. 9D, bottom), suggesting that miR-199a-5p impairs the transport of cathepsin D to lysosomes. We also examined the degradation kinetics of epidermal growth factor receptor (EGFR), as the receptor-ligand complex is internalized from the cell surface into endosomes, which ultimately fuse with lysosomes for degradation of the protein (37). We found that the total levels of EGFR protein were unaffected in cells with augmented miR-199a-5p levels (Fig. 9E). However, the degradation of EGFR was markedly attenuated in miR-199a-5p-transfected HeLa cells compared with its degradation in CM-transfected cells when EGFR was internalized upon EGF treatment for 45 min (Fig. 9F, bottom). Collectively, these results are consistent with a role of miR-199a-5p in regulating retromer function and M6PR sorting to lysosomes.
FIG 9.
miR-199a-5p regulates endolysosomal trafficking. (A) Confocal microscopy immunofluorescence images showing subcellular localization of CD63 subjected to LysoTracker internalization for 30 min at 37°C in HeLa cells transfected with CM or miR-199a-5p. Magnif, magnification of areas in dashed boxes. Scale bar = 15 μm. (B) Flow cytometry analysis of LysoTracker internalization at 37°C in HeLa cells treated as described in the legend to panel A. Data are expressed as the geometric mean values (Gmean) from three independent experiments. Data are expressed as geometric mean values (Gmean) ± SEM from three independent experiments. (C) Results of flow cytometry analysis of CM- or miR-199a-5p-transfected HeLa cells that were cultured in the presence of 200 μg/ml fluorescent DQ green BSA for 1 h at 37°C and then incubated at 37°C for 2 h. Data are expressed as fold change of Gmean ± SEM. (D) HeLa cells were transfected with CM and miR-199a-5p, and 24 h after transfection, cells were rinsed with PBS and incubated in serum-free culture medium for 24 h. The medium was collected and precipitated with trichloroacetic acid (TCA), and the resulting pellets were analyzed by 4-to-20% acrylamide gradient SDS-PAGE and immunoblotted with rabbit polyclonal antibody against cathepsin D (CatD). Blots were also probed with antibody to Hsp90 as a loading control. The positions of molecular weight markers (MW; in thousands) and of the precursor (p), intermediate (i), and mature (m) forms of cathepsin D are indicated. Bottom, quantification of the results of 3 independent experiments. (E) Western blot analysis of EGFR, p62, LC3B-I/LC3B-II, and Vps26A protein levels in CM- and miR-199a-5p-transfected cells. Bottom, quantification of the results. (F) EGFR degradation assay in HeLa cells transfected with CM or miR-199a-5p and stimulated with EGF for the indicated times. Right, quantification of the results. (D to F) Error bars indicate SEM. *, P ≤ 0.05. (G) Immunofluorescence analysis of LC3B in HeLa cells transfected with CM or miR-199a-5p. Scale bar = 15 μm.
Because autophagic and endocytic pathways converge at the endosome prior to lysosome-mediated degradation (38), we finally asked whether miR-199a-5p plays a role in the autophagy pathway by means of regulation of endolysosomal transport. Upon the induction of autophagy, soluble microtubule-associated protein light chain 3B (LC3B-I) is converted to a lipidated form (LC3B-II) that preferentially associates with the growing autophagosome membrane in punctate formations. Interestingly, we found an increase in LC3B puncta in cells transfected with miR-199-5p compared to the quantity of puncta in CM-transfected cells (Fig. 9G). Similarly, Western blot analysis demonstrated that miR-199 overexpression enhanced the expression of LC3B-II and p62/SQSTM, an autophagic substrate (Fig. 9E, bottom). These results indicate that autophagic degradation activity is significantly attenuated by miR-199a-5p. Together, our findings uncover an important, novel mechanism for the regulation of RT and the endolysosomal system by miR-199-5p (Fig. 10).
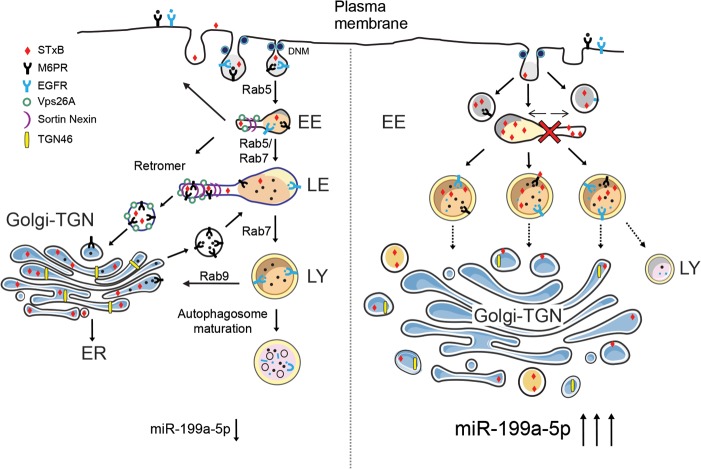
FIG 10.
Proposed model of regulation of retrograde transport by DNM/mir-199a-5p. Left, schematic model summarizing the effects on endolysosomal system of basal miR-199a-5p expression, in which normal flow of endocytic routes provides cargoes to different cell compartments to maintain cell homeostasis. Right, the effect of miR-199a-5p overexpression, which suppresses RT and endolysosomal trafficking, causing failure of proper organelle maintenance, including Golgi apparatus and lysosomes. EE, early endosome; LE, late endosome; LY, lysosome.
DISCUSSION
miR-199a-5p has been shown to be involved in a wide variety of cellular processes, such as the development and progression of various cancers, protection of cardiomyocytes, and skeletal formation (39, 40). The results of the present work identify miR-199a-5p as a major regulator of RT, controlling the posttranscriptional expression of numerous components of the RT machinery, including Vps26A and Rab9B, and cargoes, such as M6PR. We demonstrate that overexpression of miR-199a-5p impairs RT and protects against StxB-mediated inhibition of protein biosynthesis. Mechanistically, we found that miR-199a-5p influences RT by suppressing the expression of Vps26A. Of note, our results also suggest that miR-199a-5p overexpression using synthetic oligonucleotides could trigger a first defense against virus and/or other pathogens, preventing entry and consequently limiting infection. Future studies will clarify whether or not miR-199a-5p could also regulate the internalization of other pathogens, such as Salmonella, that also utilize RT to enter intracellular compartments (41).
Given the overall effect of miR-199a-5p in intracellular trafficking, we expect that miR-199a-5p might have direct and indirect effects on RT protein machinery. As an example, we can speculate that the observed disassembly of the Golgi apparatus structure in miR-199a-5p cells occurs because of impaired traffic to the TGN from endosomes in a retrograde manner. In this sense, we noticed that some defects in Golgi apparatus structure caused by miR-199a-5p expression were also partially restored using the rescue-of-function strategy with Vps26 expression. However, we cannot exclude the possibility of a direct effect of miR-199a-5p in the expression of multiple proteins resident in the Golgi apparatus, involved in both maintenance of Golgi structure and glycosylation. In fact, we observed that miR-199a-5p overexpression in HeLa cells caused deglycosylation of endogenous RT cargoes, such as TGN46. Although we have not explored the mechanism behind this effect, either for direct inhibition of any glycosylase(s) or for an indirect effect through disorganization of the Golgi apparatus and, hence, malfunctions in glycosylation, we observed that the expression of Vps26A, but not the expression of Rab9 (the retromer-independent pathway), is sufficient to partially restore TGN46 glycosylation levels, likely due to improved RT to the TGN.
In addition to regulating the expression of proteins associated with RT machinery, miR-199a-5p also controls the intracellular levels of natural RT cargoes, such as M6PR, thus suggesting that miR-199a-5p regulates the entire endolysosomal functioning. Indeed, HeLa cells transfected with miR-199a-5p mimics showed reduced expression and internalization of M6PR from the plasma membrane and, therefore, effects on the normal function of lysosomes, as shown by impaired trafficking of LysoTracker and protein cleavage. This finding was further supported by the missorting of cathepsin D observed in cells transfected with miR-199a-5p. Another demonstration that lysosomal function is compromised in cells overexpressing miR-199a-5p is the delayed lysosomal degradation of EGFR. Taken together, these findings suggest that miR-199a-5p influences lysosomal function by suppressing M6PR expression and inhibiting RT and endocytosis (27). Finally, we also demonstrate that miR-199a-5p reduces autophagosomal degradation, which is required for proper lysosomal function (42). This effect is most likely mediated by the impairment of endocytic trafficking through inhibition of Rab5, Rab7, and Rab9 GTPases, which have been reported to play an important role in regulating autophagy (43, 44) (45). However, the suppression of autophagy could be more complex, as recent studies have demonstrated an inhibitory effect of miR-199a-5p on autophagy by directly targeting autophagic regulators, including Atg7, AtgL14, and Beclin-1 (46, 47). These findings could also explain the inhibitory effect of miR-199a-5p on Rab5 and Rab7 expression. In addition to this perturbed endosomal trafficking, our results indicated that miR-199a-5p expression leads to defective RT, which also may account for the lysosome malfunction and, ultimately, autophagy blockage.
While our study established miR-199a-5p as a novel regulator of RT, further experiments will be important to elucidate the molecular mechanism that controls the endogenous expression of miR-199a/b-5p and regulates RT. In this regard, a number of reports have demonstrated that miR-199a-5p expression is upregulated in response to hypoxia and ER stress (48–50). Interestingly, these findings suggest that the induction of miR-199a-5p expression during ER stress might prevent the retrieval of ER export factors from the ER to the Golgi apparatus, avoiding exocytosis of misfolded proteins. Finally, our study also identified miR-199a-5p as a potential therapeutic approach to prevent deleterious effects caused by bacterial toxins.
MATERIALS AND METHODS
Materials.
Chemicals were obtained from Sigma-Aldrich unless otherwise noted. EGF was obtained from EMD/Calbiochem (Gibbstown, NJ). Mouse monoclonal antibodies against heat shock protein 90 (HSP90), GM130, and EEA1 were purchased from BD Biosciences. Rabbit antibodies against Vps26A, EGFR, and TGN46 were from Abcam. Rabbit polyclonal antibodies to Rab9, LC3B-II, and p62 were purchased from Cell Signaling Technology. M6PR monoclonal antibody was purchased from Calbiochem. Rabbit antibody to cathepsin D was from Epitomics. Monoclonal antiactin antibody was from Sigma. LysoTracker red, DQ red BSA, and fluorescently labeled secondary antibodies were from Molecular Probes (Invitrogen). miRNA mimics and inhibitors were obtained from Dharmacon. Vps26A-GFP, M6PR-GFP, and Rab9-YFP plasmids were kindly provided by Juan Bonifacino (NIH, Bethesda, MD). Cy3-StxB was obtained as described in reference 51.
Cell culture.
Cervix carcinoma (HeLa) and monkey kidney fibroblast (COS7) cells were obtained from American Type Culture Collection (ATCC) and were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 2% penicillin-streptomycin in 10-cm2 dishes at 37°C and 5% CO2. For the EGFR degradation assay, subconfluent HeLa cells transfected with CM or miR-199a-5p were switched to serum-free medium and cultured for approximately 12 h. For EGF stimulation, recombinant EGF (EMD) was added to the medium for 45 min at a final concentration of 100 ng/ml, and cells were cultured further for the times indicated.
Bioinformatics analysis of miRNA target genes.
Predicted mRNA targets for miR-199a/b were identified using an online target prediction bioinformatics algorithm, miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/), which provides target interaction information from eight different prediction algorithms. Specifically, the programs miRanda, miRWalk, and TargetScan were used. The putative mRNA targets for miR-199a-5p identified using these algorithms were assessed in the PANTHER gene classification system, version 8.0 (http://www.pantherdb.org), to identify target genes that were mapped to the transport process (GO:0006810). The functional interactions of these predicted mRNA targets for miR-199a/b-5p, as described in STRING, version 9.05 (http://string-db.org), were then combined with the functional annotation groups as described in DAVID. MatLab and Cytoscape, version 2.8.3, were used to create the visualization networks, as previously described (52). STRING interactions with a confidence score of 0.4 or higher were added and are highlighted in boldface in the interaction analysis scheme in Fig. 1B. Smaller annotation clusters and unconnected genes were left out of the visualization due to space constraints.
miRNA mimic/inhibitor transfections.
HeLa cells were transfected with 40 nM mirIDIAN miRNA mimics (miR-199a-5p) or with 60 nM mirIDIAN miRNA inhibitors (Inh-199a-5p) (Dharmacon) using RNAimax (Invitrogen) or Lipofectamine 2000 (Invitrogen) for cotransfection experiments with the Vps26A-GFP plasmid. All experimental control samples were treated with an equal concentration of a nontargeting control mimic sequence (CM) or negative-control inhibitor sequence (CI). Verification of miR-199a-5p overexpression and inhibition was determined using quantitative real-time PCR, as described below.
RNA isolation and quantitative real-time PCR.
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. For mRNA quantification, cDNA was synthesized using iScript reverse transcription supermix (Bio-Rad), following the manufacturer's protocol. Quantitative real-time PCR analysis was performed in triplicate using iQ SYBR green supermix (Bio-Rad) on an iCycler real-time detection system (Eppendorf). The mRNA level was normalized to that of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene as a housekeeping gene. The human primer sequences used were as follows: GAPDH, 5′-TTGATTTTGGAGGGATCTCG-3′ and 5′-CAATGACCCCTTCATTGACC-3′; SNX6, 5′-GAAGCCCCATGCCGCCTGTC-3′ and 5′-GGTGCACTGTCTGAGCACGGG-3′; M6PR, 5′-GTGTGCCGGGAAGCTGGCAA-3′ and 5′-CCACGCTCCTCAGACACAGGGT-3′; Rab9B, 5′-AGCCAGAACTGGGACCCCACA-3′ and 5′-AGGCCCCAGGTCTCATGCACT-3′; Vps26A, 5′-TGCTTGTTGATGAGGAAGACCGGAG-3′ and 5′-GCCTTTTTCCGCCCCCTCCA-3′; and Rab7A, 5′-GGGGCTGCTTTTCTAACCCA-3′ and 5′-TTTGCTAGGTCGGCCTTGTT-3′.
Western blot analysis.
Western blot analysis was performed as we described previously (27). Briefly, cells were lysed in ice-cold buffer containing 50 mM Tris-HCl, pH 7.5, 125 mM NaCl, 1% NP-40, 5.3 mM NaF, 1.5 mM NaP, 1 mM orthovanadate, 1 mg/ml protease inhibitor cocktail (Roche), and 0.25 mg/ml AEBSF (4-benzenesulfonyl fluoride hydrochloride; Roche). Then, cell lysates were rotated at 4°C for 1 h before the insoluble material was removed by centrifugation at 12,000 × g for 10 min. After normalizing for equal protein concentration, cell lysates were resuspended in SDS sample buffer before separation by SDS-PAGE. Following overnight transfer of the proteins onto nitrocellulose membranes, the membranes were probed with antibodies to the following proteins: Vps26A (1:1,000; Novus), Rab7 (1:500; Abcam), Rab9 (1:1,000; Abcam), EEA-1 (1:1,000; Abcam), GM130 (1:1,000; Abcam), TGN46 (1:1,000; Abcam), actin (1:1,000; Santa Cruz), and HSP90 (1:1,000; BD Biosciences). Protein bands were visualized using the Odyssey infrared imaging system (LI-COR Biotechnology). Densitometry analysis of the gels was carried out using ImageJ software from the NIH (http://rsbweb.nih.gov/ij/).
HeLa cells and intoxication assay.
HeLa cells were maintained at 37°C under 5% CO2 in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, 4.5 g/liter glucose, 0.01% penicillin-streptomycin, 4 mM glutamine, and 5 mM pyruvate. HeLa cells were seeded at 20,000 cells per well in 96-well plates and grown overnight. After transfection with 40 μM CM and miR-199a-5p mimics for 48 h, cells were challenged with increasing doses of Stx for 1 h. Protein biosynthesis was determined 1 h later by measuring the incorporation of radiolabeled methionine into acid-precipitable material, as previously described (53).
Fluorescence microscopy.
For anti-M6PR antibody internalization, HeLa cells were grown on coverslips and transfected with an miR-199a-5p mimic and a negative-control mimic (CM) in DMEM containing 10% FBS. At 48 h posttransfection, cells were cooled to 4°C for 20 min to stop membrane internalization. Then, cells were incubated with anti-M6PR monoclonal antibody (2G11) (Calbiochem) for 40 min at 4°C. Following incubation, cells were gently washed twice with cold medium and shifted to 37°C to allow internalization of anti-M6PR antibody complexes for the indicated times and fixed with 4% paraformaldehyde (PFA). After 5 min of treatment with 0.2% Triton X-100 for permeabilization and 15 min of blocking (3% PBS–BSA), cells were stained with anti-mouse antibodies conjugated with Alexa Fluor 488 (Molecular Probes) to detect M6PR, with Alexa Fluor 594-phalloidin to visualize F-actin, and with TO-PRO-3 (Life Technologies) for 1 h at room temperature. After this, cells were washed twice with 1× PBS and mounted on glass slides with ProLong gold (Life Technologies).
For Vps26A-GFP and M6PR-GFP rescue experiments, HeLa cells were grown on coverslips and cotransfected with 1 μg Vps26A-GFP and 40 nM CM or miR-199a-5p mimic. At 48 h posttransfection, cells were incubated or not with Cy3-StxB as described in Fig. 2A, 4A and B, and 7D. Then, cells were washed twice with 1× PBS, fixed with 4% PFA, and blocked (3% BSA in 1× PBS) for 15 min. Following this, cells were washed twice and mounted on glass slides with ProLong gold (Life Technologies). All images were analyzed using a confocal microscope (Leica SP5 II) equipped with a 63× Plan Apo lens. All gains were maintained at constant settings for the acquisition of comparable images. Analysis of different images was performed using ImageJ (NIH) and Adobe Photoshop CS5.
Image analysis and quantification.
MacBiophotonics ImageJ was used for image quantification and analysis. To analyze Golgi apparatus morphology, images of GM130 for immunofluorescence analysis were digitally recorded in 8-bit gray scale at 1,024-by-1,024 resolution. The Golgi apparatus-labeling image threshold was set at 90 on a 0-to-255 black-to-white scale (all pixels with a value under 90 were excluded from the quantification) to remove background pixels from measurement. A region of interest was defined for each cell, and the number of individual Golgi apparatus fragments with a minimum area of three pixels was measured. To quantify colocalization between Cy3-StxB and GM130 or EEA1, we used the intensity correlation analysis plug-in developed for the ImageJ 1.36b software (NIH). After image thresholding, the extent of colocalization was obtained by calculating the Pearson coefficient (r1) and the Manders overlap coefficient (shown in Fig. 2D and E, 4A, 5A, and 6A) with the corresponding standard deviation (54).
3′-UTR–luciferase reporter assays.
cDNA fragments corresponding to the entire 3′ UTRs of human Vps26A, Rab9B, SNX6, Rab7A, and M6PR were amplified by reverse transcription-PCR from total RNA extracted from Huh7 cells with XhoI and NotI linkers. The PCR product was directionally cloned downstream from the Renilla luciferase open reading frame of the psiCHECK2 vector (Promega) that also contains a constitutively expressed firefly luciferase gene, which is used to normalize transfections. Point mutations in the seed region of the predicted miR-199a binding sites within all the above-named 3′ UTRs were generated using the QuikChange multisite-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. All constructs were confirmed by sequencing. COS7 cells were plated into 12-well plates (Costar) and cotransfected with 1-μg amounts of the indicated 3′-UTR luciferase reporter vectors and miR-199a-5p mimics or CM (Dharmacon) utilizing Lipofectamine 2000 (Invitrogen) (see Fig. 1C and 7A). Luciferase activity was measured using the Dual-Glo luciferase assay system (Promega). Renilla luciferase activity was normalized to the corresponding firefly luciferase activity and plotted as a percentage of that in the control (cells cotransfected with the corresponding concentration of control mimic). Experiments were performed in triplicate wells of a 12-well plate and repeated at least three times.
LysoTracker internalization assay.
For analysis of LysoTracker red internalization, cells were incubated for 30 and 60 min at 37°C in serum-free DMEM. First, cells were incubated on serum-free medium for 30 min. The cells were washed to remove noninternalized LysoTracker and analyzed by fluorescence-activated cell sorting (FACS) experiments or fixed in 4% PFA for 15 min for fluorescence microscopy analysis.
DQ green BSA degradation assay.
To measure the lysosomal degradation of soluble proteins, we used a DQ green BSA degradation assay. DQ green BSA is a BSA labeled with a self-quenching fluorescent dye. The hydrolysis of DQ green BSA into single dye-labeled peptides by lysosomal proteases relieves the self-quenching, thus allowing us to measure the lysosomal DQ green BSA transport. The assay was done according to the manufacturer's protocol (Molecular Probes/Invitrogen, Eugene, OR). Briefly, CM- or miR-199a-5p-transfected HeLa cells were loaded with 200 μg/ml DQ green BSA for 1 h at 37°C. The cells were then washed to remove extracellular DQ green BSA and incubated at 37°C for 2 h. The DQ green BSA fluorescence was then monitored by flow cytometry.
Statistics.
All data are expressed as mean values ± standard errors of the means (SEM). Statistical differences were measured using an unpaired Student t test. A P value of ≤0.05 was considered statistically significant. Data analysis was performed using Prism software, version 6.03 (GraphPad, San Diego, CA).
ACKNOWLEDGMENTS
We thank Juan Bonifacino for generously providing the Vps26A-GFP and M6PR-GFP plasmids. We also thank Carlos Estella for critical discussions and comments about this work.
This work was supported by grants from the National Institutes of Health, R35HL135820 to C.F.-H., the Foundation Leducq (MIRVAD network) to C.F.-H., and American Heart Association Established Investigator award (16EIA27550004) to C.F.-H.
REFERENCES
- 1.Wang S, Sun H, Tanowitz M, Liang XH, Crooke ST. 2017. Intra-endosomal trafficking mediated by lysobisphosphatidic acid contributes to intracellular release of phosphorothioate-modified antisense oligonucleotides. Nucleic Acids Res 45:5309–5322. doi: 10.1093/nar/gkx231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johannes L, Wunder C. 2016. Retromer sets a trap for endosomal cargo sorting. Cell 167:1452–1454. doi: 10.1016/j.cell.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Short B. 2015. Sorting out endosome form and function. J Cell Biol 210:870. doi: 10.1083/jcb.2106fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Rojas R. 2006. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 5.Amessou M, Carrez D, Patin D, Sarr M, Grierson DS, Croisy A, Tedesco AC, Maillard P, Johannes L. 2008. Retrograde delivery of photosensitizer (TPPp-O-beta-GluOH)3 selectively potentiates its photodynamic activity. Bioconjug Chem 19:532–538. doi: 10.1021/bc7003999. [DOI] [PubMed] [Google Scholar]
- 6.Johannes L, Popoff V. 2008. Tracing the retrograde route in protein trafficking. Cell 135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. 2004. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol 165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiba Y, Romer W, Mardones GA, Burgos PV, Lamaze C, Johannes L. 2010. AGAP2 regulates retrograde transport between early endosomes and the TGN. J Cell Sci 123:2381–2390. doi: 10.1242/jcs.057778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia PZ, Gasnereau I, Lieu ZZ, Gleeson PA. 2011. Rab9-dependent retrograde transport and endosomal sorting of the endopeptidase furin. J Cell Sci 124:2401–2413. doi: 10.1242/jcs.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popoff V, Mardones GA, Tenza D, Rojas R, Lamaze C, Bonifacino JS, Raposo G, Johannes L. 2007. The retromer complex and clathrin define an early endosomal retrograde exit site. J Cell Sci 120:2022–2031. doi: 10.1242/jcs.003020. [DOI] [PubMed] [Google Scholar]
- 11.Liu TT, Gomez TS, Sackey BK, Billadeau DD, Burd CG. 2012. Rab GTPase regulation of retromer-mediated cargo export during endosome maturation. Mol Biol Cell 23:2505–2515. doi: 10.1091/mbc.E11-11-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chia PZ, Gleeson PA. 2011. The regulation of endosome-to-Golgi retrograde transport by tethers and scaffolds. Traffic 12:939–947. doi: 10.1111/j.1600-0854.2011.01185.x. [DOI] [PubMed] [Google Scholar]
- 13.Hierro A, Gershlick DC, Rojas AL, Bonifacino JS. 2015. Formation of tubulovesicular carriers from endosomes and their fusion to the trans-Golgi network. Int Rev Cell Mol Biol 318:159–202. doi: 10.1016/bs.ircmb.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 14.van Rahden VA, Brand K, Najm J, Heeren J, Pfeffer SR, Braulke T, Kutsche K. 2012. The 5-phosphatase OCRL mediates retrograde transport of the mannose 6-phosphate receptor by regulating a Rac1-cofilin signalling module. Hum Mol Genet 21:5019–5038. doi: 10.1093/hmg/dds343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiba Y, Kametaka S, Waguri S, Presley JF, Randazzo PA. 2013. ArfGAP3 regulates the transport of cation-independent mannose 6-phosphate receptor in the post-Golgi compartment. Curr Biol 23:1945–1951. doi: 10.1016/j.cub.2013.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH. 2007. Functional architecture of the retromer cargo-recognition complex. Nature 449:1063–1067. doi: 10.1038/nature06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojas R, Kametaka S, Haft CR, Bonifacino JS. 2007. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol 27:1112–1124. doi: 10.1128/MCB.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seaman MN. 2004. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol 165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. 2008. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol 183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Weering JR, Verkade P, Cullen PJ. 2012. SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation. Traffic 13:94–107. doi: 10.1111/j.1600-0854.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- 21.Lieu ZZ, Derby MC, Teasdale RD, Hart C, Gunn P, Gleeson PA. 2007. The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network. Mol Biol Cell 18:4979–4991. doi: 10.1091/mbc.E07-06-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, Hirose S, Ikehara Y, Oda K. 2010. Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic 11:1552–1566. doi: 10.1111/j.1600-0854.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 23.Laufman O, Hong W, Lev S. 2011. The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport. J Cell Biol 194:459–472. doi: 10.1083/jcb.201102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Victoria FJ, Mardones GA, Bonifacino JS. 2008. Requirement of the human GARP complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol Biol Cell 19:2350–2362. doi: 10.1091/mbc.E07-11-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong B, Kakihara K, Otani T, Wada H, Hayashi S. 2013. Rab9 and retromer regulate retrograde trafficking of luminal protein required for epithelial tube length control. Nat Commun 4:1358. doi: 10.1038/ncomms2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandvig K, Garred O, Prydz K, Kozlov JV, Hansen SH, van Deurs B. 1992. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- 27.Aranda JF, Canfran-Duque A, Goedeke L, Suarez Y, Fernandez-Hernando C. 2015. The miR-199-dynamin regulatory axis controls receptor-mediated endocytosis. J Cell Sci 128:3197–3209. doi: 10.1242/jcs.165233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ. 2007. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci 120:45–54. doi: 10.1242/jcs.03302. [DOI] [PubMed] [Google Scholar]
- 29.Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. 2006. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Sandvig K, Ryd M, Garred O, Schweda E, Holm PK, van Deurs B. 1994. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J Cell Biol 126:53–64. doi: 10.1083/jcb.126.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganley IG, Espinosa E, Pfeffer SR. 2008. A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells. J Cell Biol 180:159–172. doi: 10.1083/jcb.200707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prescott AR, Lucocq JM, James J, Lister JM, Ponnambalam S. 1997. Distinct compartmentalization of TGN46 and beta 1,4-galactosyltransferase in HeLa cells. Eur J Cell Biol 72:238–246. [PubMed] [Google Scholar]
- 33.Ludwig T, Ovitt CE, Bauer U, Hollinshead M, Remmler J, Lobel P, Ruther U, Hoflack B. 1993. Targeted disruption of the mouse cation-dependent mannose 6-phosphate receptor results in partial missorting of multiple lysosomal enzymes. EMBO J 12:5225–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuliawat R, Klumperman J, Ludwig T, Arvan P. 1997. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J Cell Biol 137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medigeshi GR, Schu P. 2003. Characterization of the in vitro retrograde transport of MPR46. Traffic 4:802–811. doi: 10.1034/j.1600-0854.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 36.Hasanagic M, Waheed A, Eissenberg JC. 2015. Different pathways to the lysosome: sorting out alternatives. Int Rev Cell Mol Biol 320:75–101. doi: 10.1016/bs.ircmb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Ceresa BP, Bahr SJ. 2006. rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J Biol Chem 281:1099–1106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- 38.Huotari J, Helenius A. 2011. Endosome maturation. EMBO J 30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su SF, Chang YW, Andreu-Vieyra C, Fang JY, Yang Z, Han B, Lee AS, Liang G. 2013. miR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene 32:4694–4701. doi: 10.1038/onc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander MS, Kawahara G, Motohashi N, Casar JC, Eisenberg I, Myers JA, Gasperini MJ, Estrella EA, Kho AT, Mitsuhashi S, Shapiro F, Kang PB, Kunkel LM. 2013. MicroRNA-199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death Differ 20:1194–1208. doi: 10.1038/cdd.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGourty K, Thurston TL, Matthews SA, Pinaud L, Mota LJ, Holden DW. 2012. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science 338:963–967. doi: 10.1126/science.1227037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, Shen HM. 2013. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res 23:508–523. doi: 10.1038/cr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. 2004. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 44.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. 2009. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 45.Ravikumar B, Imarisio S, Sarkar S, O'Kane CJ, Rubinsztein DC. 2008. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci 121:1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi H, Liang B, Jia J, Liang N, Xu H, Ju G, Ma S, Liu X. 2013. Differential roles of miR-199a-5p in radiation-induced autophagy in breast cancer cells. FEBS Lett 587:436–443. doi: 10.1016/j.febslet.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 47.Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F, Xia Q. 2012. Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem Biophys Res Commun 423:826–831. doi: 10.1016/j.bbrc.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 48.Dai BH, Geng L, Wang Y, Sui CJ, Xie F, Shen RX, Shen WF, Yang JM. 2013. microRNA-199a-5p protects hepatocytes from bile acid-induced sustained endoplasmic reticulum stress. Cell Death Dis 4:e604. doi: 10.1038/cddis.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding G, Huang G, Liu HD, Liang HX, Ni YF, Ding ZH, Ni GY, Hua HW. 2013. miR-199a suppresses the hypoxia-induced proliferation of non-small cell lung cancer cells through targeting HIF1alpha. Mol Cell Biochem 384:173–180. doi: 10.1007/s11010-013-1795-3. [DOI] [PubMed] [Google Scholar]
- 50.Li B, He L, Zuo D, He W, Wang Y, Zhang Y, Liu W, Yuan Y. 2017. Mutual regulation of MiR-199a-5p and HIF-1alpha modulates the Warburg effect in hepatocellular carcinoma. J Cancer 8:940–949. doi: 10.7150/jca.17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. 1998. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J Cell Biol 143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 53.Amessou M, Fradagrada A, Falguières T, Lord JM, Smith DC, Roberts LM, Lamaze C, Johannes L. 2007. Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J Cell Sci 120:1457–1468. doi: 10.1242/jcs.03436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolte S, Cordelieres FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]