ABSTRACT
Death-associated protein 5 (DAP5) is an atypical isoform of the translation initiation scaffolds eukaryotic initiation factor 4GI (eIF4GI) and eIF4GII (eIF4GI/II), which recruit mRNAs to ribosomes in mammals. Unlike eIF4GI/II, DAP5 binds eIF2β, a subunit of the eIF2 complex that delivers methionyl-tRNA to ribosomes. We discovered that DAP5:eIF2β binding depends on specific stimuli, e.g., protein kinase C (PKC)–Raf–extracellular signal-regulated kinase 1/2 (ERK1/2) signals, and determines DAP5's influence on global and template-specific translation. DAP5 depletion caused an unanticipated surge of hypoxia-inducible factor 1α (HIF-1α), the transcription factor and master switch of the hypoxia response. Physiologically, the hypoxia response is tempered through HIF-1α hydroxylation by the oxygen-sensing prolyl hydroxylase-domain protein 2 (PHD2) and subsequent ubiquitination and degradation. We found that DAP5 regulates HIF-1α abundance through DAP5:eIF2β-dependent translation of PHD2. DAP5:eIF2-induced PHD2 translation occurred during hypoxia-associated protein synthesis repression, indicating a role as a safeguard to reverse HIF-1α accumulation and curb the hypoxic response.
KEYWORDS: DAP5, eIF2β, HIF-1α, PHD2, translation initiation, hypoxia
INTRODUCTION
Multifaceted control of protein synthesis enables rapid cellular adaptation to instant changes in growth or survival conditions. This is particularly important when stressors require global protein synthesis repression, but cells must ramp up translation of select stress response modifiers, as exemplified by the response to oxygen deprivation. The hypoxia response is dominated by hypoxia-inducible factor 1α (HIF-1α) and its activator role in the HIF-1α/β complex, HIF-1 (1, 2). HIF-1 transcriptional activity induces >1,000 genes (3, 4) involved in cell proliferation, cell invasion/metastasis, or glucose metabolism programs (5). To restore homeostasis with normoxic balancing, HIF-1α is rapidly hydroxylated by prolyl hydroxylase-domain proteins (PHDs), ubiquitinated by von Hippel-Lindau protein (VHL), and degraded by the proteasome (6–8). The PHDs rapidly hydroxylate HIF-1α at prolines 402/564 in an oxygen-dependent manner, inducing rapid HIF-1α degradation (6, 9, 10). This process hinges on the main oxygen sensor in the cell, PHD2, since neither PHD1 nor PHD3 can functionally compensate for PHD2 depletion (11).
The eIF4G isoforms are key to adaptive protein synthesis because they combine control over the association of translation initiation machinery with mRNAs, the unwinding/scanning of the 5′ untranslated region (UTR), and eukaryotic initiation factor 3 (eIF3)/40S ribosomal subunit recruitment. There are three eIF4G isoforms in mammals, eIF4GI, eIF4GII, and death-associated protein 5 (DAP5) (12). They share similar roles in core initiation functions, mediated by association with mRNA, anchoring the eIF4A:4B translation initiation helicase cofactors and recruiting eIF3/40S ribosomal subunits. DAP5 is homologous to the C-terminal approximate two-thirds of eIF4GI/II and lacks their N-terminal domain, which contains binding motifs for the 5′ 7-methyl-guanosine cap-binding protein (eIF4E) and the poly(A) binding protein (PABP) (Fig. 1). Unlike eIF4GI/II, DAP5 binds to eIF2β (13), which in a complex with eIF2γ and eIF2α (comprising eIF2) delivers Met-tRNA to 40S ribosomal subunits (14). DAP5 has been implicated both in homeostatic global protein synthesis (13, 15) and in context-specific, m7G-cap independent initiation at select templates (16). DAP5's role in translation is unique, since eIF4GI overexpression cannot compensate for DAP5 depletion (17). The eIF4G isoforms are ubiquitously expressed; the eIF4GI/eIF4GII/DAP5 ratio in HeLa cells is approximately 10:1:45 (18). The significance of the eIF4G isoform spectrum, their functional/structural variances, and their expression divergences is not understood.
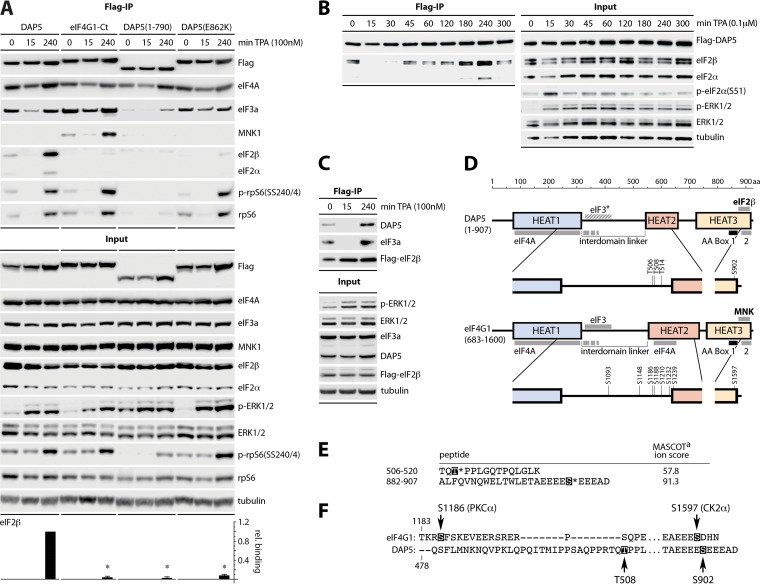
FIG 1.
Phorbol ester stimulation promotes DAP5:eIF2β binding. (A to C) HEK293 cells expressing Flag-tagged DAP5, eIF4G1-Ct, DAP5(1-790), and DAP5(E862K) (A), Flag-DAP5 (B), or Flag-eIF2β (C) were induced with dox (16 h) before stimulation with TPA, as shown. Cell lysates were analyzed by immunoblotting or subjected to anti-Flag immunoprecipitation/immunoblotting. Data bars in panel A depict the mean ratio of eIF2β to Flag signal after 240 min of TPA from three assays. Experiments were normalized by setting the Flag-DAP5 (wt) ratio for each experiment to 1; error bars represent the SEM. *, significant P value (P < 0.05). (D) Schematic representation of DAP5 and eIF4GI(683-1600) domain arrangements, protein interactions, and major Ser/Thr phosphorylation sites. (E) HEK293 cells were dox induced for Flag-DAP5 expression (16 h), treated with TPA (240 min), harvested, and subjected to Flag-DAP5 IP. Immunoprecipitation-isolated complexes were digested with trypsin, followed by TiO2 enrichment and LC-tandem MS (LC-MS/MS) analysis. Amino acid sequences of phosphopeptides identified by LC-MS/MS are shown; the highlighted amino acids before the asterisks indicate phosphorylated amino acids. The MASCOT ion score was calculated using the following equation: −10 log10 (P), where P is defined as the absolute probability of the observed match being a random event (56). DAP5(T508) and DAP5(S902) were identified by this analysis by using a cutoff MASCOT score of 20. (F) Location of DAP5(T508)/(S902) relative to eIF4GI(S1186)/(S1597) phosphorylation sites, known to control TPA-induced association with MNK (22).
Our studies show that inducible DAP5:eIF2β binding is required for DAP5-mediated translation. They reveal that DAP5:eIF2β has a defining role in controlling translation of the principal oxygen sensor of the cell, PHD2, upon oxygen deprivation. DAP5 depletion caused a surprising, paradoxical increase in HIF-1α protein due to a reduction in DAP5-dependent translation of PHD2. DAP5's role in controlling PHD2 is evident in cells exposed to hypoxic conditions, where low oxygen prompted DAP5:eIF2β binding and DAP5-mediated PHD2 biosynthesis. We confirmed the recently reported role of PHD2 in tempering AKT signaling (19) and, accordingly, defined a role for DAP5 in controlling AKT's activation status. Our findings indicate that DAP5-mediated translation is induced despite global translation repression in hypoxia, possibly due to unique structural arrangements and protein interactions that distinguish it from eIF4GI/II.
RESULTS
DAP5:eIF2β binding is inducible, e.g., by protein kinase C (PKC)-Raf-ERK1/2 signaling.
Previous studies identified binding between DAP5 and eIF2β that may be involved in DAP5-mediated translation initiation (13, 20). Binding of the mitogen-activated protein (MAPK)-interacting kinase (MNK) to eIF4GI (21), which is analogous to eIF2β association with DAP5, occurs at two conserved C-terminal aromatic and acidic (AA) boxes (Fig. 1). Since eIF4GI:MNK binding strongly responds to PKC–Raf–extracellular signal-regulated kinase 1/2 (ERK1/2) signals (21–23), we hypothesized that DAP5:eIF2β binding might be similarly regulated. We created stable HEK293 cell lines with doxycycline (dox)-inducible expression of Flag-tagged (i) wild-type (wt) DAP5; (ii) eIF4GI-Ct(683-1600), i.e., the C-terminal portion of eIF4GI homologous to DAP5; (iii) DAP5(1-790), i.e., DAP5 lacking the C-terminal AA boxes; and (iv) DAP5(E862K), a DAP5 point mutant that lacks eIF2β binding (20) (Fig. 1A and D). The cells were dox induced, treated with 12-O-tetradecanoylphorbol-13-actetate (TPA) for PKC activation, and processed for Flag immunoprecipitation (IP). TPA-responsive eIF2β and eIF2α binding occurred only with wt DAP5 (Fig. 1A). TPA induced eIF4GI-Ct binding to MNK1 but not eIF2β (Fig. 1A). DAP5(1-790) and DAP5(E862K) lost eIF2β binding (Fig. 1A); however, DAP5(1-790) also displayed significantly decreased TPA-responsive rpS6 and eIF3a coimmunoprecipitation (Co-IP), suggesting that the C-terminal truncation broadly compromises DAP5 functional integrity (Fig. 1A). Co-IP of both eIF2β and eIF2α at the same rate implies that the entire eIF2 is precipitated with DAP5, since eIF2α is unable to bind to DAP5 directly and eIF2α and eIF2β are held in complex by the third eIF2 subunit eIF2γ.
TPA treatment for 15 min induced ERK1/2 phosphorylation but diminished ribosomal protein S6 (rpS6) and eIF3a Co-IP with all DAP5 and eIF4GI variants, indicating reduced association with eIF3/40S ribosomal subunits (Fig. 1A). This coincided with similarly reduced MNK1:eIF4GI and wt DAP5:eIF2β binding (Fig. 1A). Detailed kinetics of the TPA response indicated transient interruption of translation activity, possibly related to a short-lived peak of p-eIF2α(S51) at 15 min after TPA (Fig. 1B). We believe that this accounts for the temporary slump of DAP5/eIF4GI interactions with eIF2β/MNK/rpS6/eIF3a at 15 min after TPA (Fig. 1A). Binding of all DAP5 variants/eIF4GI to eIF4A was only slightly variable throughout the period of TPA stimulation (Fig. 1A); eIF4A interactions with DAP5/eIF4GI HEAT1 do not respond to PKC-Raf-ERK1/2 (Fig. 1D) (24). The same pattern of TPA-responsive interactions occurred upon inverse Co-IP of endogenous DAP5 and eIF3a with dox-inducible Flag-eIF2β (Fig. 1C).
DAP5 is phosphorylated upon TPA stimulation.
MNK binding to the AA boxes of eIF4GI is in part regulated by PKC-Raf-ERK1/2 signals to eIF4GI (21, 22, 24). High-throughput phosphoproteomics identified putative priority DAP5 phosphosites (https://www.phosphosite.org); however, none of these have been confirmed biologically. Using a targeted, unbiased approach, we performed liquid chromatography-mass spectrometry (LC-MS) phosphoproteomics of IP-purified Flag-DAP5 from dox-induced, TPA/mock-stimulated HEK293 cells. Two prominent phosphosites were detected only in the TPA-stimulated samples threonine 508 and serine 902 (Fig. 1E). These are among few commonly identified DAP5 phosphosites in reported high-throughput mass spectrometry studies (https://www.phosphosite.org). DAP5(T508) is located in the interdomain linker (connecting HEAT1 and HEAT2 domains; Fig. 1D), a region of low sequence similarity to eIF4GI. The corresponding region in eIF4GI has clusters of multiple mitogen-responsive phosphosites, including S1186, which is phosphorylated by PKCα upon TPA stimulation, and influences MNK binding to eIF4GI (Fig. 1F) (22). DAP5(S902) is located at the C terminus, within the binding motif for eIF2β. This region is highly conserved between DAP5 and eIF4GI with eIF4GI(S1597) sharing an almost identical location and amino acid sequence context (Fig. 1F). We sought to determine whether these phosphorylation events control DAP5 association with eIF2β.
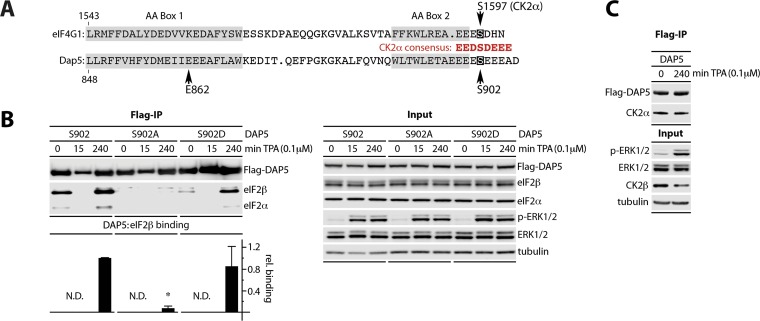
DAP5(S902) is phosphorylated by CK2α.
DAP5(S902) and eIF4G1(S1597) are within ideal consensus for casein kinase 2-alpha (CK2α) (Fig. 2A) (25). Indeed, previous investigations showed that eIF4GI(S1597) is phosphorylated by CK2α (24). To test whether S902 phosphorylation regulates DAP5:eIF2β binding, HEK293 cells with dox-inducible expression of a non-phospho-mutant DAP5(S902A) or a phospho-mimic (S902D) were derived. DAP5(S902A) did not bind eIF2β/α, whereas the S902D phospho-mimic retained TPA-responsive binding (Fig. 2B). An analogous approach demonstrated that CK2α catalyzed p-eIF4GI(S1597) controls TPA/anisomycin-induced eIF4GI:MNK1 interactions (data not shown). Co-IP of CK2α with DAP5/eIF4GI further supports its involvement in phosphorylation of S902/S1597 (Fig. 2C). Our findings suggest that a conserved mechanism regulates DAP5:eIF2β and eIF4GI:MNK1 binding via CK2α sites within the binding regions for eIF2β/MNK1. CK2 is considered constitutively active, with no known stimuli (25), suggesting that S902 (DAP5) and S1597 (eIF4GI) do not contribute to TPA-induced binding of eIF2β or MNK1.
FIG 2.
Phosphorylation of DAP5(S902) by CK2α is required for eIF2β binding. (A) S902/S1597 anchor consensus CK2α substrates at the DAP5/eIF4G1 C termini. (B) HEK293 cells expressing dox-inducible Flag-tagged DAP5, DAP5(S902A), or DAP5(S902D) were dox treated (16 h) before stimulation with TPA. Cell lysates were analyzed by immunoblotting or subjected to anti-Flag immunoprecipitation/immunoblotting. The ratio of eIF2β to Flag signal after TPA (4 h) was quantified across three repeat tests. HEK293 cells treated with TPA (4 h) were normalized to 1, with the SEM indicated. *, significant P value (P < 0.05). (C) Dox-inducible Flag-DAP5-expressing HEK293 cells were treated with dox (16 h) and TPA as shown. Lysates were tested as in panel B. All experiments were repeated three times with representative series as shown.
MEK1-ERK1/2 induces DAP5:eIF2β binding.
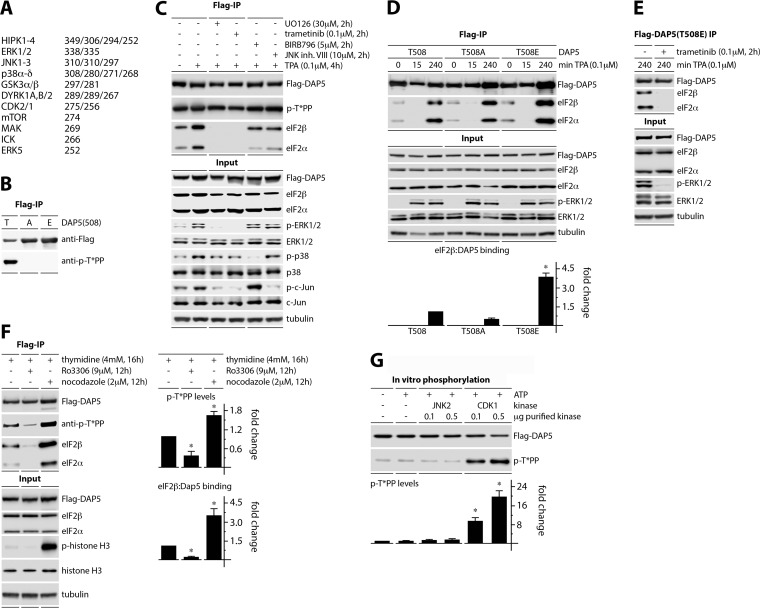
We next addressed a possible role for p-DAP5(T508) in TPA-induced DAP5:eIF2β binding. Kinase prediction (http://www.phosphonet.ca) indicated DAP5(T508) as a likely target for MAPKs or cyclin-dependent kinases (CDKs) (Fig. 3A). First, to reliably detect p-DAP5(T508), we validated a p-T*PP antibody; this probe only detected signal in Flag-IP of wt p-DAP5(T508) but not the T508A/E mutants (Fig. 3B). Second, we investigated the role of ERK1/2, p38α-δ, and JNK1/2 MAPKs in DAP5(T508) phosphorylation and DAP5:eIF2β binding. HEK293 cells with dox-inducible Flag-DAP5 expression were pretreated (2 h) with DMSO(−), the MEK1/2 inhibitors UO126 or trametinib, the p38α-δ inhibitor BIRB796, or the JNK1/2 inhibitor VIII (Fig. 3C) prior to TPA stimulation (4 h). The inhibitors exhibited the expected signaling effects; both MEK1/2 inhibitors also blocked JNK signaling (Fig. 3C, input).
FIG 3.
DAP5:eIF2β binding induced by MEK1-ERK1/2 signaling or during mitosis. (A) The top 24 predicted kinases for DAP5(T508) (http://www.phosphonet.ca; kinase predictor V2 scores). (B) Flag-DAP5(508T/A/E) immunoprecipitations show the specificity of an anti-p-T*PP antibody for T508. (C) HEK293 cells expressing Flag-DAP5 were dox induced (16 h) and pretreated with UO126, trametinib, BIRB 796, JNK inhibitor VIII, or DMSO(−) (2 h) prior to TPA stimulation. Cell lysates were analyzed by immunoblotting or anti-Flag immunoprecipitation/immunoblotting. (D) HEK293 cells expressing Flag-tagged DAP5, DAP5(T508A), or DAP5(T508E) were dox induced (16 h) before stimulation with TPA. Lysates were analyzed as in panels C and D. Data bars represent the average ratios of eIF2β to Flag immunoprecipitation signal at 4 h TPA, normalizing each experiment by setting the eIF2β/Flag ratio for DAP5(T508) to 1. (E) HEK293 cells with dox-inducible Flag-DAP5(T508E) were treated with dox (16 h), mock pretreated or pretreated with trametinib (2 h) prior to TPA stimulation and analyzed as in panel C. (F) HEK293 cells were treated with thymidine (4 mM; 16 h), followed by a medium change, and Ro3306, nocodazole, or DMSO(−), as shown. Dox was maintained (16 h) prior to harvest. Lysates were analyzed as in panels C to E. Data bars (right panels) represent mean ratio of p-T*PP/Flag and eIF2β/Flag, normalizing between experiments by setting the ratios for cells treated with thymidine block only to 1. (G) In vitro phosphorylation assay of Flag-DAP5 with recombinant JNK2 (control) or CDK1. Data bars represent the mean ratios of p-T*PP [p-DAP5(T508)] to Flag, normalizing between experiments by setting the −kinase/−ATP control to 1. All experiments were repeated three times; data bars represent the means from three tests; error bars denote the SEM. *, significant P value (P < 0.05).
Our assay revealed that none of the MAPK inhibitors altered p-DAP5(T508) levels (Fig. 3C). However, the MEK1/2 inhibitors completely abolished DAP5:eIF2β binding (Fig. 3C). These data suggest that DAP5:eIF2β association upon TPA stimulation is controlled by ERK1/2 but does not hinge on DAP5(T508) phosphorylation. Tests in TPA-stimulated (240 min) HEK293 cells with dox-inducible expression of DAP5 featuring either 508T/-A or 508T/-E showed possible correlation of eIF2β binding with p-T508 (Fig. 3D). To confirm ERK1/2-regulation of DAP5:eIF2β binding independent of DAP5(T508), HEK293 cells with dox-inducible DAP5(T508E) were pretreated (2 h) with DMSO(−) or trametinib (Fig. 3E). ERK1/2 inhibition virtually blocked DAP5(T508E):eIF2β binding (Fig. 3E). There are a large number of confirmed and putative MEK1/2-ERK1/2-responsive phosphorylation sites in multiple translation initiation factors (24, 26–29). This complexity makes defining the precise MEK1/2-ERK1/2-induced events that promote DAP5:eIF2β association a daunting enterprise, one that is beyond the scope of the present investigation.
Phosphorylation of DAP5(T508) and DAP5:eIF2β binding is induced during mitosis.
Since DAP5(T508) is not a MAPK substrate, we tested whether T508 phosphorylation and DAP5:eIF2β binding respond to CDK1 (Fig. 3A). The specific activity of DAP5 during mitosis has been suggested previously (16). HEK293 cells with dox-inducible Flag-DAP5 expression were treated with dox and synchronized using thymidine block, prior to release into fresh growth media. The cells were arrested in G2, using the CDK1 inhibitor Ro3306 (30) (Fig. 3F), or arrested in mitosis with nocodazole, as confirmed by an increase in p-histone H3 (Fig. 3F). G2 arrest decreased p-DAP5(T508) phosphorylation and DAP5:eIF2β binding; in contrast, mitotic arrest strongly enhanced both events (Fig. 3F). To test whether CDK1 phosphorylates T508, Flag-DAP5 was purified from dox-induced HEK293 cells and incubated with active recombinant JNK2 or CDK1 in the presence of ATP (Fig. 3G). DAP5(T508) was phosphorylated only upon incubation with CDK1 (Fig. 3G). Collectively, our data indicate that DAP5:eIF2β binding is inducible and strongly context specific (e.g., phorbol ester activation, mitosis), occurs with MEK1/2-ERK1/2 or CDK1 activation, and may correlate with DAP5(T508) phosphorylation.
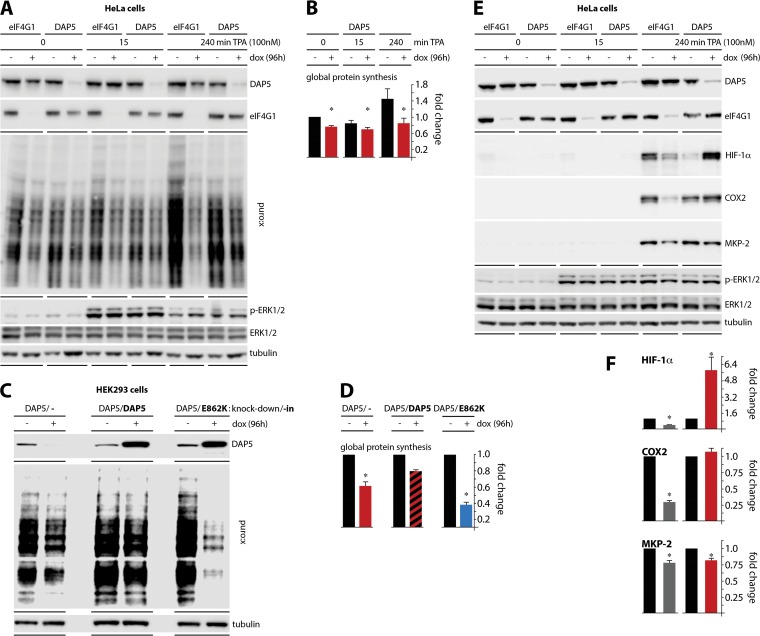
Loss in DAP5:eIF2β association results in decreased global translation.
To assay for DAP5:eIF2β binding's role in translation, we used stable HeLa cell lines with dox-inducible eIF4GI or DAP5 depletion (see Materials and Methods). Treatment with dox (96 h) resulted in similarly robust reduction of eIF4GI or DAP5 levels (Fig. 4A). The cells were stimulated with TPA (15 min, 240 min) and, 15 min prior to harvest, puromycin (PMY) was added. The abundance of puromycylated product was quantified by immunoblotting (Fig. 4B) (31). DAP5 depletion reduced global translation by ∼20% in unstimulated cells and strongly inhibited (∼40% at 4 h) the translation stimulatory effect of TPA (Fig. 4A and B). Confirming the exalted role of eIF4GI in global protein synthesis, ∼90% eIF4GI depletion induced a robust decrease of TPA-induced translation.
FIG 4.
DAP5 depletion attenuates global translation and paradoxically induces HIF-1α. (A) HeLa cell lines with dox-inducible eIF4GI or DAP5 depletion were treated with or without dox (96 h), stimulated with TPA as indicated, and treated with PMY (5 μM; 15 min) prior to harvesting. Puromycylation was stopped by washing cells with cold PBS and the addition of lysis buffer. (B) The results of mean quantification of the PMY/tubulin ratio from three experiments are shown, normalizing between experiments by setting the zero-time-point, no-dox value to 1. (C) HEK293 cells with dox-inducible DAP5 depletion combined with mock (−), wt DAP5, or DAP5(E862K) reconstitution were dox treated (96 h) prior to PMY. (D) Mean quantification of the PMY/tubulin ratio is shown for three experiments, normalizing by setting the no-dox ratio to 1. (E) HeLa cells with dox-inducible eIF4GI or DAP5 depletion were dox treated and TPA stimulated, and their lysates were subjected to immunoblotting as shown. (F) Mean HIF-1α, COX2, and MKP-2/tubulin ratios were quantified from three experiments, normalizing by setting the mock (eIF4GI)-depleted, no-dox ratio to 1. All assays were repeated three times, and a representative series for each panel is shown. Error bars denote the SEM. *, significant P value (P < 0.05).
The pattern of the global protein synthesis response to TPA closely mirrored DAP5:eIF2β binding (Fig. 4A; compare to Fig. 1A to C). To test directly whether DAP5:eIF2β binding determines DAP5's role in global translation, we established HEK293 cells with dox-inducible DAP5 knockdown combined with simultaneous wt DAP5 or DAP5(E862K) knock-in. We previously reported on this approach with dox-inducible (endogenous) eIF4GI knockdown/knock-in of Flag-tagged eIF4GI variants (26). Wild-type DAP5 reconstitution in DAP5-depleted cells rescued protein synthesis, while knock-in of the DAP5(E862K) mutant severely repressed global translation, beyond levels seen with DAP5 depletion alone (Fig. 4C and D). Our observations indicate that DAP5 participates in mediating the adaptive protein synthesis response to PKC-Raf-ERK1/2 signaling and that DAP5:eIF2β binding is required for this function.
DAP5 depletion causes paradoxical induction of HIF-1α.
Since DAP5:eIF2β facilitate a surge of global translation following Raf-ERK1/2 stimulation (Fig. 4A to D), we next tested DAP5's effects on translation of specific, known TPA-responsive templates. Since DAP5's structural arrangement and protein-protein interactions clearly distinguish it from eIF4GI/II, we compared the effect of DAP5 depletion on induction of TPA-responsive mRNAs with a corresponding knockdown of eIF4GI, mirroring our approach with global translation (Fig. 4A). Cells with dox-inducible eIF4GI or DAP5 depletion were treated with dox and TPA and harvested as for the analyses of global protein synthesis (Fig. 4E). Cell lysates were analyzed for translation of the known TPA-inducible proteins HIF-1α (32), cyclooxygenase 2 (COX2) (33), and MAPK phosphatase 2 (MKP-2) (34).
As expected, these proteins were potently induced by TPA (4 h) (Fig. 4E). eIF4GI depletion partially reversed this effect for all three proteins, mirroring its role in coordinating global translation responses to PKC activation. Surprisingly, DAP5 depletion had a strong obverse effect specifically on HIF-1α, producing >5-fold induction (Fig. 4E and F). We also saw a modest increase in COX2 levels upon DAP5 depletion (Fig. 4E and F). This likely is a direct consequence of HIF-1α induction, since COX2 abundance is linked to HIF-1α activity (35). The TPA response of MKP-2 to eIF4GI and DAP5 depletion was comparable (Fig. 4E and F). This unexpected finding suggests a paradoxical relation of DAP5 abundance to HIF-1α levels, indicating a connection between DAP5 and HIF-1α regulation that is inverse to DAP5's effect on global translation.
DAP5 knockdown increases HIF-1α through reduced PHD2 levels.
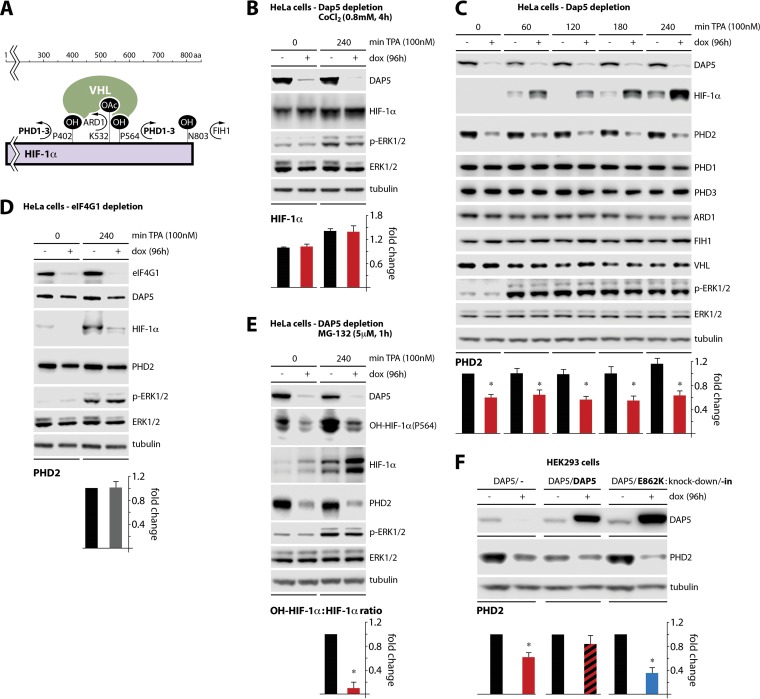
HIF-1α is translated at low levels in normoxia and the protein is highly labile in normal conditions. This is due to a number of posttranslational modifications, such as hydroxylation by the PHDs and FIH1, and acetylation by ARD1, which facilitate HIF-1α binding to the E3 ubiquitin ligase VHL, and leads to HIF-1α ubiquitination and degradation (Fig. 5A) (36). Thus, a likely scenario to explain the effect of DAP5 depletion on TPA-induced HIF-1α was a role for DAP5 in controlling the HIF-1α degradation apparatus. Therefore, to determine whether DAP5 regulates HIF-1α through hydroxylation, the hydroxylase inhibitor CoCl2 was added to DAP5-depleted HeLa cells prior to addition of TPA (Fig. 5B). CoCl2 abolished DAP5 depletion-mediated HIF-1α hyperinduction in TPA-stimulated cells (Fig. 5B; compare to Fig. 4E and F). This suggested that DAP5 may regulate HIF-1α by controlling its hydroxylation levels rather than acetylation or ubiquitination.
FIG 5.
DAP5 depletion induces HIF-1α by depressing PHD2 levels. (A) Schematic overview of posttranslational modifications and interactions of HIF-1α. (B) HeLa cells with dox-inducible DAP5 depletion were dox treated (96 h) and pretreated with CoCl2 (4 h) before TPA stimulation. The cells were harvested for immunoblotting. The mean HIF-1α/tubulin ratio was quantified, normalizing between experiments by setting the no-dox, no-TPA control to 1. (C) HeLa cells with dox-inducible DAP5 depletion were dox treated (96 h) and lysed for immunoblotting with the antibodies shown. The mean PHD2/tubulin ratios were quantified, normalizing between experiments by setting the no-dox, no-TPA time point to 1. (D) HeLa cells with dox-inducible eIF4GI depletion were dox treated as shown and mock or TPA stimulated (240 min). The mean PHD2/tubulin ratio was quantified, normalizing by setting the 240-min TPA, no-dox ratio to 1 for each experiment. (E) The assay described for panel D was repeated with the addition of MG-132 pretreatment (5 μM; 1 h) prior to TPA stimulation. The mean OH-HIF-1α (P564)/HIF-1α ratio was quantified, normalizing by setting the no-dox value to 1. (F) HEK293 cells with dox-inducible knockdown/knock-in as described for Fig. 4C were dox treated as shown and analyzed by immunoblotting. The mean PHD2/tubulin ratios were quantified, normalizing by setting the no-dox values to 1. All data bars represent the means of three independent experiments; error bars display the SEM. *, significant P value (P < 0.05).
To investigate possible effects of DAP5 depletion on HIF-1α hydroxylation factors, HeLa cells with dox-inducible DAP5 depletion were TPA stimulated and tested throughout a multistep time course to monitor PHD1, PHD2, PHD3, ARD1, FIH1, and VHL expression (Fig. 5A and C). The only factor with a detectable expression response to DAP5 depletion was PHD2, the master HIF-1α hydroxylation factor (Fig. 5C); PHD2 abundance did not respond to eIF4GI depletion (Fig. 5D). To test directly whether DAP5 depletion alters HIF-1α hydroxylation levels (via decreased PHD2 activity), HeLa cells with dox-inducible DAP5 depletion were treated with the proteasome inhibitor MG-132 (to stabilize hydroxylated HIF-1α) (Fig. 5E). We probed for hydroxylation of HIF-1α(P564), a principal PHD2 target that destines HIF-1α for rapid ubiquitination and degradation (Fig. 5E; also Fig. 5A). Upon TPA stimulation, DAP5 depletion hyperinduced HIF-1α. Simultaneously, DAP5 depletion decreased the ratio of P564-hydroxylated HIF-1α to total HIF-1α by >80% (Fig. 5E). This effect was accompanied by the suppression of PHD2 levels in DAP5-depleted cells (Fig. 5E).
Reconstitution with wt DAP5 in cells with dox-inducible DAP5 depletion rescued global protein synthesis; meanwhile, reconstitution with the DAP5(E862K) variant, devoid of eIF2β binding, had a negative effect on global translation (Fig. 4C and D). This indicated that DAP5:eIF2β binding is required for DAP5's effect on global translation. We used the same assay to query whether DAP5:eIF2β binding mediates translation of PHD2 (Fig. 5F). The results of this assay mirrored the effects seen with DAP5/DAP5(E862K) reconstitution on global translation, suggesting that PHD2 translation is controlled by the DAP5:eIF2β complex (Fig. 5F). Our data indicate a direct link between DAP5 and PHD2, the master oxygen sensor and coordinator of HIF-1α degradation. PHD2 knockdown alone is capable of stabilizing HIF-1α during normoxia; PHD2 is functionally nonredundant with other HIF1-α regulatory proteins and is also the most abundant of the PHDs (11, 37).
DAP5 regulates translation of PHD2.
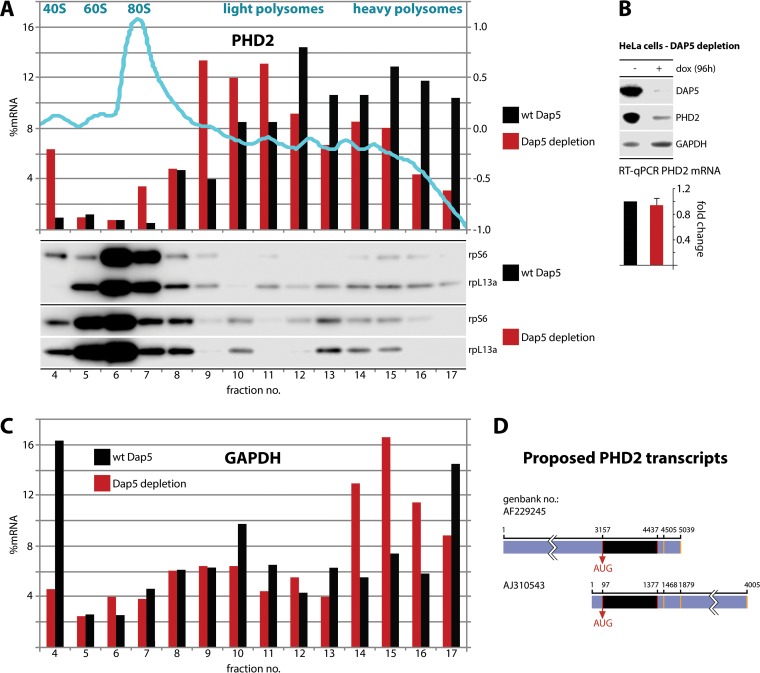
Our research strongly implicated DAP5 in control over PHD2 levels but does not demonstrate an involvement in translation initiation at the PHD2 template. Therefore, we conducted polysome profile analysis to probe for shifts of PHD2 mRNA cosedimentation with polysomal fractions upon DAP5 depletion (Fig. 6). We performed quantitative reverse transcription-PCR (RT-qPCR) to detect PHD2 template in individual polysome fractions and plotted its distribution in the profile (Fig. 6A). DAP5 depletion shifted the distribution of PHD2 RNA in the polysome profile toward 80S ribosomes and light polysomes, suggesting a reduction in PHD2 translation. The distribution of control (GAPDH) mRNA was not similarly affected by DAP5 depletion (Fig. 6C).
FIG 6.
DAP5 is involved in PHD2 translation. (A and C) HeLa cells with dox-inducible DAP5 depletion were dox treated (96 h), and lysates were subjected to polysome profiling. (Top panel) A representative absorbance (260:280) profile of the fractionated gradient is shown in light blue with the peaks identified. Total RNA was purified from the fractions, RT-qPCR analyses for PHD2 (A) and GAPDH (C) mRNA were performed, and the percentages of the PHD2/GAPDH mRNA signal were determined for each fraction. This experiment was repeated twice and yielded similar results in each series; representative profiles are shown. Proteins from each fraction were TCA precipitated and analyzed by immunoblotting. (B) HeLa cells with dox-inducible DAP5 depletion were treated with dox (96 h) or left untreated. Total polyadenylated RNA was isolated from the cells and analyzed by RT-qPCR. The average of two assays with the uninduced controls was normalized to 1, and the SEM values are shown. Representative immunoblots from lysates are shown. (D) Schematic view of the ambiguously annotated 3′ and 5′ UTRs of PHD2. Alternative transcriptional start sites and polyadenylation signals (orange bars) are indicated (see the text for references).
In addition, DAP5 depletion did not reduce total PHD2 mRNA levels (Fig. 6B), further suggesting regulation at the level of translation. Template-specific translation control involves regulatory features in the 5′ and/or 3′ UTRs of select mRNAs. Unfortunately, the 5′ and 3′ UTRs of the PHD2 message are not annotated: (i) distinct proposed 5′ UTRs of >3,000 nucleotides (nt) and <100 nt and (ii) proposed alternative polyadenylation sites in the 3′ UTR were reported (38–40) (Fig. 6D). To investigate DAP5's potential role in template-specific translation regulation of PHD2, extensive further work is required for mapping the definitive transcriptional start site(s), annotate the 5′ UTR(s), and determine the nature and prevalence of alternative 3′ UTRs of the PHD2 message. This study is beyond the scope of this investigation.
DAP5:eIF2β-mediated control of PHD2 modulates AKT phosphorylation.
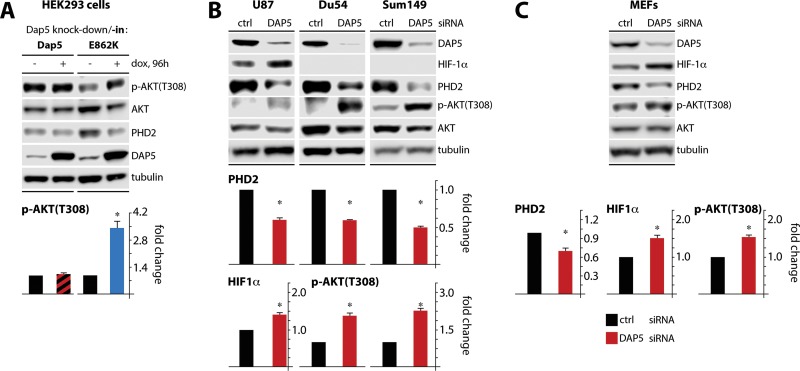
Recently, PHD2 has been shown to hydroxylate AKT, provoking AKT-VHL binding, and block of AKT(T308) phosphorylation (19). Thus, if DAP5 depletion diminished PHD2, it should also, indirectly, depress AKT activation. To explore such a link, we used our empirical system with dox-inducible DAP5 depletion combined with DAP5/DAP(E862K) knock-in (Fig. 4C; Fig. 5F). Knock-in of wt DAP5 in DAP5-depleted cells had no effect on p-AKT(T308), while knock-in of eIF2β-binding defective DAP5(E862K) reduced the abundance of PHD2 and elevated p-AKT(T308) levels (Fig. 7A). These observations confirm claims by Guo et al. (19) about PHD2's involvement in control over AKT activity and reinforce DAP5's role in controlling PHD2 translation. Since our assays so far relied on dox-inducible systems in HeLa or HEK293 cells, we tested the effects of transient DAP5 depletion (upon small interfering RNA [siRNA] transfection) in a range of cancer lines: U87 cells, Du54 malignant glioma cells, and Sum149 breast cancer cells (Fig. 7B) and in mouse embryonic fibroblasts (MEFs) (Fig. 7C). In all four lines, DAP5 depletion diminished PHD2 levels (Fig. 7B and C). Only U87 and MEF cells spontaneously expressed abundant HIF-1α; DAP5 depletion in these cells mediated an increase in HIF-1α levels (Fig. 7B and C). All four lines exhibited increased p-AKT(T308) levels upon DAP5 depletion (Fig. 7B and C). Collectively, our findings suggest that DAP5 controls baseline PHD2 abundance in diverse malignant cell types and in MEFs.
FIG 7.
DAP5 modulates AKT(T308) phosphorylation. (A) HEK293 cells with dox-inducible (endogenous) DAP5 depletion and either wild-type DAP5 or DAP5(E862K) reconstitution were treated with dox as shown (96 h). Lysates were subjected to immunoblotting with the indicated antibodies; the results of a representative test are shown. The p-AKT(T308)/AKT signal ratio from three independent series was quantified. The uninduced controls were normalized to 1. (B and C) Two malignant glioma cell lines (Du54 and U87) and one breast cancer cell line (Sum149) (B), as well as MEFs (C), were transiently transfected with siRNA targeting DAP5. Lysates were analyzed by immunoblotting. Mean HIF-1α/tubulin, PHD2/tubulin, and/or p-AKT(T308)/AKT1 signal ratios from three independent assays are shown, normalizing the control siRNA sample values to 1; error bars denote the SEM. HIF-1α (Du54 and Sum149 cells) and p-AKT (U87 cells) levels could not be quantified due to insufficient signal. *, significant P value (P < 0.05).
DAP5 controls the response to hypoxia.
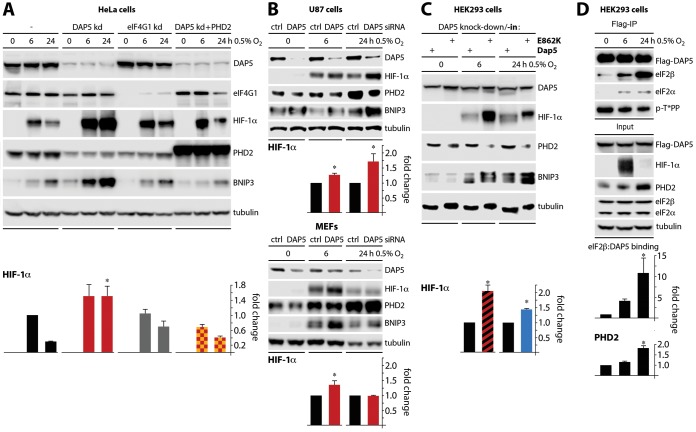
DAP5's influence over PHD2 biosynthesis, and its downstream effects on HIF-1α accumulation or AKT activity, indicate important functions in cellular responses to oxygen deprivation. We performed tests to confirm this in cells where a physiologic response to hypoxia was induced by low-oxygen growth conditions. Unmodified HeLa cells or HeLa cells with (i) dox-inducible depletion of DAP5, (ii) dox-inducible depletion of eIF4GI, or (iii) combined dox-inducible DAP5 depletion/ectopic PHD2 overexpression were cultured in 0.5% oxygen (6 and 24 h) (Fig. 8A). All cell lines responded with HIF-1α induction to 6 h under hypoxia; this response was consistently more pronounced in DAP5-depleted cells (Fig. 8A). More importantly, all cell lines (except the DAP5-depleted cultures) succeeded in containing the hypoxic response by downregulating HIF-1α at 24 h after hypoxia (Fig. 8A). In DAP5-depleted cultures, the hypoxia-induced surge of HIF-1α was inappropriately sustained (Fig. 8A). Ectopic PHD2 compensated for the loss of DAP5 and restored a physiologic pattern of HIF-1α regulation, confirming prior assays implicating DAP5 in control over PHD2 translation (Fig. 8A). As suggested by earlier assays (Fig. 4E), eIF4GI does not appear to be involved in these processes (Fig. 8A).
FIG 8.
DAP5:eIF2β binding is induced during hypoxia and regulates the cellular hypoxia response through PHD2. (A) HeLa cells with dox-inducible DAP5 depletion, eIF4GI depletion, or DAP5 depletion, combined with PHD2 overexpression, were dox treated (96 h) as shown and placed in a chamber containing 0.5% O2 for 6 or 24 h. Lysates were prepared and analyzed by immunoblotting. The mean HIF-1α/tubulin signal was quantified, normalizing between experiments by setting the (mock-depleted) HeLa cell 6-h value to 1. (B) U87 (top panels) and MEF (bottom panels) cells were transfected with DAP5 or control siRNA and cultured in 0.5% O2. The mean HIF-1α/tubulin signal was quantified, normalizing between experiments by setting control siRNA samples to 1. (C) The HEK293 knockdown/knock-in cells described for Fig. 4C and 5F were treated with dox as shown and placed in 0.5% O2. The mean HIF-1α/tubulin ratios were determined, normalizing by setting DAP5 depletion/DAP5 reconstitution samples to 1. (D) HEK293 cells with dox-inducible Flag-DAP5 expression were treated with dox (16 h); lysates were prepared and analyzed by immunoblotting or anti-Flag immunoprecipitation/immunoblotting as shown. The mean eIF2β/Flag ratio from immunoprecipitations and the mean PHD2/tubulin ratio from the input signal were quantified, normalizing by setting 0-h samples to 1. All data bars represent the averages from three independent tests; error bars indicate the SEM. *, significant P value (P < 0.05).
We probed for a well-known transcriptional target of HIF-1α, BNIP3, to assess whether the HIF-1α response in our assays is functionally relevant. This revealed BNIP3 accumulation in step with HIF-1α levels (Fig. 8A). Prolonged BNIP3 induction during hypoxia leads to cell death (41, 42). Thus, DAP5 depletion not only exaggerated acute HIF-1α induction but also intercepted physiologic means of ceasing this response (Fig. 8A). This corresponded to the known role of PHD2 in controlling HIF-1α levels. Similar effects on HIF-1α dynamics were observed upon transient DAP5 depletion from U87 malignant glioma cells (Fig. 8B, top panels).
The response of (nonmalignant) MEFs to DAP5 depletion diverged from U87 malignant glioma cells at 24 h under hypoxia (Fig. 8B, bottom panels). This indicates that coopting DAP5 for containing damage from the hypoxic response may be a function of malignancy.
DAP5:eIF2β binding controls DAP5 activity during hypoxia.
Our investigations reported above strongly implicated inducible eIF2β binding in DAP5's role in translation control, e.g., of the PHD2 message. To analyze the role of DAP5:eIF2β in the hypoxic response, HEK293 cells with dox-inducible depletion combined with wt DAP5/DAP5(E862K) reconstitution were exposed to oxygen deprivation, as shown in Fig. 8A and B.
Reconstitution with DAP5(E862K) led to sustained, inappropriate induction of HIF-1α and BNIP3 at 6 and 24 h in 0.5% oxygen relative to wt DAP5 reconstituted cells (Fig. 8C). This effect correlated with reduced PHD2 levels (Fig. 8C). Lastly, probing for DAP5 in the physiologic response to oxygen deprivation in HEK293 cells with dox-inducible Flag-DAP5 revealed key insight into the DAP5:PHD2:HIF-1α axis. Hypoxia induced DAP5:eIF2β binding and enhanced PHD2 levels (Fig. 8D). This suggests that hypoxia spurs DAP5-dependent protein synthesis, since conditions that increase binding to eIF2β corresponded with increased translational activity of DAP5 (Fig. 4). Tests for p-DAP5(T508) with the anti p-T*PP motif antibody showed no response to hypoxia (Fig. 8D), corroborating our data shown in Fig. 3C.
DISCUSSION
In mammals, translation initiation requires a bridge from mRNA to eIF3/40S ribosomal subunits that, according to current knowledge, can only be provided by any of the three eIF4G isoforms (43). Considering the dominant role of protein synthesis control in gene expression adjustment to sudden physiologic challenges, e.g., hypoxia (44), it is intuitively obvious that this critical node is subject to tight regulation. Our work focused on the eIF4G isoform DAP5, which is unique in lacking eIF4GI/II's N terminus (binding to eIF4E/PABP) and being capable of binding eIF2β. We determined (i) that DAP5:eIF2β association is strongly inducible and occurs in specific biological programs with profound gene expression shifts, (ii) that DAP5:eIF2β binding favors translation activity of DAP5, and (iii) that DAP5 is a key factor in the hypoxic response by controlling translation of the oxygen sensor and HIF-1α destabilizer, PHD2.
We used phorbol ester-mediated PKC-Raf-ERK1/2 stimulation to decipher principles of DAP5 function. However, DAP5:eIF2β binding occurs in diverse circumstances not associated with active ERK1/2, e.g., hypoxia or mitosis. Such convergence is common in translation control due to an exceedingly broad biological repertoire carried out by a limited number of ubiquitous initiation factors. For example, phosphorylation of eIF4GI(S1232) by ERK1/2 (24) or by CDK1 (26) controls the activity of the eIF4G:4A:4B translation initiation helicase in mitogenic stimulation and in mitosis, respectively.
Mammalian eIF2 does not interact with eIF3 but directly contacts 40S ribosomal subunits (45). Thus, DAP5 bound to eIF2 may have unique contacts with 40S subunits (eIF4GI/II bridge 40S subunits exclusively via eIF3). eIF4GI/II only indirectly assemble with the ternary complex (eIF2–GTP–Met-tRNAiMet, via eIF3/40S subunits); DAP5 can bind either to eIF2 or to the ternary complex directly. The DAP5:eIF2β complex and its unique range of contacts in the translation apparatus may offer noncanonical means of initiation complex assembly and function that are critical in times of global protein synthesis adaptation (e.g., hypoxia or mitosis). DAP5:eIF2β binding occurs in concert with other dynamic interactions, e.g., with mRNA, eIF3 and eIF4A:4B, involved in the convoluted process of 5′ UTR scanning/unwinding. Also, DAP5's HEAT2 domain, unlike eIF4GI/II's, does not bind eIF4A (Fig. 1D) (12), suggesting an involvement of other, currently unidentified DAP5 interaction partners.
eIF2β binds DAP5 at an AA box that is conserved in other eIF2β binding partners in the translation initiation apparatus, eIF2Bε and eIF5 (46). eIF2β binding to DAP5 could precede association with eIF5, within a processive model of PIC and 48S interactions (14, 47–49). Since eIF2Bε:eIF2β binding occurs outside translation initiation, it is unclear whether DAP5 competes with eIF2Bε for eIF2β binding. Deciphering the precise contributions of DAP5:eIF2β binding to shaping “hypoxic” translation initiation complexes is a major undertaking that requires wide-ranging new studies.
eIF4GI/II can bind mRNAs through eIF4E/the m7G-cap, in a template-nonspecific manner. However, all eIF4G isoforms can recruit ribosomes independently of an m7G-cap, by directly engaging with mRNAs (50–52). The extent of the latter in global protein synthesis, and the factors governing the involvement of eIF4G isoforms at specific templates remain unknown. An involvement of DAP5:eIF2β in translation of PHD2 indicates that the physiologic context of oxygen deprivation and sequence-specific features of the PHD2 message drive template specificity of DAP5. Due to the conflicting and puzzling annotation of the PHD2 5′ and 3′ UTRs (38–40) extensive future work, which would be beyond the scope of the current study, is required to unravel how DAP5:eIF2β specifically targets PHD2 transcripts for translation.
In normoxia, similar basal PHD2:HIF-1α regulation through DAP5 was observed in cancerous or nontransformed cells. However, prolonged hypoxia revealed a survival benefit mediated by engaging DAP5 in malignant cells but not in MEFs. Distinct responses to DAP5 depletion (under hypoxia) of primary MEFs versus malignant glioma cells suggest that DAP5-mediated control of PHD2 may have special significance in cancer. Neoplastic cells may exploit DAP5 for managing chronic oxygen deprivation, possibly contributing to their adaptation to growth/proliferation under hypoxia.
PHD2 levels increase during prolonged periods of low oxygen, possibly because it is a transcriptional target for HIF-1. However, transcription control may not be able to mediate the sudden changes in protein abundance required to contain HIF-1α and the potentially deleterious hypoxia response. Indeed, system-wide analyses suggest that the response to hypoxia is accompanied by far-reaching translation adjustment (44, 53). DAP5:eIF2β-mediated control of PHD2 provides for powerful, rapid regulatory adaptation that does not require new transcription.
MATERIALS AND METHODS
Cell lines, inhibitors, and stimulants.
U87, Du54, and Sum149 lines were gifts from D. Bigner and S. Nair (Duke University). MEFs were a gift from R. Fukunaga (54). Cells were grown in 10% fetal bovine serum (FBS)-containing Dulbecco modified Eagle medium (DMEM; Invitrogen) or, for Sum149, DMEM–F-12 (Lonza). Stable dox-inducible HeLa (50) and HEK293 (21) cell lines were grown in DMEM supplemented with 10% FBS, blasticidin S (5 μg/ml for HeLa cells and 15 μg/ml for HEK293 cells; Sigma-Aldrich), and hygromycin B (100 μg/ml; Invitrogen). HEK293 and HeLa cells expressing a shRNA targeting DAP5 or eIF4G1 were grown with G418 (500 μg/ml; Gibco). Doxycycline (dox; Sigma-Aldrich) was dissolved in water and used at a concentration of 1 μg/ml. 12-O-Tetradecanoylphorbol-13-acetate (TPA; Tocris) was dissolved in dimethyl sulfoxide (DMSO). Inhibitors of MEK (UO126 [Promega]; trametinib [Selleckchem]), p38 (BIRB [Selleckchem]), JNK (JNK VIII [Calbiochem]), CDK1 inhibitor (Ro-3306 [Tocris]), microtubule polymerization (nocodazole [Tocris]), and the proteasome (MG-132 [Tocris]) were dissolved in DMSO and used as described. Cell cycle inhibitor thymidine (Alfa Aesar), hydroxylation inhibitor [Cobalt(III) chloride hexahydrate, CoCl2; Sigma], and protein synthesis inhibitors (puromycin [Sigma-Aldrich]) were dissolved in water and used as described.
Expression plasmids and stable cell lines.
The construction of myc- and Flag-tagged eIF4GI expression plasmids [eIF4GI, eIF4GI(682-1600), and eIF4GI(197-1600)] was described previously (22). The pCI-Flag-DAP5 vector was a generous gift from Martin Holcik (University of Ottawa, Ottawa, Canada); the PHD2 [EGLN1(myc-DDK-tagged) pCMV6-entry] and eIF2β [eIF2S2 (myc-DDK-tagged) pCMV6-entry] vectors were obtained from Origene (catalog no. RC215158 and RC201563, respectively). DAP5, PHD2, and eIF2β were cloned from original vectors into pcDNA5/FRT/TO expression plasmid. The DAP5 truncation variant was generated by PCR amplification of the corresponding fragment. Single amino acid mutations were introduced by overlapping PCR, as described earlier (22). The generation of stable HEK293 and HeLa cell lines with dox-inducible expression of myc/Flag-tagged eIF4GI and HEK293 expressing Flag-tagged DAP5 has been described previously (55). DAP5 depletion/knock-in HeLa and HEK293 cell lines were established similarly to eIF4GI, as described earlier (23, 26). The primers used in this study are listed in Table 1.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| DAP5_1360_Stu_5 | CGAAGGATATGCCACCTCGG |
| BGH_Rev | TAGAAGGCACAGTCGAGG |
| DAP5(E862K)_anti | CTTCTTTAATAATTTCCATGTCATAG |
| DAP5(E862K)_sense | GGAAATTATTAAAGAAGAAGCTTTCTTGGC |
| DAP5(1–790)_Not1_3′ | ATGCGGCCGCTTATTATTCATCGCTGGGTGGGTTTACTTCAC |
| DAP5(S902A)_Not1_3′ | GTGCGGCCGCTTAGTCAGCTTCTTCCTCTGCTTCTTCTTCTTCAGC |
| DAP5(S902D)_Not1_3′ | GTGCGGCCGCTTAGTCAGCTTCTTCCTCATCTTCTTCTTCTTCAG |
| eIF4G1(4250)_Pml_5′ | CCCACGTGTGGCTCTACCTAGC |
| DAP5(T508A)_anti | GAGGTGGTGCTTGAGTGCGTGGTGGTT |
| DAP5(T508A)_sense | CACTCAAGCACCACCTCTGGGACAG |
| DAP5(T508E)_anti | GAGGTGGTTCTTGAGTGCGTGGTGGTT |
| DAP5(T508E)_sense | CACTCAAGAACCACCTCTGGGACA |
| DAP5(3039)_shRNA_5′ | GCCTCGAGATCTGCGGGCCAAAGCCGTAAATTGTGCAAGTGAAGCCACAGATG |
| DAP5(3039)_shRNA_3′ | GCCTCGAGGATCCGCAAGCCAAAGCCTTAAATTGTGCAACATCTGTGGCTTCAC |
| DAP5(3366)_shRNA_5′ | GCCTCGAGATCTGCGGAGCAACACTTAATACTGTAGAAGTGAAGCCACAGATG |
| DAP5(3366)_shRNA_3′ | GCCTCGAGGATCCGCATAGCAACACTGAATACTGTAGAACATCTGTGGCTTCAC |
Antibodies, immunoprecipitation, immunoblots, siRNA transfections, and RT-qPCR.
Antibodies to eIF4A, eIF3a, MNK1, eIF2α, p-eIF2α(S51), rpS6, p-rpS6(240/244), DAP5, eIF4GI, p-ERK1/2(T202/Y204), ERK1/2, CK2α, GAPDH, p-p38(T108/Y182), p38, pT*PP substrate, p-cJun(S73), cJun, p-histone H3(S10), histone H3, p-JNK(T183,Y185), COX2, MKP-2, HIF-1α, PHD2, FIH1, ARD1, VHL, OH-HIF1α(P564), rpL13a, p-Akt(T308), Akt1, and BNIP3 (Cell Signaling Technologies); eIF2β, CK2β, and PHD3 (Novus); Flag, tubulin, and myc (Sigma-Aldrich); puromycin (EMD Millipore); and PHD1 (R&D Systems) were used. Two ∼70% confluent 150-mm dishes were treated as described in the figures and legends, and immunoprecipitations were performed as previously described (24). Immunoblotting was performed as described previously (22). Immunoblot signals were obtained on and quantified using the Li-COR Odyssey FC2 imaging system and Image Studio software. DAP5 depletion from U87, Du54, and Sum149 was performed using siRNAs. For this, cells in six-well plates were transfected with 2 μl of 20 μM DAP5 (GE Dharmacon) or All-Stars control siRNA (Qiagen) using jetPRIME transfection reagent (Polyplus Transfections) according to the manufacturer's protocol. For RT-qPCR analysis, an RNA-to-CT one-step kit (Invitrogen) was used according to the manufacturer's protocol on a 7900 HT TaqMan machine; samples were measured in triplicate, and the average value was used for further calculation. For analysis of PHD2 mRNA levels following DAP5 depletion, data were analyzed by using the 2−ΔΔCT method using GAPDH as a reference. To analyze polysome profile-derived RNA, the 2−CT value of each fraction was divided by the sum of 2−CT values for all samples and multiplied by 100 to determine the percent RNA in each fraction.
In vitro phosphorylation assays and phosphoproteomic analysis.
For in vitro phosphorylation and proteomic analyses, Flag-tagged DAP5 was immunoprecipitated from dox-induced cells and eluted from beads using Flag-peptide (Sigma-Aldrich) in NT-2 buffer (50 mM Tris-HCI [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 0.05% Igepal CA-630) at 4°C (16 h). Flag-affinity beads were removed using 30 μM chromatography columns (Thermo-Fisher), and eluted proteins were concentrated using a 10-kDa cutoff protein concentration column (Millipore). In vitro phosphorylation of recombinant Flag-tagged DAP5 by recombinant JNK2, or CDK1/cyclin A1 (SignalChem) was performed in 15 μl in kinase assay buffer (SignalChem) with 2 mM ATP, 0.2 mM dithiothreitol, and 2× HALT protease and phosphatase inhibitor cocktail (Thermo Scientific). The reaction mixture was incubated at room temperature with 1 or 5 μl of recombinant kinase (0.1 μg/μl; 1 h). Samples were mixed with 5 μl of 4× LDS buffer (Invitrogen) with 2.5% β-mercaptoethanol and heated for 1 min at 95°C to stop the enzymatic reaction, followed by immunoblotting. Fifteen ∼70% confluent 150-mm dishes of cells were treated with TPA as described in the figures and figure legends. Flag IP, followed by Flag elution purification, was performed with final purification and concentration of each sample into 50 mM NH4HCO3 (pH 8.0). Proteomic analyses were carried out at the Duke University Proteomic Core Facility as previously described (26).
Polysome profiling.
Cells in confluent 100-mm3 plates treated with or without dox were harvested and lysed in 1 ml of lysis buffer (50 mM HEPES [pH 7.2], 200 mM potassium acetate, 0.5% NP-40 [Sigma], 1× HALT protease and phosphatase inhibitor, 1× RNaseOUT [Thermo Fisher Scientific], 50 μg/ml cycloheximide, 10 mM MgCl2). Sucrose gradients (15 to 50%) were prepared in lysis buffer without detergent; ∼80% of total lysate (800 μl) was loaded onto the gradients and spun at 35,000 rpm (using an SW41 rotor) for 180 min at 4°C. A Teledyne instrument was used to analyze and create 500-μl fractions. RNA was isolated using TRIzol (Invitrogen), followed by RNeasy column purification (Qiagen). RNA was suspended in 25 μl of RNase-free water and stored at −80°C. Protein was extracted by first diluting a split fraction 1:1 with sterile water and then adding 0.11 volumes of trichloroacetic acid, followed by incubation at 4°C (1 h). Samples were pelleted at 16,000 × g for 25 min at 4°C, and the supernatant was discarded. The pellets were washed in ice-cold acetone, spun again for 10 min, dried, and processed for immunoblotting.
Hypoxia treatment.
Cells were grown in six-well plates or 150-mm dishes (for immunoprecipitation) and treated prior to hypoxia in a chamber (Coy Laboratory Products, Inc.) as described in the figures and figure legends. Cells were then moved into the inner hypoxia chambers which maintained cells at 37°C, 5% CO2, and 0.5% oxygen. The inner chamber is located within a larger chamber with arm-ports that is maintained at 8% oxygen. Once the cells are placed in the inner chambers and the desired oxygen concentration is reached, the inner chamber remained closed, and the cells were not manipulated until lysis. To lyse the cells, the growth media were rapidly removed, and 150 μl of LDS buffer or 800 μl of polysomal lysis buffer (PLB [for immunoprecipitation]; 10 mM HEPES [pH 7.4], 100 mM KCl, 5 mM MgCl2, 0.5% Igepal CA-630 [Sigma], 3 mM dithiothreitol, 1× HALT phosphatase inhibitor cocktail) was added to the cells. The lysates were placed on dry ice.
Statistics.
Statistical analysis was performed using JMP (v.13) software (SAS). All error bars represent the standard errors of the mean (SEM). For multiple group comparisons, all groups from each experimental repeat were compared using analysis of variance (ANOVA). If the ANOVA result was significant (P < 0.05), a post hoc Tukey honestly significant difference test was performed. Paired t tests were used for data comparing only two groups.
ACKNOWLEDGMENTS
We thank Erik Soderblom and the Proteomics and Metabolomics core facility (Duke University) for analyzing the phosphoproteomic assays in this study. We thank Lianxin Hu (University of North Carolina, Chapel Hill, NC) in Qing Zhang's lab for help with setting up experiments in the hypoxia chamber. We also thank Christopher Nicchitta and Alyson Hoffman (Duke University) for access to equipment and technical advice on polysome profiling.
This study was supported by PHS grant CA124756 (M.G.).
REFERENCES
- 1.Wang GL, Jiang BH, Rue EA, Semenza GL. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GL, Semenza GL. 1995. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 3.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. 2005. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 4.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. 2011. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. 2014. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 6.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 7.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. 2001. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 8.Yu F, White SB, Zhao Q, Lee FS. 2001. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A 98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. 2001. Caenorhabditis elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 10.Jiang BH, Semenza GL, Bauer C, Marti HH. 1996. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 271:C1172–C1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 11.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. 2003. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellsolell L, Cho-Park PF, Poulin F, Sonenberg N, Burley SK. 2006. Two structurally atypical HEAT domains in the C-terminal portion of human eIF4G support binding to eIF4A and Mnk1. Structure 14:913–923. doi: 10.1016/j.str.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, McCormick F. 2006. p97/DAP5 is a ribosome-associated factor that facilitates protein synthesis and cell proliferation by modulating the synthesis of cell cycle proteins. EMBO J 25:4008–4019. doi: 10.1038/sj.emboj.7601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano K, Clayton J, Shalev A, Hinnebusch AG. 2000. A multifactor complex of eukaryotic initiation factors, eIF1, eIF2, eIF3, eIF5, and initiator tRNAMet is an important translation initiation intermediate in vivo. Genes Dev 14:2534–2546. doi: 10.1101/gad.831800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanaka S, Zhang XY, Maeda M, Miura K, Wang S, Farese RV Jr, Iwao H, Innerarity TL. 2000. Essential role of NAT1/p97/DAP5 in embryonic differentiation and the retinoic acid pathway. EMBO J 19:5533–5541. doi: 10.1093/emboj/19.20.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marash L, Liberman N, Henis-Korenblit S, Sivan G, Reem E, Elroy-Stein O, Kimchi A. 2008. DAP5 promotes cap-independent translation of Bcl-2 and CDK1 to facilitate cell survival during mitosis. Mol Cell 30:447–459. doi: 10.1016/j.molcel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ. 2008. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol 181:293–307. doi: 10.1083/jcb.200710215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaraj N, Wisniewski JR, Geiger T, Cox J, Kircher M, Kelso J, Paabo S, Mann M. 2011. Deep proteome and transcriptome mapping of a human cancer cell line. Mol Syst Biol 7:548. doi: 10.1038/msb.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J, Chakraborty AA, Liu P, Gan W, Zheng X, Inuzuka H, Wang B, Zhang J, Zhang L, Yuan M, Novak J, Cheng JQ, Toker A, Signoretti S, Zhang Q, Asara JM, Kaelin WG Jr, Wei W. 2016. pVHL suppresses kinase activity of Akt in a proline-hydroxylation-dependent manner. Science 353:929–932. doi: 10.1126/science.aad5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberman N, Gandin V, Svitkin YV, David M, Virgili G, Jaramillo M, Holcik M, Nagar B, Kimchi A, Sonenberg N. 2015. DAP5 associates with eIF2β and eIF4AI to promote internal ribosome entry sit-driven translation. Nucleic Acids Res 43:3764–3775. doi: 10.1093/nar/gkv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shveygert M, Kaiser C, Bradrick SS, Gromeier M. 2010. Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1-eIF4G interaction. Mol Cell Biol 30:5160–5167. doi: 10.1128/MCB.00448-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrikov M, Dobrikova E, Shveygert M, Gromeier M. 2011. Phosphorylation of eukaryotic translation initiation factor 4G1 (eIF4G1) by protein kinase Cα regulates eIF4G1 binding to Mnk1. Mol Cell Biol 31:2947–2959. doi: 10.1128/MCB.05589-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown MC, Gromeier M. 2017. MNK controls mTORC1:substrate association through regulation of TELO2 binding with mTORC1. Cell Rep 18:1444–1457. doi: 10.1016/j.celrep.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobrikov MI, Dobrikova EY, Gromeier M. 2013. Dynamic regulation of the translation initiation helicase complex by mitogenic signal transduction to eukaryotic translation initiation factor 4G. Mol Cell Biol 33:937–946. doi: 10.1128/MCB.01441-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litchfield DW. 2003. Protein kinase CK2: structure, regulation, and role in cellular decisions of life and death. Biochem J 369:1–15. doi: 10.1042/bj20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrikov MI, Shveygert M, Brown MC, Gromeier M. 2014. Mitotic phosphorylation of eukaryotic initiation factor 4G1 (eIF4G1) at Ser1232 by Cdk1:cyclin B inhibits eIF4A helicase complex binding with RNA. Mol Cell Biol 34:439–451. doi: 10.1128/MCB.01046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux PP, Blenis J. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahbazian D, Parsyan A, Petroulakis E, Hershey J, Sonenberg N. 2010. eIF4B controls survival and proliferation and is regulated by proto-oncogenic signaling pathways. Cell Cycle 9:4106–4109. doi: 10.4161/cc.9.20.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahbazian D, Parsyan A, Petroulakis E, Topisirovic I, Martineau Y, Gibbs BF, Svitkin Y, Sonenberg N. 2010. Control of cell survival and proliferation by mammalian eukaryotic initiation factor 4B. Mol Cell Biol 30:1478–1485. doi: 10.1128/MCB.01218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. 2006. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci U S A 103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aviner R, Geiger T, Elroy-Stein O. 2013. Novel proteomic approach (PUNCH-P) reveals cell cycle-specific fluctuations in mRNA translation. Genes Dev 27:1834–1844. doi: 10.1101/gad.219105.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page EL, Robitaille GA, Pouyssegur J, Richard DE. 2002. Induction of hypoxia-inducible factor-1α by transcriptional and translational mechanisms. J Biol Chem 277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 33.Liu XH, Rose DP. 1996. Differential expression and regulation of cyclooxygenase-1 and -2 in two human breast cancer cell lines. Cancer Res 56:5125–5127. [PubMed] [Google Scholar]
- 34.Misra-Press A, Rim CS, Yao H, Roberson MS, Stork PJ. 1995. A novel mitogen-activated protein kinase phosphatase: structure, expression, and regulation. J Biol Chem 270:14587–14596. doi: 10.1074/jbc.270.24.14587. [DOI] [PubMed] [Google Scholar]
- 35.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. 2003. Coordinate activation of HIF-1 and NF-κB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci 44:4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. 2003. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 37.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. 2004. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 38.Dupuy D, Aubert I, Duperat VG, Petit J, Taine L, Stef M, Bloch B, Arveiler B. 2000. Mapping, characterization, and expression analysis of the SM-20 human homologue, c1orf12, and identification of a novel related gene, SCAND2. Genomics 69:348–354. doi: 10.1006/geno.2000.6343. [DOI] [PubMed] [Google Scholar]
- 39.Metzen E, Stiehl DP, Doege K, Marxsen JH, Hellwig-Burgel T, Jelkmann W. 2005. Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln-1) gene: identification of a functional hypoxia-responsive element. Biochem J 387:711–717. doi: 10.1042/BJ20041736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor MS. 2001. Characterization and comparative analysis of the EGLN gene family. Gene 275:125–132. doi: 10.1016/S0378-1119(01)00633-3. [DOI] [PubMed] [Google Scholar]
- 41.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. 2008. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. 2002. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A 99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hentze MW. 1997. eIF4G: a multipurpose ribosome adapter? Science 275:500–501. doi: 10.1126/science.275.5299.500. [DOI] [PubMed] [Google Scholar]
- 44.Ho JJD, Wang M, Audas TE, Kwon D, Carlsson SK, Timpano S, Evagelou SL, Brothers S, Gonzalgo ML, Krieger JR, Chen S, Uniacke J, Lee S. 2016. Systemic reprogramming of translation efficiencies on oxygen stimulus. Cell Rep 14:1293–1300. doi: 10.1016/j.celrep.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonetti A, Brito Querido J, Myasnikov AG, Mancera-Martinez E, Renaud A, Kuhn L, Hashem Y. 2016. eIF3 peripheral subunits rearrangement after mRNA binding and start-codon recognition. Mol Cell 63:206–217. doi: 10.1016/j.molcel.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 46.Asano K, Krishnamoorthy T, Phan L, Pavitt GD, Hinnebusch AG. 1999. Conserved bipartite motifs in yeast eIF5 and eIF2Bε, GTPase-activating and GDP-GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J 18:1673–1688. doi: 10.1093/emboj/18.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alone PV, Dever TE. 2006. Direct binding of translation initiation factor eIF2γ-G domain to its GTPase-activating and GDP-GTP exchange factors eIF5 and eIF2Bε. J Biol Chem 281:12636–12644. doi: 10.1074/jbc.M511700200. [DOI] [PubMed] [Google Scholar]
- 48.Luna RE, Arthanari H, Hiraishi H, Nanda J, Martin-Marcos P, Markus MA, Akabayov B, Milbradt AG, Luna LE, Seo HC, Hyberts SG, Fahmy A, Reibarkh M, Miles D, Hagner PR, O'Day EM, Yi T, Marintchev A, Hinnebusch AG, Lorsch JR, Asano K, Wagner G. 2012. The C-terminal domain of eukaryotic initiation factor 5 promotes start codon recognition by its dynamic interplay with eIF1 and eIF2β. Cell Rep 1:689–702. doi: 10.1016/j.celrep.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh CR, Watanabe R, Chowdhury W, Hiraishi H, Murai MJ, Yamamoto Y, Miles D, Ikeda Y, Asano M, Asano K. 2012. Sequential eukaryotic translation initiation factor 5 (eIF5) binding to the charged disordered segments of eIF4G and eIF2β stabilizes the 48S preinitiation complex and promotes its shift to the initiation mode. Mol Cell Biol 32:3978–3989. doi: 10.1128/MCB.00376-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaiser C, Dobrikova EY, Bradrick SS, Shveygert M, Herbert JT, Gromeier M. 2008. Activation of cap-independent translation by variant eukaryotic initiation factor 4G in vivo. RNA 14:2170–2182. doi: 10.1261/rna.1171808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Breyne S, Yu Y, Unbehaun A, Pestova TV, Hellen CU. 2009. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc Natl Acad Sci U S A 106:9197–9202. doi: 10.1073/pnas.0900153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hundsdoerfer P, Thoma C, Hentze MW. 2005. Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc Natl Acad Sci U S A 102:13421–13426. doi: 10.1073/pnas.0506536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. 2006. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. 2004. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol 24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown MC, Bryant JD, Dobrikova EY, Shveygert M, Bradrick SS, Chandramohan V, Bigner DD, Gromeier M. 2014. Induction of viral, 7-methyl-guanosine cap-independent translation and oncolysis by mitogen-activated protein kinase-interacting kinase-mediated effects on the serine/arginine-rich protein kinase. J Virol 88:13135–13148. doi: 10.1128/JVI.01883-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. 2006. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]