ABSTRACT
Environmental exposure to arsenic is linked to adverse health effects, including cancer and diabetes. Pleiotropic cellular effects are observed with arsenic exposure. Previously, we demonstrated that arsenic dysregulated the autophagy pathway at low, environmentally relevant concentrations. Here we show that arsenic blocks autophagy by preventing autophagosome-lysosome fusion. Specifically, arsenic disrupts formation of the STX17-SNAP29-VAMP8 SNARE complex, where SNAP29 mediates vesicle fusion through bridging STX17-containing autophagosomes to VAMP8-bearing lysosomes. Mechanistically, arsenic inhibits SNARE complex formation, at least in part, by enhancing O-GlcNAcylation of SNAP29. Transfection of O-GlcNAcylation-defective, but not wild-type, SNAP29 into clustered regularly interspaced short palindromic repeat (CRISPR)-mediated SNAP29 knockout cells abolishes arsenic-mediated autophagy inhibition. These findings reveal a mechanism by which low levels of arsenic perturb proteostasis through inhibition of SNARE complex formation, providing a possible therapeutic target for disease intervention in the more than 200 million people exposed to unsafe levels of arsenic.
KEYWORDS: autophagy, STX17, SNAP29, VAMP8, arsenic, O-GlcNAc, SNARE complex
INTRODUCTION
More than 200 million people worldwide are exposed to arsenic at levels that cause adverse health effects (1). Chronic exposure to inorganic arsenic, mainly via contaminated drinking water, is associated with the development of skin, lung, and bladder cancer, as well as cardiovascular disease, respiratory disease, kidney disease, and type II diabetes (2–8). While the correlation between high levels of arsenic exposure and increased disease risk has been well established, the pathological role of low levels of arsenic is still a matter of debate, with more studies investigating the effects of environmentally relevant concentrations of arsenic being needed (9).
The level of arsenic contamination in food and drinking water can vary greatly depending on the location and source of exposure. The World Health Organization (WHO)- and Environmental Protection Agency (EPA)-recommended limit of arsenic in drinking water is 10 μg/liter (10 ppb). However, the levels of arsenic contamination in certain areas of the United States, Mexico, Taiwan, China, India, and Bangladesh have been reported to significantly exceed this limit, ranging anywhere from 50 to >1,000 ppb (2, 10–15). Many studies investigating the pathological effects of arsenic utilize concentrations in the range of >10 μM, i.e., ∼750 ppb of sodium arsenite [As(III)], representing a higher end concentration even for the more severely affected areas. To better understand the effects of lower concentrations, our group has utilized As(III) concentrations ranging from 0.5 to 5 μM [37.5 to 375 ppb], representing a more accurate range of what might be found, at least in an environmental context. Testing the cellular pathways affected by arsenic in this range will help clarify relevant molecular mechanisms that underlie the pathogenesis associated with low-level arsenic exposure.
Multiple mechanisms have been described for arsenic mode of action. Many studies have reported increased generation of reactive oxygen and nitrogen species (ROS/RNS) as a result of cellular arsenic metabolism and induction of NADPH oxidase activity (16–19). It has also been reported that arsenic-induced ROS deplete glutathione pools, cause oxidative modifications to lipids and proteins, affect mitochondrial integrity and function, and result in mutations or chromosomal aberrations, which may be exacerbated by the arsenic-mediated inhibition of DNA repair enzymes (20–23). However, a recent publication from our group demonstrates that exposure to acute, low levels of arsenic does not induce ROS and that ROS generation typically occurs only following exposure to higher concentrations (24). Arsenic also causes epigenetic modifications that affect gene expression. Metabolic conversion of arsenic to methylated forms consumes S-adenosylmethionine (SAM), a methyl group donor necessary for DNA and histone methylation (25). Moreover, recent studies have correlated arsenic exposure to alterations in microRNA (miRNA) expression (17, 26).
Another possible mechanism of arsenic-induced dysfunction involves direct interaction of arsenic with sulfhydryl groups in cysteine residues of proteins, thus altering their conformation, function, and protein-protein or protein-DNA interactions (27). At very high (and possibly supraphysiological) concentrations, arsenate can substitute phosphate in molecules such as ATP, affecting metabolism and other energy-dependent cellular processes (28). All of these mechanisms have been proposed as the underlying molecular alterations driving arsenic-related pathologies; however, some of these effects are observed only at high concentrations and, as mentioned above, are thus not sufficient to explain the pathogenesis of populations exposed chronically to low concentrations of arsenic. Previously, our group demonstrated that arsenic at very low concentrations, such as those used in this study, inhibits autophagic flux at the final step of the autophagy-lysosome pathway (29), a key cellular degradation pathway responsible for protein and organelle turnover. However, the detailed molecular mechanism by which arsenic inhibits this step of the autophagy pathway had not been determined.
The three main stages of the autophagy pathway, initiation, elongation, and fusion, are mediated by a number of tightly regulated protein-protein and protein-lipid interactions. Initiation and elongation of the phagophore involve phosphorylation of unc-51- like autophagy activating kinase 1 (ULK1) by upstream activators, such as the mammalian target of rapamycin (mTOR) or AMP-activated protein kinase (AMPK) (30), which then initiates the formation of the beclin-1/phosphoinositide-3-kinase (PI3K) complex and activation of two autophagy-related (ATG) protein-dependent ubiquitin-like conjugation systems, ATG5-12 conjugation mediated by ATG7 and ATG10 and the conjugation of microtubule-associated protein light chain 3 (MAP1LC3A/B) to phosphatidylethanolamine via ATG3 and ATG10 (31). The elongating phagophore then engulfs its cargo to form a double-membrane-bound autophagosome, which fuses with the lysosome. Autophagosome-lysosome fusion is mediated, at least in part, by the interaction between three soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins, syntaxin 17 (STX17), synaptosome-associated protein 29 (SNAP29), and vesicle-associated membrane protein 8 (VAMP8), with SNAP29 linking STX17 in the membrane of the completed autophagosome to VAMP8 in the lysosomal membrane (32). Interestingly, O-GlcNAcylation of SNAP29 has been shown to prevent the interaction of STX17 with VAMP8, preventing autophagosome-lysosome fusion and proper flux through the autophagy pathway (33).
In the present study, we revealed a molecular mechanism by which low levels of arsenic perturb proteostasis. Arsenic blocks autophagosome-lysosome fusion by disrupting formation of the STX17-SNAP29-VAMP8 SNARE protein complex. Furthermore, decreased SNARE complex formation is a result of increased O-GlcNAcylation of SNAP29. These data support a novel mechanism of arsenic-based autophagy inhibition and provide a new subset of possible therapeutic targets that could be utilized for treating arsenic and autophagic dysfunction-promoted diseases.
RESULTS
Arsenic inhibits autophagic flux by blocking autophagosome-lysosome fusion.
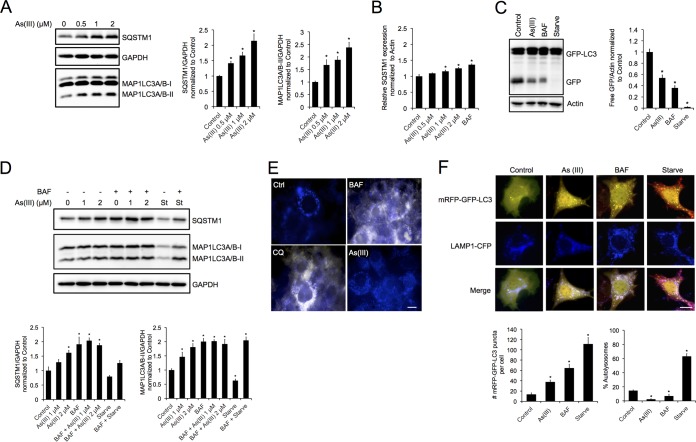
We have shown previously that treatment with sodium arsenite [As(III)] induced autophagic dysfunction, resulting in the accumulation of autophagosomes (29). Furthermore, the accumulation of autophagosomes in response to As(III) is due not to autophagy upregulation but to the blockage of autophagic flux. Bafilomycin A1 (BAF), which blocks autophagic flux by inhibiting vacuolar ATPases, was used as a positive control for blockage of autophagy. Consistent with our previous results, HeLa cells treated with increasing concentrations of As(III) (0 to 2 μM) for 4 h showed a significant increase in the levels of SQSTM1/p62, an autophagy adaptor protein and substrate, as well as MAP1LC3A/B-I and MAP1LC3A/B-II, indicating blockage of autophagic flux (Fig. 1A). Since previous reports have indicated that arsenic can induce SQSTM1 at the transcript level, we measured SQSTM1 mRNA via quantitative real-time PCR (qRT-PCR) (Fig. 1B). Both BAF and As(III) treatments resulted in a slight, but significant, increase in SQSTM1 mRNA levels; however, this mild increase in mRNA is probably not enough to fully account for the up to 2-fold increase observed in the protein level at 4 h.
FIG 1.
Arsenic inhibits autophagic flux by blocking autophagosome-lysosome fusion. (A) HeLa cells were left untreated or treated with 0.5, 1, or 2 μM sodium arsenite [As(III)] for 4 h. Immunoblot analysis of SQSTM1 and MAP1LC3A/B-I and MAP1LC3A/B-II protein levels was performed. (B) HeLa cells were left untreated or treated with 0.5, 1, or 2 μM As(III) or 100 nM bafilomycin A1 (BAF) for 4 h. mRNA levels were measured using quantitative real time-PCR. β-Actin was used as an internal control. (C) iBMKs stably expressing GFP-LC3 were either left untreated or treated with 1× Hanks' balanced salt solution (HBSS; Starve) or 2 μM As(III) for 4 h. Immunoblot analysis of free GFP levels was then performed. (D) NIH 3T3 cells were treated with 1 or 2 μM As(III), HBSS, 100 nM BAF, or As(III) plus BAF or HBSS plus BAF. Immunoblot analysis of MAP1LC3A/B-I and MAP1LC3A/B-II protein levels was performed. Actin and GAPDH were used as internal loading controls. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the control (Student's t test). (E) NIH 3T3 cells were treated with 2 μM As(III), 100 nM BAF, or 40 μM chloroquine (CQ) for 4 h. Following treatment, lysosomal pH was detected using LysoSensor Yellow/Blue DND-160. Blue to yellow/white indicates an increase in lysosomal pH. Scale bar = 10 μm. (F) NIH 3T3 cells were transfected with 1 μg of the mRFP-GFP-LC3 tandem fluorescent reporter and 1 μg of LAMP1-CFP for 24 h and left untreated or treated with 2 μM As(III), HBSS, or 100 nM BAF for 4 h. Cells were subjected to immunofluorescence analysis to detect autophagosomes (yellow), lysosomes (blue), and autolysosomes (pink). mRFP-GFP-positive puncta and percent autolysosomes were determined by quantifying puncta from five cells, and repeating three times. Data are means ± SEMs (n = 5). *, P < 0.05 compared to the value for the control (Student's t test). Scale bar = 10 μm.
To validate that autophagy flux was inhibited by As(III), immortalized baby mouse kidney cells (iBMKs) stably expressing green fluorescent protein (GFP)-LC3 were left untreated, treated with 2 μM As(III) or 100 nM BAF, or starved using 1× Hanks' balanced salt solution (HBSS). HBSS lacks glucose and amino acids, preventing cells from obtaining any nutrients. Following treatment, the release of free GFP was assessed by immunoblot analysis. Free GFP indicates lysosomal protease activity, as the MAP1LC3A/B portion of the fusion protein is sensitive to lysosomal degradation, whereas GFP is more resistant to lysosomal proteases (34). Free GFP in As(III)- and BAF-treated cells was lower than in control cells, indicating a lack of exposure to active lysosomal proteases (Fig. 1C). Interestingly, starvation resulted in total degradation of GFP, which can result from potent activators of autophagy, such as starvation (35). To further confirm that As(III) inhibits autophagy flux at the fusion step, changes in MAP1LC3A/B-I and MAP1LC3A/B-II, as well as SQSTM1, protein levels were measured following As(III) treatment, or HBSS in the presence or absence of BAF, in NIH 3T3 cells. While cells treated with 1 or 2 μM As(III) showed an increase in MAP1LC3A/B-II and SQSTM1, indicating a blockage of autophagic flux, MAP1LC3A/B-II and SQSTM1 levels did not further increase in cells treated with As(III) plus BAF, implying that As(III) and BAF inhibit autophagy at the same step (Fig. 1D).
To determine if As(III) was blocking flux by altering lysosomal pH, NIH 3T3 cells were treated with As(III), BAF, or chloroquine (CQ), which also inhibits flux by increasing lysosomal pH, and then lysosomal acidification was measured using the pH-sensitive probe Lysosensor Yellow/Blue DND-160. Interestingly, while both BAF and CQ treatment increased lysosomal pH (blue puncta to white/yellow puncta), As(III)-treated cells showed blue puncta that were comparable to those for the control (Fig. 1E), indicating that As(III) blocks autophagic flux by a mechanism independent of lysosomal pH alteration. Collectively, these results indicate that As(III) most likely blocks autophagic flux by inhibiting the fusion of the autophagosome with the lysosome.
To test this hypothesis, NIH 3T3 cells were cotransfected with the monomeric red fluorescent protein (mRFP)-GFP-LC3 fluorescent tandem reporter (autophagosome marker, yellow; autolysosome marker, red) and LAMP1-CFP (lysosomal marker, blue) constructs. Starved cells (HBSS treated) showed a significant overlap of red and blue puncta (pink), indicating increased autolysosome formation. Conversely, distinct yellow and blue puncta were observed in As(III)-treated cells, indicating that arsenic treatment prevents autolysosome formation, and light blue/white puncta (indicating overlap of yellow and blue, but no red, puncta) were observed in BAF-treated cells, indicating fusion but improper acidification of the autolysosome (Fig. 1F). Furthermore, the total number of mRFP-GFP-positive puncta increased significantly across groups; however, only starvation resulted in a significant increase in the formation of autolysosomes compared to that of the control (Fig. 1F). Together, these results indicate that although As(III) and BAF are both able to inhibit autolysosomal function, the molecular mechanisms of inhibition are different.
Arsenic disrupts formation of the STX17-SNAP29-VAMP8 SNARE fusion complex.
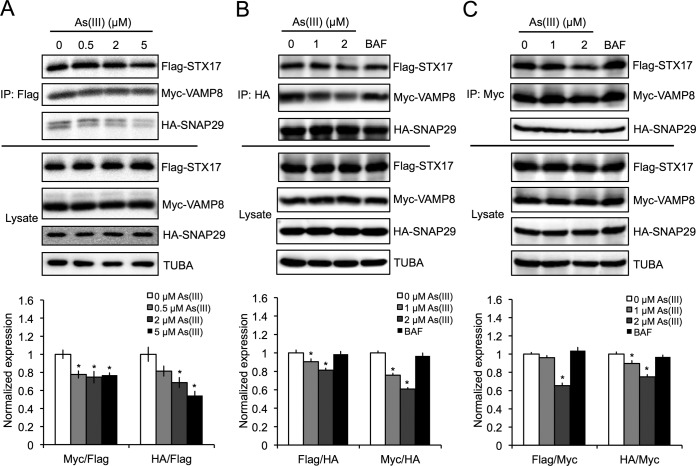
It was reported recently that three key SNARE proteins, STX17, SNAP29, and VAMP8, mediate the fusion of the autophagosome with the lysosome (32). Since arsenic-mediated inhibition of autophagic flux is independent of increased lysosomal pH, indicating blocked autophagosome-lysosome fusion, the effect of As(III) on the formation of the SNARE fusion complex was assessed. HEK293 cells were ectopically cotransfected with Flag-STX17, hemagglutinin (HA)-SNAP29, and myc-VAMP8 and treated with increasing concentrations of As(III). STX17-SNAP29-VAMP8 complex formation was then measured by coimmunoprecipitation analyses. As(III) reduced the amount of HA-SNAP29 and myc-VAMP8 that coimmunoprecipitated with Flag-STX17 in a concentration-dependent manner (Fig. 2A). As(III) also reduced the levels of Flag-STX17 and myc-VAMP8 in HA-SNAP29-immunoprecipitated complexes, as well as HA-SNAP29 and Flag-STX17 in myc-VAMP8-immunoprecipitated complexes, with BAF treatment having no detectable effects in both cases tested (Fig. 2B and C). These results indicate that arsenic-mediated inhibition of autophagosome-lysosome fusion is through decreased SNARE complex formation.
FIG 2.
Arsenic disrupts formation of the STX17-SNAP29-VAMP8 SNARE fusion complex. HEK293 cells were cotransfected with 1 μg of Flag-STX17, HA-SNAP29, and myc-VAMP8 for 24 h and then treated with the indicated concentrations of As(III) or BAF for 4 h. SNARE complexes were coimmunoprecipitated (IP) with an anti-Flag, anti-HA, or anti-myc antibody and immunoblotted for Flag, myc, or HA protein levels. Tubulin (TUBA) was used as an internal loading control. Immunoblot analysis of Flag-STX17 (A), HA-SNAP29 (B), and myc-VAMP8 (C) coimmunoprecipitated complexes was performed.
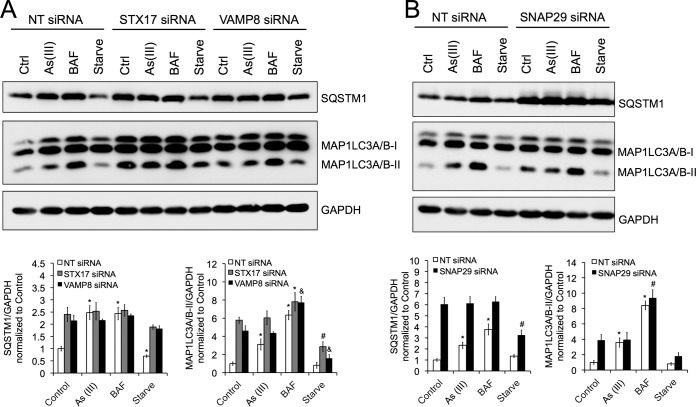
Knockdown of STX17, SNAP29, or VAMP8 mimics arsenic-mediated autophagy inhibition and abolishes arsenic effect.
To further confirm that arsenic blocks autophagosome-lysosome fusion by disrupting SNARE complex formation, the effects of small interfering RNA (siRNA)-mediated knockdown of STX17, SNAP29, or VAMP8 in the absence or presence of arsenic were determined. HeLa cells were transfected with a 5 nM concentration of either a negative control, nontargeted (NT) siRNA, which does not target a protein in the cell, or siRNA against individual genes encoding each SNARE protein. Following knockdown, cells were treated with 2 μM As(III), 100 nM BAF, or HBSS, and SQSTM1 and MAP1LC3A/B-II protein levels were detected by immunoblot analyses to assess autophagy inhibition. As expected, while As(III) and BAF increased the levels of SQSTM1 and MAP1LC3A/B-II in the NT siRNA cells, indicating autophagy inhibition, siRNA knockdowns of STX17, SNAP29, and VAMP8 all resulted in higher levels of SQSTM1 and MAP1LC3A/B-II in the nontreated groups, with As(III) no longer causing an increase in autophagy inhibition compared to the internal control group (Fig. 3). SQSTM1 and MAP1LC3A/B-II levels were also increased in HBSS-treated cells among all three SNARE siRNA-transfected cells compared to the NT siRNA control, indicating that both basal autophagy and starvation-induced autophagy are inhibited in the absence of these SNARE complex proteins. These results demonstrate that arsenic-mediated inhibition of autophagy occurs in an STX17-SNAP29-VAMP8-dependent manner.
FIG 3.
Knockdown of STX17, SNAP29, or VAMP8 mimics arsenic-mediated autophagy inhibition and abolishes arsenic effect. HeLa cells were transfected with 5 nM either nontargeted (NT) siRNA or siRNA against STX17, SNAP29, or VAMP8 for 48 h. Following transfection, cells were either left untreated (control) or treated with 2 μM As(III), 100 nM BAF, or HBSS for 4 h. (A) Immunoblot analysis of SQSTM1 and MAP1LC3A/B-I and MAP1LC3A/B-II levels following siRNA knockdown of STX17 and VAMP8. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the untreated NT siRNA control; #, P < 0.05 compared to the value for the untreated STX17 siRNA control; &, P < 0.05 compared to the value for the untreated VAMP8 siRNA control (Student's t test). (B) Immunoblot analysis of SQSTM1and MAP1LC3A/B-I and MAP1LC3A/B-II levels following siRNA knockdown of SNAP29. GAPDH was used as an internal loading control. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the untreated NT siRNA control; #, P < 0.05 compared to the value for the SNAP29 siRNA control (Student's t test).
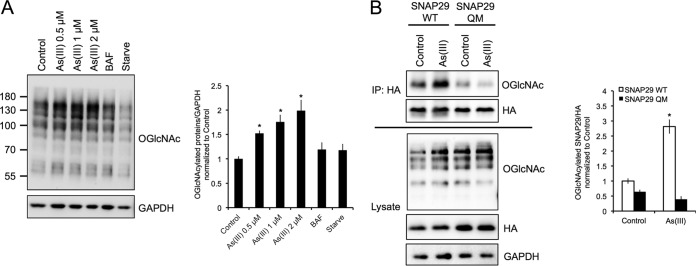
Arsenic promotes the O-GlcNAcylation of SNAP29.
A recent study found that O-GlcNAcylation of four key serine/threonine (Ser/Thr) residues in SNAP29 prevents its interaction with STX17 and VAMP8, resulting in autophagy inhibition (33). Therefore, a mutated SNAP29 (SNAP29-QM) with all four key Ser/Thr residues changed to alanine (S2A, S61G, T130A, and S153G) was generated. First, the effects of As(III) on global protein O-GlcNAcylation levels were investigated. HeLa cells were treated with 0, 0.5, 1, or 2 μM As(III), 100 nM BAF, or HBSS, and protein O-GlcNAcylation was detected using an anti-N-acetylglucosamine antibody. As(III) significantly increased protein O-GlcNAcylation, whereas starvation caused a slight decrease in the level of O-GlcNAcylated proteins (Fig. 4A). Protein O-GlcNAcylation in BAF-treated cells was similar to that in the control group. To determine if the O-GlcNAcylation of SNAP29 was specifically enhanced by As(III) treatment, HEK293 cells were transfected with HA-tagged wild-type SNAP29 (SNAP-WT) or mutated HA-SNAP29 (SNAP29-QM) and treated with 0.5 μM As(III). SNAP29 O-GlcNAcylation was detected by immunoprecipitation/immunoblot analysis. As shown in Fig. 4B, As(III) treatment significantly enhanced the O-GlcNAcylation of SNAP29-WT. Furthermore, mutation of the four key Ser/Thr residues (SNAP29-QM) significantly reduced basal levels of SNAP29 O-GlcNAcylation and abolished the As(III)-mediated increase in SNAP29 O-GlcNAcylation (Fig. 4B). These results indicate that arsenic enhances O-GlcNAcylation of SNAP29, with these four Ser/Thr residues as the major UDP-GlcNAc-accepting sites.
FIG 4.
Arsenic promotes the O-GlcNAcylation of SNAP29. (A) HeLa cells were left untreated or treated with 0.5, 1, or 2 μM As(III), 100 nM BAF, or HBSS for 4 h. Protein O-GlcNAcylation was then assessed via immunoblot analysis of Ser/Thr residue O-GlcNAcylation using the N-acetylglucosamine antibody. GAPDH was used as an internal loading control. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the control (Student's t test). (B) HEK293 cells were transfected with wild-type HA-tagged SNAP29 (WT) or HA-tagged SNAP29 where four key Ser/Thr residues that are O-GlcNAcylated were mutated (SNAP29-QM). Cells were then treated with 0.5 μM As(III) for 4 h and immunoprecipitated using the anti-HA antibody. Equal amounts of HA-SNAP29 were loaded for immunoblot analysis of O-GlcNAcylated SNAP29 using the N-acetylglucosamine antibody. GAPDH was used as an internal loading control. The graph presents the level of O-GlcNAcylated SNAP29 WT versus SNAP29-QM normalized to HA. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the untreated SNAP29 WT control (Student's t test).
Knockdown of OGT abolishes arsenic-mediated autophagy blockage.
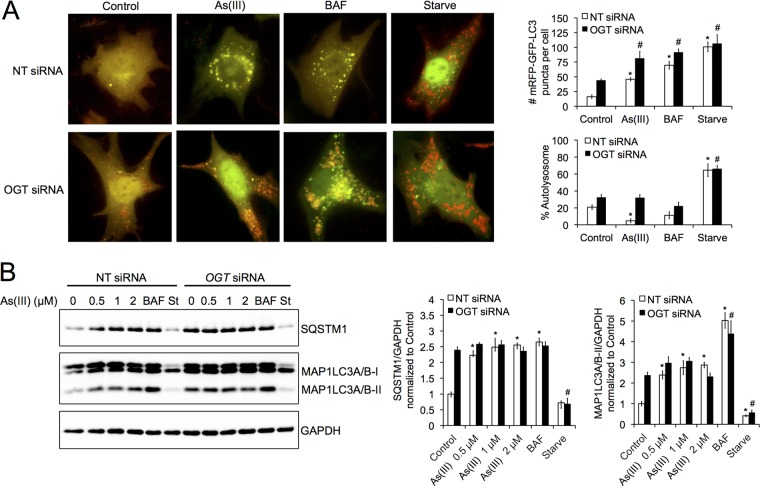
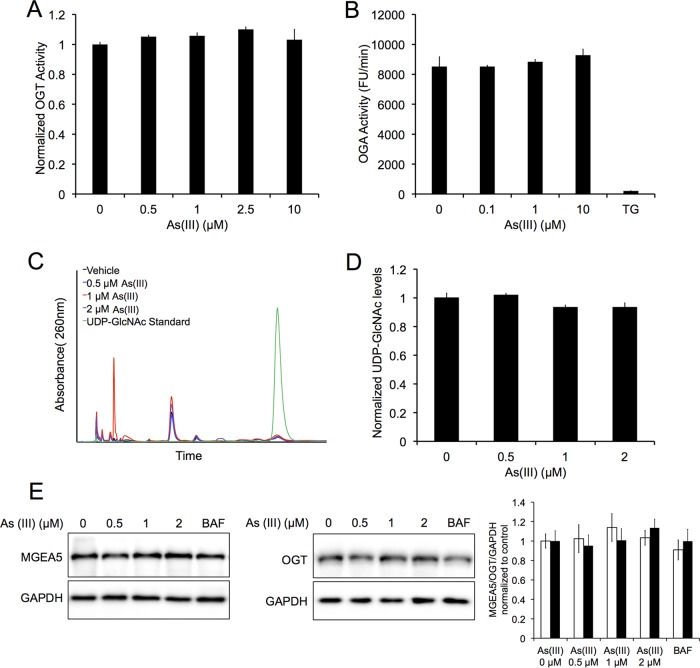
O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) and O-GlcNAcase (MGEA5/OGA) are the enzymes that catalyze the addition or removal of UDP-GlcNac, the substrate utilized for O-GlcNAcylation, to Ser/Thr residues of proteins. Knockdown of OGT in mammalian systems has been shown to prevent SNAP29 O-GlcNAcylation and restore proper autophagic flux (33). To determine if knockdown of OGT could also abolish arsenic-mediated autophagy inhibition, NIH 3T3 cells were transfected with NT or OGT siRNA, and the mRFP-GFP-LC3 fluorescent tandem, followed by treatment with either 0.5 μM As(III), 100 nM BAF, or HBSS, and autophagic flux was assessed. As expected, both As(III) and BAF treatments resulted in autophagy blockage and the accumulation of yellow puncta (autophagosomes), with starved cells showing a significant increase in the number of red puncta (autolysosomes) in the NT siRNA-transfected cells (Fig. 5A). However, cells transfected with OGT siRNA demonstrated both an increased number of mRFP-GFP-positive puncta and autolysosome formation in the control and As(III)-treated groups. OGT siRNA caused a slight increase in autolysosome number in the control and BAF-treated cells but had no obvious effect on starvation-induced autophagosome/autolysosome formation (Fig. 5A). Effects of OGT on arsenic-mediated autophagy blockage were further analyzed using immunoblot analysis (Fig. 5B). In OGT siRNA-transfected cells, As(III) treatment resulted in increased MAP1LC3A/B-II and SQSTM1 protein levels compared to those in the NT siRNA cells (Fig. 5B); however, protein levels of SQSTM1 and MAP1LC3A/B-II did not increase with increasing concentrations of As(III), indicating a higher basal autophagy, with knockdown of OGT abolishing arsenic-mediated autophagy inhibition. Knockdown of OGT also enhanced autophagy in the presence of autophagy blockage with 100 nM BAF, as well as activation with HBSS (Fig. 5B). Interestingly, both the protein level and activity of OGT and MGEA5, as well as the levels of UDP-GlcNAc, were not significantly altered by As(III) treatment (Fig. 6). Collectively, these results indicate that the effects of As(III) on autophagy inhibition are associated with protein O-GlcNAcylation.
FIG 5.
Knockdown of OGT abolishes arsenic-mediated autophagy blockage. (A) NIH 3T3 cells were transfected with a 5 nM concentration of either nontargeted (NT) or OGT siRNA for 48 h and then transfected with 1 μg of the mRFP-GFP-LC3 plasmid for an additional 24 h. Following transfection, cells were treated with 0.5 μM As(III), 100 nM BAF, or HBSS for 4 h and imaged to determine changes in autophagic flux. mRFP-GFP-positive puncta and percent autolysosomes were determined by quantifying puncta from five cells, with repetition three times. Data are means ± SEMs (n = 5). *, P < 0.05 compared to the value for the untreated NT siRNA control; #, P < 0.05 compared to the value for the untreated OGT siRNA control (Student's t test). (B) HeLa cells were transfected with 5 nM NT or OGT siRNA for 48 h and left untreated or treated with 0.5, 1, or 2 μM As(III), 100 nM BAF, or HBSS for 4 h. Protein levels of SQSTM1, MAP1LC3A/B-I, and MAP1LC3A/B-II were then determined by immunoblot analysis. GAPDH was used as an internal loading control. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the untreated NT siRNA control; #, P < 0.05 compared to the value for the untreated OGT siRNA control (Student's t test).
FIG 6.
OGT and OGA protein level and activity, as well as UDP-GlcNAc levels, are not affected by arsenic treatment. (A) Recombinant OGT was exposed to 0, 0.5, 1, 2.5, or 10 μM As(III) for 1 h and OGT activity was assessed based on the levels of released phosphate as indicated by changes in malachite green absorbance at 660 nm. The graph presents results normalized to the control. Data are means ± SEMs (n = 3). (B) Recombinant MGEA5 was exposed to a vehicle or 0.1, 1, or 10 μM As(III), and activity was assessed over a 5-min period by measuring the fluorescence of the fluorogenic substrate 4-methylumbelliferyl N-acetyl-β-d-glucosaminide (4-MU). A 10 μM concentration of thiamet G (TG) was used as a positive control. The graph presents MGEA5 activity (RFU/min) normalized to the control. Data are means ± SEMs (n = 3). (C) HeLa cells were treated with 0, 0.5, 1, or 2 μM As(III) for 4 h. UDP-GlcNAc levels were then measured using high-performance liquid chromatography. The chromatogram indicates UDP-GlcNAc peak following As(III) treatment compared to a diluted UDP-GlcNAc standard. The graph presents UDP-GlcNAc levels normalized to the control. Data are means ± SEMs (n = 3). (D) HeLa cells were treated with 0, 0.5, 1, or 2 μM As(III), 100 nM BAF, or HBSS for 4 h. Immunoblot analysis of OGT or MGEA5 protein levels was conducted following treatment. GAPDH was used as an internal loading control. Data are means ± SEMs (n = 3).
O-GlcNAcylation-defective SNAP29 abolishes arsenic-mediated autophagy inhibition.
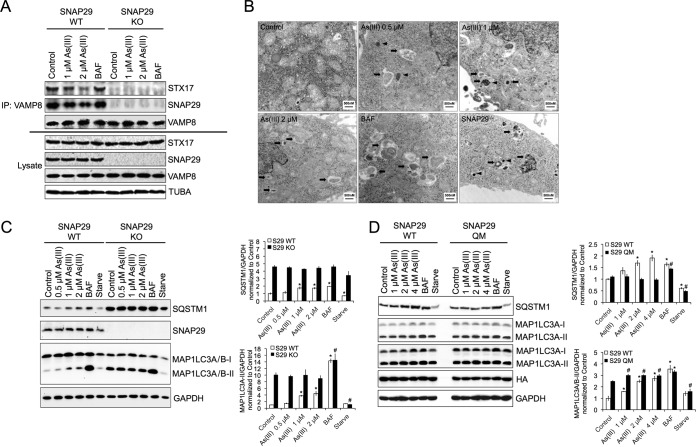
To further confirm the importance of SNAP29 O-GlcNAcylation in arsenic-mediated autophagic dysfunction, a SNAP29 knockout (SNAP29 KO) HeLa cell line was generated using the clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 system. The effect of As(III) on endogenous SNARE complex formation in HeLa wild-type (WT) and KO cell lines was then measured by immunoprecipitation/immunoblot analysis. Treatment with 1 or 2 μM As(III) disrupted the STX17-SNAP29-VAMP8 SNARE complex in a concentration-dependent manner, whereas BAF had no effect. Importantly, knockout of SNAP29 completely abolished the STX17-VAMP8 interaction (Fig. 7A). Interestingly, SNAP29 KO cells exhibit autophagosome accumulation and protein aggregation similar to those in As(III)-treated WT HeLa cells (Fig. 7B). Next, the effects of As(III) on autophagy flux were measured in SNAP29 KO cells. Similar to the case with siRNA-transfected cells (Fig. 3), increased basal levels of SQSTM1 and MAP1LC3A/B-II compared to WT HeLa cells were observed, with As(III) treatment displaying no further autophagy inhibition compared to the nontreated KO cells (Fig. 7C). In contrast, BAF treatment resulted in a further increase of MAP1LC3A/B-II levels in KO cells, implying a different mechanism of inhibition other than disrupting SNARE fusion complex formation. Next, SNAP29 KO cells were transfected with a plasmid containing either HA-tagged SNAP29-WT or the O-GlcNAcylation-defective HA-SNAP29-QM. Transfection of the O-GlcNAcylation-defective SNAP29 mutant into SNAP29 KO cells rescued arsenic-mediated autophagy inhibition, with SQSTM1 protein levels being lower than in As(III)-treated KO cells transfected with wild type SNAP29 and MAP1LC3A/B-I and MAP1LC3A/B-II levels no longer increasing following As(III) treatment (Fig. 7D). It is interesting that MAP1LC3A/B-I and MAP1LC3A/B-II levels were higher in SNAP29-QM transfected cells, possibly indicating a change in basal autophagy. These findings demonstrate that inhibition of SNAP29 O-GlcNAcylation can abolish arsenic-induced autophagy blockage.
FIG 7.
O-GlcNAcylation-defective SNAP29 abolishes arsenic-mediated autophagy inhibition. (A) HeLa or CRISPR-generated SNAP29 knockout HeLa cells were left untreated or treated with 1 or 2 μM As(III) or 100 nM BAF for 4 h. Coimmunoprecipitation was then performed on cell lysates using the anti-VAMP8 antibody, and samples were subjected to immunoblot analysis for STX17, SNAP29, and VAMP8 protein levels. (B) SNAP29 KO or WT KO HeLa cells were left untreated or treated with 0.5, 1, or 2 μM As(III) or 100 nM BAF for 4 h. Following treatment, cells were fixed and imaged using transmission electron microscopy. Scale bars = 500 nm. Arrows indicate autophagosomes. Arrowheads indicate protein aggregates. (C) WT and SNAP29 KO HeLa cells were treated with 0, 0.5, 1, or 2 μM As(III), 100 nM BAF, or HBSS for 4 h. SQSTM1, SNAP29, MAP1LC3A/B-I, and MAP1LC3A/B-II protein levels were then determined via immunoblotting. GAPDH was used as an internal loading control. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the untreated S29 WT control; #, P < 0.05 compared to the value for the untreated SNAP29 KO control (Student's t test). (D) Immunoblot analysis of SQSTM1, MAP1LC3A/B-I, MAP1LC3A/B-II, and HA-SNAP29 in SNAP29 KO HeLa cells transfected with SNAP29-WT or SNAP29-QM for 24 h. Following transfection, cells were left untreated or treated with 1 or 2 μM As(III), 100 nM BAF, or HBSS for 4 h. GAPDH was used as an internal loading control. Data are means ± SEMs (n = 3). *, P < 0.05 compared to the value for the S29 WT control; #, P < 0.05 compared to the value for the untreated SNAP29 QM control (Student's t test).
DISCUSSION
Chronic exposure to inorganic arsenic is associated with an enhanced risk of developing a number of diseases. Integral to developing effective therapeutic strategies for the treatment of diseases associated with arsenic exposure is determining the mechanisms associated with arsenic-induced dysfunction. While a number of studies have investigated the pathological consequences and intracellular events associated with both acute and chronic arsenic exposure, many of these studies utilized concentrations above the exposure levels found in nature. Studies centered on arsenic concentrations in the nanomolar to low micromolar range, which is more comparable to concentrations found in contaminated areas, are important to progressing our understanding of arsenic-linked pathologies. Among the key cellular pathways affected by low-level arsenic is the autophagy lysosome pathway, which our group previously demonstrated is inhibited by treatment with sodium arsenite at low nanomolar concentrations. While the inhibitory effects of arsenic on the autophagy pathway have been clearly demonstrated, the mechanism by which arsenic blocks autophagic flux had not yet been determined.
In this study, we demonstrated that arsenic blocks fusion of the autophagosome with the lysosome (Fig. 1), by inhibiting formation of the STX17-SNAP29-VAMP8 SNARE complex (Fig. 2). This mechanism of autophagy inhibition differs from that of BAF or CQ, which blocks autophagy via disruption of lysosomal pH, as As(III) had no effect on the pH of the lysosome. In this study, we found that As(III) blocks SNARE complex formation via enhancing O-GlcNAcylation of SNAP29.
Arsenic-mediated inhibition of the autophagy pathway is specifically dependent on preventing SNARE complex formation, as siRNA-mediated knockdown of STX17, SNAP29, or VAMP8 abolished the effect of arsenic on autophagy blockage (Fig. 3). Another interesting finding is that arsenic disrupted the interaction of STX17, SNAP29, and VAMP8 by increasing O-GlcNAcylation of four key Ser/Thr residues in SNAP29. It was reported previously that the O-GlcNAcylation of SNAP29 prevented its interaction with STX17 and VAMP8, thus blocking the fusion of the autophagosome with the lysosome (33). Here, we also show that As(III) increased global protein O-GlcNAcylation, including the O-GlcNAcylation of SNAP29 (Fig. 4), with knockdown of OGT, the enzyme that catalyzes the addition of UDP-GlcNAc to proteins, abolishing arsenic-mediated autophagy inhibition (Fig. 5). Intriguingly, OGT knockdown increased both MAP1LC3A/B-II and SQSTM1 levels, contrary to prior reports (6); however, autolysosomes were observed in all OGT siRNA groups, indicating increased autophagic flux (Fig. 5). One possibility is that SQSTM1 transcription is increased to accommodate the increase in overall flux following OGT knockdown. The effect of OGT knockdown on SQSTM1 transcript or protein stability will be investigated in future studies. Generation of a SNAP29 knockout cell line using the CRISPR/Cas9 gene editing system also resulted in autophagy inhibition that could not be further enhanced by treatment with As(III) (Fig. 7). Moreover, replacement of endogenous SNAP29 with the O-GlcNAcylation-defective SNAP29 mutant (S2A, S51G, T130A, and S153G) rescued arsenic-mediated autophagy inhibition (Fig. 7). To our knowledge, these are the first findings that demonstrate a specific mechanism by which arsenic dysregulates the autophagy pathway.
In this study, we have clearly established the importance of decreased SNARE complex formation in arsenic-mediated autophagy inhibition, with the O-GlcNAcylation of SNAP29 playing a key role. However, how arsenic enhances O-GlcNAcylation of SNAP29 has yet to be determined. A number of possibilities exist, including that the altered autophagy that results from introducing an O-GlcNAc-deficient SNAP29 affects overall UDP-GlcNAc levels, or the autophagic turnover of O-GlcNAcylated proteins. It is also possible that the localization of OGT or OGA is somehow altered by arsenite treatment, possibly by affecting AMPK, which has been shown to regulate OGT localization via phosphorylation (36). Arsenic also results in prolonged activation of NRF2 (29), which controls a number of metabolic enzymes, including those in the pentose phosphate pathway (37), and therefore could redirect glucose through alternative pathways, including the hexosamine biosynthesis pathway. Further studies should clarify the exact mechanism by which arsenic affects these key pathways.
In addition, the biochemical data demonstrating that the activities of OGT and MGEA5 were unaffected by sodium arsenite even at very high concentrations (Fig. 6) should be interpreted with caution, since it is highly possible that the active arsenic species in the cell enhancing protein O-GlcNAcylation through modulation of MGEA5/OGT are the metabolites of inorganic arsenic, such as monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). Further in-depth research dissecting the mechanisms by which these two events occur in response to arsenic exposure is warranted. Importantly, while arsenic has been shown to affect a number of key intracellular pathways and targets at higher concentrations, this study identifies key targets of arsenic in the autophagy pathway that are affected at lower levels.
One of the biggest limitations in arsenic-based research is determining relevant intracellular concentrations of arsenic that adequately represent affected populations. Many of the studies indicating enhanced risk of disease following arsenic exposure measure levels of the inorganic and organic forms in the urine, blood, or hair of exposed individuals. Consequently, the intracellular concentrations of arsenic in its many forms are not entirely clear. However, lower concentrations of arsenite, similar to those chosen for this study, should better reflect what the intracellular levels present in individuals chronically exposed to unsafe levels of arsenic might be and therefore represent an important range for studying the pathogenic effects associated with environmental arsenic exposure. Another important task is determining if prolonged autophagic dysfunction is a key driving force of arsenic-based diseases. The role of autophagy and autophagic dysfunction in cancer progression, as well as many other diseases, is well established (38, 39), including SNARE protein deficiency, as SNAP29 mutations lead to cerebral dysgenesis-neuropathy-ichthyosis-keratoderma (CEDNIK) syndrome (40) and loss of VAMP8 results in pancreatic dysfunction (41). However, determining whether chronic inhibition of autophagy is the main cause of or merely enhances arsenic-associated diseases is an important area of future study.
MATERIALS AND METHODS
Chemicals, antibodies, and reagents.
Sodium arsenite (S7400), bafilomycin A1 (B1793), anti-HA–agarose beads (A2095), anti-Flag M2 affinity gel beads (A2220), 4-methylumbelliferyl N-acetyl-β-d-glucosaminide (4-MU; M2133), and primary antibodies against MAP1LC3A/B (L7543), STX17 (SAB1304559), Flag (F3165), OGT (O6264), and MGEA5/OGA (SAB4200267) were purchased from Sigma. The c-myc antibody conjugated to agarose beads (sc-40 A-C) and primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sc-32233), SNAP29 (sc-19370-R), tubulin (TUBA; sc-8035), and myc (sc-40) as well as horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-rabbit [sc-2004] and goat anti-mouse [sc-2005] antibodies) were purchased from Santa Cruz. The HA primary antibody (901502) was obtained from Biolegend. The primary antibody against green fluorescent protein (GFP; 42060) was purchased from GeneTex. The primary antibody against SQSTM1/p62 (89-015-843) was obtained from Abnova. The primary antibody against O-linked N-acetylglucosamine (O-GlcNAc; ab2739) and VAMP8 (ab76021) were purchased from Abcam. Lipofectamine 3000 (L3000075) and Lysosensor Yellow/Blue DND-160 (L7545) were purchased from Thermo Fisher. STX17 (GS55014), SNAP29 (GS9342), VAMP8 (GS8673), and OGT (GS8473) Flexitube siRNAs were purchased from Qiagen. Protein A-agarose beads (15918014) were obtained from Invitrogen. Recombinant Bacteroides thetaiotaomicron O-GlcNAcase (6779-GH-020) and recombinant human O-GlcNAc transferase (8446-GT-010) were purchased from R&D Systems. The OGT peptide substrate (AS-63726) was purchased from AnaSpec. Calf intestinal phosphatase (M0290S) was obtained from New England BioLabs. Mouse NIH 3T3 fibroblasts and human cervical carcinoma (HeLa) and human embryonic kidney (HEK293) cell lines were purchased from the American Type Culture Collection (ATCC). GFP-LC3 immortalized baby mouse kidney cells (iBMKs) were a gift from Eileen White. Dulbecco's modified Eagle's medium (DMEM; MT10014CV) was purchased from Corning. Hanks' balanced salt solution (HBSS; SH30268.01), which contains no glucose or amino acids, was purchased from HyClone. Fetal bovine serum (FBS; S11150H) was purchased from Atlanta Biological. l-Glutamine (25030081) and penicillin-streptomycin (15140722) were obtained from Gibco.
Transfection and live cell fluorescent imaging.
NIH 3T3 cells were seeded in glass bottom 35-mm dishes. Cells were cotransfected with 1 μg of mRFP-GFP-LC3 and 1 μg of LAMP1-CFP using Lipofectamine 3000 according to the manufacturer's instructions. Twenty-four hours later, cells were either left untreated (control) or treated with 0.5, 1, or 2 μM As(III), BAF (100 nM), or HBSS (for starvation) for 4 h. Prior to imaging, cells were gently washed with 1× phosphate-buffered saline (PBS), and DMEM without phenol red was added. Images were taken with a Zeiss Observer.Z1 microscope using the Slidebook 4.2.0.11 software (Intelligent Imaging Innovations, Inc.). The total number of mRFP-GFP-positive puncta and percent autolysosomes were determined using ImageJ. Graphs present quantification of five cells from one experiment, with each group being repeated three times.
Coimmunoprecipitation.
For coimmunoprecipitation of exogenous SNARE proteins, an ∼90% confluent 35-mm dish of HEK293 cells was cotransfected with 1 μg of Flag-tagged STX17, 1 μg of HA-tagged SNAP29, and 1 μg of myc-tagged VAMP8 for 24 h. Cells were then left untreated or treated with 0.5, 1, 2, or 5 μM As(III) or 100 nM BAF for 4 h. Following treatment, cells were washed twice with ice-cold PBS and harvested in radioimmunoprecipitation (RIPA) buffer (50 mM Tris [pH 7.8], 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, and 0.1% SDS) containing 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and a protease inhibitor cocktail. Prior to immunoprecipitation, 20 μl of 2× sample buffer (100 mM Tris-Cl [pH 6.8 to 8], 4% SDS, 20% glycerol, 200 mM DTT, and 0.1% bromophenol blue) was added to 20 μl of cell lysate as an input control. The remaining lysate was then incubated with the appropriate beads and rotated overnight at 4°C. The next day, immunoprecipitated complexes were washed three times with RIPA buffer containing DTT, PMSF, and the protease inhibitor cocktail. Protein was then eluted into 2× sample buffer by boiling the beads for 5 min. Protein samples were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to immunoblot analysis. For endogenous SNARE proteins, 80 to 90% confluent D100 dishes of either HeLa or CRISPR-generated SNAP29 knockout HeLa cells were left untreated (control) or treated with 1 or 2 μM As(III) or 100 nM BAF for 4 h. Following treatment, cells were washed with 1× PBS and harvested in the same RIPA buffer as used for exogenous protein immunoprecipitation. Twenty microliters of 2× sample buffer was added to 20 μl of cell lysate as an input control. The remaining cell lysates were then incubated with protein A-agarose beads bound to 1 μg of anti-VAMP8 antibody and rotated overnight at 4°C. The next day, immunoprecipitated complexes were washed with the same RIPA buffer containing DTT, PMSF, and the protease inhibitor cocktail three times. Protein was then eluted into 1× sample buffer, boiled, and resolved by SDS-PAGE.
Immunoblot analysis:.
To detect protein expression, 3 × 105 cells were seeded in 12-well plates and 24 h later were either left untreated or treated with the desired concentrations of As(III), BAF, or HBSS (for starvation) for 4 h. Following treatment, cells were washed in 1× PBS, harvested in 1× NuPAGE LDS sample buffer with 1× NuPAGE reducing agent (Thermo Fisher Scientific; NP0007) or 1× Laemmli buffer (31.25 mM [Tris-Cl], 1.5% SDS, 5% glycerol, 50× β-mercaptoethanol, and 0.05% bromophenol blue), and boiled for 5 min. Cell lysates were then resolved by SDS-PAGE and subjected to immunoblot analysis with the appropriate antibodies. All immunoblot images were taken using the Bio-Rad Chemidoc system and analyzed using the Bio-Rad Quantity One software v4.6.1.
TEM.
For transmission electron microscopy (TEM), 6 × 105 either WT or SNAP29 KO HeLa cells were seeded in 6-well plates and 24 h later were either left untreated or treated with 0.5, 1, or 2 μM As(III) or 100 nM BAF for 4 h. Cells were then washed 3× with PBS, processed, and imaged.
siRNA-mediated knockdown of SNARE proteins and OGT.
To knock down protein levels of STX17, SNAP29, VAMP8, or OGT, 1 × 105 cells were seeded in 12-well plates and 24 h later transfected with 5 nM nontargeted (NT), STX17, SNAP29, VAMP8, or OGT siRNA using Qiagen Hiperfect according to the manufacturer's instructions. Four siRNA constructs against each target of interest were obtained from Qiagen (Flexitube), and the construct that obtained the maximum knockdown was utilized for further study. Following 48 h of transfection, knockdown cells were left untreated or treated with the desired concentrations of As(III), BAF, or HBSS for 4 h and subjected to immunoblot or immunofluorescence analysis.
Mutagenesis of SNAP29.
Mutagenesis PCRs were set up as follows: 1 μl of DNA template (10 to 20 ng/μl), 0.5 μl of forward primer (50 pmol/μl), 0.5 μl of reverse primer (50 pmol/μl), 1 μl of deoxynucleoside triphosphates (dNTPs; 10 mM), 5 μl of 10× PFU buffer, 1 μl of PFU DNA polymerase, and 41 μl of H2O to obtain a 50-μl total volume. PCR products were then generated using the following program: 95°C for 30 s, followed by 25 cycles of 95°C for 30 s, 60°C for 30 s, and 68°C for 10 min. Products were then digested with 1 μl of the restriction enzyme DpnI at 37°C for 1 h. Following digestion, 5 μl of the reaction mixture was transformed into competent DH5α, and the next day, colonies were picked and purified, and successful mutation of the desired amino acid residues was verified via sequencing. The following primers were used to mutate the indicated residues (mutated nucleotides are indicated in bold): for S2A, F (GCCCGAATTCGGATGGCAGCTTACCCTAAAAGC) and R (GTAGCTTTTAGGGTAAGCTGCCATCCGAATTCGG); for S61G, F2 (ACGGCCGCCAGCACCGGCAGGTCCCTGGCCCTCAT) and R2 (ATGAGGGCCAGGGACCTGCCGGTGCTGGCGGCCGTG); for T130A, F3 (ATCCAAACCAGTAGAGGCCCCACCTGAACAGAATGG) and R3 (CTGTTCAGGTG GGGCCTCTACTGGTTTGGATTTG); and for S153G, F4 (GCTATAAGTACAAGGTAAAGAACAGGAAGC) and R4 (TTCGCCTGTTCTTTACCTGTACTTATAGCTTC).
Immunoprecipitation of O-GlcNAcylated SNAP29.
For immunoprecipitation of exogenous wild-type HA-SNAP29 (SNAP29-WT) and the HA-SNAP29 with four Ser/Thr mutations (SNAP29-QM), an ∼90% confluent D35 dish of HEK293 cells was transfected with 1 μg of either SNAP29-WT or SNAP29-QM for 24 h. Cells were then left untreated or treated with 0.5 μM As(III) for 4 h. Following treatment, cells were washed twice with ice-cold PBS and harvested in RIPA buffer. Prior to immunoprecipitation, 20 μl of 2× sample buffer was added to 20 μl of cell lysate as an input control. The remaining lysate was then incubated with anti-HA-agarose beads and rotated overnight at 4°C. The next day, immunoprecipitated complexes were washed three times with RIPA buffer and eluted into 2× sample buffer by boiling the beads for 5 min. Protein samples were resolved by SDS-PAGE and subsequent immunoblotting.
Generation of the HeLa SNAP29 CRISPR knockout cell line.
The CRISPR (clustered regularly interspaced short palindromic repeat)/Cas9 (CRISPR-associated genes) system was used to generate a homozygous SNAP29 knockout (KO) cell line using HeLa cells. Briefly, single guide RNA (sgRNA) oligonucleotides against human SNAP29 were designed, annealed, and ligated into the pSpCas9(BB)-2A-GFP vector. The sgRNA-Cas9 plasmid was then amplified using PCR, and 5 μl of the PCR product was used to transform Stbl4 competent cells. The next day, at least 2 individual colonies were picked, and DNA was purified using the GeneJet plasmid miniprep kit as per the manufacturer's instructions. One microgram of the sgRNA-Cas9 plasmid was then transfected into a 70 to 80% confluent 35-mm dish of HeLa cells using Lipofectamine 3000. The next day, cells were plated at low confluence and allowed to grow until colonies formed from individual cells. Twenty to 30 colonies were then trypsinized and replated in a 24-well plate, with successful knockout of SNAP29 being confirmed by PCR, sequencing, and immunoblot analysis.
Measurement of OGT and MGEA5 activity.
For OGT activity, a truncated (amino acid residues 353 to 1041) recombinant human O-GlcNAc transferase (OGT) was diluted in ATPase buffer (25 mM HEPES [pH 7.4], 10 mM CaCl2, and 10 mM MgCl2) at a concentration of 400 nM. An additional solution of 1.6 mM UDP-GlcNAc, 1 U of calf intestinal phosphatase (CIP), and 0.8 mM OGT substrate peptide was also made in the ATPase buffer. Ten microliters of diluted OGT was then added to wells of a 384-well microplate containing 5 μl of 4 times the desired As(III) concentrations (desired concentrations, 0, 0.5, 1, 2.5, and 10 μM) in ATPase buffer. After 15 min of incubation at room temperature, 5 μl of the substrate solution was added to each well. The mixture was then incubated at 37°C for 45 min, and 50 μl of a malachite green solution was added to each well. Absorbance was then read at 660 nm using a Biotek Synergy 2 microplate reader, and activity was normalized to the vehicle control. For MGEA5 activity, recombinant B. thetaiotaomicron O-GlcNAcase (MGEA5/OGA) was diluted to 2 ng/μl in assay buffer containing 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) and 100 mM NaCl (pH 5.5). Fifty microliters of diluted OGA was then added to 50 μl of 2 mM 4-methylumbelliferyl N-acetyl-β-d-glucosaminide (4-MU), and fluorescence (365 nm/445 nm) was measured over a 5-min period. Specific activity was calculated using the following equation: adjusted Vmax (RFU/minute) × conversion factor (picomoles/RFU)/amount of enzyme (micrograms), where RFU is relative fluorescence units. The conversion factor was derived using a calibration standard of 4-MU.
Determination of UDP-GlcNAc levels.
To measure levels of UDP-GlcNAc following As(III) treatment, sugar nucleotides were extracted from HeLa cells as previously reported (42). Purified sugar nucleotides were then run on an HP 1100 high-performance liquid chromatography system equipped with an Agilent Zorbax C18 column with dimensions of 3 by 150 mm. Using 5 mM tetrabutylammonium sulfate (TBAS)–50 mM ammonium acetate, pH 5.0 (A), and 5 mM TBAS-methanol (B), a gradient was run as follows: 2% B for 15 min, linear gradient to 15% B over 15 min, holding at 15% B for 3 min, and reequilibration at 2% B for 7 min, all with a flow rate of 0.75 ml/min at 30°C. A serial dilution of a UDP-GlcNAc standard was conducted to verify elution time and peak size. After runs, peaks were integrated using MassLynx v4.1 software (Waters) and normalized internally to the largest peak and externally to the untreated or time zero signal.
Statistics.
Results are expressed as means ± standard errors of the means (SEMs) from three independent experiments. Student's t tests were used to determine statistical significance.
ACKNOWLEDGMENTS
We are funded by the following grants from the National Institutes of Health: ES015010 (D.D.Z.), DK109555 (D.D.Z.), ES023758 (E.C. and D.D.Z.), GM008804 (A.J.A.; T32 training grant), and ES006694 (a center grant).
We have no conflicts of interest to disclose.
REFERENCES
- 1.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karagas MR, Gossai A, Pierce B, Ahsan H. 2015. Drinking water arsenic contamination, skin lesions, and malignancies: a systematic review of the global evidence. Curr Environ Health Rep 2:52–68. doi: 10.1007/s40572-014-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmaus C, Ferreccio C, Yuan Y, Acevedo J, Gonzalez F, Perez L, Cortes S, Balmes JR, Liaw J, Smith AH. 2014. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am J Epidemiol 180:1082–1087. doi: 10.1093/aje/kwu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinmaus CM, Ferreccio C, Romo JA, Yuan Y, Cortes S, Marshall G, Moore LE, Balmes JR, Liaw J, Golden T, Smith AH. 2013. Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev 22:623–630. doi: 10.1158/1055-9965.EPI-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng CH, Chong CK, Tseng CP, Hsueh YM, Chiou HY, Tseng CC, Chen CJ. 2003. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett 137:15–21. doi: 10.1016/S0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YY, Huang NC, Chang YT, Sung JM, Shen KH, Tsai CC, Guo HR. 2017. Associations between arsenic in drinking water and the progression of chronic kidney disease: a nationwide study in Taiwan. J Hazard Mater 321:432–439. doi: 10.1016/j.jhazmat.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. 2012. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120:1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. 2008. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 9.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorg TJ, Chen AS, Wang L. 2014. Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Res 48:156–169. doi: 10.1016/j.watres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Mendez MA, Gonzalez-Horta C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Ceron RH, Morales DV, Terrazas FA, Ishida MC, Gutierrez-Torres DS, Saunders RJ, Drobna Z, Fry RC, Buse JB, Loomis D, Garcia-Vargas GG, Del Razo LM, Styblo M. 2016. Chronic exposure to arsenic and markers of cardiometabolic risk: a cross-sectional study in Chihuahua, Mexico. Environ Health Perspect 124:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida T, Yamauchi H, Fan Sun G. 2004. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol 198:243–252. doi: 10.1016/j.taap.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Harvey CF, Swartz CH, Badruzzaman AB, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF. 2002. Arsenic mobility and groundwater extraction in Bangladesh. Science 298:1602–1606. doi: 10.1126/science.1076978. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, Basu GC, Chanda CR, Lodh D, Saha KC, Mukherjee SK, Roy S, Kabir S, Quamruzzaman Q, Chakraborti D. 2000. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect 108:393–397. doi: 10.1289/ehp.00108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buschmann J, Berg M, Stengel C, Winkel L, Sampson ML, Trang PT, Viet PH. 2008. Contamination of drinking water resources in the Mekong delta floodplains: arsenic and other trace metals pose serious health risks to population. Environ Int 34:756–764. doi: 10.1016/j.envint.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M. 2011. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107. [DOI] [PubMed] [Google Scholar]
- 17.Pratheeshkumar P, Son YO, Divya SP, Wang L, Zhang Z, Shi X. 2016. Oncogenic transformation of human lung bronchial epithelial cells induced by arsenic involves ROS-dependent activation of STAT3-miR-21-PDCD4 mechanism. Sci Rep 6:37227. doi: 10.1038/srep37227. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Valko M, Morris H, Cronin MT. 2005. Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 19.Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. 2004. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A 101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prakash C, Soni M, Kumar V. 2016. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: a review. J Appl Toxicol 36:179–188. doi: 10.1002/jat.3256. [DOI] [PubMed] [Google Scholar]
- 21.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. 2011. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrew AS, Burgess JL, Meza MM, Demidenko E, Waugh MG, Hamilton JW, Karagas MR. 2006. Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ Health Perspect 114:1193–1198. doi: 10.1289/ehp.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Chen F. 2016. Oxidative stress, epigenetics, and cancer stem cells in arsenic carcinogenesis and prevention. Curr Pharmacol Rep 2:57–63. doi: 10.1007/s40495-016-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodson M, Rojo de la Vega EM, Harder B, Castro-Portuguez R, Rodrigues SD, Wong PK, Chapman E, Zhang DD. 2018. Low-level arsenic causes proteotoxic stress and not oxidative stress. Toxicol Appl Pharmacol 341:106–113. doi: 10.1016/j.taap.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoen A, Beck B, Sharma R, Dube E. 2004. Arsenic toxicity at low doses: epidemiological and mode of action considerations. Toxicol Appl Pharmacol 198:253–267. doi: 10.1016/j.taap.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Michailidi C, Hayashi M, Datta S, Sen T, Zenner K, Oladeru O, Brait M, Izumchenko E, Baras A, VandenBussche C, Argos M, Bivalacqua TJ, Ahsan H, Hahn NM, Netto GJ, Sidransky D, Hoque MO. 2015. Involvement of epigenetics and EMT-related miRNA in arsenic-induced neoplastic transformation and their potential clinical use. Cancer Prev Res (Phila) 8:208–221. doi: 10.1158/1940-6207.CAPR-14-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen S, Li XF, Cullen WR, Weinfeld M, Le XC. 2013. Arsenic binding to proteins. Chem Rev 113:7769–7792. doi: 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng CH. 2004. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol 197:67–83. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, Zhang DD. 2013. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol 33:2436–2446. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Kundu M, Viollet B, Guan KL. 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. 2010. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 32.Itakura E, Kishi-Itakura C, Mizushima N. 2012. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Guo B, Liang Q, Li L, Hu Z, Wu F, Zhang P, Ma Y, Zhao B, Kovacs AL, Zhang Z, Feng D, Chen S, Zhang H. 2014. O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nat Cell Biol 16:1215–1226. doi: 10.1038/ncb3066. [DOI] [PubMed] [Google Scholar]
- 34.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algul H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, Alonso C, Altan-Bonnet N, Altieri DC, Alvarez S, Alvarez-Erviti L, Alves S, Amadoro G, Amano A, Amantini C, Ambrosio S, Amelio I, Amer AO, Amessou M, Amon A, An Z, et al. . 2016. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX. 2011. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy 7:188–204. doi: 10.4161/auto.7.2.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bullen JW, Balsbaugh JL, Chanda D, Shabanowitz J, Hunt DF, Neumann D, Hart GW. 2014. Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK). J Biol Chem 289:10592–10606. doi: 10.1074/jbc.M113.523068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SB, Sellers BN, DeNicola GM. 2017. The regulation of NRF2 by nutrient-responsive signaling and its role in anabolic cancer metabolism. Antioxid Redox Signal doi: 10.1089/ars.2017.7356. [DOI] [PubMed] [Google Scholar]
- 38.Choi AM, Ryter SW, Levine B. 2013. Autophagy in human health and disease. N Engl J Med 368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 39.White E. 2012. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs-Telem D, Stewart H, Rapaport D, Nousbeck J, Gat A, Gini M, Lugassy Y, Emmert S, Eckl K, Hennies HC, Sarig O, Goldsher D, Meilik B, Ishida-Yamamoto A, Horowitz M, Sprecher E. 2011. CEDNIK syndrome results from loss-of-function mutations in SNAP29. Br J Dermatol 164:610–616. [DOI] [PubMed] [Google Scholar]
- 41.Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. 2004. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev Cell 7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Tomiya N, Ailor E, Lawrence SM, Betenbaugh MJ, Lee YC. 2001. Determination of nucleotides and sugar nucleotides involved in protein glycosylation by high-performance anion-exchange chromatography: sugar nucleotide contents in cultured insect cells and mammalian cells. Anal Biochem 293:129–137. doi: 10.1006/abio.2001.5091. [DOI] [PubMed] [Google Scholar]